Environmentally Friendly Melt-Processed Chitosan/Starch Composites Modified with PVA and Lignin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
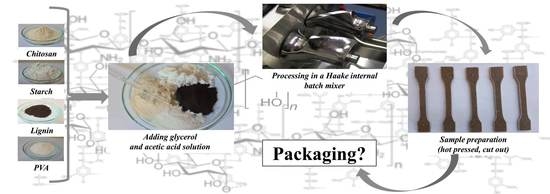
2.2. Sample Preparation
2.3. Moisture Content (MC), Swelling Degree (SD), and Total Soluble Matter (TSM)
2.4. Mechanical Properties
2.5. Scanning Electron Microscopy (SEM)
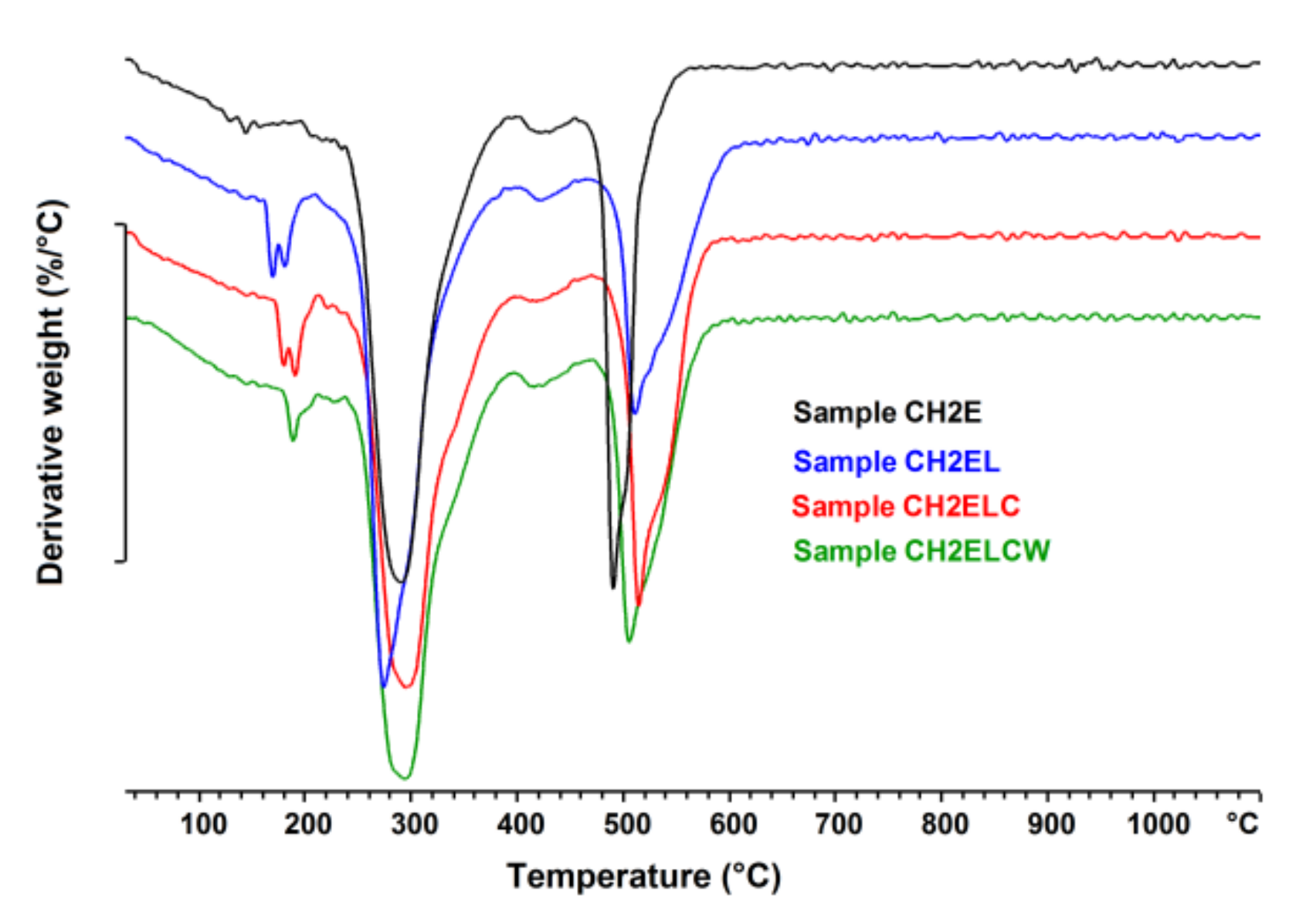
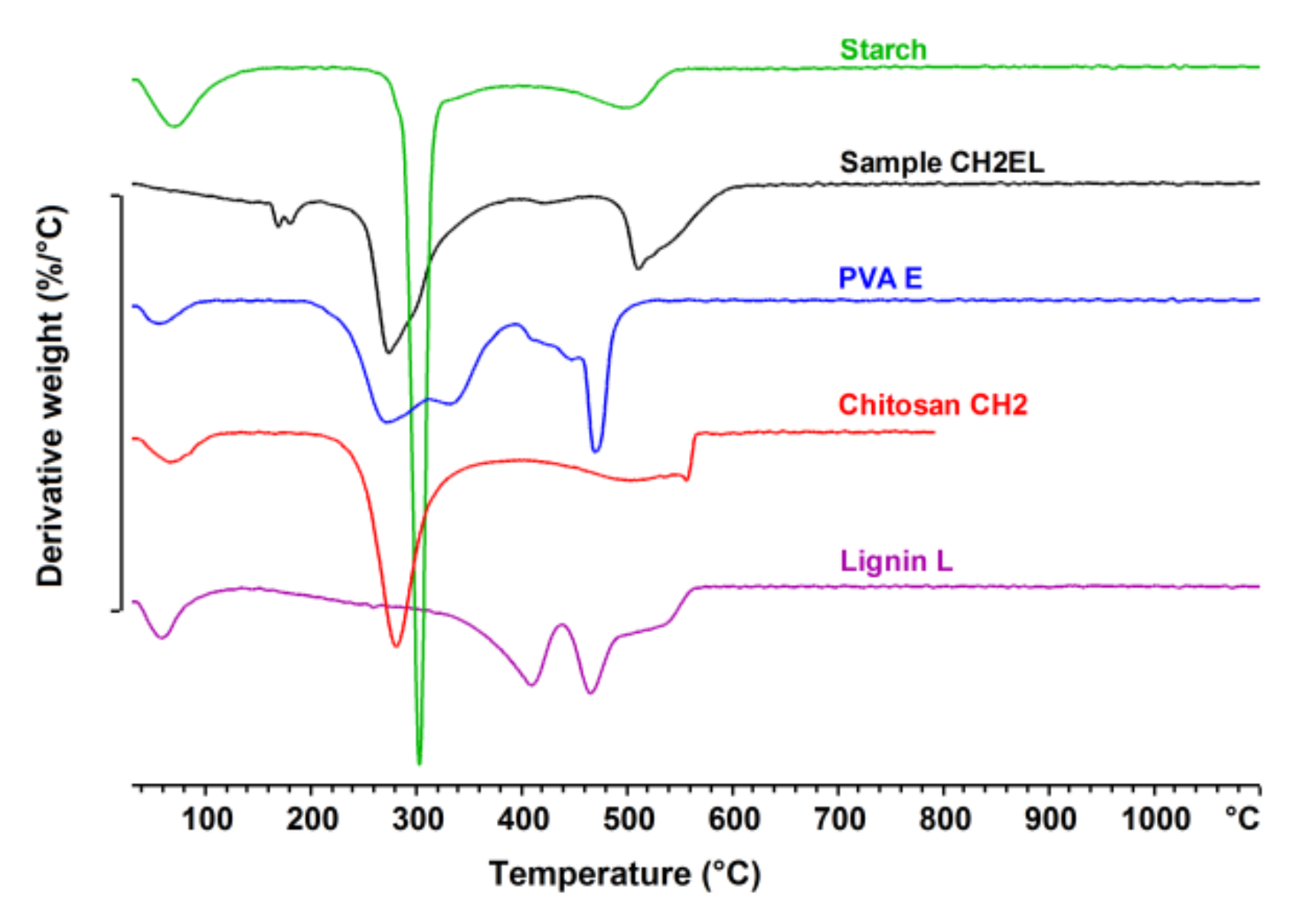
2.6. Thermogravimetric Analysis (TGA)
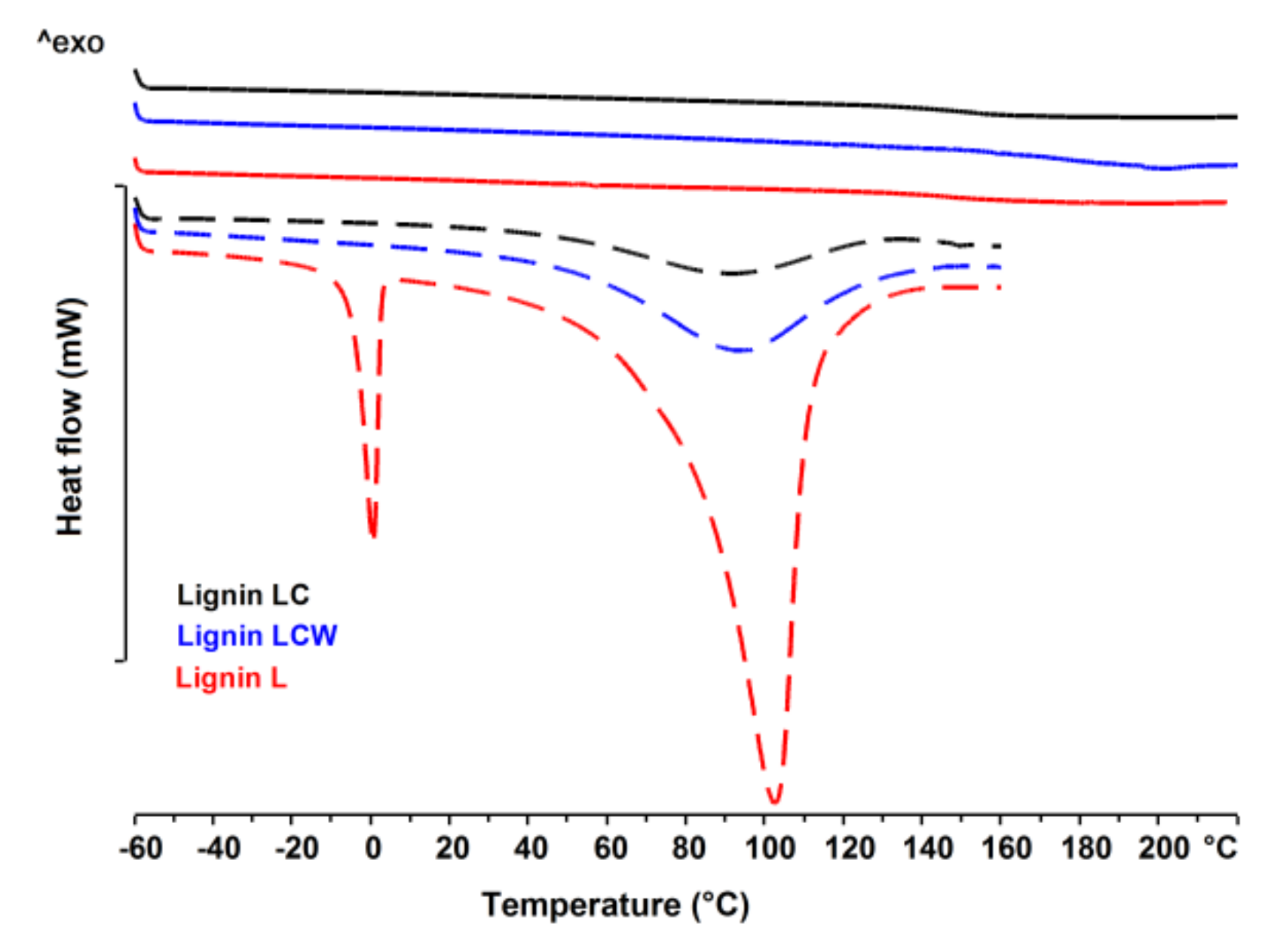
2.7. Differential Scanning Calorimetry (DSC)
3. Results
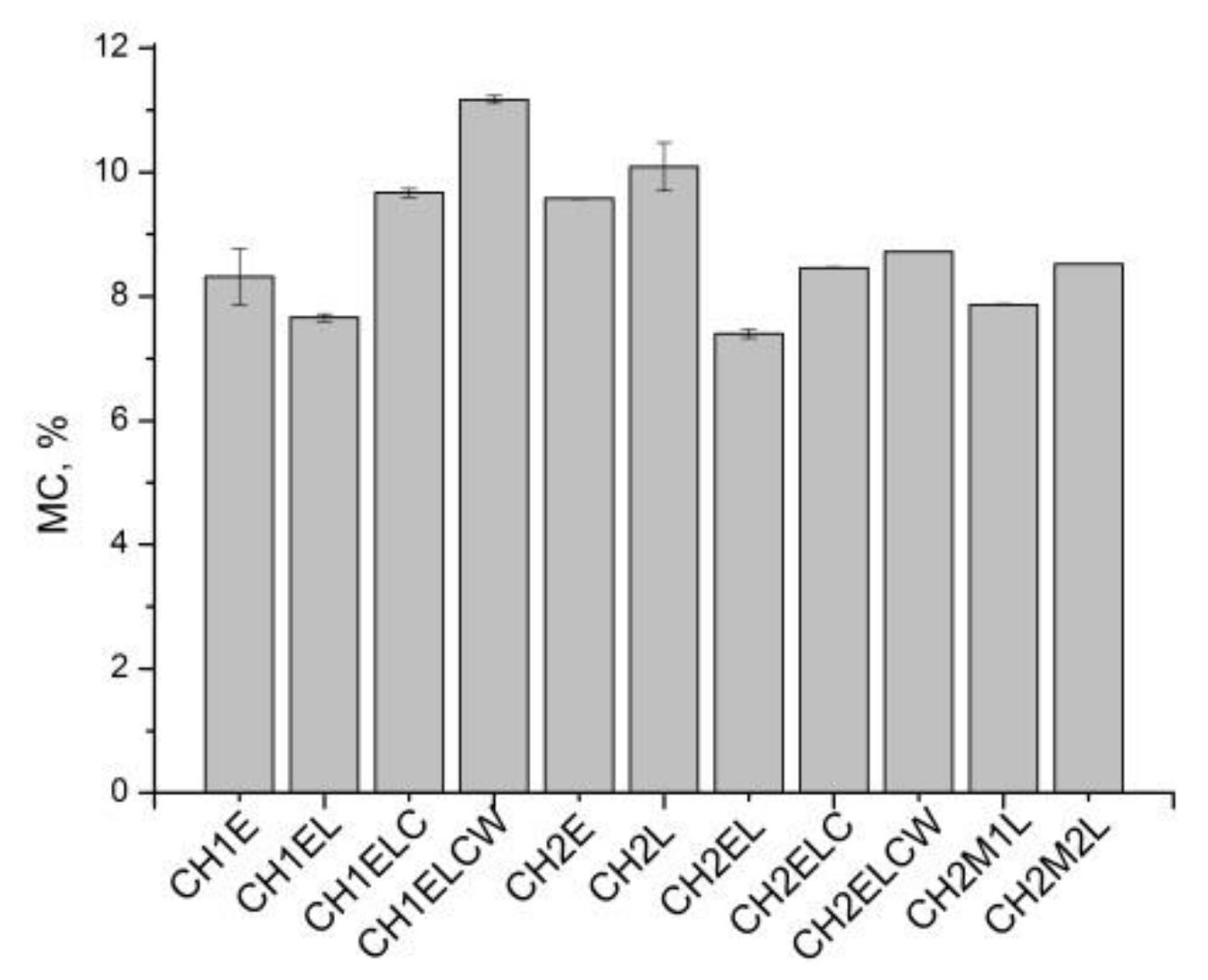
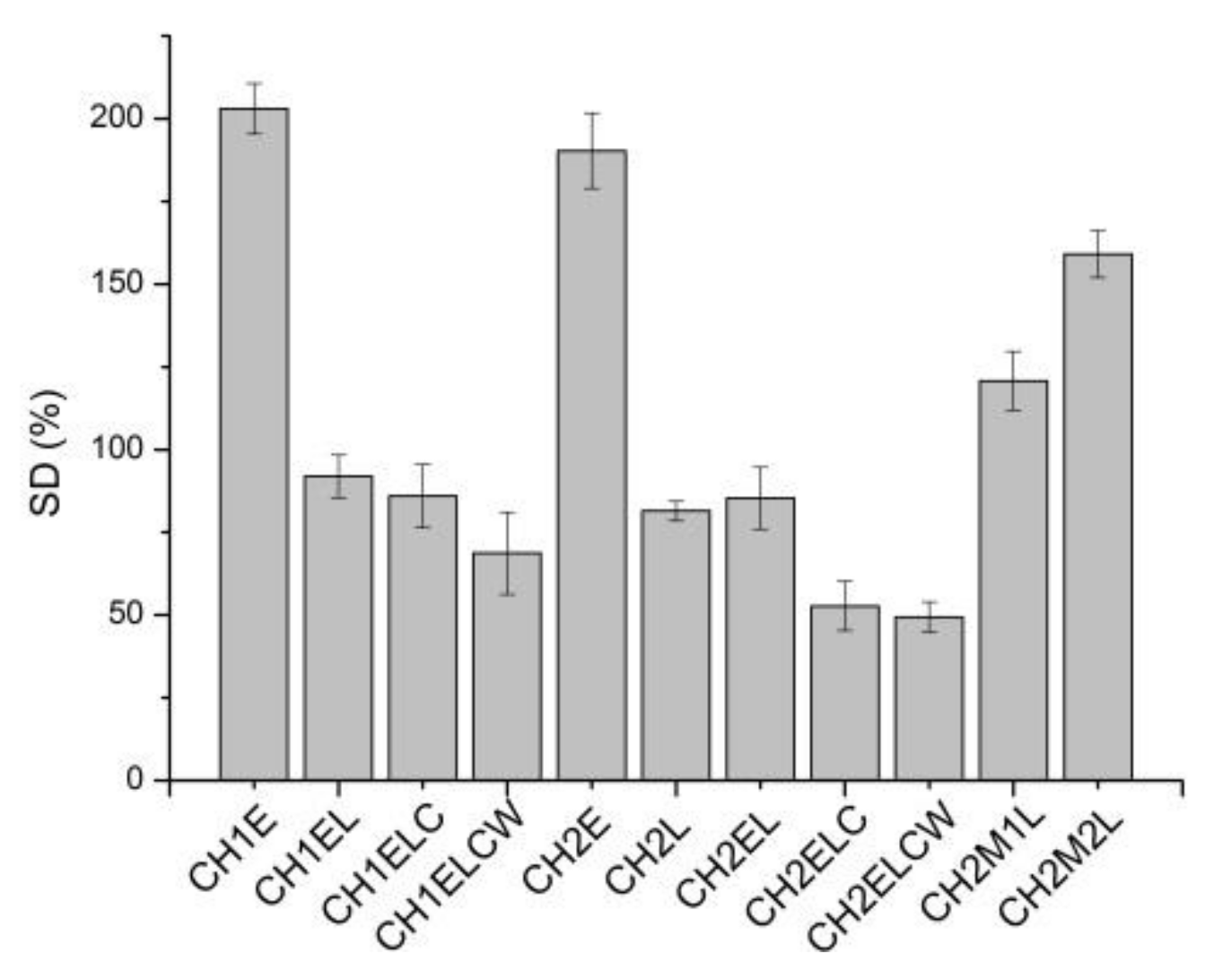
3.1. Moisture Content, Swelling Degree, and Total Soluble Matter
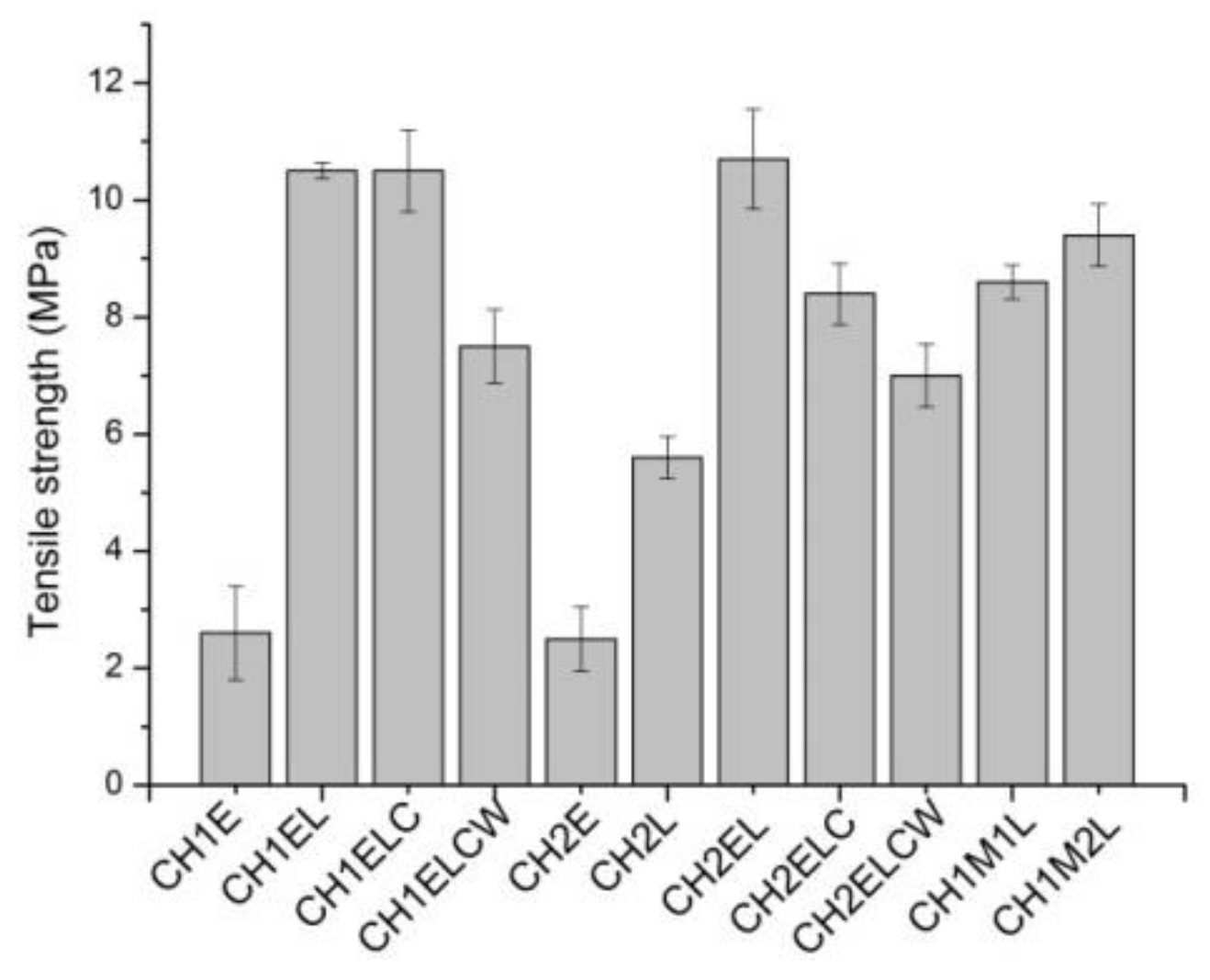
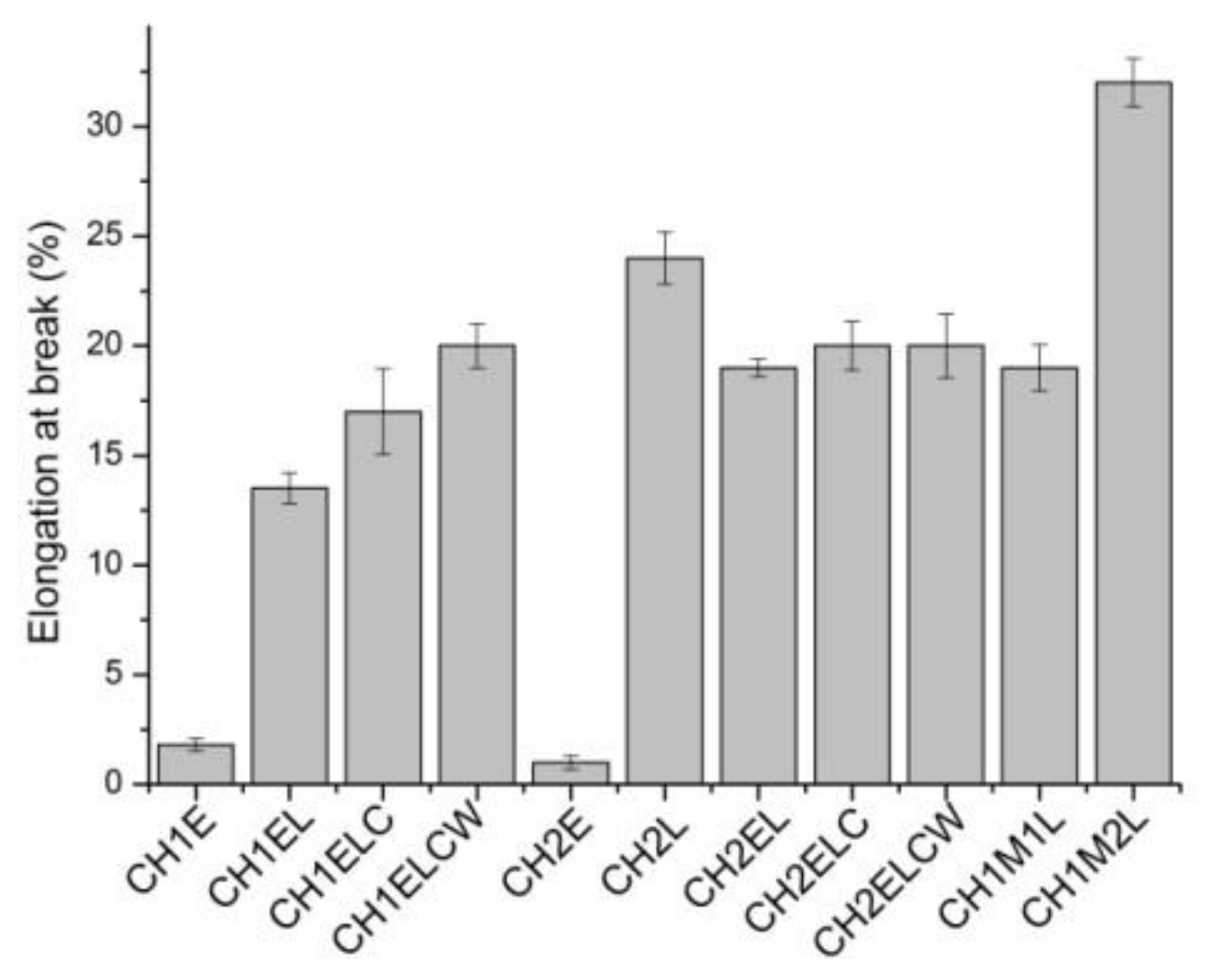
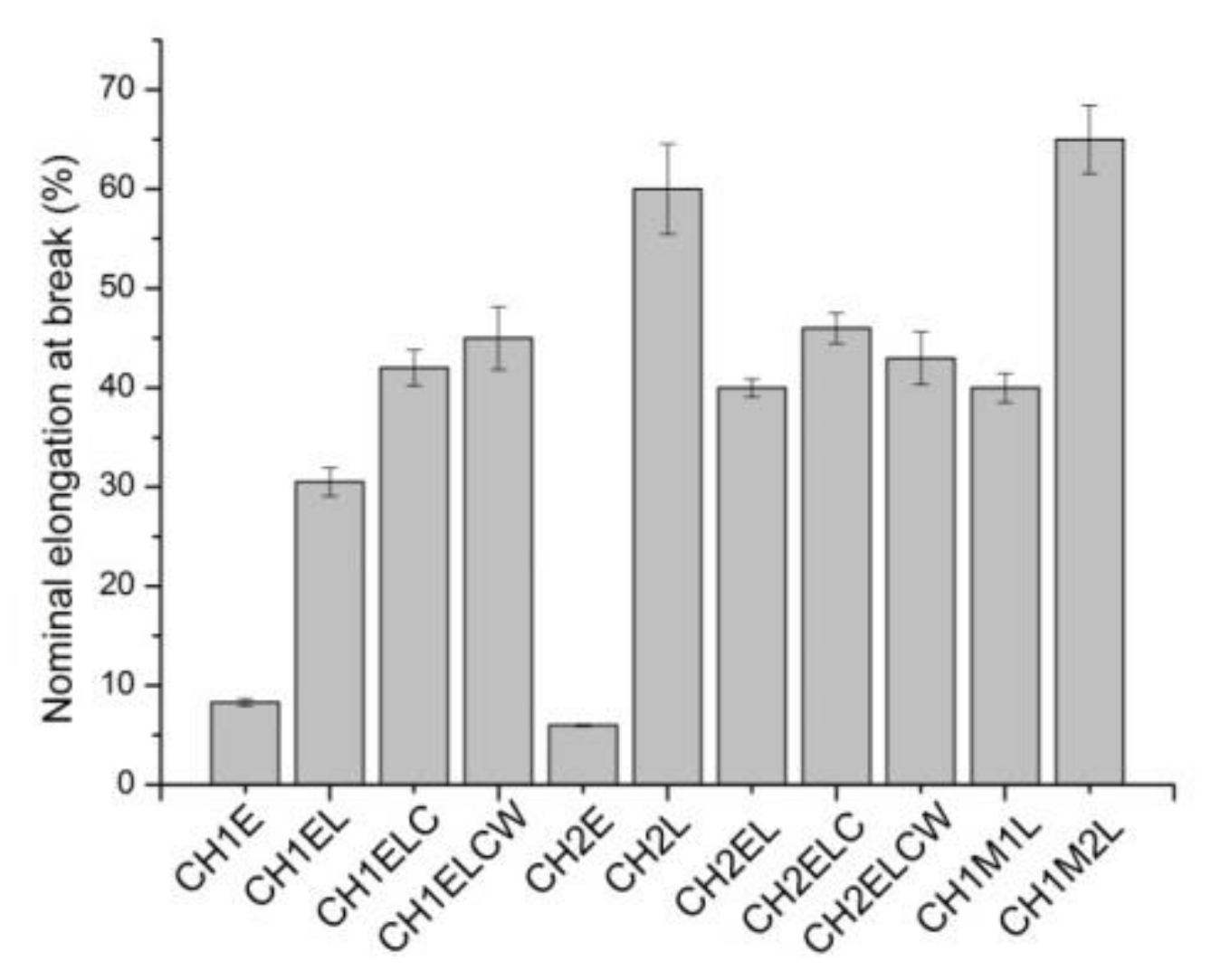
3.2. Mechanical Properties
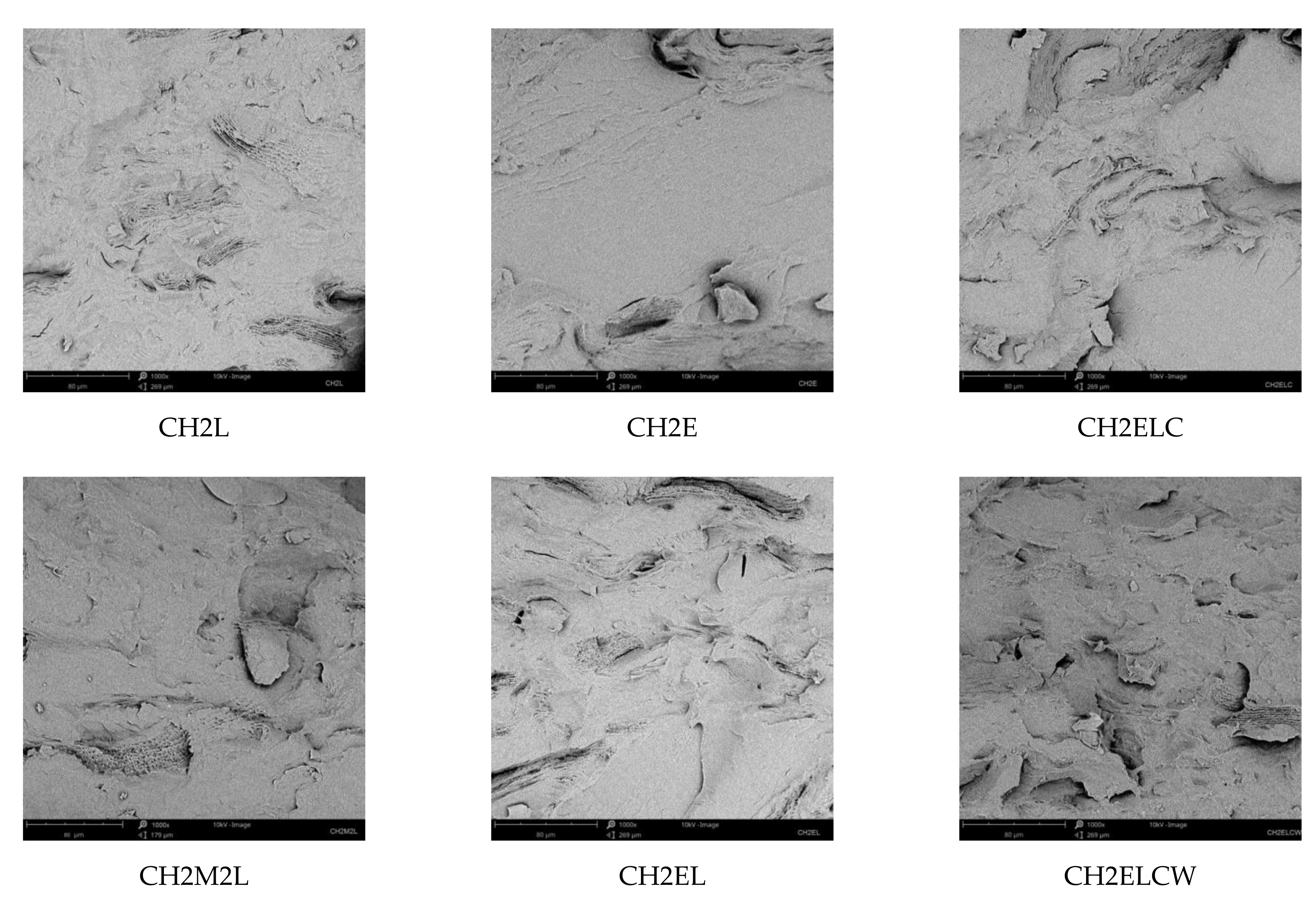
3.3. Morphology
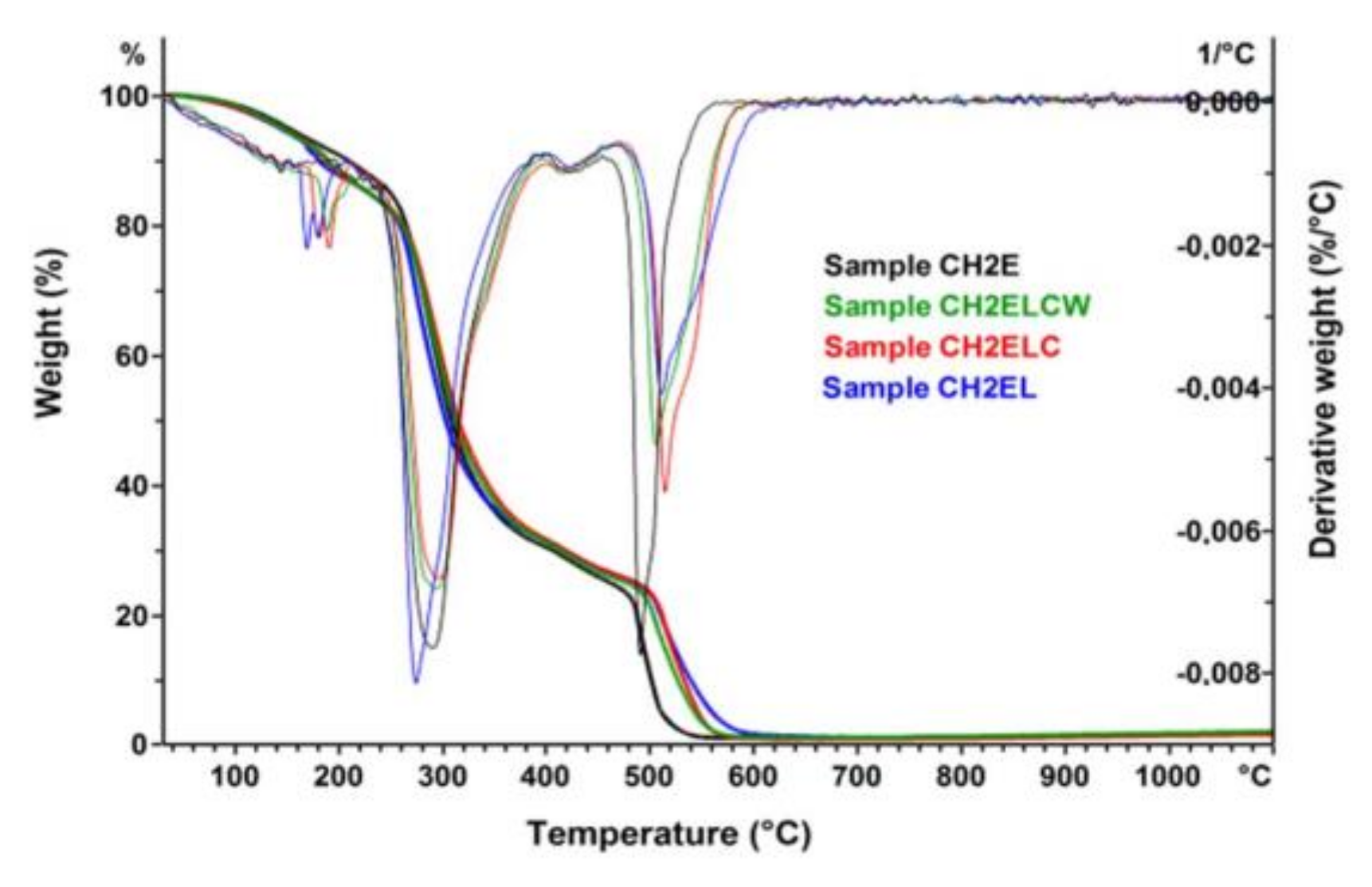
3.4. Thermal Analysis
4. Conclusions
5. Patents
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumari, S.; Kumar Annamareddy, S.H.; Abanti, S.; Kumar Rath, P. Physicochemical Properties and Characterization of Chitosan Synthesized from Fish Scales, Crab and Shrimp Shells. Int. J. Biol. Macromol. 2017, 104, 1697–1705. [Google Scholar] [CrossRef]
- Leceta, I.; Guerrero, P.; de la Caba, K. Functional Properties of Chitosan-Based Films. Carbohydr. Polym. 2013, 93, 339–346. [Google Scholar] [CrossRef]
- Ke, C.-L.; Deng, F.-S.; Chuang, C.-Y.; Lin, C.-H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef] [PubMed]
- Souza, V.G.L.; Pires, J.R.A.; Rodrigues, C.; Coelhoso, I.M.; Fernando, A.L. Chitosan Composites in Packaging Industry—Current Trends and Future Challenges. Polymers 2020, 12, 417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van den Broek, L.A.M.; Knoop, R.J.I.; Kappen, F.H.J.; Boeriu, C.G. Chitosan Films and Blends for Packaging Material. Carbohydr. Polym. 2015, 116, 237–242. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, B.-L.; Wang, L.-J.; Deng, Y.-H.; Zhou, S.-Y.; Feng, J.-W. Preparation, Structure and Properties of Acid Aqueous Solution Plasticized Thermoplastic Chitosan. Polymers 2019, 11, 818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casey, L.; Wilson, L. Investigation of Chitosan-PVA Composite Films and Their Adsorption Properties. J. Geosci. Environ. Prot. 2015, 3, 78–84. [Google Scholar] [CrossRef]
- Choo, K.; Ching, Y.C.; Chuah, C.H.; Julai, S.; Liou, N.-S. Preparation and Characterization of Polyvinyl Alcohol-Chitosan Composite Films Reinforced with Cellulose Nanofiber. Materials 2016, 9, 644. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.; Gherissi, A. Synthesis and Characterization of the Composite Material PVA/Chitosan/5% Sorbitol with Different Ratio of Chitosan. Int. J. Mech. Mechatron. Eng. 2017, 17, 15–28. [Google Scholar]
- Sakkara, S.; Nataraj, D.; Venkatesh, K.; Reddy, N. Influence of Alkali Treatment on the Physicochemical and Mechanical Properties of Starch Chitosan Films. Starch—Stärke 2019, 71, 1800084. [Google Scholar] [CrossRef]
- Flores-Hernández, C.G.; Colin-Cruz, A.; Velasco-Santos, C.; Castaño, V.M.; Almendarez-Camarillo, A.; Olivas-Armendariz, I.; Martínez-Hernández, A.L. Chitosan–Starch–Keratin Composites: Improving Thermo-Mechanical and Degradation Properties Through Chemical Modification. J. Polym. Environ. 2018, 26, 2182–2191. [Google Scholar] [CrossRef]
- Chen, L.; Tang, C.; Ning, N.; Wang, C.; Fu, Q.; Zhang, Q. Peparation and Properties of Chitosan/Lignin Composite Films. Chin. J. Polym. Sci. 2009, 27, 739–746. [Google Scholar] [CrossRef]
- Pandharipande, S.L.; Pujari, N.; Deshmukh, K. Synthesis and Characterisation of Biocomposite Films of Chitosan and Lignin. Int. J. Eng. Res. Technol. 2016, 5, 626–636. [Google Scholar] [CrossRef]
- Kopania, E.; Wiśniewska-Wrona, M. Biopolymer Composites Based on Lignin and Microcrystalline Chitosan. Prog. Chem. Appl. Chitin Its Deriv. 2016, 21, 122–134. [Google Scholar] [CrossRef] [Green Version]
- Rosova, E.; Smirnova, N.; Dresvyanina, E.; Smirnova, V.; Vlasova, E.; Ivan’kova, E.; Sokolova, M.; Maslennikova, T.; Malafeev, K.; Kolbe, K.; et al. Biocomposite Materials Based on Chitosan and Lignin: Preparation and Characterization. Cosmetics 2021, 8, 24. [Google Scholar] [CrossRef]
- Gomes, A.M.M.; da Silva, P.L.; e Moura, C.D.L.; da Silva, C.E.M.; Ricardo, N.M.P.S. Study of the Mechanical and Biodegradable Properties of Cassava Starch/Chitosan/PVA Blends. Macromol. Symp. 2011, 299, 220–226. [Google Scholar] [CrossRef]
- Karua, C.S.; Sahoo, A. Synthesis and Characterization of Starch/Chitosan Composites. Mater. Today Proc. 2020, 33, 5179–5183. [Google Scholar] [CrossRef]
- Kochkina, N.E.; Lukin, N.D. Structure and Properties of Biodegradable Maize Starch/Chitosan Composite Films as Affected by PVA Additions. Int. J. Biol. Macromol. 2020, 157, 377–384. [Google Scholar] [CrossRef]
- Epure, V.; Griffon, M.; Pollet, E.; Avérous, L. Structure and Properties of Glycerol-Plasticized Chitosan Obtained by Mechanical Kneading. Carbohydr. Polym. 2011, 83, 947–952. [Google Scholar] [CrossRef]
- Matet, M.; Heuzey, M.-C.; Pollet, E.; Ajji, A.; Avérous, L. Innovative Thermoplastic Chitosan Obtained by Thermo-Mechanical Mixing with Polyol Plasticizers. Carbohydr. Polym. 2013, 95, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Heuzey, M.-C.; Carreau, P.J. Hierarchical Structure and Physicochemical Properties of Plasticized Chitosan. Biomacromolecules 2014, 15, 1216–1224. [Google Scholar] [CrossRef]
- Chen, P.; Xie, F.; Tang, F.; McNally, T. Unexpected Plasticization Effects on the Structure and Properties of Polyelectrolyte Complexed Chitosan/Alginate Materials. ACS Appl. Polym. Mater. 2020, 2, 2957–2966. [Google Scholar] [CrossRef]
- Mendes, J.F.; Paschoalin, R.T.; Carmona, V.B.; Sena Neto, A.R.; Marques, A.C.P.; Marconcini, J.M.; Mattoso, L.H.C.; Medeiros, E.S.; Oliveira, J.E. Biodegradable Polymer Blends Based on Corn Starch and Thermoplastic Chitosan Processed by Extrusion. Carbohydr. Polym. 2016, 137, 452–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matet, M.; Heuzey, M.-C.; Ajji, A.; Sarazin, P. Plasticized Chitosan/Polyolefin Films Produced by Extrusion. Carbohydr. Polym. 2015, 117, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Flores-Hernandez, C.G.; Martinez-Hernandez, A.L.; Colin-Cruz, A.; Martinez-Bustos, F.; Castaño, V.M.; Olivas-Armendariz, I.; Almendarez-Camarillo, A.; Velasco-Santos, C. Starch Modified With Chitosan and Reinforced With Feather Keratin Materials Produced by Extrusion Process: An Alternative to Starch Polymers. Starch—Stärke 2018, 70, 1700295. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Yamashita, F.; Grossmann, M.V.E. Extrusion Parameters Related to Starch/Chitosan Active Films Properties. Int. J. Food Sci. Technol. 2011, 46, 702–710. [Google Scholar] [CrossRef]
- Liu, R.; Dai, L.; Xu, C.; Wang, K.; Zheng, C.; Si, C. Lignin-Based Micro- and Nanomaterials and Their Composites in Biomedical Applications. ChemSusChem 2020, 13, 4266–4283. [Google Scholar] [CrossRef] [PubMed]
- Puspitasari, T.; Raja, K.M.L.; Pangerteni, D.S.; Patriati, A.; Putra, E.G.R. Structural Organization of Poly(vinyl alcohol) Hydrogels Obtained by Freezing/Thawing and J-Irradiation Processes: A Small-Angle Neutron Scattering (SANS) Study. Procedia Chem. 2012, 4, 186–193. [Google Scholar] [CrossRef] [Green Version]
- Konduri, M.; Kong, F.; Fatehi, P. Production of carboxymethylated lignin and its application as a dispersant. Eur. Polym. J. 2015, 70, 371–383. [Google Scholar] [CrossRef]
- Yu, O.; Kim, K.H. Lignin to Materials: A Focused Review on Recent Novel Lignin Applications. Appl. Sci. 2020, 10, 4626. [Google Scholar] [CrossRef]
- Nasalapure, A.V.; Chalannavar, R.K.; Malabadi, R.B. Preparation of Poly(Vinyl Alcohol)/Chitosan/Starch Blends and Studies on Thermal and Surface Properties. AIP Conf. Proc. 2018, 1953, 100060. [Google Scholar] [CrossRef]
- Wang, K.; Loo, L.S.; Goh, K.L. A Facile Method for Processing Lignin Reinforced Chitosan Biopolymer Microfibres: Optimising the Fibre Mechanical Properties through Lignin Type and Concentration. Mater. Res. Express 2016, 3, 035301. [Google Scholar] [CrossRef]
- Arora, S.; Lal, S.; Sharma, C.; Aneja, K. Synthesis, Thermal and Antimicrobial Studies of Chitosan/Starch/Poly(Vinyl Alcohol) Ternary Blend Films. Chem. Sin. 2011, 2, 75–86. [Google Scholar]
- Grande, G.; Pessan, L.A.; Carvahlo, A. Thermoplastic blends of chitosan: A method for the preparation of high thermally blends with polyesters. Carboh. Polym. 2018, 191, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wan, Y.; Cao, X.; Wu, Q. Interlocked chitosan/poly(DL-lactide) blends. Mater. Lett. 2008, 62, 330–334. [Google Scholar] [CrossRef]
- Almazrouei, M.; Samad, T.E.; Janajreh, I. Thermogravimetric Kinetics and High Fidelity Analysis of Crude Glycerol. Energy Procedia 2017, 142, 1699–1705. [Google Scholar] [CrossRef]
- Manley, T.R. Calorimetry and Thermal Analysis of Polymers, Edited by V. B. F. Mathot. Hanser, Munich, 1994. pp. 377. ISBN 3-446-17511-3. Polym. Int. 1995, 37, 83. [Google Scholar] [CrossRef]




| Sample | Composition, wt.% | |||||
|---|---|---|---|---|---|---|
| Chitosan | Starch | PVA | Lignin | Glycerol | AAAS | |
| CH1E | 30.30CH1 | 15.76 | 21.52E | - | 10.90 | 21.52 |
| CH2E | 30.30CH2 | 15.76 | 21.52E | - | 10.90 | 21.52 |
| CH1EL | 28.74CH1 | 14.94 | 20.40E | 5.17L | 10.35 | 20.40 |
| CH2EL | 28.74CH2 | 14.94 | 20.40E | 5.17L | 10.35 | 20.40 |
| CH1ELC | 28.74CH1 | 14.94 | 20.40E | 5.17LC | 10.35 | 20.40 |
| CH2ELC | 28.74CH2 | 14.94 | 20.40E | 5.17LC | 10.35 | 20.40 |
| CH1ELCW | 28.74CH1 | 14.94 | 20.40E | 5.17LCW | 10.35 | 20.40 |
| CH2ELCW | 28.74CH2 | 14.94 | 20.40E | 5.17LCW | 10.35 | 20.40 |
| CH2L | 36.10CH2 | 18.77 | - | 6.50L | 13.00 | 25.63 |
| CH2M1L | 28.74CH2 | 14.94 | 20.40M1 | 5.17L | 10.35 | 20.40 |
| CH2M2L | 28.74CH2 | 14.94 | 20.40M2 | 5.17L | 10.35 | 20.40 |
| Sample | Tg (°C) | ∆Cp (J·g−1·K−1) |
|---|---|---|
| Lignin LC | 148.4 | 0.374 |
| Lignin LCW | 154.2 | 0.325 |
| Lignin L | 170.7 | 0.658 |
| Chitosan CH2 | 89.8 | 0.457 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janik, W.; Wojtala, A.; Pietruszka, A.; Dudek, G.; Sabura, E. Environmentally Friendly Melt-Processed Chitosan/Starch Composites Modified with PVA and Lignin. Polymers 2021, 13, 2685. https://doi.org/10.3390/polym13162685
Janik W, Wojtala A, Pietruszka A, Dudek G, Sabura E. Environmentally Friendly Melt-Processed Chitosan/Starch Composites Modified with PVA and Lignin. Polymers. 2021; 13(16):2685. https://doi.org/10.3390/polym13162685
Chicago/Turabian StyleJanik, Weronika, Anna Wojtala, Anna Pietruszka, Gabriela Dudek, and Ewa Sabura. 2021. "Environmentally Friendly Melt-Processed Chitosan/Starch Composites Modified with PVA and Lignin" Polymers 13, no. 16: 2685. https://doi.org/10.3390/polym13162685
APA StyleJanik, W., Wojtala, A., Pietruszka, A., Dudek, G., & Sabura, E. (2021). Environmentally Friendly Melt-Processed Chitosan/Starch Composites Modified with PVA and Lignin. Polymers, 13(16), 2685. https://doi.org/10.3390/polym13162685