Cobalt-Mediated Radical Copolymerization of Vinylidene Fluoride and 2,3,3,3-Trifluoroprop-1-ene
Abstract
:1. Introduction
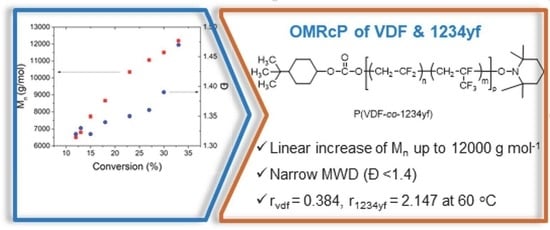
2. Results and Discussion
Copolymerization of VDF with 1234yf
3. Experimental Section
3.1. Materials and Methods
3.2. Characterizations
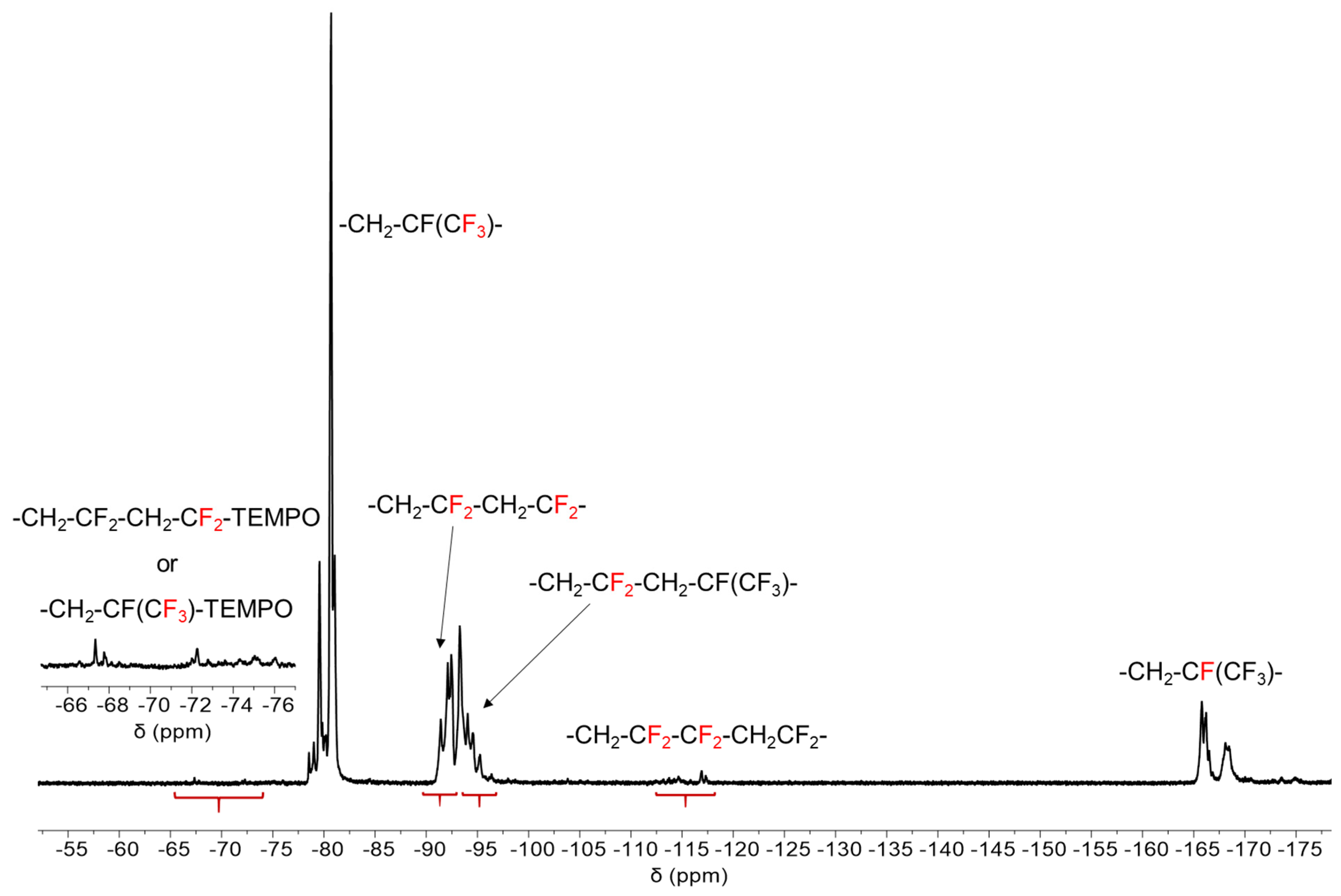
3.2.1. Nuclear Magnetic Resonance (NMR) Spectroscopy
3.2.2. Gel Permeation Chromatography (GPC)
3.2.3. OMRP of VDF with 1234yf Initiated by P16 in the Presence of Co(acac)2
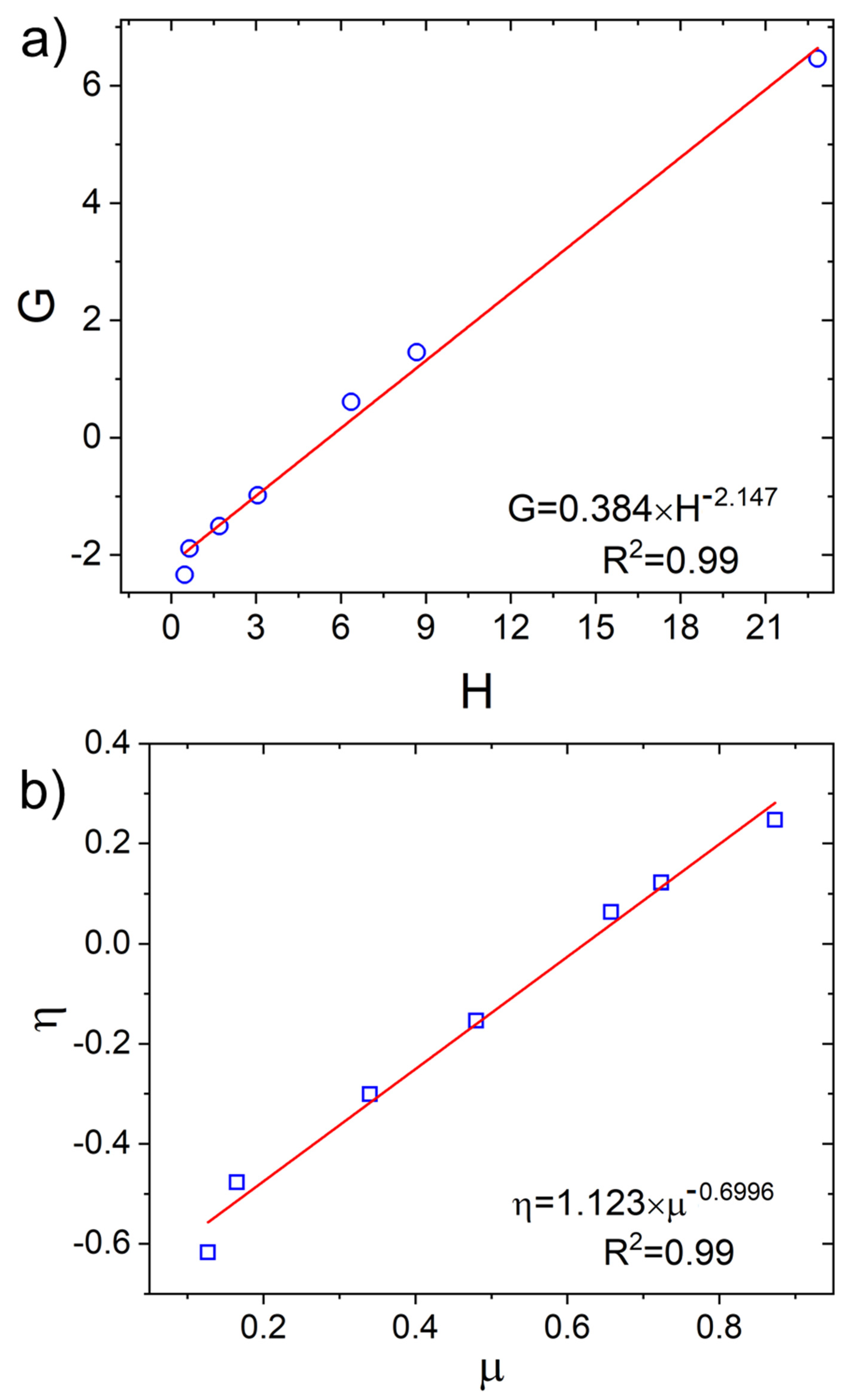
3.2.4. Determination of the Reactivity Ratios of VDF and 1234yf
3.2.5. Determination of Reactivity Ratios with the Fineman-Ross and Kelen-Tüdös Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Smith, D.W.; Iacono, S.T.; Iyer, S.S. Handbook of Fluoropolymer Science and Technology; Wiley: New York, NY, USA, 2014. [Google Scholar]
- Goldbach, J.T.; Amin-Sanayei, R.; He, W.; Henry, J.; Kosar, W.; Lefebvre, A.; O’Brien, G.; Vaessen, D.; Wood, K.; Zerafati, S. Chapter 6 Commercial Synthesis and Applications of Poly(Vinylidene Fluoride). In Fluorinated Polymers: Volume 2: Applications; The Royal Society of Chemistry: London, UK, 2017; Volume 2, pp. 127–157. [Google Scholar]
- Ameduri, B.; Fomin, S. Fascinating Fluoropolymers and Their Applications; Elsevier: Oxford, UK, 2020; p. 494. [Google Scholar]
- Ebnesajjad, S. Non-Melt Processible Fluoroplastics; William Andrew Publishing: Norwich, UK; New York, NY, USA, 2000. [Google Scholar]
- Puts, G.J.; Crouse, P.; Ameduri, B.M. Polytetrafluoroethylene: Synthesis and Characterization of the Original Extreme Polymer. Chem. Rev. 2019, 119, 1763–1805. [Google Scholar] [CrossRef]
- Ameduri, B. From Vinylidene Fluoride (VDF) to the Applications of VDF-Containing Polymers and Copolymers: Recent Developments and Future Trends. Chem. Rev. 2009, 109, 6632–6686. [Google Scholar] [CrossRef] [Green Version]
- Asandei, A.D. Photomediated Controlled Radical Polymerization and Block Copolymerization of Vinylidene Fluoride. Chem. Rev. 2016, 116, 2244–2274. [Google Scholar] [CrossRef]
- Costa, C.M.; Silva, M.M.; Lanceros-Méndez, S. Battery separators based on vinylidene fluoride (VDF) polymers and copolymers for lithium ion battery applications. RSC Adv. 2013, 3, 11404–11417. [Google Scholar] [CrossRef]
- Velders, G.J.M.; Fahey, D.W.; Daniel, J.S.; Andersen, S.O.; McFarland, M. Future atmospheric abundances and climate forcings from scenarios of global and regional hydrofluorocarbon (HFC) emissions. Atmos. Environ. 2015, 123, 200–209. [Google Scholar] [CrossRef]
- Velders, G.J.M.; Fahey, D.W.; Daniel, J.S.; McFarland, M.; Andersen, S.O. The large contribution of projected HFC emissions to future climate forcing. Proc. Natl. Acad. Sci. USA 2009, 106, 10949–10954. [Google Scholar] [CrossRef] [Green Version]
- WMO (World Meteorological Organization). Scientific Assessment of Ozone Depletion: 2018; Global Ozone Research and Monitoring Project–Report No. 58; World Meteorological Organization: Geneva, Switzerland, 2018; p. 588. [Google Scholar]
- Patil, Y.; Alaaeddine, A.; Ono, T.; Ameduri, B. Novel Method to Assess the Molecular Weights of Fluoropolymers by Radical Copolymerization of Vinylidene Fluoride with Various Fluorinated Comonomers Initiated by a Persistent Radical. Macromolecules 2013, 46, 3092–3106. [Google Scholar] [CrossRef]
- Soulestin, T.; Ladmiral, V.; Lannuzel, T.; Santos, F.D.D.; Améduri, B. Differences in electroactive terpolymers based on VDF, TrFE and 2,3,3,3-tetrafluoropropene prepared by batch solution and semi-continuous aqueous suspension polymerizations. Polym. Chem. 2017, 8, 735–747. [Google Scholar] [CrossRef]
- Durali, M.C.; Mountz, D.A. Vinylidene Fluoride/2,3,3,3-Tetrafluoropropene Copolymers. U.S. Patent 10,259,899, 16 April 2019. [Google Scholar]
- Banerjee, S.; Zaghloul, S.; Alaaeddine, A.; Ameduri, B. Kinetic and mechanistic aspects of the iodine transfer copolymerization of vinylidene fluoride with 2,3,3,3-tetrafluoro-1-propene and functionalization into ω-hydroxy fluorinated copolymers. Polym. Chem. 2016, 7, 6099–6109. [Google Scholar] [CrossRef]
- Ameduri, B.; Alaaeddine, A. Controlled Radical Copolymerization of Trifluoroethylene. U.S. Patent 2017/9708419, 18 July 2021. [Google Scholar]
- Falireas, P.G.; Ladmiral, V.; Debuigne, A.; Detrembleur, C.; Poli, R.; Ameduri, B. Straightforward Synthesis of Well-Defined Poly(vinylidene fluoride) and Its Block Copolymers by Cobalt-Mediated Radical Polymerization. Macromolecules 2019, 52, 1266–1276. [Google Scholar] [CrossRef]
- Banerjee, S.; Ladmiral, V.; Debuigne, A.; Detrembleur, C.; Poli, R.; Améduri, B. Organometallic-Mediated Radical Polymerization of Vinylidene Fluoride. J. Am. Chem. Soc. 2018, 57, 2934–2937. [Google Scholar] [CrossRef]
- Demarteau, J.; Debuigne, A.; Detrembleur, C. Organocobalt Complexes as Sources of Carbon-Centered Radicals for Organic and Polymer Chemistries. Chem. Rev. 2019, 119, 6906–6955. [Google Scholar] [CrossRef]
- Wang, P.; Wang, H.; Dong, Q.; Bai, R. Cobalt-Mediated Radical Copolymerization of Chlorotrifluoroethylene and Vinyl Acetate. Polymers 2019, 11, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asandei, A.D.; Adebolu, O.I.; Simpson, C.P. Mild-Temperature Mn2(CO)10-Photomediated Controlled Radical Polymerization of Vinylidene Fluoride and Synthesis of Well-Defined Poly(vinylidene fluoride) Block Copolymers. J. Am. Chem. Soc. 2012, 134, 6080–6083. [Google Scholar] [CrossRef] [PubMed]
- Debuigne, A.; Caille, J.-R.; Jérôme, R. Highly Efficient Cobalt-Mediated Radical Polymerization of Vinyl Acetate. Angew. Chem. Int. Ed. 2005, 44, 1101–1104. [Google Scholar] [CrossRef] [PubMed]
- Destarac, M.; Matyjaszewski, K.; Silverman, E.; Ameduri, B.; Boutevin, B. Atom Transfer Radical Polymerization Initiated with Vinylidene Fluoride Telomers. Macromolecules 2000, 33, 4613–4615. [Google Scholar] [CrossRef]
- Girard, E.; Marty, J.-D.; Ameduri, B.; Destarac, M. Direct Synthesis of Vinylidene Fluoride-Based Amphiphilic Diblock Copolymers by RAFT/MADIX Polymerization. ACS Macro Lett. 2012, 1, 270–274. [Google Scholar] [CrossRef]
- Golzari, N.; Adams, J.; Beuermann, S. Inducing β Phase Crystallinity in Block Copolymers of Vinylidene Fluoride with Methyl Methacrylate or Styrene. Polymers 2017, 9, 306. [Google Scholar] [CrossRef] [Green Version]
- Bryaskova, R.; Detrembleur, C.; Debuigne, A.; Jérôme, R. Cobalt-Mediated Radical Polymerization (CMRP) of Vinyl Acetate Initiated by Redox Systems: Toward the Scale-Up of CMRP. Macromolecules 2006, 39, 8263–8268. [Google Scholar] [CrossRef]
- Guerre, M.; Rahaman, S.M.W.; Améduri, B.; Poli, R.; Ladmiral, V. Limits of Vinylidene Fluoride RAFT Polymerization. Macromolecules 2016, 49, 5386–5396. [Google Scholar] [CrossRef]
- Fineman, M.; Ross, S.D. Linear method for determining monomer reactivity ratios in copolymerization. J. Polym. Sci. 1950, 5, 259–262. [Google Scholar] [CrossRef]
- Kelen, T.; Tüdos, F. Analysis of the Linear Methods for Determining Copolymerization Reactivity Ratios. I. A New Improved Linear Graphic Method. J. Macromol. Sci. Part A Chem. 1975, 9, 1–27. [Google Scholar] [CrossRef]
- Hosemann, B.; Siegmann, R.; Beuermann, B. Supercritical Carbon Dioxide as Reaction Medium for Fluoropolymer Synthesis and Kinetic Investigations into Radical Polymerizations of VDF and HFP. In Fluorinated Polymers: Synthesis, Properties Processing and Simulation; Ameduri, B., Sawada, H., Eds.; RSC Polymer Chemistry Series No. 23; The Royal Society of Chemistry: London, UK, 2017; Volume 1, Chapter 7; pp. 211–232. [Google Scholar]
- Boyer, C.; Ameduri, B.; Hung, M.H. Telechelic Diiodopoly(VDF-co-PMVE) Copolymers by Iodine Transfer Copolymerization of Vinylidene Fluoride (VDF) with Perfluoromethyl vinyl ether (PMVE). Macromolecules 2010, 43, 3652–3663. [Google Scholar] [CrossRef]
- Alfrey, T.; Price, C.C. Relative reactivities in vinyl copolymerization. J. Polym. Sci. 1947, 2, 101–106. [Google Scholar] [CrossRef]
- Brandrup, J.; Immergut, E.H.; Grulke, E.A. Polymer Handbook, 4th ed.; Wiley: New York, NY, USA; Chichester, UK, 2004. [Google Scholar]
- Igarashi, S. Representation of composition and blockiness of the copolymer by a triangular coordinate system. J. Polym. Sci. Part B Polym. Lett. 1963, 1, 359–363. [Google Scholar] [CrossRef]
- Elias, H.G. Macromolecules: Structure and Properties; Plenum Press: New York, NY, USA, 1971; Volume 2. [Google Scholar]





| Entry | Polymerization Time (h) | Conv. (%) a | Mn (g/mol) b | Ð b |
|---|---|---|---|---|
| 1 | 0.25 | 12 | 6500 | 1.33 |
| 2 | 0.5 | 13 | 6800 | 1.16 |
| 3 | 0.75 | 15 | 7700 | 1.32 |
| 4 | 1 | 18 | 8600 | 1.34 |
| 5 | 2 | 26 | 10,300 | 1.29 |
| 6 | 4 | 27 | 11,000 | 1.36 |
| 7 | 6 | 30 | 11,500 | 1.39 |
| 8 | 8 | 33 | 12,200 | 1.47 |
| fVDF | f1234yf | FVDF | F1234yf |
|---|---|---|---|
| 90 | 10 | 79 | 21 |
| 79 | 21 | 62 | 38 |
| 74 | 26 | 57 | 43 |
| 57 | 43 | 38 | 62 |
| 43 | 57 | 14 | 86 |
| 23 | 77 | 8 | 92 |
| 16 | 84 | 1 | 99 |
| Method | VDF/1234yf |
|---|---|
| Fineman-Ross | rvdf = 0.384 ± 0.013, r1234yf = 2.147 ± 0.129 |
| Kelen-Tüdös | rvdf = 0.434 ± 0.054, r1234yf = 2.285 ± 0.054 |
| FVDF | F1234yf | VDF-VDF | 1234yf-1234yf | VDF-1234yf | μVDF | μ1234yf |
|---|---|---|---|---|---|---|
| 0.79 | 0.21 | 0.5977 | 0.0177 | 0.3846 | 2.4445 | 1.5707 |
| 0.62 | 0.38 | 0.3454 | 0.1054 | 0.5491 | 1.6265 | 2.3159 |
| 0.57 | 0.43 | 0.2899 | 0.1498 | 0.5603 | 1.5090 | 2.6196 |
| 0.38 | 0.62 | 0.1921 | 0.4321 | 0.3758 | 1.2353 | 4.5030 |
| 0.14 | 0.86 | 0.0821 | 0.8021 | 0.1158 | 1.0625 | 14.1887 |
| 0.08 | 0.92 | 0.0482 | 0.8882 | 0.0636 | 1.0333 | 25.6905 |
| 0.01 | 0.99 | 0.0062 | 0.9862 | 0.0076 | 1.0038 | 213.5535 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falireas, P.G.; Ameduri, B. Cobalt-Mediated Radical Copolymerization of Vinylidene Fluoride and 2,3,3,3-Trifluoroprop-1-ene. Polymers 2021, 13, 2676. https://doi.org/10.3390/polym13162676
Falireas PG, Ameduri B. Cobalt-Mediated Radical Copolymerization of Vinylidene Fluoride and 2,3,3,3-Trifluoroprop-1-ene. Polymers. 2021; 13(16):2676. https://doi.org/10.3390/polym13162676
Chicago/Turabian StyleFalireas, Panagiotis G., and Bruno Ameduri. 2021. "Cobalt-Mediated Radical Copolymerization of Vinylidene Fluoride and 2,3,3,3-Trifluoroprop-1-ene" Polymers 13, no. 16: 2676. https://doi.org/10.3390/polym13162676
APA StyleFalireas, P. G., & Ameduri, B. (2021). Cobalt-Mediated Radical Copolymerization of Vinylidene Fluoride and 2,3,3,3-Trifluoroprop-1-ene. Polymers, 13(16), 2676. https://doi.org/10.3390/polym13162676