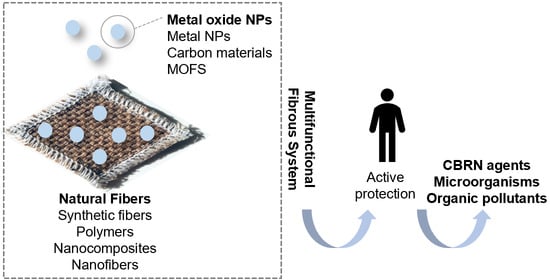
Protective Multifunctional Fibrous Systems Based on Natural Fibers and Metal Oxide Nanoparticles
Abstract
:1. Introduction
2. Personal Protective Equipment
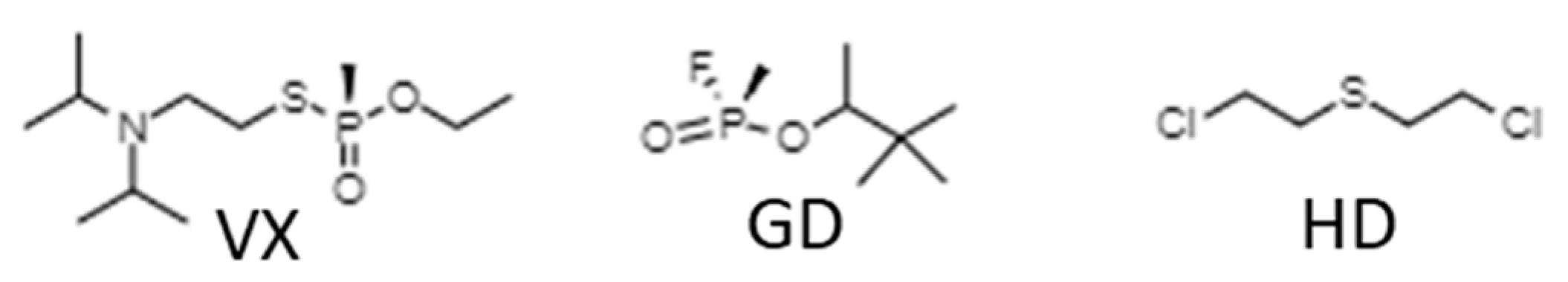
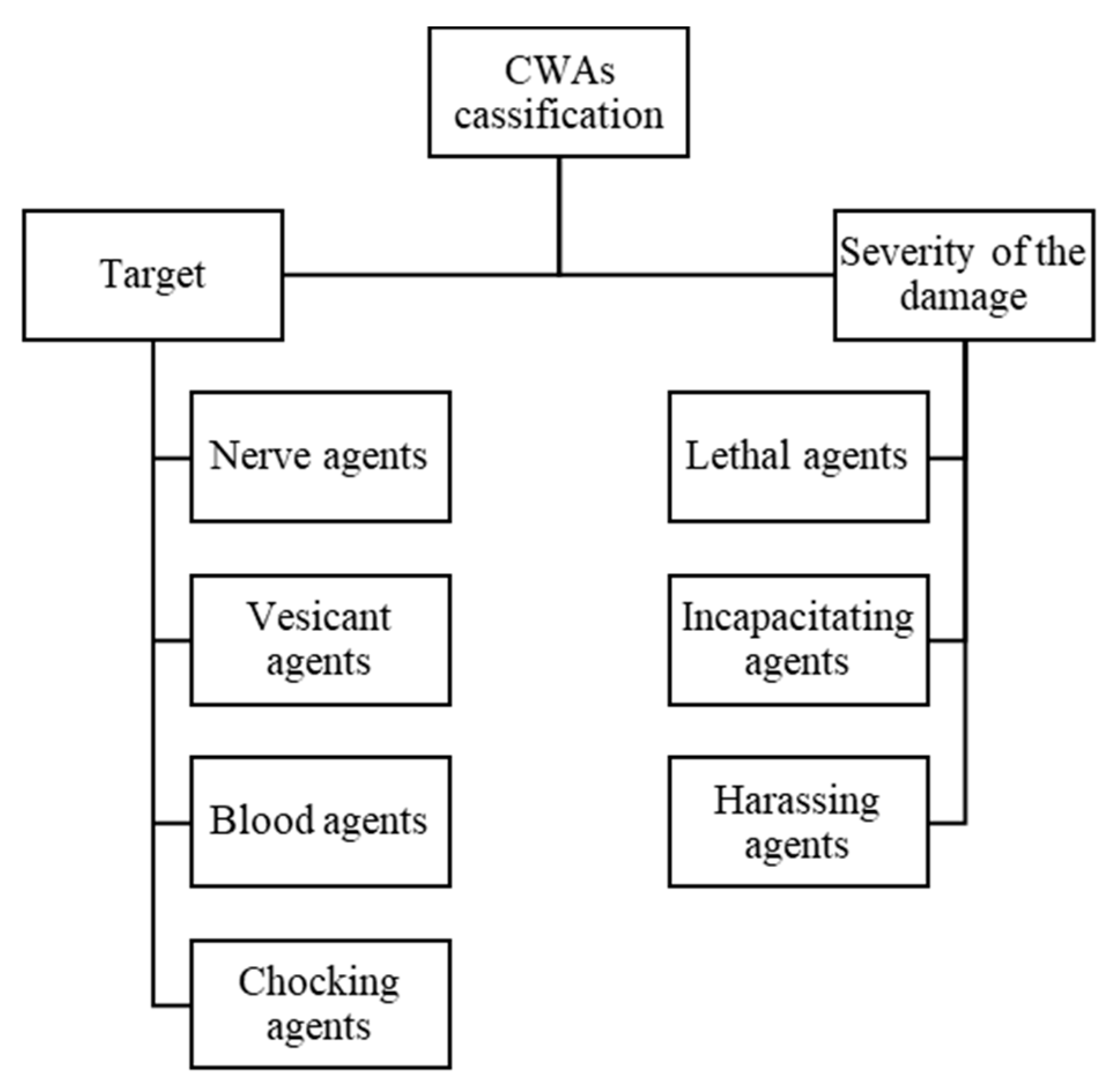
2.1. Chemical Warfare Agents: Incidents and Classification
2.2. Biological Warfare Agents: Incidents and Classification
2.3. Chemical and Biological Protective Textiles
3. Nanoparticles
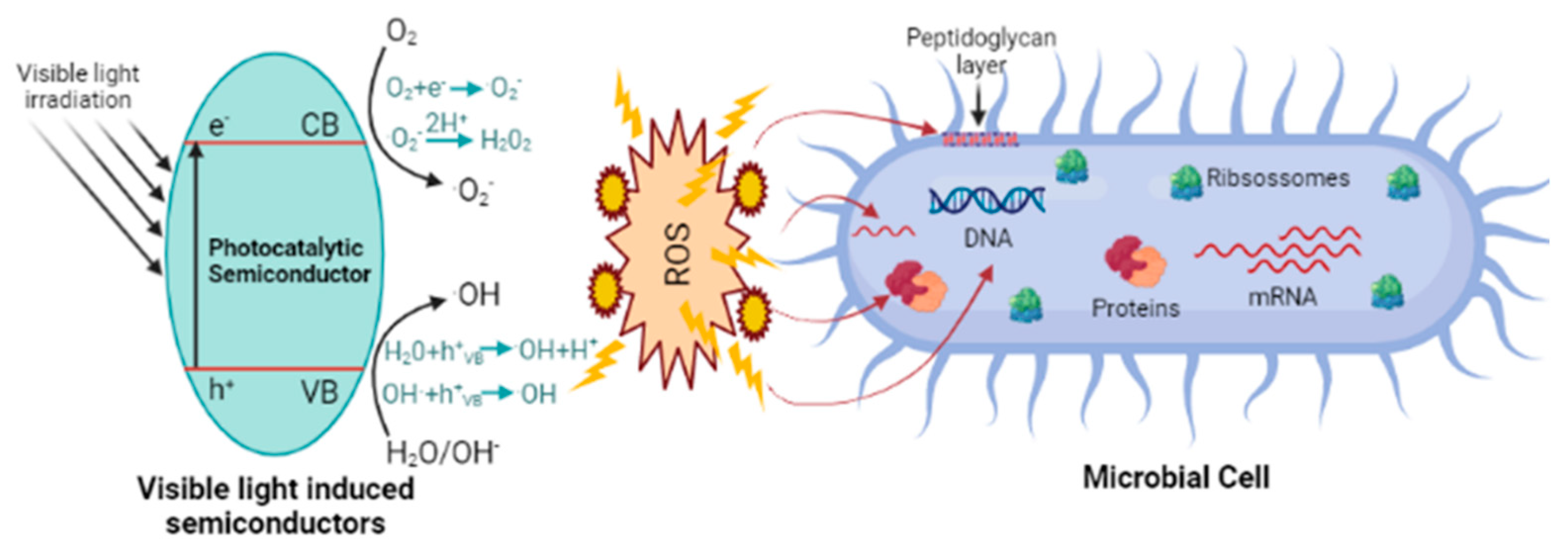
Metal Oxide Nanoparticles and Photocatalysis
4. Fibrous Structures Functionalized with Nanomaterials
4.1. Metal and Metal Oxides
4.2. Metal Organic Frameworks (MOFs)
4.3. Carbon Nanomaterials
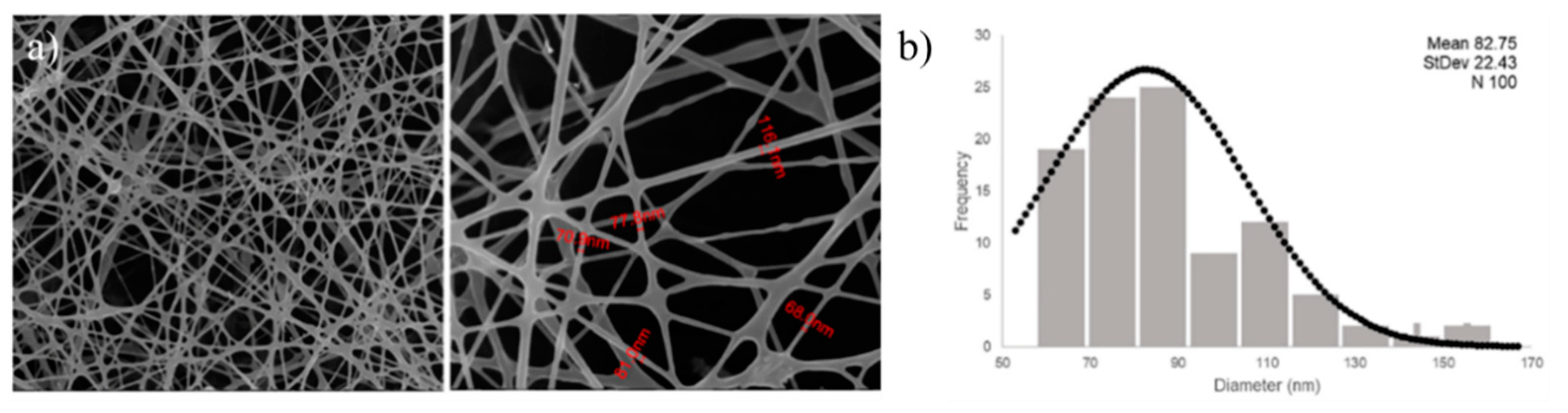
4.4. Nanofibers Produced by Electrospinning: New Trend
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bhuiyan, M.A.R.; Wang, L.; Shaid, A.; Shanks, R.A.; Ding, J. Advances and applications of chemical protective clothing system. J. Ind. Text. 2018, 1–42. [Google Scholar] [CrossRef]
- Binder, M.K.; Ackerman, G.A. Pick Your POICN: Introducing the Profiles of Incidents involving CBRN and Non-State Actors (POICN) Database. Stud. Confl. Terror. 2019, 1–25. [Google Scholar] [CrossRef]
- Bakowski, P. CBRN Terrorism: Threats and the EU Response. 2015. Available online: https://www.europarl.europa.eu/thinktank/en/document.html?reference=EPRS_BRI(2015)545724 (accessed on 20 July 2020).
- Vu, A.; Ho, K.; Lee, C. Removal of gaseous sulfur and phosphorus compounds by carbon-coated porous magnesium oxide composites. Chem. Eng. J. 2016, 283, 1234–1243. [Google Scholar] [CrossRef]
- Szinicz, L. History of chemical and biological warfare agents. Toxicology 2005, 214, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Gusain, R.; Gupta, K.; Joshi, P.; Khatri, O.P. Adsorptive removal and photocatalytic degradation of organic pollutants using metal oxides and their composites: A comprehensive review. Adv. Colloid Interface Sci. 2019, 272, 102009. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, K.; Varadharajan, V.; Kadirvelu, K.G. Photocatalytic Decontamination of Organic Pollutants Using Advanced Materials. In Modern Age Waste Water Problems; Oves, M., Ansari, M.O., Khan, M.Z., Shahdat, M., Ismail, I.M.I., Eds.; Springer International Publishing: Berlin, Germany, 2020; pp. 195–212. [Google Scholar]
- Gopinath, A.; Krishna, K. Dual role of chemically functionalized activated carbon fibres: Investigation of parameters influencing the degradation of organophosphorus compounds and antibacterial behaviour. J. Chem. Technol. Biotechnol. 2019, 94, 611–617. [Google Scholar] [CrossRef]
- Delfino, R.T.; Ribeiro, T.S.; Figueroa-villar, J.D. Organophosphorus Compounds as Chemical Warfare Agents: A Review. J. Braz. Chem. Soc. 2009, 20, 407–428. [Google Scholar] [CrossRef]
- Schreuder-Gibson, H.L.; Truong, Q.; Walker, J.E.; Owens, J.R.; Wander, J.D.; Jr, W.E.J. Chemical and Biological Protection and Detection in Fabrics for Protective Clothing. MRS Bull. 2003, 28, 574–578. [Google Scholar] [CrossRef] [Green Version]
- Olczyk, J.; Sójka-Ledakowicz, J.; Walawska, A.; Antecka, A.; Siwińska-Ciesielczyk, K.; Zdarta, J.; Jesionowski, T. Antimicrobial Activity and Barrier Properties against UV Radiation of Alkaline and Enzymatically Treated Linen Woven Fabrics Coated with Inorganic Hybrid Material. Molecules 2020, 25, 5701. [Google Scholar] [CrossRef]
- Joshi, M.; Adak, B. Advances in Nanotechnology Based Functional, Smart and Intelligent Textiles: A Review; Elsevier Ltd.: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Araújo, J.C.; Ferreira, D.P.; Teixeira, P.; Fangueiro, R. In-situ synthesis of CaO and SiO2 nanoparticles onto jute fabrics: Exploring the multifunctionality. Cellulose 2020. [Google Scholar] [CrossRef]
- Kozlowski, R.M.; Kajzar, F.; Nyszko, G.; Wertejuk, Z.; Majewski, K.; Mackiewicz-Talarczyk, M.; Małkowski, Z. Special textiles with multifunctional systems of human protection against contamination, fire, cold & UV radiation. Mol. Cryst. Liq. Cryst. 2017, 655, 195–203. [Google Scholar] [CrossRef]
- Truong, Q.; Wilusz, E. 13-Advances in chemical and biological protective clothing. In Woodhead Publishing Series in Textiles; Chapman, R., Ed.; Woodhead Publishing: Cambridge, UK, 2013; pp. 364–377. [Google Scholar]
- Sakurada, K.; Ohta, H. No promising antidote 25 years after the Tokyo subway sarin attack: A review. Leg. Med. 2020, 47, 101761. [Google Scholar] [CrossRef]
- Sellwood, C.; Wapling, A. Health Emergency Preparedness and Response; Centre for Agriculture and Bioscience International: Wallingford, UK, 2016; Volume 53. [Google Scholar]
- Turaga, U.; Kendall, R.J.; Singh, V.; Lalagiri, M.; Ramkumar, S.S. 12-Advances in materials for chemical, biological, radiological and nuclear (CBRN) protective clothing. In Woodhead Publishing Series in Textiles; Sparks, E., Ed.; Woodhead Publishing: Cambridge, UK, 2012; pp. 260–287. [Google Scholar]
- Altmann, H.J.; Richardt, A. Decontamination of Chemical Warfare Agents. In Decontamination of Warfare Agents: Enzymatic Methods for the Removal of B/C Weapon; Richardt, A., Blum, M., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; pp. 35–54. [Google Scholar]
- Sundarrajan, S.; Chandrasekaran, A.R.; Ramakrishna, S. An update on nanomaterials-based textiles for protection and decontamination. J. Am. Ceram. Soc. 2010, 93, 3955–3975. [Google Scholar] [CrossRef]
- Václav, Š.; Jiří, H.; Pavel, J.; Miroslav, S. Nanostructured Metal Oxides for Stoichiometric Degradation of Chemical Warfare Agents. In Reviews of Environmental Contamination and Toxicology; De Voogt, P., Ed.; Springer International Publishing: Berlin, Germany, 2015; Volume 236, pp. 1–297. [Google Scholar]
- Ganesan, K.; Raza, S.K.; Vijayaraghavan, R. Chemical warfare agents. J. Pharm. Bioallied Sci. 2010, 2, 166. [Google Scholar] [CrossRef] [PubMed]
- Schwenk, M. Chemical warfare agents. Classes and targets. Toxicol. Lett. 2018, 293, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Bartelt-Hunt, S.L.; Knappe, D.R.U.; Barlaz, M.A. A review of chemical warfare agent simulants for the study of environmental behavior. Crit. Rev. Environ. Sci. Technol. 2008, 38, 112–136. [Google Scholar] [CrossRef]
- Ciotti, M.; Ciccozzi, M.; Terrinoni, A.; Jiang, W.-C.; Wang, C.-B.; Bernardini, S. The COVID-19 pandemic. Crit. Rev. Clin. Lab. Sci. 2020, 57, 365–388. [Google Scholar] [CrossRef]
- Ivanova, K.; Sandler, T. CBRN incidents: Political regimes, perpetrators, and targets. Terror. Polit. Violence 2006, 18, 423–448. [Google Scholar] [CrossRef]
- Flora, S.J.S. Biological warfare agents: History and modern-day relevance. In Handbook on Biological Warfare Preparedness; Flora, S.J.S., Pachauri, V., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 1–11. [Google Scholar]
- Russmann, H.; Richardt, A. Biological Warfare Agents. In Decontamination of Warfare Agents Enzymatic Methods for the Removal of B/C Weapon; Richardt, A., Blum, M., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; pp. 35–54. [Google Scholar]
- Lister, A.P.; Sellors, W.J.; Howle, C.R.; Mahajan, S. Raman Scattering Techniques for Defense and Security Applications. Anal. Chem. 2020, 93, 417–429. [Google Scholar] [CrossRef]
- Oudejans, L.; O’Kelly, J.; Evans, A.S.; Wyrzykowska-Ceradini, B.; Touati, A.; Tabor, D.; Snyder, E.G. Decontamination of personal protective equipment and related materials contaminated with toxic industrial chemicals and chemical warfare agent surrogates. J. Environ. Chem. Eng. 2016, 4, 2745–2753. [Google Scholar] [CrossRef]
- Lukey, B.J.; Romano Jr, J.A.; Salem, H. Chemical Warfare Agents: Biomedical and Psychological Effects, Medical Countermeasures, and Emergency Response; CRC Press: Oca Raton, FL, USA, 2019. [Google Scholar]
- Cao, H. Smart technology for personal protective equipment and clothing. In Woodhead Publishing Series in Textiles; Woodhead Publishin: Cambridge, UK, 2013; pp. 229–243. [Google Scholar]
- Mao, N. Textile materials for protective textiles. In High Performance Technical Textiles; Paul, R., Ed.; Wiley: Hoboken, NJ, USA, 2019. [Google Scholar]
- Cekovic, B.; Dieter, R. A Fresh Approach: Review of the Production Development of the CBRN/HAZMAT Equipment. In Cyber and Chemical, Biological, Radiological, Nuclear, Explosives Challenges. Terrorism, Security, and Computation; Martellini, M., Malizia, A., Eds.; Springer: Berlin, Germany, 2017. [Google Scholar]
- Zhang, X.; Liu, Y.; Yong, H.; Qin, Y.; Liu, J.; Liu, J. Development of multifunctional food packaging films based on chitosan, TiO2 nanoparticles and anthocyanin-rich black plum peel extract. Food Hydrocoll. 2019, 94, 80–92. [Google Scholar] [CrossRef]
- Villalobos-Hernández, J.R.; Müller-Goymann, C.C. Sun protection enhancement of titanium dioxide crystals by the use of carnauba wax nanoparticles: The synergistic interaction between organic and inorganic sunscreens at nanoscale. Int. J. Pharm. 2006, 322, 161–170. [Google Scholar] [CrossRef]
- Zhou, Q.; Lv, J.; Cai, L.; Ren, Y.; Chen, J.; Gao, D.; Lu, Z.; Wang, C. Preparation and characterization of ZnO/AGE MNPs with aloe gel extract and its application on linen fabric. J. Text. Inst. 2017, 108, 1371–1378. [Google Scholar] [CrossRef]
- Seok, J.K.; Kwak, J.Y.; Choi, G.W.; An, S.M.; Kwak, J.; Seo, H.; Suh, H.; Boo, Y.C. Scutellaria radix extract as a natural UV protectant for human skin. Phyther. Res. 2016, 30, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, M.; Hashimi, S. Graphene as a Material–An Overview of Its Properties and Characteristics and Development Potential for Practical Applications; Elsevier Ltd.: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Tolasz, J.; Stengl, V.; St, M.; David, Z. Graphene oxide/MnO2 nanocomposite as destructive adsorbent of nerve-agent simulants in aqueous media. Appl. Surf. Sci. 2017, 412, 19–28. [Google Scholar] [CrossRef]
- Sayago, I.; Matatagui, D.; Jesús, M.; Luis, J.; Jurewicz, I.; Garriga, R.; Muñoz, E. Graphene oxide as sensitive layer in Love-wave surface acoustic wave sensors for the detection of chemical warfare agent simulants. Talanta 2016, 148, 393–400. [Google Scholar] [CrossRef]
- Jeong, S.; Yeo, S.; Yi, S. The effect of filler particle size on the antibacterial properties of compounded polymer/silver fibers. J. Mater. Sci. 2005, 5407–5411. [Google Scholar] [CrossRef]
- Ki, H.Y.; Kim, J.H.; Kwon, S.C. A study on multifunctional wool textiles treated with nano-sized silver. J. Mater. Sci. 2007, 42, 8020–8024. [Google Scholar] [CrossRef]
- Teli, M.D.; Sheikh, J. International Journal of Biological Macromolecules Modified bamboo rayon–copper nanoparticle composites as antibacterial textiles. Int. J. Biol. Macromol. 2013, 61, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Becheri, A.; Durr, M.; Nostro, P.; Baglioni, P. Synthesis and characterization of zinc oxide nanoparticles: Application to textiles as UV-absorbers. J. Nanoparticle Res. 2008, 10, 679–689. [Google Scholar] [CrossRef]
- Duan, W.; Xie, A.; Shen, Y.; Wang, X.; Wang, F.; Zhang, Y.; Li, J. Fabrication of Superhydrophobic Cotton Fabrics with UV Protection Based on CeO2 Particles. Ind. Eng. Chem. Res. 2011, 50, 4441–4445. [Google Scholar] [CrossRef]
- Pakdel, E.; Wang, J.; Kashi, S.; Sun, L.; Wang, X. Advances in photocatalytic self-cleaning, superhydrophobic and electromagnetic interference shielding textile treatments. Adv. Colloid Interface Sci. 2020, 277, 102116. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Wang, K.; Hou, S.; Wan, L.; Lv, J.; Zhang, Y.; Qu, X.; Chen, S.; Xu, J. H2O2 and/or TiO2 photocatalysis under UV irradiation for the removal of antibiotic resistant bacteria and their antibiotic resistance genes. J. Hazard. Mater. 2017, 323, 710–718. [Google Scholar] [CrossRef] [Green Version]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Regmi, C.; Joshi, B.; Ray, S.K.; Gyawali, G.; Pandey, R.P. Understanding Mechanism of Photocatalytic Microbial Decontamination of Environmental Wastewater. Front. Chem. 2018, 6, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raj, V.B.; Singh, H.; Nimal, A.T.; Sharma, M.U.; Gupta, V. Oxide thin films (ZnO, TeO2, SnO2, and TiO2) based surface acoustic wave (SAW) E-nose for the detection of chemical warfare agents. Sens. Actuators Chem. 2013, 178, 636–647. [Google Scholar] [CrossRef]
- Sadeghi, M.; Yekta, S.; Ghaedi, H. Decontamination of chemical warfare sulfur mustard agent simulant by ZnO nanoparticles. Int. Nano Lett. 2016, 6, 161–171. [Google Scholar] [CrossRef] [Green Version]
- Neatu, S.; Cojocaru, B.; Alvaro, M.; Garcia, H. Visible-light C–heteroatom bond cleavage and detoxification of chemical warfare agents using titania-supported gold nanoparticles as photocatalyst. J. Mater. Chem. 2010, 20, 4050–4054. [Google Scholar] [CrossRef]
- Alvaro, M.; Cojocaru, B.; Ismail, A.A.; Petrea, N.; Ferrer, B.; Harraz, F.A.; Parvulescu, V.I.; Garcia, H. Environmental Visible-light photocatalytic activity of gold nanoparticles supported on template-synthesized mesoporous titania for the decontamination of the chemical warfare agent Soman. Applied Catal. Environ. 2010, 99, 191–197. [Google Scholar] [CrossRef]
- Petrea, N.; Petre, R.; Toader, C.; Neațu, F.; Florea, M.; Abramiuc, L.E.; Teodorescu, C.M.; Șomoghi, V. Photocatalytic degradation of sulfur mustard over NiO-ZnO/TiO2 composites. Semant. Sch. 2018, 42, 5–6. [Google Scholar]
- Štengl, V.; Št’astný, M.; Janoš, P.; Mazanec, K.; Perez-Diaz, J.L.; Štenglová-Netíková, I.R. From the Decomposition of Chemical Warfare Agents to the Decontamination of Cytostatics. Ind. Eng. Chem. Res. 2018, 57, 2114–2122. [Google Scholar] [CrossRef]
- Wagner, G.W.; Peterson, G.W.; Mahle, J.J. Effect of Adsorbed Water and Surface Hydroxyls on the Hydrolysis of VX, GD, and HD on Titania Materials: The Development of Self-Decontaminating Paints. Ind. Eng. Chem. Res. 2012, 51, 3598–3603. [Google Scholar] [CrossRef]
- Denet, E.; Espina-Benitez, M.B.; Pitault, I.; Pollet, T.; Blaha, D.; Bolzinger, M.-A.; Rodriguez-Nava, V.; Briançon, S. Metal oxide nanoparticles for the decontamination of toxic chemical and biological compounds. Int. J. Pharm. 2020, 583, 119373. [Google Scholar] [CrossRef] [PubMed]
- Das, B.; Moumita, S.; Ghosh, S.; Khan, M.I.; Indira, D.; Jayabalan, R.; Tripathy, S.K.; Mishra, A.; Balasubramanian, P. Biosynthesis of magnesium oxide (MgO) nanoflakes by using leaf extract of Bauhinia purpurea and evaluation of its antibacterial property against Staphylococcus aureus. Mater. Sci. Eng. 2018, 91, 436–444. [Google Scholar] [CrossRef]
- Ogunyemi, S.O.; Abdallah, Y.; Zhang, M.; Fouad, H.; Hong, X.; Ibrahim, E.; Masum, M.M.I.; Hossain, A.; Mo, J.; Li, B. Green synthesis of zinc oxide nanoparticles using different plant extracts and their antibacterial activity against Xanthomonas oryzae pv. oryzae. Artif. Cells Nanomed. Biotechnol. 2019, 47, 341–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, L.; Chen, J.; Liu, Z.; Wang, H.; Yang, H.; Ding, W. Magnesium Oxide Nanoparticles: Effective Agricultural Antibacterial Agent Against Ralstonia solanacearum. Front. Microbiol. 2018, 9, 790. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, S.; Koper, O.; Decker, S.; Klabunde, K.J. Nanocrystalline Metal Oxides as Destructive Adsorbents for Organophosphorus Compounds at Ambient Temperatures. Chem. Eur. J. 2002, 4, 2602–2607. [Google Scholar] [CrossRef]
- Bakardjieva, S.; Subrt, J.; Ol, M. Reaction of sulfur mustard gas, soman and agent VX with nanosized anatase TiO2 and ferrihydrite. J. Chem. Technol. Biotechnol. 2005, 758, 754–758. [Google Scholar] [CrossRef]
- Paukku, Y.; Michalkova, A.; Leszezynkski, J. Adsorption of dimethyl methylphosphonate and trimethyl phosphate on calcium oxide: An ab initio study. Struct. Chem. 2008, 2, 307–320. [Google Scholar] [CrossRef]
- Gershonov, E.; Columbus, I.; Zafrani, Y. Facile Hydrolysis-Based Chemical Destruction of the Warfare Agents VX, GB, and HD by Alumina-Supported Fluoride Reagents. J. Org. Chem. 2009, 74, 329–338. [Google Scholar] [CrossRef]
- Ramakrishna, S.S.Æ.S. Fabrication of nanocomposite membranes from nanofibers and nanoparticles for protection against chemical warfare stimulants. J. Mater. Sci. 2007, 42, 8400–8407. [Google Scholar] [CrossRef]
- Ramaseshan, R.; Sundarrajan, S. Functionalized polymer nanofibre membranes for protection from chemical. Nanotechnology 2006, 17, 2947. [Google Scholar] [CrossRef]
- Lee, S.; Obendorf, S.K. Use of Electrospun Nanofiber Web for Protective Textile Materials as Barriers to Liquid Penetration. Text. Res. J. 2014, 77, 696–702. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Prabhu, R.; Muruganantham, K.; Shanmuganathan, R.; Natarajan, S. Anticancer, antimicrobial and photocatalytic activities of green synthesized magnesium oxide nanoparticles (MgONPs) using aqueous extract of Sargassum wightii. J. Photochem. Photobiol. Biol. 2018, 190, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.P.; Cruz, J.; Fangueiro, R. Surface modification of natural fibers in polymer composites. In Green Composites for Automotive Applications; Koronis, G., Silva, A., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 3–41. [Google Scholar]
- Sundarrajan, S.; Ramakrishna, S. 4-The use of nanomaterials in smart protective clothing. In Smart Textiles for Protection; Chapman, R.A., Ed.; Woodhead Publishing Series in Textiles; Woodhead Publishing: Cambridge, UK, 2013; pp. 127–147. [Google Scholar]
- Grandcolas, M.; Sinault, L.; Mosset, F.; Louvet, A.; Keller, N.; Keller, V. Self-decontaminating layer-by-layer functionalized textiles based on WO3-modified titanate nanotubes. Application to the solar photocatalytic removal of chemical warfare agents. Appl. Catal. Gen. 2011, 391, 455–467. [Google Scholar] [CrossRef]
- Wang, S.; Ding, H.; Zhao, Y.; Li, Y.; Wang, W. Fabrication of Protective Textile with N-doped TiO2 Embedded Citral Microcapsule Coating and Its Air Purification Properties. Fibers Polym. 2020, 21, 334–342. [Google Scholar] [CrossRef]
- Mihailović, D.; Šaponjić, Z.; Radoičić, M.; Radetić, T.; Jovančić, P.; Nedeljković, J.; Radetić, M. Functionalization of polyester fabrics with alginates and TiO2 nanoparticles. Carbohydr. Polym. 2010, 79, 526–532. [Google Scholar] [CrossRef]
- Mihailović, D.; Šaponjić, Z.; Vodnik, V.; Potkonjak, B.; Jovančić, P.; Nedeljković, J.M.; Radetić, M. Multifunctional PES fabrics modified with colloidal Ag and TiO2 nanoparticles. Polym. Adv. Technol. 2011, 22, 2244–2249. [Google Scholar] [CrossRef]
- Abdelrahman, M.S.; Nassar, S.H.; Mashaly, H.; Mahmoud, S.; Maamoun, D.; El-Sakhawy, M.; Khattab, T.A.; Kamel, S. Studies of Polylactic Acid and Metal Oxide Nanoparticles-Based Composites for Multifunctional Textile Prints. Coatings 2020, 10, 58. [Google Scholar] [CrossRef] [Green Version]
- Prasad, V.; Arputharaj, A.; Bharimalla, A.K.; Patil, P.G.; Vigneshwaran, N. Durable multifunctional finishing of cotton fabrics by in situ synthesis of nano-ZnO. Appl. Surf. Sci. 2016, 390, 936–940. [Google Scholar] [CrossRef]
- Zhu, C.; Shi, J.; Xu, S.; Ishimori, M.; Sui, J.; Morikawa, H. Design and characterization of self-cleaning cotton fabrics exploiting zinc oxide nanoparticle-triggered photocatalytic degradation. Cellulose 2017, 24, 2657–2667. [Google Scholar] [CrossRef]
- Costa, S.M.; Ferreira, D.P.; Ferreira, A.; Vaz, F.; Fangueiro, R. Multifunctional Flax Fibres Based on the Combined Effect of Silver and Zinc Oxide (Ag/ZnO) Nanostructures. Nanomaterials 2018, 8, 1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, J.F.; Ferreira, D.P.; Pinho, E.; Fangueiro, R. Chemical and Biological Warfare Protection and Self-Decontaminating Flax Fabrics Based on CaO Nanoparticles. Key Eng. Mater. 2019, 812, 75–83. [Google Scholar] [CrossRef]
- Vasantharaj, S.; Sathiyavimal, S.; Saravanan, M.; Senthilkumar, P.; Gnanasekaran, K.; Shanmugavel, M.; Manikandan, E.; Pugazhendhi, A. Synthesis of ecofriendly copper oxide nanoparticles for fabrication over textile fabrics: Characterization of antibacterial activity and dye degradation potential. J. Photochem. Photobiol. Biol. 2019, 191, 143–149. [Google Scholar] [CrossRef]
- Attia, N.F.; Moussa, M.; Sheta, A.M.F.; Taha, R.; Gamal, H. Synthesis of effective multifunctional textile based on silica nanoparticles. Prog. Org. Coatings 2017, 106, 41–49. [Google Scholar] [CrossRef]
- Gao, L.-Z.; Bao, Y.; Cai, H.-H.; Zhang, A.-P.; Ma, Y.; Tong, X.-L.; Li, Z.; Dai, F.-Y. Multifunctional silk fabric via surface modification of nano-SiO2. Text. Res. J. 2020, 90, 1616–1627. [Google Scholar] [CrossRef]
- Ferreira, D.P.; Costa, S.M.; Felgueiras, H.P.; Fangueiro, R. Smart and Sustainable Materials for Military Applications Based on Natural Fibres and Silver Nanoparticles. Key Eng. Mater. 2019, 812, 66–74. [Google Scholar] [CrossRef]
- Ferreira, D.P.; Ferreira, A.; Fangueiro, R. Searching for Natural Conductive Fibrous Structures via a Green Sustainable Approach Based on Jute Fibers and Silver Nanoparticles. Polymers 2018, 10, 63. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Yang, Z.-Y.; Tang, R.-C. Green and facile fabrication of AgNPs@silk for colorful and multifunctional textiles using baicalin as a natural reductant. J. Clean. Prod. 2018, 170, 940–949. [Google Scholar] [CrossRef]
- Li, G.; Liu, H.; Zhao, H.; Gao, Y.; Wang, J.; Jiang, H.; Boughton, R.I. Chemical assembly of TiO2 and TiO2@Ag nanoparticles on silk fiber to produce multifunctional fabrics. J. Colloid Interface Sci. 2011, 358, 307–315. [Google Scholar] [CrossRef]
- Rehan, M.; Mashaly, H.M.; Mowafi, S.; Abou El-Kheir, A.; Emam, H.E. Multi-functional textile design using in-situ Ag NPs incorporation into natural fabric matrix. Dye. Pigment. 2015, 118, 9–17. [Google Scholar] [CrossRef]
- Shahid-ul-Islam; Butola, B.S.; Gupta, A.; Roy, A. Multifunctional finishing of cellulosic fabric via facile, rapid in-situ green synthesis of AgNPs using pomegranate peel extract biomolecules. Sustain. Chem. Pharm. 2019, 12, 100135. [Google Scholar] [CrossRef]
- Shabbir, M.; Mohammad, F. Multifunctional AgNPs@Wool: Colored, UV-protective and antioxidant functional textiles. Appl. Nanosci. 2018, 8, 545–555. [Google Scholar] [CrossRef]
- Rac-Rumijowska, O.; Maliszewska, I.; Fiedot-Toboła, M.; Karbownik, I.; Teterycz, H. Multifunctional Nanocomposite Cellulose Fibers Doped in Situ with Silver Nanoparticles. Polymers 2019, 11, 562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rehan, M.; Barhoum, A.; Van Assche, G.; Dufresne, A.; Gätjen, L.; Wilken, R. Towards multifunctional cellulosic fabric: UV photo-reduction and in-situ synthesis of silver nanoparticles into cellulose fabrics. Int. J. Biol. Macromol. 2017, 98, 877–886. [Google Scholar] [CrossRef] [PubMed]
- El-Naggar, M.E.; Khattab, T.A.; Abdelrahman, M.S.; Aldalbahi, A.; Hatshan, M.R. Development of antimicrobial, UV blocked and photocatalytic self-cleanable cotton fibers decorated with silver nanoparticles using silver carbamate and plasma activation. Cellulose 2021, 28, 1105–1121. [Google Scholar] [CrossRef]
- Tang, B.; Lin, X.; Zou, F.; Fan, Y.; Li, D.; Zhou, J.; Chen, W.; Wang, X. In situ synthesis of gold nanoparticles on cotton fabric for multifunctional applications. Cellulose 2017, 24, 4547–4560. [Google Scholar] [CrossRef]
- Lee, D.T.; Jamir, J.D.; Peterson, G.W.; Parsons, G.N. Protective fabrics: Metal-organic framework textiles for rapid photocatalytic sulfur mustard simulant detoxification. Matter 2020, 2, 404–415. [Google Scholar] [CrossRef] [Green Version]
- Jung, H.; Kim, M.-K.; Jang, S. Liquid-repellent textile surfaces using zirconium (Zr)-based porous materials and a polyhedral oligomeric silsesquioxane coating. J. Colloid Interface Sci. 2020, 563, 363–369. [Google Scholar] [CrossRef]
- Lee, D.T.; Zhao, J.; Peterson, G.W.; Parsons, G.N. Catalytic “MOF-Cloth” Formed via Directed Supramolecular Assembly of UiO-66-NH2 Crystals on Atomic Layer Deposition-Coated Textiles for Rapid Degradation of Chemical Warfare Agent Simulants. Chem. Mater. 2017, 29, 4894–4903. [Google Scholar] [CrossRef]
- Smith, M.K.; Mirica, K.A. Self-Organized Frameworks on Textiles (SOFT): Conductive Fabrics for Simultaneous Sensing, Capture, and Filtration of Gases. J. Am. Chem. Soc. 2017, 139, 16759–16767. [Google Scholar] [CrossRef]
- Emam, H.E.; Abdelhameed, R.M. Anti-UV Radiation Textiles Designed by Embracing with Nano-MIL (Ti, In)–Metal Organic Framework. ACS Appl. Mater. Interfaces 2017, 9, 28034–28045. [Google Scholar] [CrossRef] [PubMed]
- Bunge, M.A.; Davis, A.B.; West, K.N.; West, C.W.; Glover, T.G. Synthesis and Characterization of UiO-66-NH2 Metal–Organic Framework Cotton Composite Textiles. Ind. Eng. Chem. Res. 2018, 57, 9151–9161. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Hu, Y.; Florent, M.; Bandosz, T.J. Smart textiles of MOF/gC 3 N 4 nanospheres for the rapid detection/detoxification of chemical warfare agents. Nanoscale Horiz. 2017, 2, 356–364. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, Z.; Mao, X.; Chen, X.-L.; Li, H.-H.; Wang, Y.-Y. Multifunctional Textiles/Metal−Organic Frameworks Composites for Efficient Ultraviolet Radiation Blocking and Noise Reduction. ACS Appl. Mater. Interfaces 2020, 12, 55316–55323. [Google Scholar] [CrossRef] [PubMed]
- Jhinjer, H.S.; Singh, A.; Bhattacharya, S.; Jassal, M.; Agrawal, A.K. Metal-organic frameworks functionalized smart textiles for adsorptive removal of hazardous aromatic pollutants from ambient air. J. Hazard. Mater. 2021, 411, 125056. [Google Scholar] [CrossRef]
- Noor, N.; Mutalik, S.; Younas, M.W.; Chan, C.Y.; Thakur, S.; Wang, F.; Yao, M.Z.; Mou, Q.; Leung, P.H. Durable Antimicrobial Behaviour from Silver-Graphene Coated Medical Textile Composites. Polymers 2019, 11, 2000. [Google Scholar] [CrossRef] [Green Version]
- Jin, J.; Rafiq, R.; Gill, Y.Q.; Song, M. Preparation and characterization of high performance of graphene/nylon nanocomposites. Eur. Polym. J. 2013, 49, 2617–2626. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Macintyre, C.R.; Wen, X.; Bahl, P.; Kumar, U.; Chughtai, A.A.; Joshi, R. Nanoparticles incorporated graphene-based durable cotton fabrics. Carbon N. Y. 2020, 166, 148–163. [Google Scholar] [CrossRef]
- Cai, G.; Xu, Z.; Yang, M.; Tang, B.; Wang, X. Functionalization of cotton fabrics through thermal reduction of graphene oxide. Appl. Surf. Sci. 2017, 393, 441–448. [Google Scholar] [CrossRef]
- Stan, M.S.; Badea, M.A.; Pircalabioru, G.G.; Chifiriuc, M.C.; Diamandescu, L.; Dumitrescu, I.; Trica, B.; Lambert, C.; Dinischiotu, A. Designing cotton fibers impregnated with photocatalytic graphene oxide/Fe, N-doped TiO2 particles as prospective industrial self-cleaning and biocompatible textiles. Mater. Sci. Eng. 2019, 94, 318–332. [Google Scholar] [CrossRef]
- Cao, J.; Wang, C. Multifunctional surface modification of silk fabric via graphene oxide repeatedly coating and chemical reduction method. Appl. Surf. Sci. 2017, 405, 380–388. [Google Scholar] [CrossRef]
- Shirgholami, M.A.; Karimi, L.; Mirjalili, M. Multifunctional modification of wool fabric using graphene/TiO2 nanocomposite. Fibers Polym. 2016, 17, 220–228. [Google Scholar] [CrossRef]
- Pereira, P.; Ferreira, D.P.; Araújo, J.C.; Ferreira, A.; Fangueiro, R. The Potential of Graphene Nanoplatelets in the Development of Smart and Multifunctional Ecocomposites. Polymers 2020, 12, 2189. [Google Scholar] [CrossRef]
- Vaseashta, A.; Karagülle-bölgen, N. Loaded Nano fibers: Force Protection, Filtration, Decontamination. In Advanced Nanotechnologies for Detection and Defense against CBRN Agents; Petkov, P., Ed.; Springer: Berlin, Germany, 2018; pp. 253–258. [Google Scholar]
- Kim, S.; Ying, W.B.; Jung, H.; Ryu, S.G.; Lee, B.; Lee, K.J. Zirconium Hydroxide-coated Nanofiber Mats for Nerve Gas Decontamination. Chem. Asian J. 2017, 12, 698–705. [Google Scholar] [CrossRef]
- Bolgen, N.; Vaseashta, A. Nanocomposites of Electrospun Polymeric Materials as Protective Textiles Against Chemical and Biological Hazards. In Advanced Nanotechnologies for Detection and Defense against CBRN Agents; Petkov, P., Ed.; Springer: Berlin, Germany, 2018; pp. 253–258. [Google Scholar]
- Francavilla, P.; Ferreira, D.P.; Araújo, J.C.; Fangueiro, R. Smart Fibrous Structures Produced by Electrospinning Using the Combined Effect of PCL/Graphene Nanoplatelets. Appl. Sci. 2021, 11, 1124. [Google Scholar] [CrossRef]
- Ribeiro, A.S.; Costa, S.M.; Ferreira, D.P.; Calhelha, R.C.; Barros, L.; Stojković, D.; Soković, M.; Ferreira, I.C.F.R.; Fangueiro, R. Chitosan/nanocellulose electrospun fibers with enhanced antibacterial and antifungal activity for wound dressing applications. React. Funct. Polym. 2021, 159, 104808. [Google Scholar] [CrossRef]
- Ribeiro, A.S.; Costa, S.; P Ferreira, D.; Houcine, A.; Fangueiro, R. Development of Chitosan-Gelatin Nanofibers with Cellulose Nanocrystals for Skin Protection Applications. Key Eng. Mater. 2021, 893, 45–55. [Google Scholar] [CrossRef]
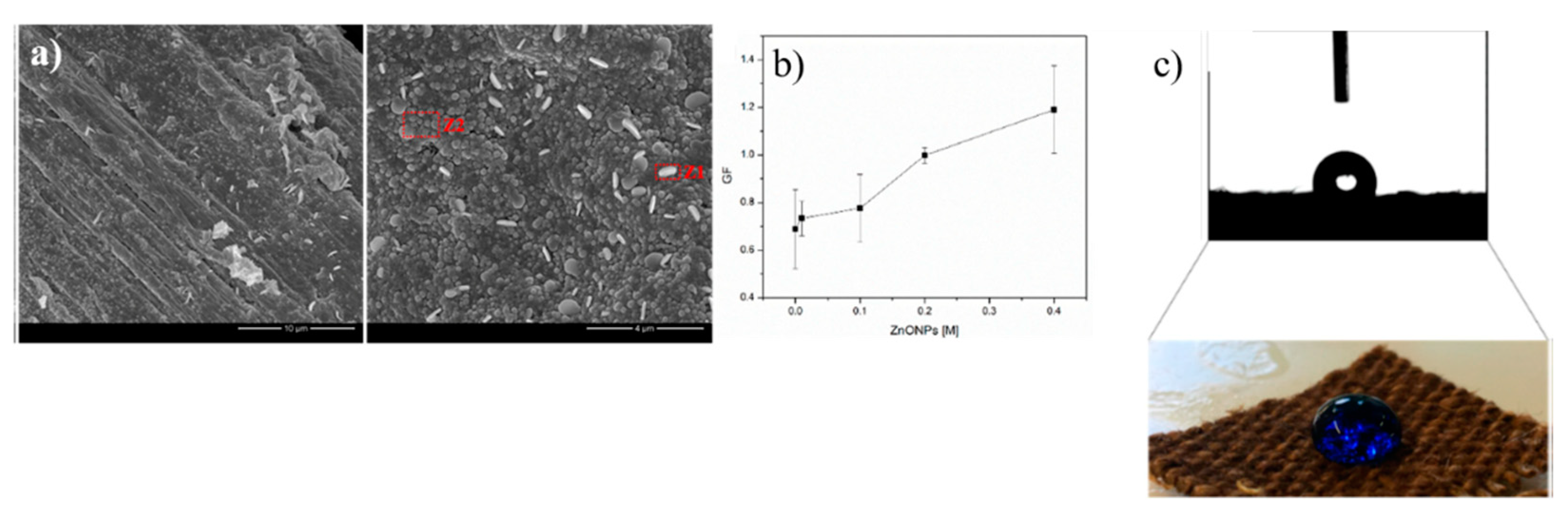

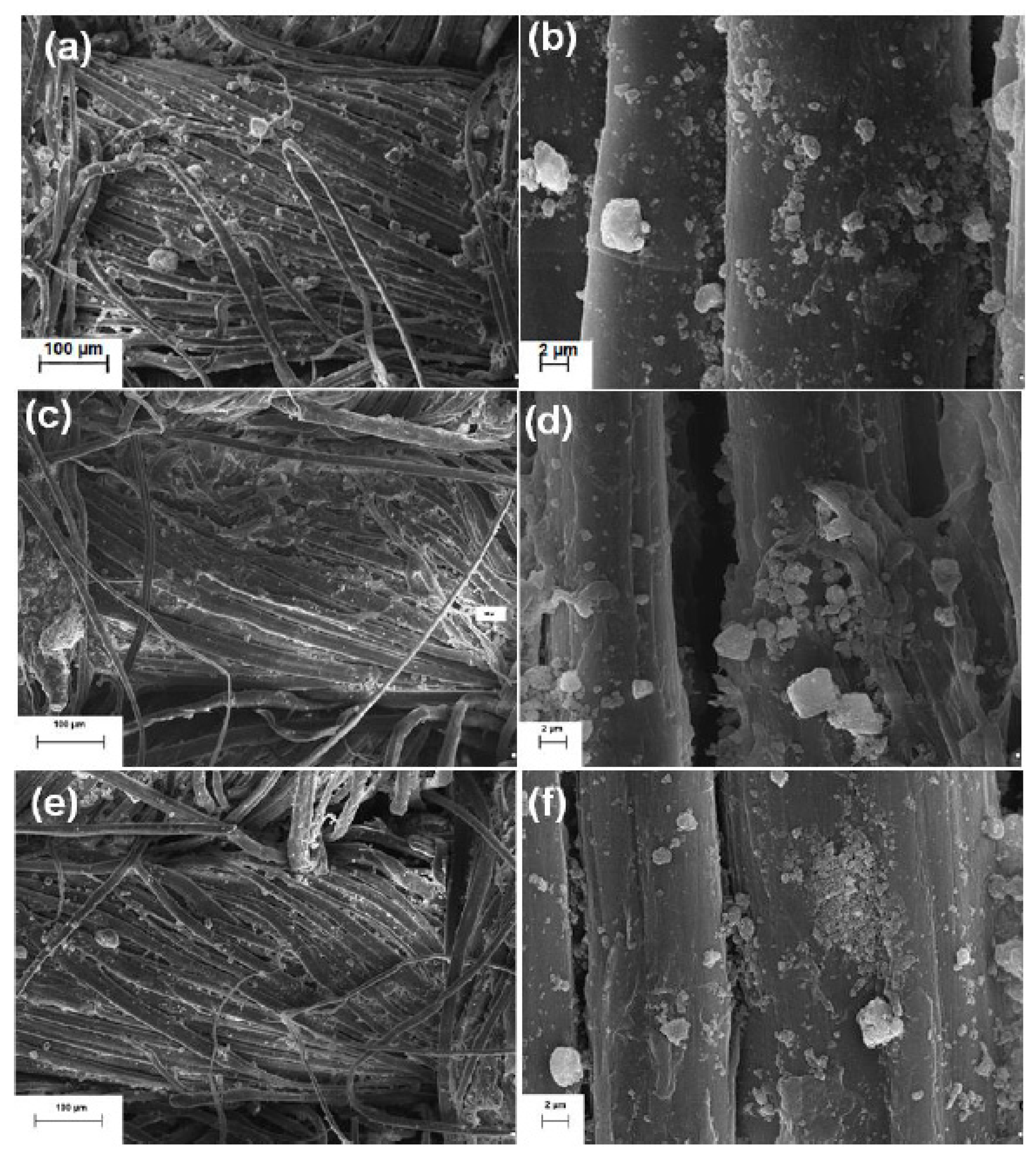

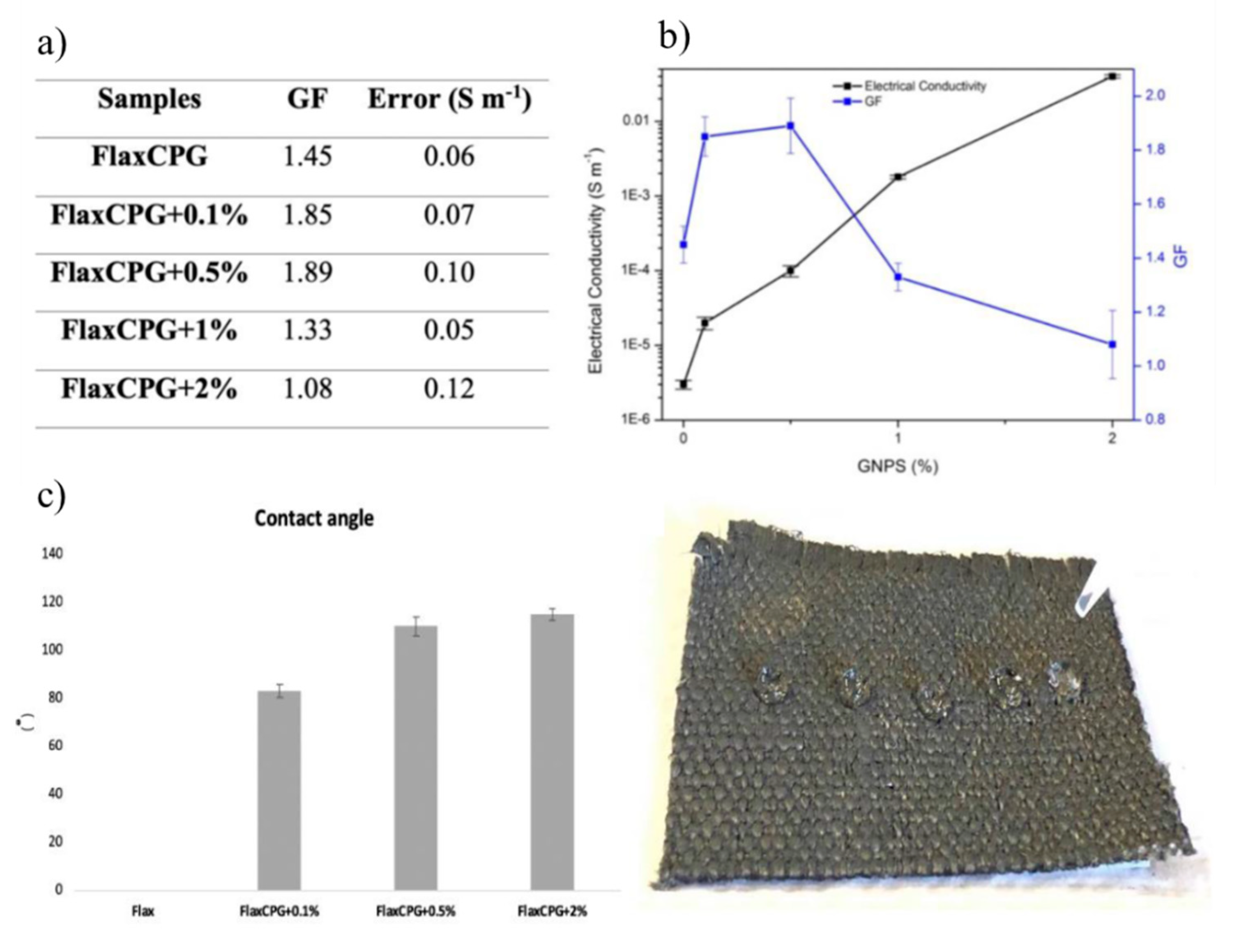
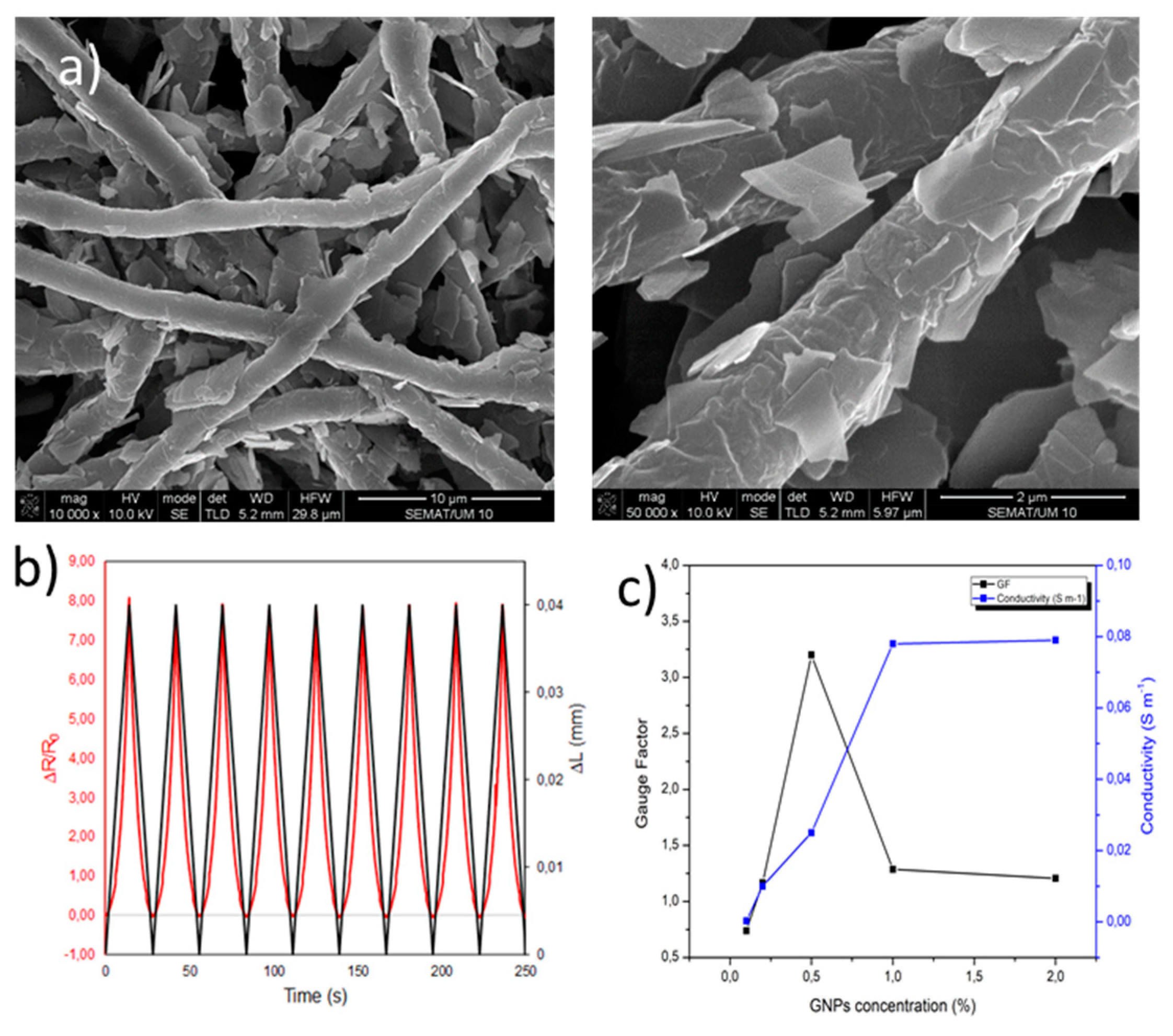
| Bacteria | Viruses | Toxins |
|---|---|---|
| Anthrax (Bacillus anthracis) Plague (Yersinia pestis) Tularemia (Francisella tularensis) Glanders (Burkholderia mallei) and meliodosis (Burkholderia pseudomallei) Brucellosis (Brucella sp.) Q fever (Coxiella burnetii) | Smallpox (Variola major) Viral encephalitis (e.g., VEE) Viral hemorrhagic fevers (e.g., Ebola, Marburg disease) | Botulinum toxin Ricin Staphylococcal enterotoxin B |
| Nanoparticles | Application | Warfare Agents or Simulants | Reference |
|---|---|---|---|
| ZnO, TeO2, SnO2 and TiO2 | Detection | DMMP, DBS, CEPS and DECP | [51] |
| ZnO | Decontamination | CEPS | [52] |
| Au/TiO2 | Decontamination | Soman, VX and sulfur mustard | [53] |
| Mesoporous TiO2/Au | Decontamination | Soman | [54] |
| NiO-ZnO/TiO2 | Decontamination | Sulfur mustard | [55] |
| Fast-Act® (MgO and TiO2) | Decontamination | Soman and VX | [56] |
| Self-decontamination paints (TiO2) | Decontamination | VX, GD and HD | [57] |
| ZnO and MgO | Antimicrobial activity | Pseudomonas aeruginosa and Staphylococcus aureus, Xanthomonas oryzae pv. oryzae and Ralstonia solanacearum | [59,60,61] |
| Graphene oxide-MnO2 | Decontamination | DMMP | [40] |
| Graphene oxide | Detection | DMMP and DPGME | [41] |
| Sample | UPF Value | UPF Rating |
|---|---|---|
| PES | 43.0 | 40 |
| PES + TiO2 | 91.6 | 50+ |
| PES + Ag10 + TiO2 | 91.6 | 50+ |
| PES + TiO2 + Ag10 | 76.1 | 50+ |
| PES + Ag50 + TiO2 | 118.6 | 50+ |
| PES + TiO2 + Ag50 | 112.0 | 50+ |
| Samples | UVA (T%) | UVB (T%) | Blocking UVA | Blocking UVB | UPF | UPF Rate |
|---|---|---|---|---|---|---|
| Silk | 17 ± 1.8 | 6.3 ± 1.2 | 83 ± 1.8 | 93.7 ± 2.1 | 10.5 ± 1.8 | Insufficient |
| Silk/Ti-MIL-2 | 0 | 0 | 100 | 100 | >100 | Excellent |
| Cotton | 34.3 ± 2.6 | 24.3 ± 2.1 | 65.7 ± 2.8 | 75.7 ± 2.3 | 3.5 ± 0.7 | Insufficient |
| Cotton/Ti-MIL-2 | 0 | 0 | 100 | 100 | >100 | Excellent |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, J.C.; Fangueiro, R.; Ferreira, D.P. Protective Multifunctional Fibrous Systems Based on Natural Fibers and Metal Oxide Nanoparticles. Polymers 2021, 13, 2654. https://doi.org/10.3390/polym13162654
Araújo JC, Fangueiro R, Ferreira DP. Protective Multifunctional Fibrous Systems Based on Natural Fibers and Metal Oxide Nanoparticles. Polymers. 2021; 13(16):2654. https://doi.org/10.3390/polym13162654
Chicago/Turabian StyleAraújo, Joana C., Raul Fangueiro, and Diana P. Ferreira. 2021. "Protective Multifunctional Fibrous Systems Based on Natural Fibers and Metal Oxide Nanoparticles" Polymers 13, no. 16: 2654. https://doi.org/10.3390/polym13162654
APA StyleAraújo, J. C., Fangueiro, R., & Ferreira, D. P. (2021). Protective Multifunctional Fibrous Systems Based on Natural Fibers and Metal Oxide Nanoparticles. Polymers, 13(16), 2654. https://doi.org/10.3390/polym13162654