Manufacturing a First Upper Molar Dental Forceps Using Continuous Fiber Reinforcement (CFR) Additive Manufacturing Technology with Carbon-Reinforced Polyamide
Abstract
:1. Introduction
2. Materials and Methods
2.1. 3D Printing Parameters and Fabrication of Test Specimens
2.2. Material Testing
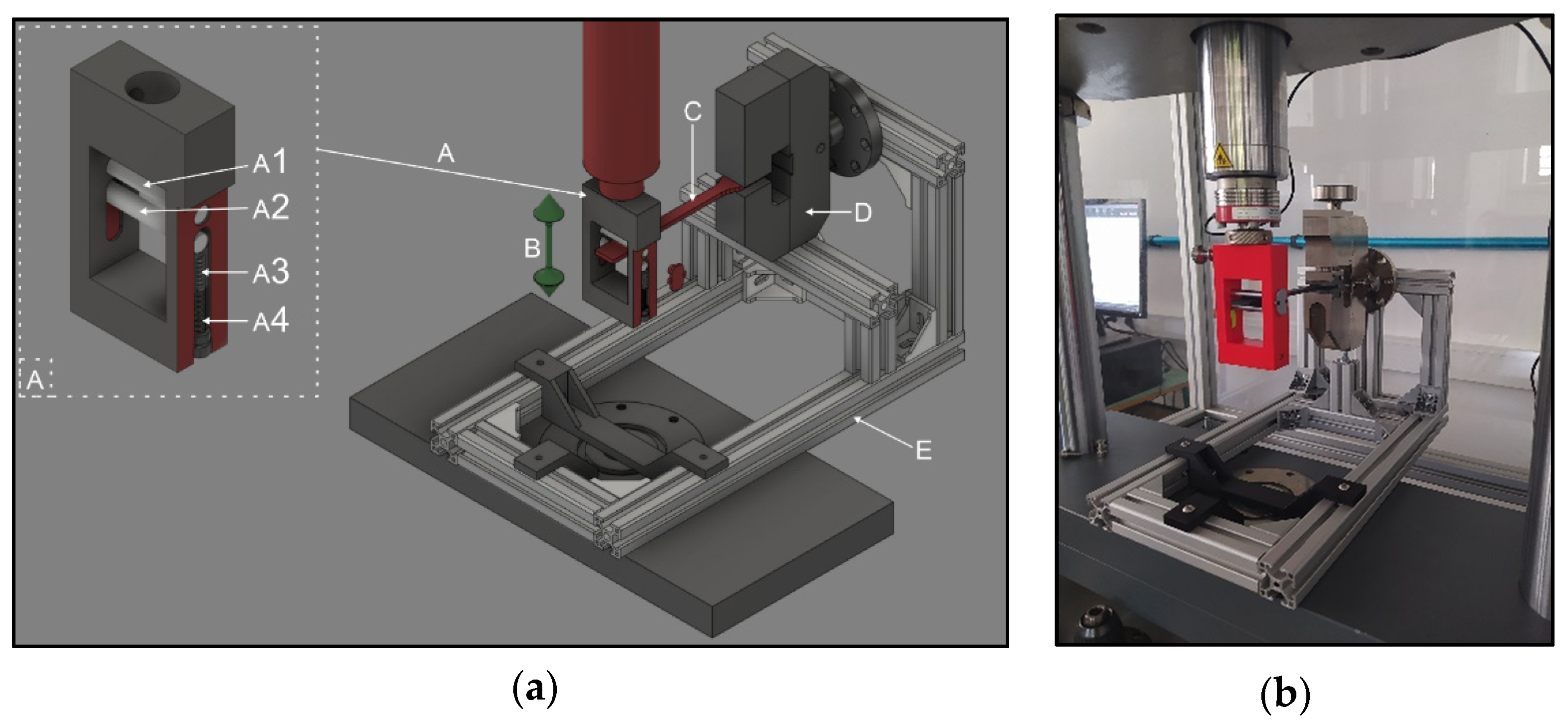
2.2.1. Flexural Fatigue Test
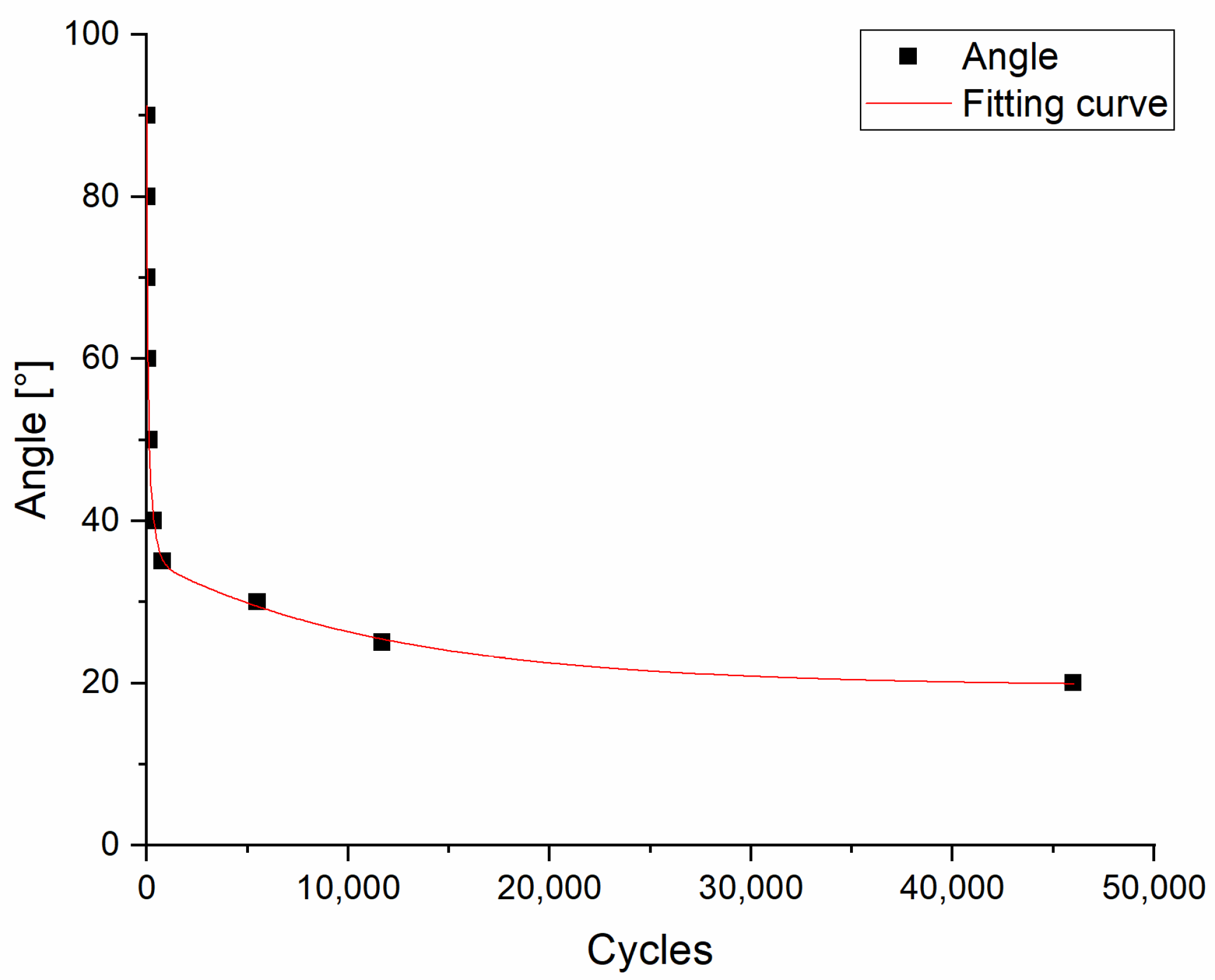
2.2.2. Torsion Fatigue Test
2.2.3. Shore D Measurements
2.2.4. Tensile Test
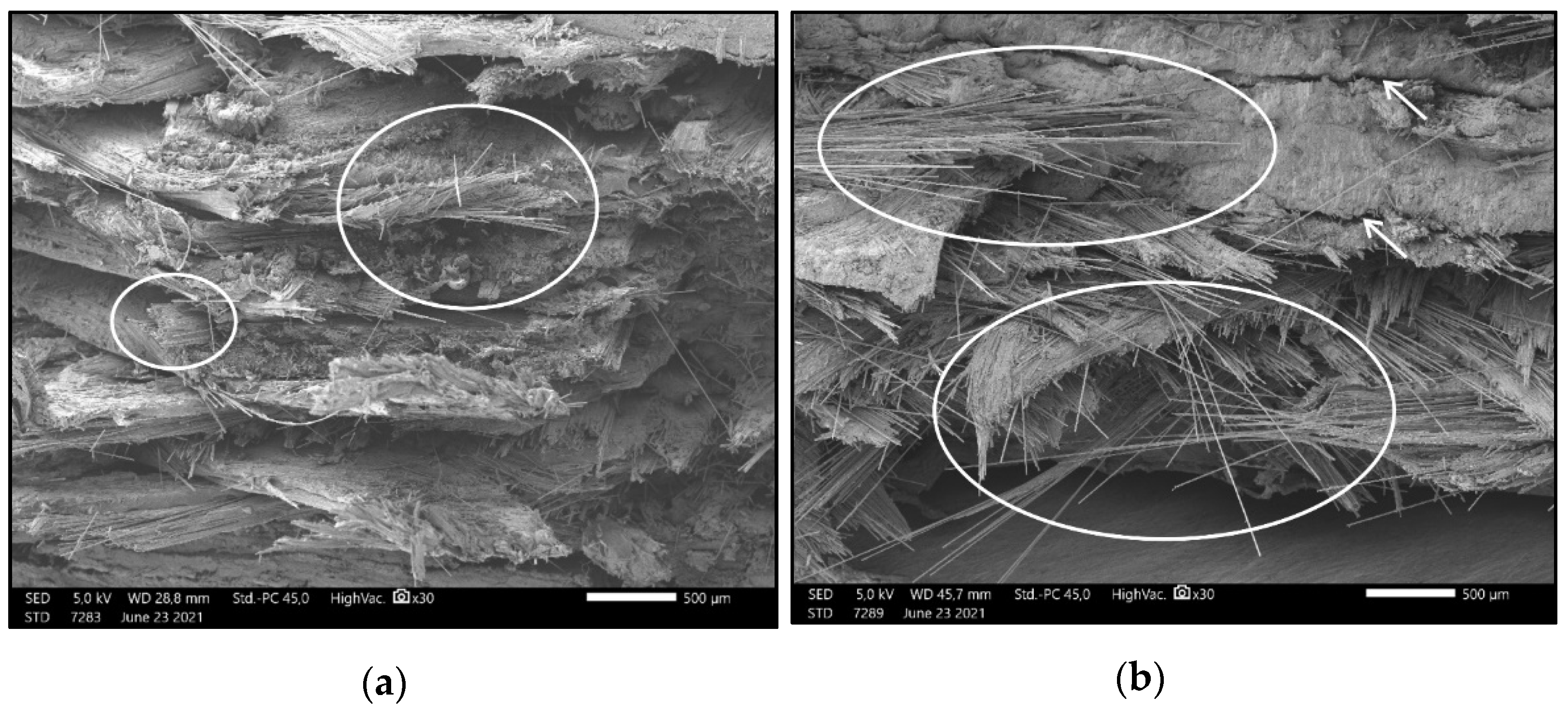
2.2.5. Scanning Electron Microscopy (SEM)
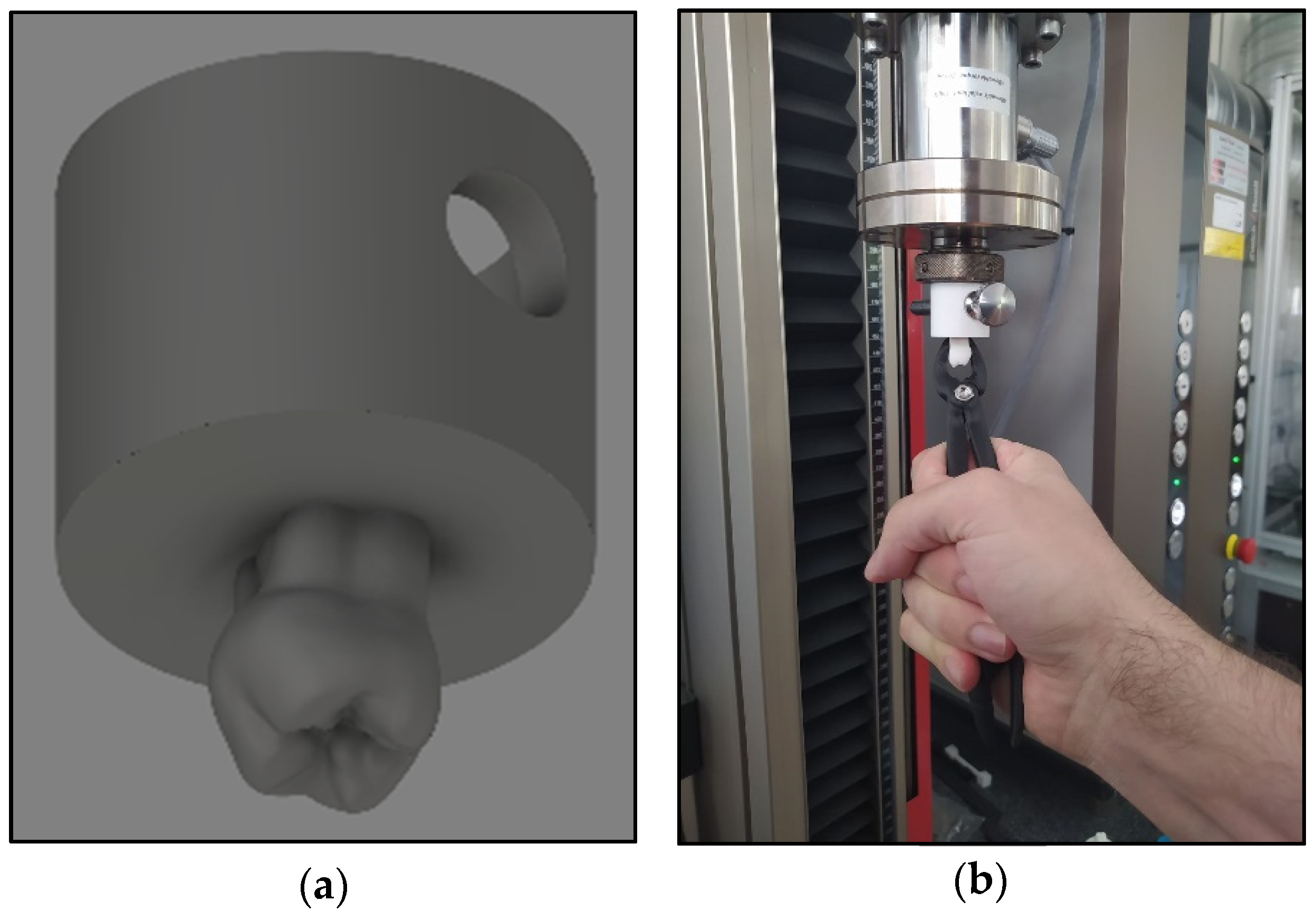
2.2.6. Upper Right First Molar Forceps Scanning, Design, and Printing
2.2.7. Clamp Test with Biaxial Tester and Likert Scale
2.2.8. Statistics and Analysis
3. Results
3.1. Material Testing
3.1.1. Flexural Fatigue Test
3.1.2. Torsion Fatigue Test
3.1.3. Shore D Hardness Measurements
3.1.4. Tensile Test
3.2. Results of Scanning Electron Microscopy Imaging
3.3. Evaluation of 3D-Printed Molar Extruder
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Dentist 1 | Dentist 2 | Dentist 3 | Dentist 4 | Dentist 5 | AVG | ||
|---|---|---|---|---|---|---|---|
| Metal Upper Right Molar Forceps | How firmly could you grab the 3D-printed tooth with the metal dental forceps? | 5 | 4 | 4 | 4 | 5 | 4.4 |
| How similar was the torque you could use to what is necessary in a clinical environment? | 5 | 4 | 4 | 4 | 4 | 4.2 | |
| How similar was the axial extraction force you could use to what is necessary in a clinical environment? | 5 | 5 | 4 | 4 | 5 | 4.6 | |
| Composite Upper Right Molar Forceps | How firmly could you place the forceps on the model? | 3 | 4 | 4 | 4 | 3 | 3.6 |
| How could you compare the similarity of the torque you used to what is necessary in a clinical environment? | 3 | 3 | 4 | 4 | 3 | 3.4 | |
| How could you compare the similarity of the axial extraction force you used to what is necessary in a clinical environment? | 3 | 4 | 3 | 4 | 4 | 3.6 | |
| To what degree could the flexibility of the forceps influence the proper positioning of the equipment during extraction? | 4 | 4 | 5 | 3 | 4 | 4.0 | |
| How convenient did you find the composite forceps during the procedure? | 4 | 5 | 4 | 5 | 4 | 4.4 | |
| Would you use such forceps during patient care? | No | Yes | No | Yes | No | 40% | |
| If you would not use our composite forceps in patient care, could it be made appropriate for you with some modifications? | Yes | Yes | Yes | Yes | Yes | 100% |
References
- Nadagouda, M.N.; Rastogi, V.; Ginn, M. A Review on 3D Printing Techniques for Medical Applications. Curr. Opin. Chem. Eng. 2020, 28, 152–157. [Google Scholar] [CrossRef]
- Sparks, D.; Kavanagh, K.R.; Vargas, J.A.; Valdez, T.A. 3D Printed Myringotomy and Tube Simulation as an Introduction to Otolaryngology for Medical Students. Int. J. Pediatr. Otorhinolaryngol. 2020, 128, 109730. [Google Scholar] [CrossRef]
- Tejo-Otero, A.; Buj-Corral, I.; Fenollosa-Artés, F. 3D Printing in Medicine for Preoperative Surgical Planning: A Review. Ann. Biomed. Eng. 2020, 48, 536–555. [Google Scholar] [CrossRef]
- Okolie, O.; Stachurek, I.; Kandasubramanian, B.; Njuguna, J. 3D Printing for Hip Implant Applications: A Review. Polymers 2020, 12, 2682. [Google Scholar] [CrossRef] [PubMed]
- Toth, L.; Schiffer, A.; Nyitrai, M.; Pentek, A.; Told, R.; Maroti, P. Developing an Anti-Spastic Orthosis for Daily Home-Use of Stroke Patients Using Smart Memory Alloys and 3D Printing Technologies. Mater. Des. 2020, 195, 109029. [Google Scholar] [CrossRef]
- Pentek, A.; Nyitrai, M.; Schiffer, A.; Abraham, H.; Bene, M.; Molnar, E.; Told, R.; Maroti, P. The Effect of Printing Parameters on Electrical Conductivity and Mechanical Properties of PLA and ABS Based Carbon Composites in Additive Manufacturing of Upper Limb Prosthetics. Crystals 2020, 10, 398. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M.; Khan, R.H.; Suman, R. 3D Printing Applications in Bone Tissue Engineering. J. Clin. Orthop. Trauma 2020, 11, S118–S124. [Google Scholar] [CrossRef]
- Wake, N.; Rosenkrantz, A.B.; Huang, R.; Park, K.U.; Wysock, J.S.; Taneja, S.S.; Huang, W.C.; Sodickson, D.K.; Chandarana, H. Patient-Specific 3D Printed and Augmented Reality Kidney and Prostate Cancer Models: Impact on Patient Education. 3D Print. Med. 2019, 5, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javaid, M.; Haleem, A. Current Status and Applications of Additive Manufacturing in Dentistry: A Literature-Based Review. J. Oral Biol. Craniofac. Res. 2019, 9, 179–185. [Google Scholar] [CrossRef]
- Lin, L.; Fang, Y.; Liao, Y.; Chen, G.; Gao, C.; Zhu, P. 3D Printing and Digital Processing Techniques in Dentistry: A Review of Literature. Adv. Eng. Mater. 2019, 21, 1801013. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Cho, J.-W.; Chang, N.-Y.; Chae, J.-M.; Kang, K.-H.; Kim, S.-C.; Cho, J.-H. Accuracy of Three-Dimensional Printing for Manufacturing Replica Teeth. Korean J. Orthod. 2015, 45, 217. [Google Scholar] [CrossRef] [Green Version]
- Turkyilmaz, I.; Wilkins, G.N. 3D Printing in Dentistry—Exploring the New Horizons. J. Dent. Sci. 2021, 16, 1037–1038. [Google Scholar] [CrossRef]
- Wong, J.Y. 3D Printing Applications for Space Missions. Aerosp. Med. Hum. Perform. 2016, 87, 580–582. [Google Scholar] [CrossRef]
- Wong, J.Y.; Pfahnl, A.C. 3D Printing of Surgical Instruments for Long-Duration Space Missions. Aviat. Space Environ. Med. 2014, 85, 758–763. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.Y.; Pfahnl, A.C. 3D Printed Surgical Instruments Evaluated by a Simulated Crew of a Mars Mission. Aerosp. Med. Hum. Perform. 2016, 87, 806–810. [Google Scholar] [CrossRef]
- Saharudin, M.S.; Hajnys, J.; Kozior, T.; Gogolewski, D.; Zmarzły, P. Quality of Surface Texture and Mechanical Properties of PLA and PA-Based Material Reinforced with Carbon Fibers Manufactured by FDM and CFF 3D Printing Technologies. Polymers 2021, 13, 1671. [Google Scholar] [CrossRef]
- Saleh Alghamdi, S.; John, S.; Roy Choudhury, N.; Dutta, N.K. Additive Manufacturing of Polymer Materials: Progress, Promise and Challenges. Polymers 2021, 13, 753. [Google Scholar] [CrossRef] [PubMed]
- Kabir, S.M.F.; Mathur, K.; Seyam, A.-F.M. A Critical Review on 3D Printed Continuous Fiber-Reinforced Composites: History, Mechanism, Materials and Properties. Compos. Struct. 2020, 232, 111476. [Google Scholar] [CrossRef]
- Torres, J.; Cole, M.; Owji, A.; DeMastry, Z.; Gordon, A.P. An Approach for Mechanical Property Optimization of Fused Deposition Modeling with Polylactic Acid via Design of Experiments. Rapid Prototyp. J. 2016, 22, 387–404. [Google Scholar] [CrossRef]
- Ayatollahi, M.R.; Nabavi-Kivi, A.; Bahrami, B.; Yazid Yahya, M.; Khosravani, M.R. The Influence of In-Plane Raster Angle on Tensile and Fracture Strengths of 3D-Printed PLA Specimens. Eng. Fract. Mech. 2020, 237, 107225. [Google Scholar] [CrossRef]
- Gomez-Gras, G.; Jerez-Mesa, R.; Travieso-Rodriguez, J.A.; Lluma-Fuentes, J. Fatigue Performance of Fused Filament Fabrication PLA Specimens. Mater. Des. 2018, 140, 278–285. [Google Scholar] [CrossRef] [Green Version]
- Landaeta, F.J.; Shiozawa, J.N.; Erdman, A.; Piazza, C. Low Cost 3D Printed Clamps for External Fixator for Developing Countries: A Biomechanical Study. 3D Print. Med. 2020, 6, 31. [Google Scholar] [CrossRef]
- Ramesh, M.; Panneerselvam, K. PLA-Based Material Design and Investigation of Its Properties by FDM. In Advances in Additive Manufacturing and Joining; Shunmugam, M.S., Kanthababu, M., Eds.; Springer: Singapore, 2020; pp. 229–241. [Google Scholar]
- Yasa, E.; Ersoy, K. Dimensional Accuracy and Mechanical Properties of Chopped Carbon Reinforced Polymers Produced by Material Extrusion Additive Manufacturing. Materials 2019, 12, 3885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietrich, T.; Schmid, I.; Locher, M.; Addison, O. Extraction Force and Its Determinants for Minimally Invasive Vertical Tooth Extraction. J. Mech. Behav. Biomed. Mater. 2020, 105, 103711. [Google Scholar] [CrossRef] [PubMed]
- Ahel, V.; Ćabov, T.; Špalj, S.; Perić, B.; Jelušić, D.; Dmitrašinović, M. Forces That Fracture Teeth during Extraction with Mandibular Premolar and Maxillary Incisor Forceps. Br. J. Oral Maxillofac. Surg. 2015, 53, 982–987. [Google Scholar] [CrossRef]
- Loy, J.; Tatham, P.; Healey, R.; Cassie, L. Tapper 3D Printing Meets Humanitarian Design Research: Creative Technologies in Remote Regions. In Creative Technologies for Multidisciplinary Applications; Connor, A.M., Marks, S., Eds.; IGI Global: Hershey, PA, USA, 2016; pp. 54–75. ISBN 978-1-5225-0016-2. [Google Scholar]
- Longhitano, G.A.; Nunes, G.B.; Candido, G.; da Silva, J.V.L. The Role of 3D Printing during COVID-19 Pandemic: A Review. Prog. Addit. Manuf. 2021, 6, 19–37. [Google Scholar] [CrossRef]
- Oladapo, B.I.; Ismail, S.O.; Afolalu, T.D.; Olawade, D.B.; Zahedi, M. Review on 3D Printing: Fight against COVID-19. Mater. Chem. Phys. 2021, 258, 123943. [Google Scholar] [CrossRef]
- Rendeki, S.; Nagy, B.; Bene, M.; Pentek, A.; Toth, L.; Szanto, Z.; Told, R.; Maroti, P. An Overview on Personal Protective Equipment (PPE) Fabricated with Additive Manufacturing Technologies in the Era of COVID-19 Pandemic. Polymers 2020, 12, 2073. [Google Scholar] [CrossRef] [PubMed]
- Culmone, C.; Smit, G.; Breedveld, P. Additive Manufacturing of Medical Instruments: A State-of-the-Art Review. Addit. Manuf. 2019, 27, 461–473. [Google Scholar] [CrossRef]
- Nikitakos, N.; Dagkinis, I.; Papachristos, D.; Georgantis, G.; Kostidi, E. Chapter 6—Economics in 3D printing. In 3D Printing: Applications in Medicine and Surgery; Tsoulfas, G., Bangeas, P.I., Suri, J.S., Eds.; Elsevier: St. Louis, MO, USA, 2020; pp. 85–95. ISBN 978-0-323-66164-5. [Google Scholar]







| Bending (mm) | Thickness at Middle Point (mm) | Thickness at Flexural Point (mm) | Difference (mm) | Permanent Deflection (mm) |
|---|---|---|---|---|
| 10 | 4.182 | 4.163 | −0.019 | 0 |
| 15 | 4.174 | 4.18 | 0.006 | 0 |
| 20 | 4.154 | 4.093 | −0.061 | 0 |
| 25 | 4.152 | 4.133 | −0.019 | 0 |
| 30 | 4.073 | 4.046 | −0.027 | 0 |
| 35 | 4.16 | 4.392 | 0.232 | 8.16 |
| 40 | 4.153 | 4.474 | 0.321 | 9.20 |
| 45 | 4.121 | 4.537 | 0.416 | 12.78 |
| 50 | 4.059 | 4.397 | 0.338 | 10.78 |
| Metal Forceps | 3D-Printed Composite Forceps | ||
|---|---|---|---|
| Dentist | Force of Normal Extraction of Tooth (N) | Force of Strong Extraction of Tooth (N) | Force Performed with Composite Forceps (N) |
| Dentist 1. | 50 | 80 | 69.0 |
| Dentist 2. | 46 | 84 | 71.5 |
| Dentist 3. | 79 | 95 | 72.8 |
| Dentist 4. | 41 | 60 | 74.8 |
| Dentist 5. | 73 | 105 | 63.4 |
| Average (N) | 57.80 | 84.80 | 70.30 |
| SD (N) | 17.05 | 16.96 | 4.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Told, R.; Marada, G.; Rendeki, S.; Pentek, A.; Nagy, B.; Molnar, F.J.; Maroti, P. Manufacturing a First Upper Molar Dental Forceps Using Continuous Fiber Reinforcement (CFR) Additive Manufacturing Technology with Carbon-Reinforced Polyamide. Polymers 2021, 13, 2647. https://doi.org/10.3390/polym13162647
Told R, Marada G, Rendeki S, Pentek A, Nagy B, Molnar FJ, Maroti P. Manufacturing a First Upper Molar Dental Forceps Using Continuous Fiber Reinforcement (CFR) Additive Manufacturing Technology with Carbon-Reinforced Polyamide. Polymers. 2021; 13(16):2647. https://doi.org/10.3390/polym13162647
Chicago/Turabian StyleTold, Roland, Gyula Marada, Szilard Rendeki, Attila Pentek, Balint Nagy, Ferenc Jozsef Molnar, and Peter Maroti. 2021. "Manufacturing a First Upper Molar Dental Forceps Using Continuous Fiber Reinforcement (CFR) Additive Manufacturing Technology with Carbon-Reinforced Polyamide" Polymers 13, no. 16: 2647. https://doi.org/10.3390/polym13162647
APA StyleTold, R., Marada, G., Rendeki, S., Pentek, A., Nagy, B., Molnar, F. J., & Maroti, P. (2021). Manufacturing a First Upper Molar Dental Forceps Using Continuous Fiber Reinforcement (CFR) Additive Manufacturing Technology with Carbon-Reinforced Polyamide. Polymers, 13(16), 2647. https://doi.org/10.3390/polym13162647








