The Influence of Hydrophobic Blocks of PEO-Containing Copolymers on Glyceryl Monooleate Lyotropic Liquid Crystalline Nanoparticles for Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. MEC Synthesis
2.2.2. Polymerization of the Block Copolymers
2.2.3. Preparation of Liquid Crystalline Nanoparticle Dispersions
2.2.4. Physicochemical and Morphological Characterization of the Liquid Crystalline Dispersions
Dynamic and Electrophoretic Light Scattering
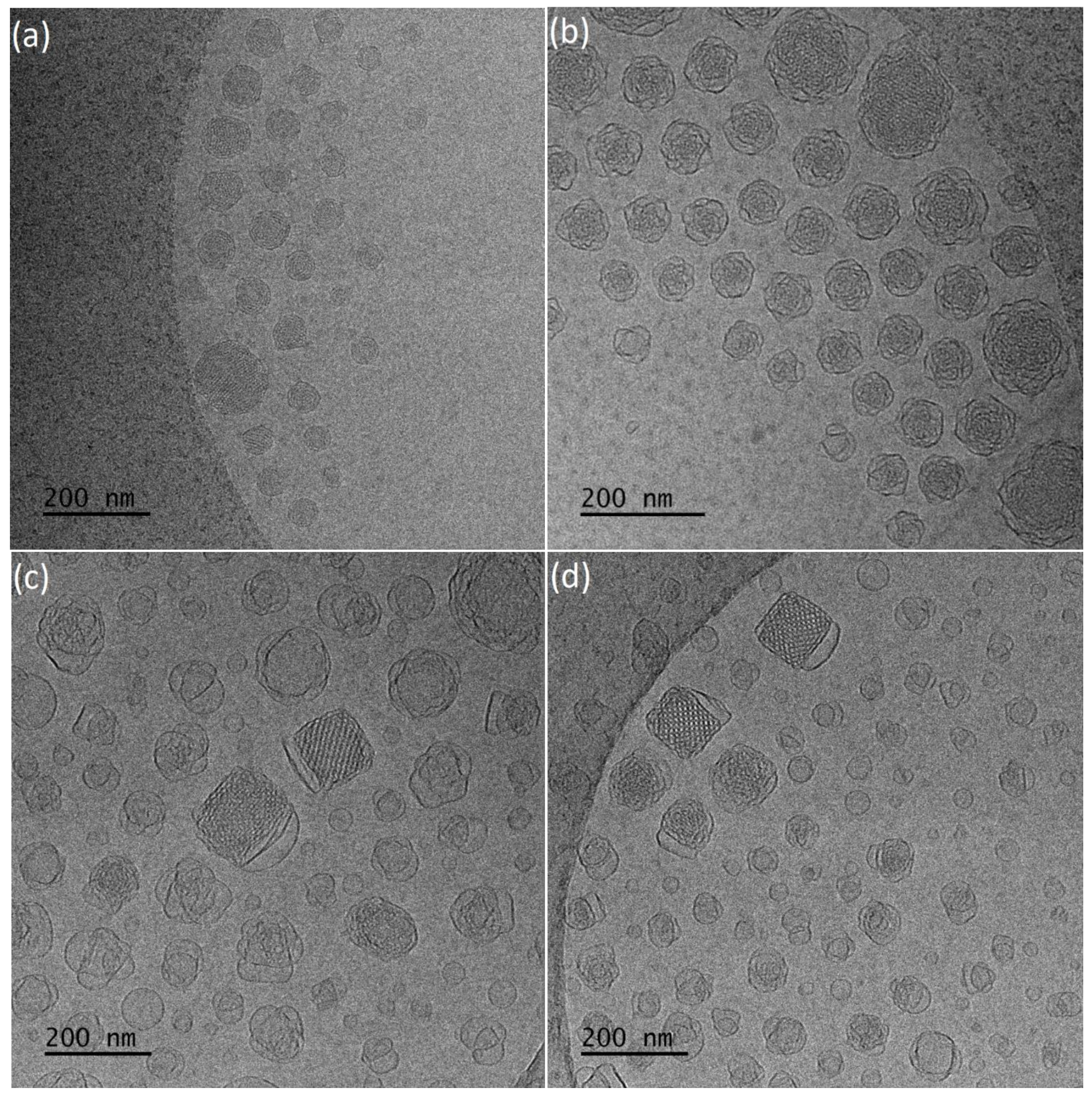
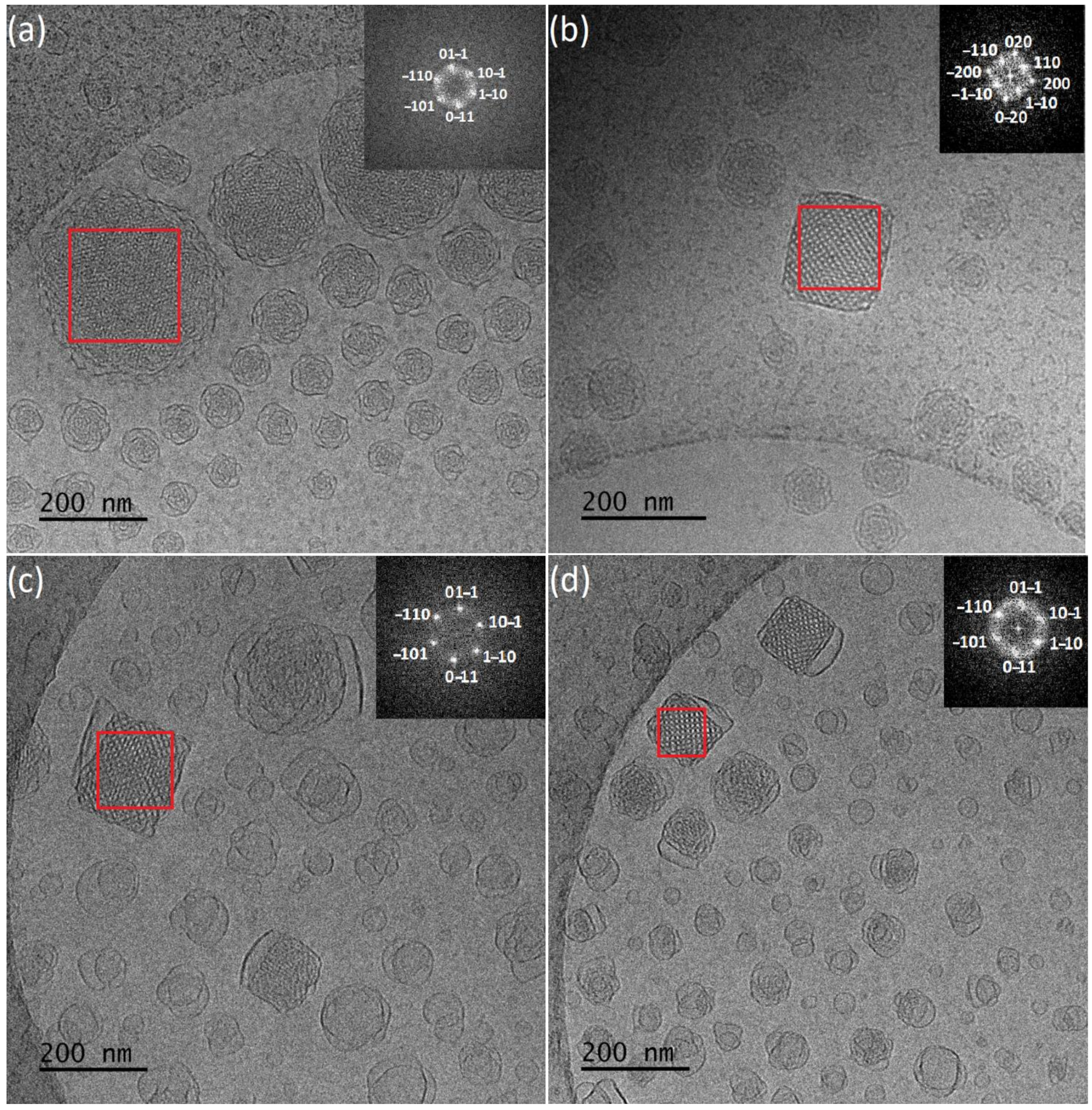
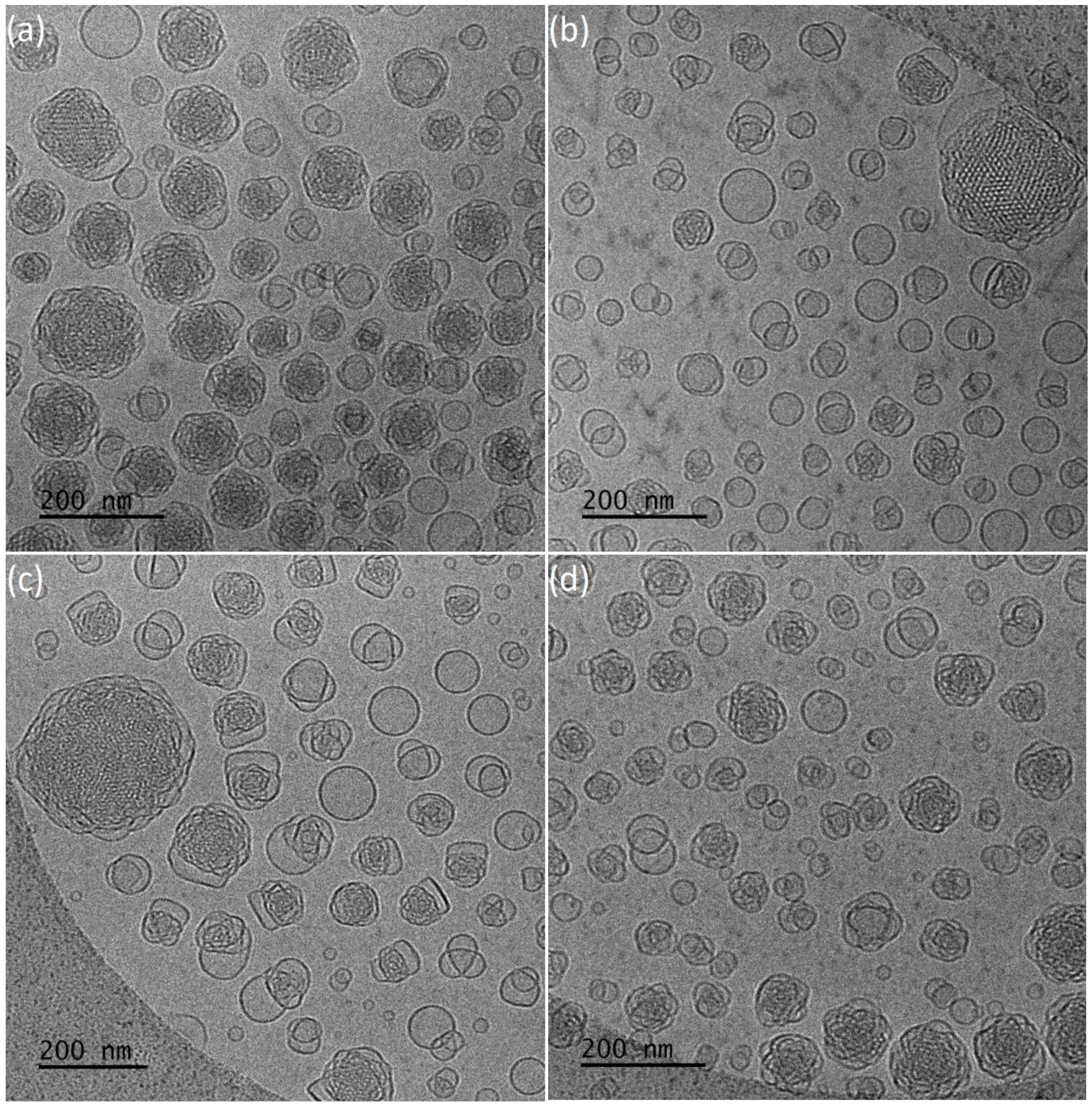
Cryogenic Transmission Electron Microscopy (Cryo-TEM)
X-ray Diffraction (XRD)
2.2.5. Characterization of the Liquid Crystalline Dispersions with Entrapped Resveratrol
Entrapment Efficiency and Drug Loading Determination
In Vitro Resveratrol Release Studies
3. Results and Discussion
3.1. Physicochemical and Morphological Characteristics of the Liquid Crystalline Nanosystems as Revealed by DLS and Cryo-TEM
3.2. X-ray Diffraction
3.3. Resveratrol Entrapment and In Vitro Release Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dierking, I.; Neto, A.M.F. Novel trends in lyotropic liquid crystals. Crystals 2020, 10, 604. [Google Scholar] [CrossRef]
- Singhvi, G.; Banerjee, S.; Khosa, A. Lyotropic Liquid Crystal Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780128136638. [Google Scholar]
- Barriga, H.M.G.; Holme, M.N.; Stevens, M.M. Cubosomes: The next generation of smart lipid nanoparticles? Angew. Chem. Int. Ed. 2019, 58, 2958–2978. [Google Scholar] [CrossRef] [Green Version]
- Nisini, R.; Poerio, N.; Mariotti, S.; De Santis, F.; Fraziano, M. The multirole of liposomes in therapy and prevention of infectious diseases. Front. Immunol. 2018, 9, 155. [Google Scholar] [CrossRef] [PubMed]
- Yaghmur, A.; Mu, H. Recent advances in drug delivery applications of cubosomes, hexosomes, and solid lipid nanoparticles. Acta Pharm. Sin. B 2021, 11, 871–885. [Google Scholar] [CrossRef] [PubMed]
- Barriga, H.M.G.; Ces, O.; Law, R.V.; Seddon, J.M.; Brooks, N.J. Engineering swollen cubosomes using cholesterol and anionic lipids. Langmuir 2019, 35, 16521–16527. [Google Scholar] [CrossRef]
- Boge, L.; Hallstensson, K.; Ringstad, L.; Johansson, J.; Andersson, T.; Davoudi, M.; Larsson, P.T.; Mahlapuu, M.; Håkansson, J.; Andersson, M. Cubosomes for topical delivery of the antimicrobial peptide LL-37. Eur. J. Pharm. Biopharm. 2019, 134, 60–67. [Google Scholar] [CrossRef]
- Karami, Z.; Hamidi, M. Cubosomes: Remarkable drug delivery potential. Drug Discov. Today 2016, 21, 789–801. [Google Scholar] [CrossRef]
- Azmi, I.D.M.; Moghimi, S.M.; Yaghmur, A. Cubosomes and hexosomes as versatile platforms for drug delivery. Ther. Deliv. 2015, 6, 1347–1364. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ma, P.; Gui, S. Cubic and hexagonal liquid crystals as drug delivery systems. Biomed. Res. Int. 2014, 2014, 116. [Google Scholar] [CrossRef] [PubMed]
- Chountoulesi, M.; Pippa, N.; Pispas, S.; Chrysina, E.D.; Forys, A.; Trzebicka, B.; Demetzos, C. Cubic lyotropic liquid crystals as drug delivery carriers: Physicochemical and morphological studies. Int. J. Pharm. 2018, 550, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Han, K.; Peng, X.; Yang, Z.; Qin, L.; Zhu, C.; Huang, X.; Shi, X.; Dian, L.; Lu, M.; et al. Nanostructed cubosomes as advanced drug delivery system. Curr. Pharm. Des. 2013, 19, 6290–6297. [Google Scholar] [CrossRef]
- Wu, H.; Li, J.; Zhang, Q.; Yan, X.; Guo, L.; Gao, X.; Qiu, M.; Jiang, X.; Lai, R.; Chen, H. A novel small odorranalectin-bearing cubosomes: Preparation, brain delivery and pharmacodynamic study on amyloid-β 25-35-treated rats following intranasal administration. Eur. J. Pharm. Biopharm. 2012, 80, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Mariani, P.; Ravani, L.; Contado, C.; Volta, M.; Bido, S.; Drechsler, M.; Mazzoni, S.; Menegatti, E.; Morari, M.; et al. Nanoparticulate lipid dispersions for bromocriptine delivery: Characterization and in vivo study. Eur. J. Pharm. Biopharm. 2012, 80, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Angelova, A.; Hu, F.; Garamus, V.M.; Peng, C.; Li, N.; Liu, J.; Liu, D.; Zou, A. PH responsiveness of hexosomes and cubosomes for combined delivery of brucea javanica oil and doxorubicin. Langmuir 2019, 35, 14532–14542. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, S.B.; Assmus, D.; Boehnke, A.; Hanley, T.; Boyd, B.J.; Rades, T.; Hook, S. Preparation of phytantriol cubosomes by solvent precursor dilution for the delivery of protein vaccines. Eur. J. Pharm. Biopharm. 2011, 79, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Conn, C.E.; Waddington, L.J.; Mudie, S.T.; Drummond, C.J. Colloidal amphiphile self-assembly particles composed of gadolinium oleate and Myverol: Evaluation as contrast agents for magnetic resonance imaging. Langmuir 2010, 26, 2383–2391. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Bye, N.; Moffat, B.A.; Wright, D.K.; Cuddihy, A.; Hinton, T.M.; Hawley, A.M.; Reynolds, N.P.; Waddington, L.J.; Mulet, X.; et al. Dual-modality NIRF-MRI cubosomes and hexosomes: High throughput formulation and in vivo biodistribution. Mater. Sci. Eng. C 2017, 71, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Caltagirone, C.; Falchi, A.M.; Lampis, S.; Lippolis, V.; Meli, V.; Monduzzi, M.; Prodi, L.; Schmidt, J.; Sgarzi, M.; Talmon, Y.; et al. Cancer-cell-targeted theranostic cubosomes. Langmuir 2014, 30, 6228–6236. [Google Scholar] [CrossRef]
- Meli, V.; Caltagirone, C.; Falchi, A.M.; Hyde, S.T.; Lippolis, V.; Monduzzi, M.; Obiols-Rabasa, M.; Rosa, A.; Schmidt, J.; Talmon, Y.; et al. Docetaxel-loaded fluorescent liquid-crystalline nanoparticles for cancer theranostics. Langmuir 2015, 31, 9566–9575. [Google Scholar] [CrossRef]
- Zhang, L.; Li, J.; Tian, D.; Sun, L.; Wang, X.; Tian, M. Theranostic combinatorial drug-loaded coated cubosomes for enhanced targeting and efficacy against cancer cells. Cell Death Dis. 2020, 11, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Akhlaghi, S.P.; Loh, W. Interactions and release of two palmitoyl peptides from phytantriol cubosomes. Eur. J. Pharm. Biopharm. 2017, 117, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Mansour, M.; Kamel, A.O.; Mansour, S.; Mortada, N.D. Novel polyglycerol-dioleate based cubosomal dispersion with tailored physical characteristics for controlled delivery of ondansetron. Colloids Surf. B Biointerfaces 2017, 156, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Noor, A.H.; Ghareeb, M.M. Formulation and evaluation of ondansetron HCl nanoparticles for transdermal delivery. Iraqi J. Pharm. Sci. 2020, 29, 70–79. [Google Scholar] [CrossRef]
- Clemente, I.; Bonechi, C.; Rodolfi, L.; Bacia-Verloop, M.; Rossi, C.; Ristori, S. Lipids from algal biomass provide new (nonlamellar) nanovectors with high carrier potentiality for natural antioxidants. Eur. J. Pharm. Biopharm. 2021, 158, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.Y.T.T.; Mulet, X.; Postma, A.; Keddie, D.J.; Waddington, L.J.; Boyd, B.J.; Drummond, C.J. Novel RAFT amphiphilic brush copolymer steric stabilisers for cubosomes: Poly(octadecyl acrylate)-block-poly(polyethylene glycol methyl ether acrylate). Soft Matter 2014, 10, 6666–6676. [Google Scholar] [CrossRef]
- Chong, J.Y.T.; Mulet, X.; Boyd, B.J.; Drummond, C.J. Steric Stabilizers for Cubic Phase Lyotropic Liquid Crystal Nanodispersions (Cubosomes), 1st ed.; Elsevier: Amsterdam, The Netherlands, 2015; Volume 21. [Google Scholar]
- Chountoulesi, M.; Perinelli, D.R.; Forys, A.; Bonacucina, G.; Trzebicka, B.; Pispas, S.; Demetzos, C. Liquid crystalline nanoparticles for drug delivery: The role of gradient and block copolymers on the morphology, internal organisation and release profile. Eur. J. Pharm. Biopharm. 2020, 158, 21–34. [Google Scholar] [CrossRef]
- Hamada, N.; Gakhar, S.; Longo, M.L. Hybrid lipid/block copolymer vesicles display broad phase coexistence region. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183552. [Google Scholar] [CrossRef]
- Khan, S.; McCabe, J.; Hill, K.; Beales, P.A. Biodegradable hybrid block copolymer—Lipid vesicles as potential drug delivery systems. J. Colloid Interface Sci. 2020, 562, 418–428. [Google Scholar] [CrossRef]
- Kulkarni, C.V.; Vishwapathi, V.K.; Quarshie, A.; Moinuddin, Z.; Page, J.; Kendrekar, P.; Mashele, S.S. Self-assembled lipid cubic phase and cubosomes for the delivery of aspirin as a model drug. Langmuir 2017, 33, 9907–9915. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Hinton, T.M.; Waddington, L.J.; Fong, C.; Tran, N.; Mulet, X.; Drummond, C.J.; Muir, B.W. Lipid-PEG conjugates sterically stabilize and reduce the toxicity of phytantriol-based lyotropic liquid crystalline nanoparticles. Langmuir 2015, 31, 10871–10880. [Google Scholar] [CrossRef]
- Zhai, J.; Suryadinata, R.; Luan, B.; Tran, N.; Hinton, T.M.; Ratcliffe, J.; Hao, X.; Drummond, C.J. Amphiphilic brush polymers produced using the RAFT polymerisation method stabilise and reduce the cell cytotoxicity of lipid lyotropic liquid crystalline nanoparticles. Faraday Discuss. 2016, 191, 545–563. [Google Scholar] [CrossRef]
- Kluzek, M.; Tyler, A.I.I.; Wang, S.; Chen, R.; Marques, C.M.; Thalmann, F.; Seddon, J.M.; Schmutz, M. Influence of a pH-sensitive polymer on the structure of monoolein cubosomes. Soft Matter 2017, 13, 7571–7577. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Wen, X.; Pan, X.; Wang, R.; Chen, B.; Wu, C. Design and in vitro evaluation of capsaicin transdermal controlled release cubic phase gels. AAPS PharmSciTech 2010, 11, 1405–1410. [Google Scholar] [CrossRef]
- Gustafsson, J.; Ljusberg-Wahren, H.; Almgren, M.; Larsson, K. Submicron particles of reversed lipid phases in water stabilized by a nonionic amphiphilic polymer. Langmuir 1997, 13, 6964–6971. [Google Scholar] [CrossRef]
- Da Dong, Y.; Larson, I.; Barnes, T.J.; Prestidge, C.A.; Allen, S.; Chen, X.; Roberts, C.J.; Boyd, B.J. Understanding the interfacial properties of nanostructured liquid crystalline materials for surface-specific delivery applications. Langmuir 2012, 28, 13485–13495. [Google Scholar] [CrossRef] [PubMed]
- D’souza, A.A.; Shegokar, R. Polyethylene Glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef]
- Pippa, N.; Kaditi, E.; Pispas, S.; Demetzos, C. PEO-b-PCL-DPPC chimeric nanocarriers: Self-assembly aspects in aqueous and biological media and drug incorporation. Soft Matter 2013, 9, 4073–4082. [Google Scholar] [CrossRef]
- Gou, M.; Wei, X.; Men, K.; Wang, B.; Luo, F.; Zhao, X.; Wei, Y.; Qian, Z. PCL/PEG copolymeric nanoparticles: Potential nanoplatforms for anticancer agent delivery. Curr. Drug Targets 2011, 12, 1131–1150. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Müller, S.S.; Frey, H. Beyond poly(ethylene glycol): Linear polyglycerol as a multifunctional polyether for biomedical and pharmaceutical applications. Biomacromolecules 2014, 15, 1935–1954. [Google Scholar] [CrossRef]
- Abuchowski, A.; McCoy, J.R.; Palczuk, N.C.; van Es, T.; Davis, F.F. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J. Biol. Chem. 1977, 252, 3582–3586. [Google Scholar] [CrossRef]
- Milton Harris, J.; Chess, R.B. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2003, 2, 214–221. [Google Scholar] [CrossRef]
- Kolate, A.; Baradia, D.; Patil, S.; Vhora, I.; Kore, G.; Misra, A. PEG—A versatile conjugating ligand for drugs and drug delivery systems. J. Control Release 2014, 192, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Södergård, A.; Stolt, M. Industrial Production of High Molecular Weight Poly(lactic acid). In Poly(Lactic Acid): Synthesis, Structures, Properties, Processing, and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 27–41. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic Acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Han, Y.; Guan, J.; Chung, S.; Wang, C.; Li, D. Poly(ethylene glycol)-polylactide micelles for cancer therapy. Front. Pharmacol. 2018, 9, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Garlotta, D. A literature review of poly (lactic acid). J. Polym. Environ. 2019, 9, 63–84. [Google Scholar] [CrossRef]
- De Queiroz, T.S.; Prado, R.F.; Aparecida, I.; De Brito, W.; De Oliveira, L.D.; Marotta, L.; De Vasconcellos, R.; Camargo, E.A. Cytotoxicity and genotoxicity of PLA and PCL membranes on osteoblasts. Acta Sci. Dent 2019, 3, 55–59. [Google Scholar]
- Fukushima, K.; Pratt, R.C.; Nederberg, F.; Tan, J.P.K.; Yang, Y.Y.; Waymouth, R.M.; Hedrick, J.L. Organocatalytic approach to amphiphillic comb-block copolymers capable of stereocomplexation and self-assembly. Biomacromolecules 2008, 9, 3051–3056. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.J.; Hendren, R.W.; Jensen, K.; Pitt, C.G. Synthesis, properties, and biodegradation of poly(1,3-trimethylene carbonate). Macromolecules 1991, 24, 1736–1740. [Google Scholar] [CrossRef]
- Liu, Z.L.; Zhou, Y.; Zhuo, R.X. Synthesis and properties of functional aliphatic polycarbonates. J. Polym. Sci. Part A Polym. Chem. 2003, 41, 4001–4006. [Google Scholar] [CrossRef]
- Keylor, M.H.; Matsuura, B.S.; Stephenson, C.R.J. Chemistry and biology of resveratrol-derived natural products. Chem. Rev. 2015, 115, 8976–9027. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A double-edged sword in health benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef] [Green Version]
- Nakano, M.; Sugita, A.; Matsuoka, H.; Handa, T. Small-angle X-ray scattering and 13C NMR investigation on the internal structure of “cubosomes”. Langmuir 2001, 17, 3917–3922. [Google Scholar] [CrossRef]
- Akhlaghi, S.P.; Ribeiro, I.R.; Boyd, B.J.; Loh, W. Impact of preparation method and variables on the internal structure, morphology, and presence of liposomes in phytantriol-Pluronic® F127 cubosomes. Colloids Surf. B Biointerfaces 2016, 145, 845–853. [Google Scholar] [CrossRef]
- Chong, J.Y.T.; Mulet, X.; Waddington, L.J.; Boyd, B.J.; Drummond, C.J. Steric stabilisation of self-assembled cubic lyotropic liquid crystalline nanoparticles: High throughput evaluation of triblock polyethylene oxide-polypropylene oxide-polyethylene oxide copolymers. Soft Matter 2011, 7, 4768–4777. [Google Scholar] [CrossRef]
- Rietveld, H.M. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Rietveld, H.M. Line profiles of neutron powder-diffraction peaks for structure refinement. Acta Crystallogr. 1967, 22, 151–152. [Google Scholar] [CrossRef]
- Pippa, N.; Merkouraki, M.; Pispas, S.; Demetzos, C. DPPC:MPOx chimeric advanced drug delivery nano systems (chi-aDDnSs): Physicochemical and structural characterization, stability and drug release studies. Int. J. Pharm. 2013, 450, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Pippa, N.; Dokoumetzidis, A.; Pispas, S.; Demetzos, C. The interplay between the rate of release from polymer grafted liposomes and their fractal morphology. Int. J. Pharm. 2014, 465, 63–69. [Google Scholar] [CrossRef]
- Chong, J.Y.T.; Mulet, X.; Waddington, L.J.; Boyd, B.J.; Drummond, C.J. High-throughput discovery of novel steric stabilizers for cubic lyotropic liquid crystal nanoparticle dispersions. Langmuir 2012, 28, 9223–9232. [Google Scholar] [CrossRef] [PubMed]
- Barauskas, J.; Misiunas, A.; Gunnarsson, T.; Tiberg, F.; Johnsson, M. “Sponge” nanoparticle dispersions in aqueous mixtures of diglycerol monooleate, glycerol dioleate, and polysorbate 80. Langmuir 2006, 22, 6328–6334. [Google Scholar] [CrossRef]
- Barauskas, J.; Johnsson, M.; Tiberg, F. Self-assembled lipid superstructures: Beyond vesicles and liposomes. Nano Lett. 2005, 5, 1615–1619. [Google Scholar] [CrossRef]
- Chountoulesi, M.; Perinelli, D.R.; Pippa, N.; Chrysostomou, V.; Forys, A.; Otulakowski, L.; Bonacucina, G.; Trzebicka, B.; Pispas, S.; Demetzos, C. Physicochemical, morphological and thermal evaluation of lyotropic lipidic liquid crystalline nanoparticles: The effect of stimuli-responsive polymeric stabilizer. Colloids Surf. A Physicochem. Eng. Asp. 2020, 595, 124678. [Google Scholar] [CrossRef]
- Tilley, A.J.; Drummond, C.J.; Boyd, B.J. Disposition and association of the steric stabilizer Pluronic® F127 in lyotropic liquid crystalline nanostructured particle dispersions. J. Colloid Interface Sci. 2013, 392, 288–296. [Google Scholar] [CrossRef]
- Sagalowicz, L.; Michel, M.; Adrian, M.; Frossard, P.; Rouvet, M.; Watzke, H.J.; Yaghmur, A.; De Campo, L.; Glatter, O.; Leser, M.E. Crystallography of dispersed liquid crystalline phases studied by cryo-transmission electron microscopy. J. Microsc. 2006, 221, 110–121. [Google Scholar] [CrossRef]
- Badie, H.; Abbas, H. Novel small self-assembled resveratrol-bearing cubosomes and hexosomes: Preparation, charachterization, and ex vivo permeation. Drug Dev. Ind. Pharm. 2018, 44, 2013–2025. [Google Scholar] [CrossRef]
- Elgindy, N.A.; Mehanna, M.M.; Mohyeldin, S.M. Self-assembled nano-architecture liquid crystalline particles as a promising carrier for progesterone transdermal delivery. Int. J. Pharm. 2016, 501, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Boyd, B.J.; Whittaker, D.V.; Khoo, S.M.; Davey, G. Lyotropic liquid crystalline phases formed from glycerate surfactants as sustained release drug delivery systems. Int. J. Pharm. 2006, 309, 218–226. [Google Scholar] [CrossRef]
- Thorn, C.R.; Clulow, A.J.; Boyd, B.J.; Prestidge, C.A.; Thomas, N. Bacterial lipase triggers the release of antibiotics from digestible liquid crystal nanoparticles. J. Control. Release 2020, 319, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Fan, Y.; Zhang, N.; Trépout, S.; Ptissam, B.; Brûlet, A.; Tang, B.Z.; Li, M.H. Fluorescent polymer cubosomes and hexosomes with aggregation-induced emission. Chem. Sci. 2021, 12, 5495–5504. [Google Scholar] [CrossRef] [PubMed]
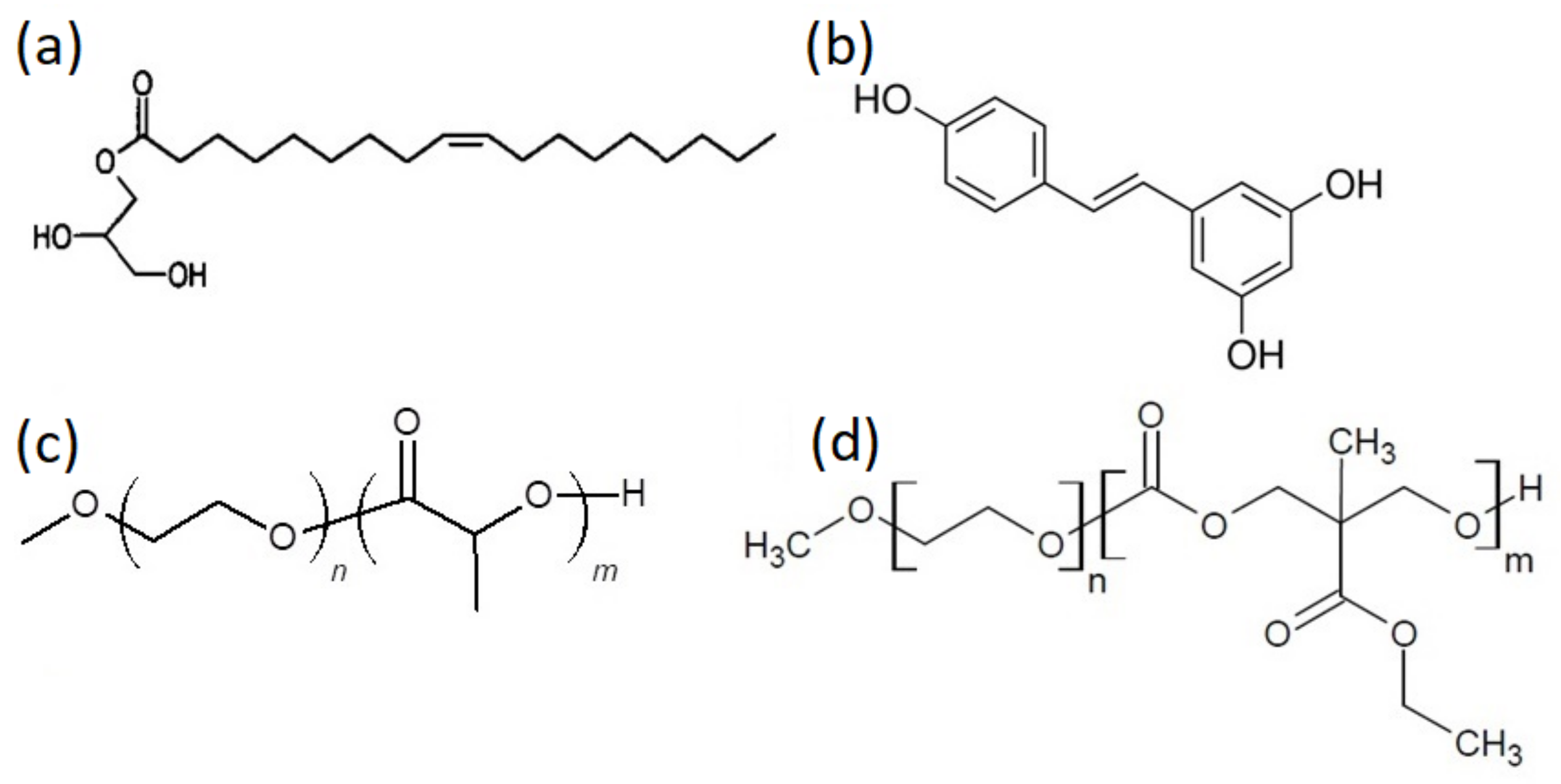
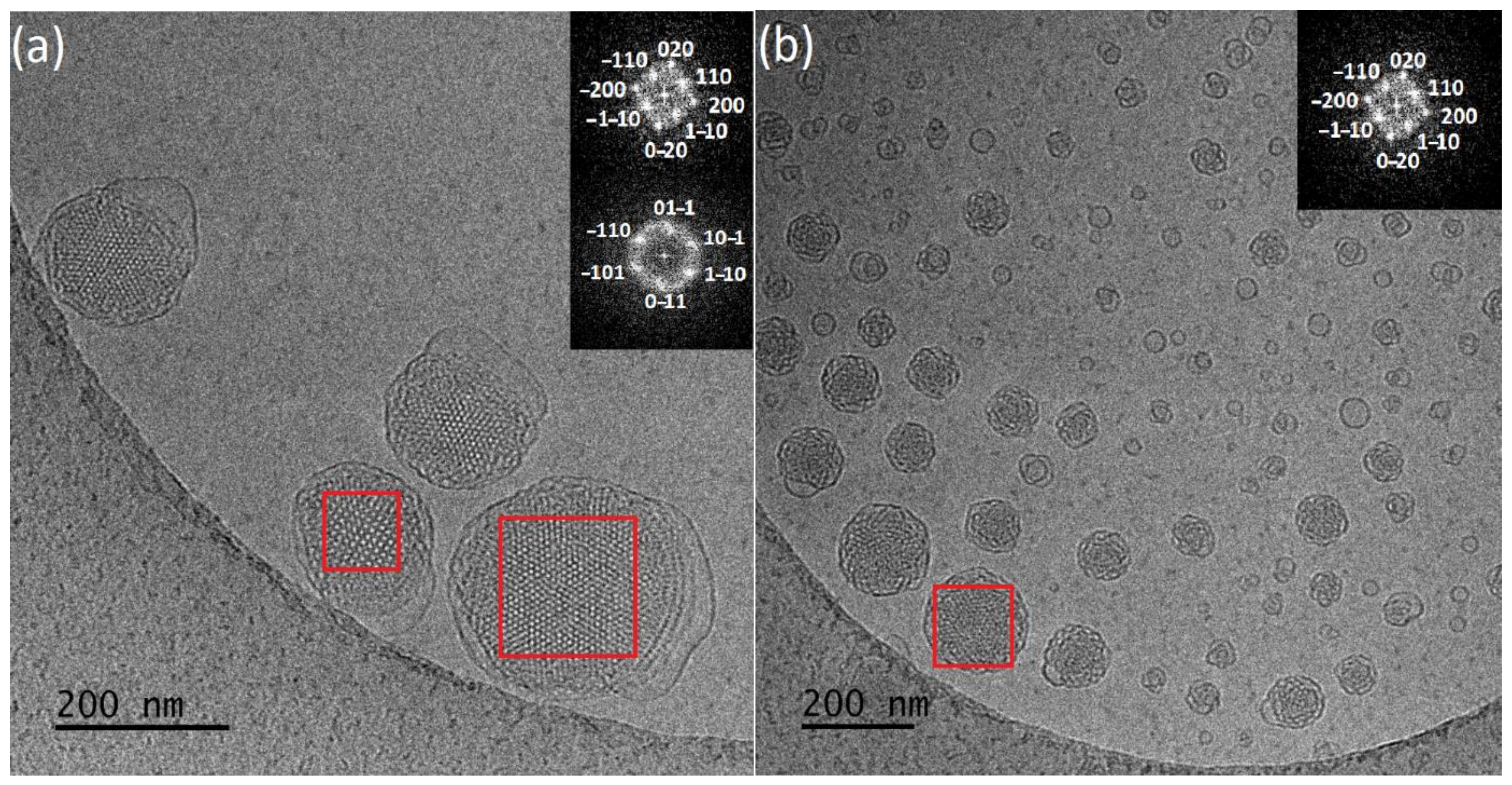
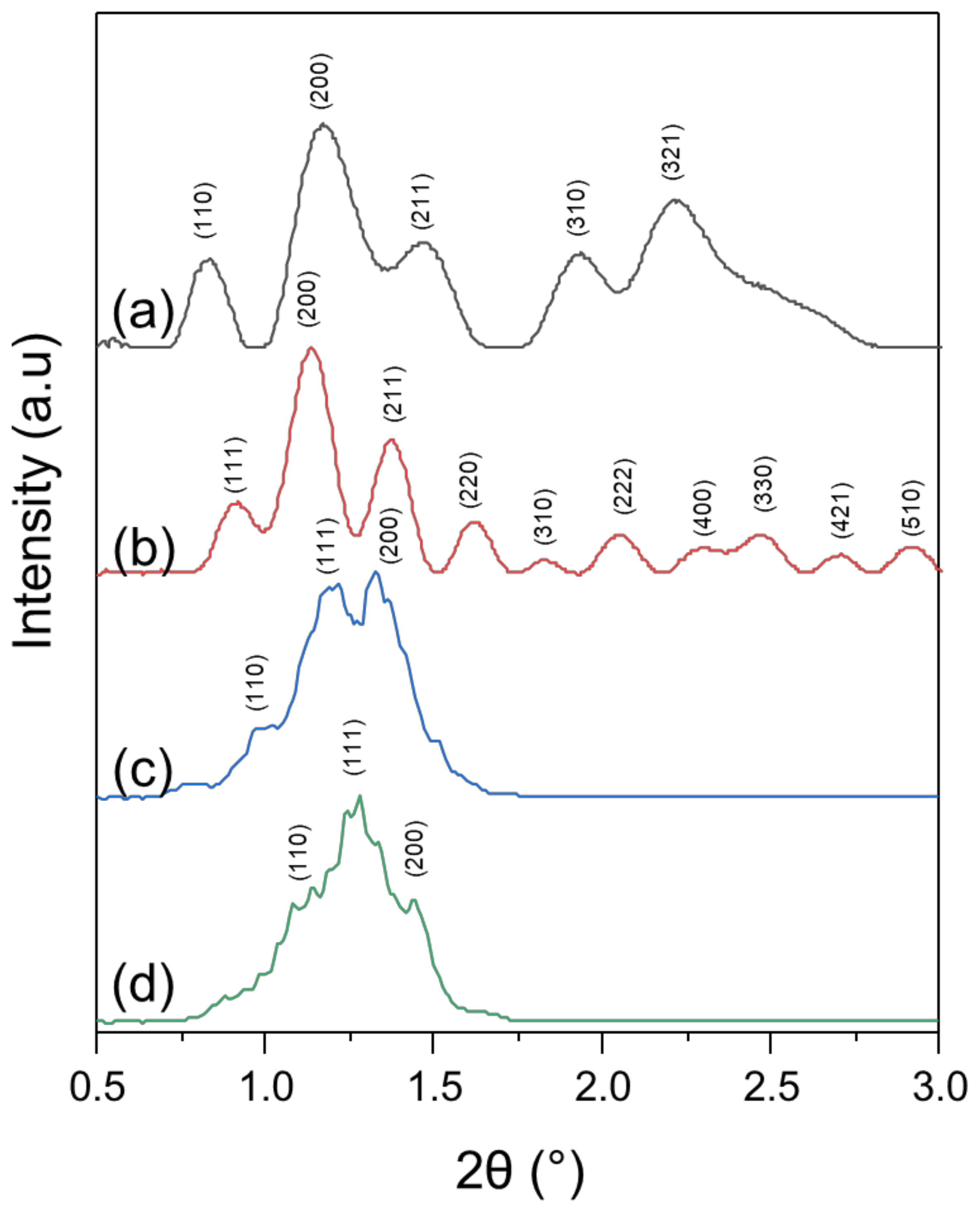
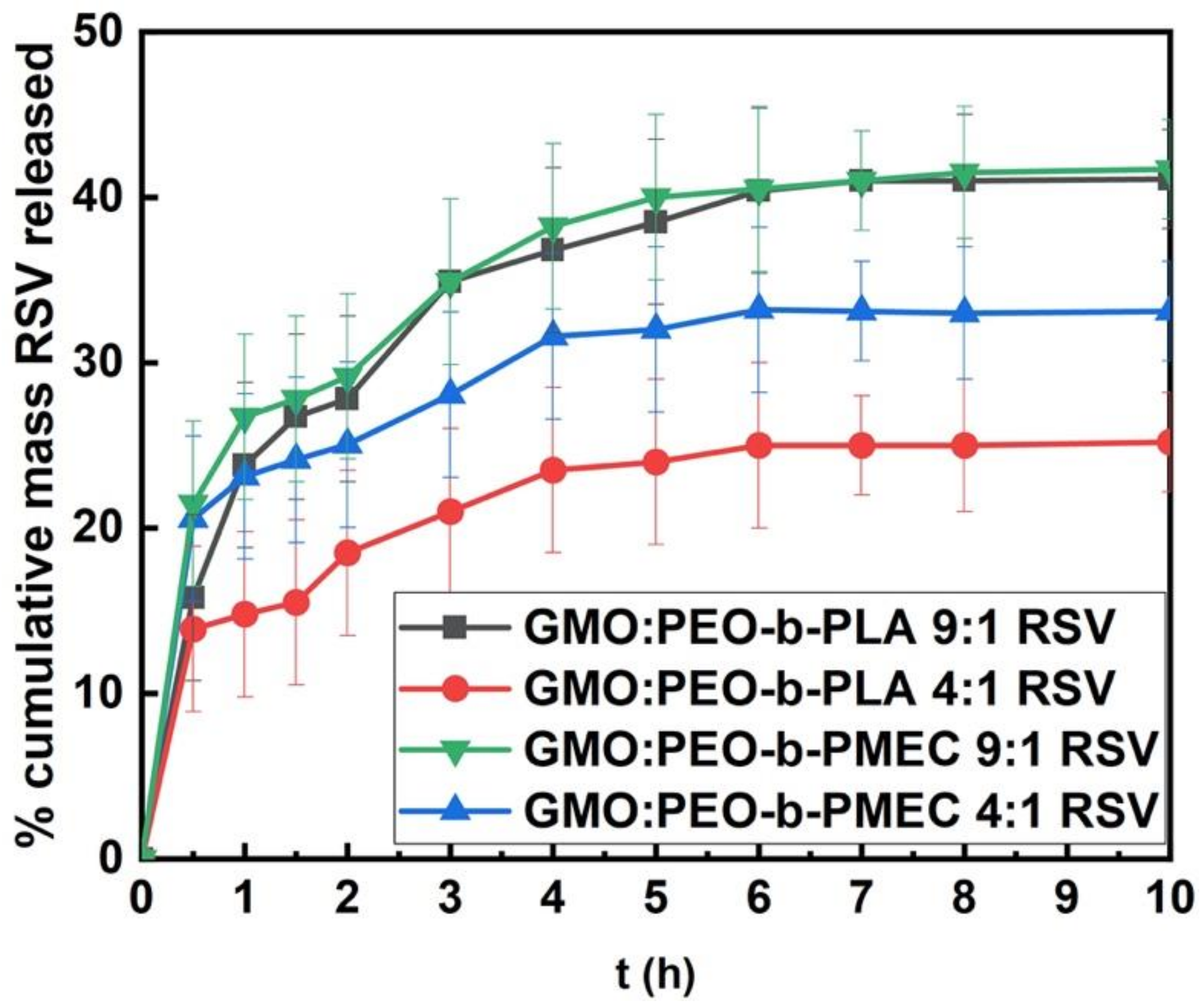
| Polymer | DP(NMR) of Hydrophobic Block | Mn(NMR) | Mw(GPC) | Mw/Mn a | %wt b |
|---|---|---|---|---|---|
| PEO-b-PLA | 18 | 6700 | 6850 | 1.06 | %wt PEO 81 |
| PEO-b-PMEC | 9 | 7100 | 6560 | 1.05 | %wt PEO 76 |
| Sample | Weight Ratio | Rh (nm) | PDI (±SD) | ζ-Pot (mV) | Average Size (nm) Cryo-TEM | Space Group XRD | Space Group FFT |
|---|---|---|---|---|---|---|---|
| GMO: PEO-b-PLA | 9:1 | 88 and 10 | 0.53 (±0.04) | −13 | 66 | Pn3m | Pn3m |
| GMO: PEO-b-PLA | 4:1 | 137 and 18 | 0.62 (±0.01) | −17 | 88 | Im3m | Im3m/Pn3m |
| GMO: PEO-b-PMEC | 9:1 | 150 and 19 | 0.63 (±0.03) | −21 | 85 | Pn3m | Pn3m |
| GMO: PEO-b-PMEC | 4:1 | 141 and 16 | 0.55 (±0.02) | −16 | 63 | Pn3m | Pn3m |
| GMO: PEO-b-PLA +resveratrol | 9:1 | 17 | 0.40 (±0.02) | −26 | 85 | - | - |
| GMO: PEO-b-PLA +resveratrol | 4:1 | 146 and 20 | 0.55 (±0.04) | −13 | 78 | - | - |
| GMO: PEO-b-PMEC +resveratrol | 9:1 | 179 and 23 | 0.58 (±0.01) | −27 | 147 | - | Im3m/Pn3m |
| GMO: PEO-b-PMEC +resveratrol | 4:1 | 157 and 14 | 0.85 (±0.04) | −18 | 57 | - | Im3m |
| Sample | Weight Ratio | Entrapment Efficiency (%) | Drug Loading (%) |
|---|---|---|---|
| GMO:PEO-b-PLA | 9:1 | 99.91 | 9.99 |
| GMO:PEO-b-PLA | 4:1 | 99.84 | 9.98 |
| GMO:PEO-b-PMEC | 9:1 | 99.84 | 9.98 |
| GMO:PEO-b-PMEC | 4:1 | 99.46 | 9.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forys, A.; Chountoulesi, M.; Mendrek, B.; Konieczny, T.; Sentoukas, T.; Godzierz, M.; Kordyka, A.; Demetzos, C.; Pispas, S.; Trzebicka, B. The Influence of Hydrophobic Blocks of PEO-Containing Copolymers on Glyceryl Monooleate Lyotropic Liquid Crystalline Nanoparticles for Drug Delivery. Polymers 2021, 13, 2607. https://doi.org/10.3390/polym13162607
Forys A, Chountoulesi M, Mendrek B, Konieczny T, Sentoukas T, Godzierz M, Kordyka A, Demetzos C, Pispas S, Trzebicka B. The Influence of Hydrophobic Blocks of PEO-Containing Copolymers on Glyceryl Monooleate Lyotropic Liquid Crystalline Nanoparticles for Drug Delivery. Polymers. 2021; 13(16):2607. https://doi.org/10.3390/polym13162607
Chicago/Turabian StyleForys, Aleksander, Maria Chountoulesi, Barbara Mendrek, Tomasz Konieczny, Theodore Sentoukas, Marcin Godzierz, Aleksandra Kordyka, Costas Demetzos, Stergios Pispas, and Barbara Trzebicka. 2021. "The Influence of Hydrophobic Blocks of PEO-Containing Copolymers on Glyceryl Monooleate Lyotropic Liquid Crystalline Nanoparticles for Drug Delivery" Polymers 13, no. 16: 2607. https://doi.org/10.3390/polym13162607
APA StyleForys, A., Chountoulesi, M., Mendrek, B., Konieczny, T., Sentoukas, T., Godzierz, M., Kordyka, A., Demetzos, C., Pispas, S., & Trzebicka, B. (2021). The Influence of Hydrophobic Blocks of PEO-Containing Copolymers on Glyceryl Monooleate Lyotropic Liquid Crystalline Nanoparticles for Drug Delivery. Polymers, 13(16), 2607. https://doi.org/10.3390/polym13162607












