Fabrication of Bio-Nanocomposite Based on HNT-Methionine for Controlled Release of Phenytoin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. HNT Purification
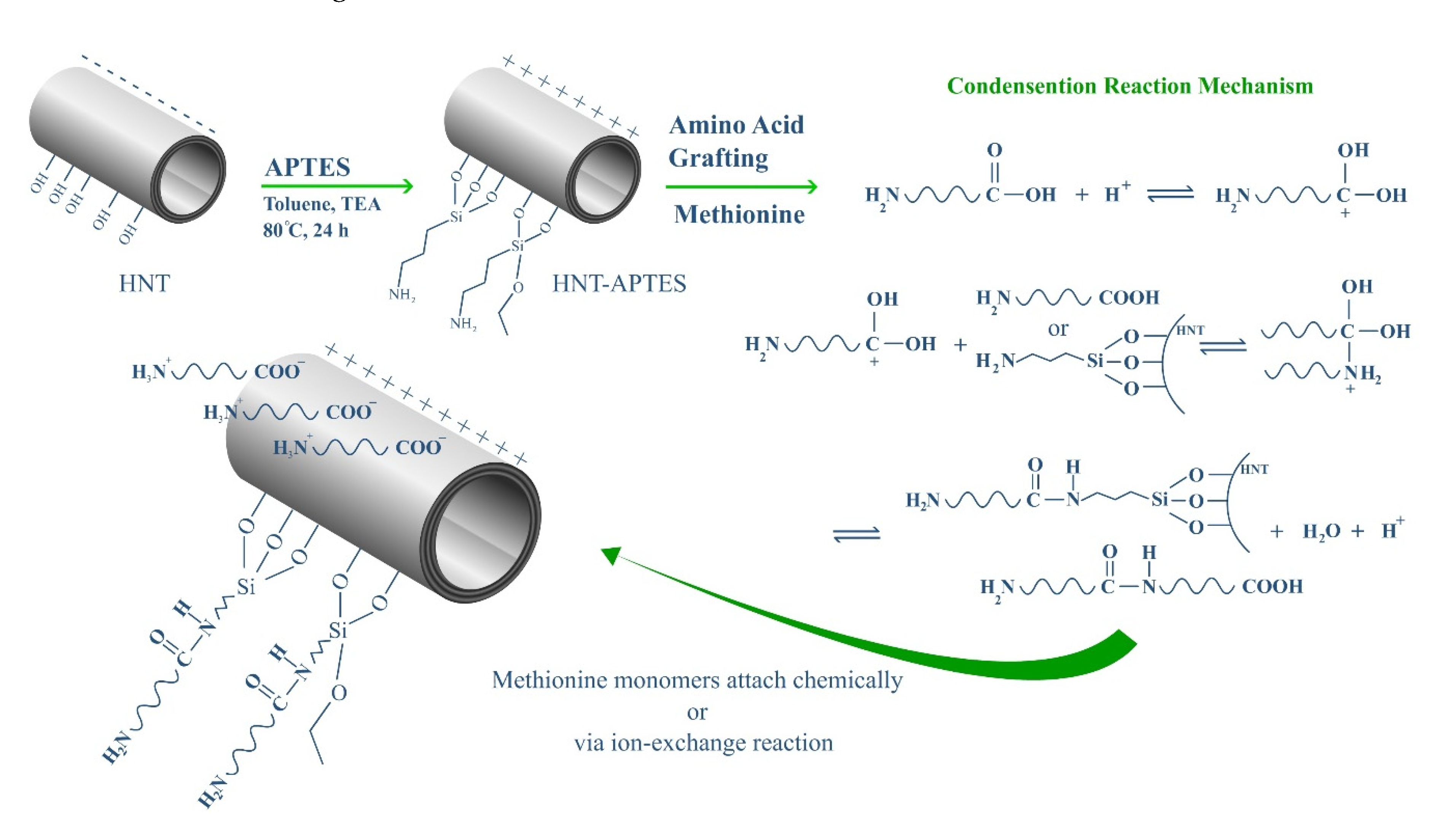
2.3. HNT Modification with APTES
2.4. Preparation of HNT-g-Met Nanocomposites
2.5. PHT Loading Studies
2.6. In Vitro Drug Release Studies
2.7. Characterization
2.8. Statistical Analysis
3. Results and Discussion
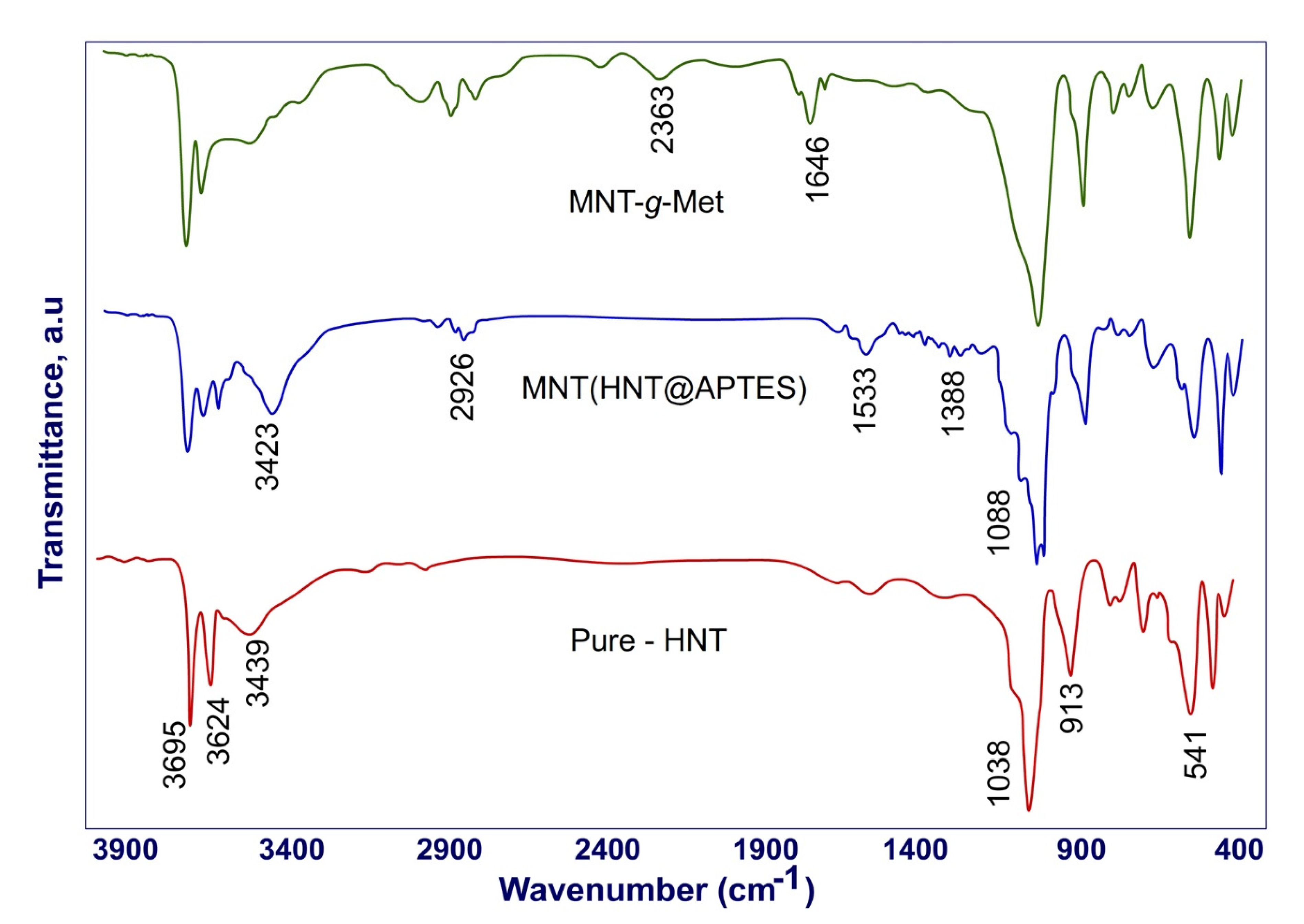
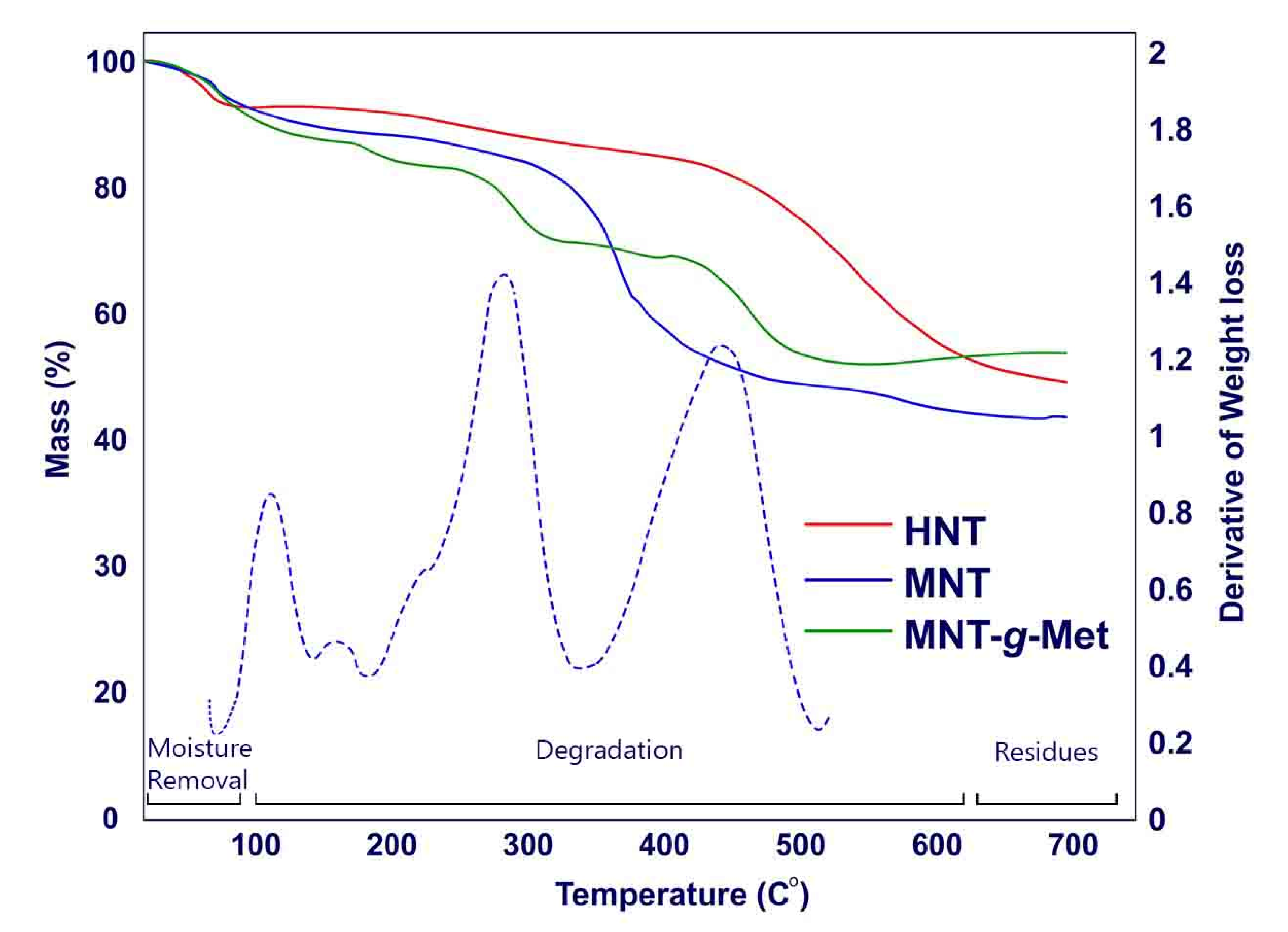
3.1. Physicochemical Studies
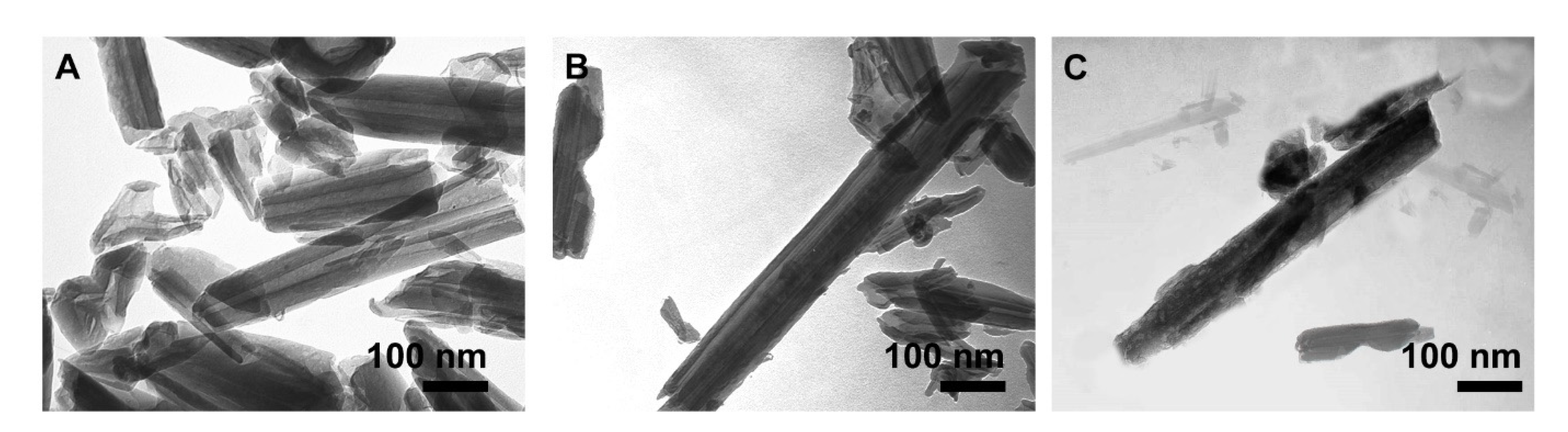
3.2. Morphology Studies
3.3. Loading Studies of PHT
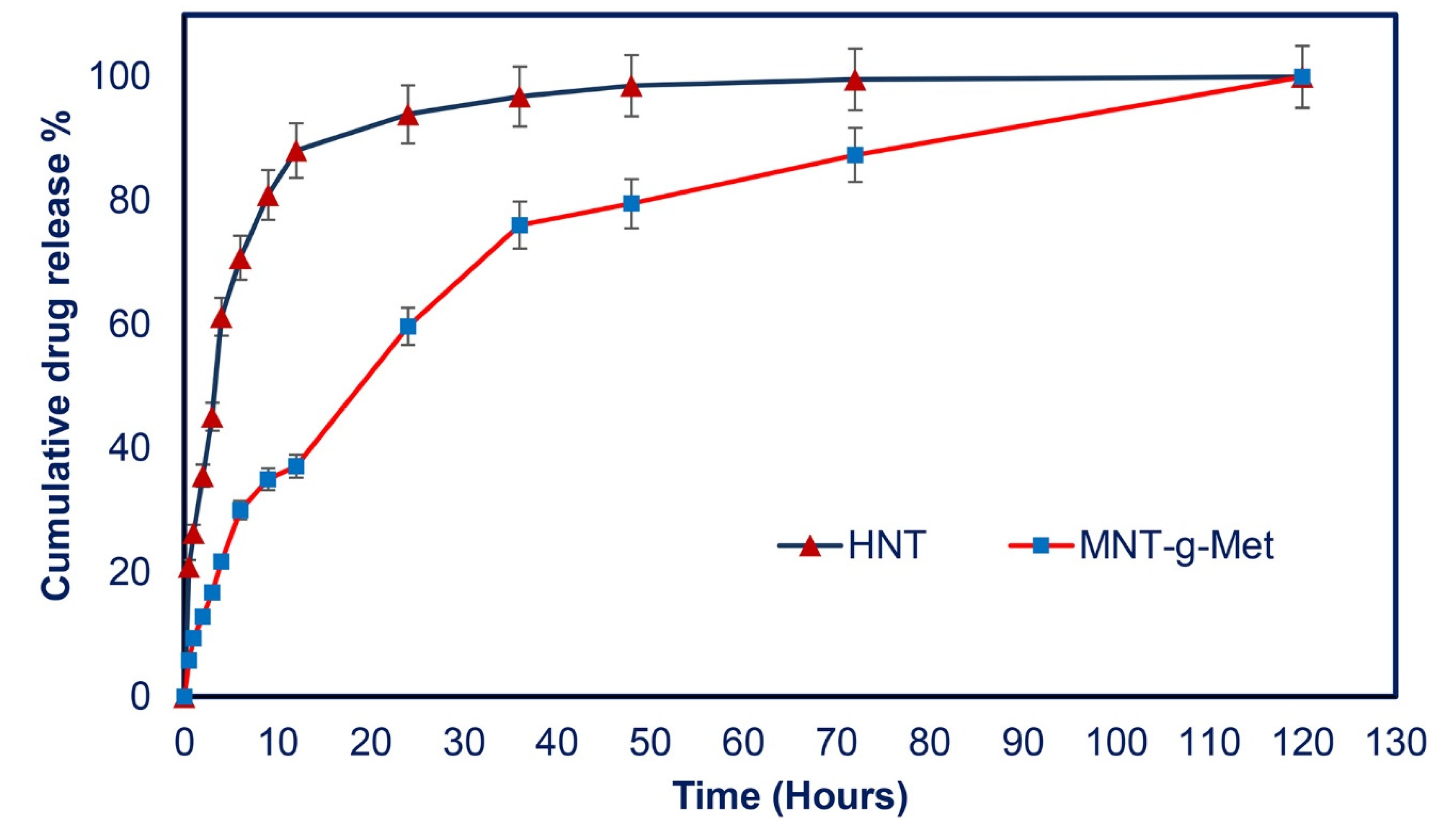
3.4. In Vitro Drug Release Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharifi, S.; Hajipour, M.J.; Gould, L.; Mahmoudi, M. Nanomedicine in Healing Chronic Wounds: Opportunities and Challenges. Mol. Pharm. 2020, 18, 550–575. [Google Scholar] [CrossRef]
- Nethi, S.K.; Das, S.; Patra, C.R.; Mukherjee, S. Recent advances in inorganic nanomaterials for wound-healing applications. Biomater. Sci. 2019, 7, 2652–2674. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef]
- Mawer, G.; Mullen, P.; Rodgers, M.; Robins, A.; Lucas, S. Phenytoin dose adjustment in epileptic patients. Br. J. Clin. Pharmacol. 1974, 1, 163–168. [Google Scholar] [CrossRef] [Green Version]
- Herranz, J.L.; Armijo, J.A.; Arteaga, R. Clinical side effects of phenobarbital, primidone, phenytoin, carbamazepine, and valproate during monotherapy in children. Epilepsia 1988, 29, 794–804. [Google Scholar] [CrossRef]
- Scheinfeld, N. Phenytoin in cutaneous medicine: Its uses and side effects. Dermatol. Online J. 2003, 9, 9. [Google Scholar] [CrossRef]
- Pendse, A.K.; Sharma, A.; Sodani, A.; Hada, S. Topical phenytoin in wound healing. Int. J. Dermatol. 1993, 32, 214–217. [Google Scholar] [CrossRef]
- Teo, S.Y.; Yew, M.Y.; Lee, S.Y.; Rathbone, M.J.; Gan, S.N.; Coombes, A.G. In vitro evaluation of novel phenytoin-loaded alkyd nanoemulsions designed for application in topical wound healing. J. Pharm. Sci. 2017, 106, 377–384. [Google Scholar] [CrossRef] [Green Version]
- Doshi, A.; McAuley, J.W.; Tatakis, D.N. Topical phenytoin effects on palatal wound healing. J. Periodontol. 2020. [Google Scholar] [CrossRef]
- Sanad, A.; Emile, S.; Thabet, W.; Ellaithy, R. A randomized controlled trial on the effect of topical phenytoin 2% on wound healing after anal fistulotomy. Colorectal Dis. 2019, 21, 697–704. [Google Scholar] [CrossRef]
- Kopsky, D.J.; Hesselink, J.M.K. Topical phenytoin for the treatment of neuropathic pain. J. Pain Res. 2017, 10, 469. [Google Scholar] [CrossRef] [Green Version]
- Motawea, A.; Abd El, A.E.-G.H.; Borg, T.; Motawea, M.; Tarshoby, M. The impact of topical phenytoin loaded nanostructured lipid carriers in diabetic foot ulceration. Foot 2019, 40, 14–21. [Google Scholar] [CrossRef]
- Mohebali, A.; Abdouss, M.; Taromi, F.A. Fabrication of biocompatible antibacterial nanowafers based on HNT/PVA nanocomposites loaded with minocycline for burn wound dressing. Mater. Sci. Eng. C 2020, 110, 110685. [Google Scholar] [CrossRef]
- Zahidah, K.A.; Kakooei, S.; Ismail, M.C.; Raja, P.B. Halloysite nanotubes as nanocontainer for smart coating application: A review. Prog. Org. Coat. 2017, 111, 175–185. [Google Scholar] [CrossRef]
- Cavallaro, G.; Danilushkina, A.A.; Evtugyn, V.G.; Lazzara, G.; Milioto, S.; Parisi, F.; Rozhina, E.V.; Fakhrullin, R.F. Halloysite nanotubes: Controlled access and release by smart gates. Nanomaterials 2017, 7, 199. [Google Scholar] [CrossRef]
- Rao, K.M.; Kumar, A.; Suneetha, M.; Han, S.S. pH and near-infrared active; chitosan-coated halloysite nanotubes loaded with curcumin-Au hybrid nanoparticles for cancer drug delivery. Int. J. Biol. Macromol. 2018, 112, 119–125. [Google Scholar] [CrossRef]
- Kumar, A.; Zo, S.M.; Kim, J.H.; Kim, S.-C.; Han, S.S. Enhanced physical, mechanical, and cytocompatibility behavior of polyelectrolyte complex hydrogels by reinforcing halloysite nanotubes and graphene oxide. Compos. Sci. Technol. 2019, 175, 35–45. [Google Scholar] [CrossRef]
- Kumar, A.; Rao, K.M.; Han, S.S. Development of sodium alginate-xanthan gum based nanocomposite scaffolds reinforced with cellulose nanocrystals and halloysite nanotubes. Polym. Test. 2017, 63, 214–225. [Google Scholar] [CrossRef]
- Tarasova, E.; Naumenko, E.; Rozhina, E.; Akhatova, F.; Fakhrullin, R. Cytocompatibility and uptake of polycations-modified halloysite clay nanotubes. Appl. Clay Sci. 2019, 169, 21–30. [Google Scholar] [CrossRef]
- Panchal, A.; Fakhrullina, G.; Fakhrullin, R.; Lvov, Y. Self-assembly of clay nanotubes on hair surface for medical and cosmetic formulations. Nanoscale 2018, 10, 18205–18216. [Google Scholar] [CrossRef]
- Bediako, E.G.; Nyankson, E.; Dodoo-Arhin, D.; Agyei-Tuffour, B.; Łukowiec, D.; Tomiczek, B.; Yaya, A.; Efavi, J.K. Modified halloysite nanoclay as a vehicle for sustained drug delivery. Heliyon 2018, 4, e00689. [Google Scholar] [CrossRef] [Green Version]
- Abdullayev, E.; Joshi, A.; Wei, W.; Zhao, Y.; Lvov, Y. Enlargement of halloysite clay nanotube lumen by selective etching of aluminum oxide. ACS Nano 2012, 6, 7216–7226. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Parisi, F.; Milioto, S.; Lazzara, G. Colloidal stability of halloysite clay nanotubes. Ceram. Int. 2019, 45, 2858–2865. [Google Scholar] [CrossRef]
- Liu, M.; Jia, Z.; Jia, D.; Zhou, C. Recent advance in research on halloysite nanotubes-polymer nanocomposite. Prog. Polym. Sci. 2014, 39, 1498–1525. [Google Scholar] [CrossRef]
- Lazzara, G.; Cavallaro, G.; Panchal, A.; Fakhrullin, R.; Stavitskaya, A.; Vinokurov, V.; Lvov, Y. An assembly of organic-inorganic composites using halloysite clay nanotubes. Curr. Opin. Colloid Interface Sci. 2018, 35, 42–50. [Google Scholar] [CrossRef]
- Saif, M.J.; Asif, H.M.; Naveed, M. Properties and modification methods of halloysite nanotubes: A state-of-The-art review. J. Chil. Chem. Soc. 2018, 63, 4109–4125. [Google Scholar] [CrossRef] [Green Version]
- Fizir, M.; Dramou, P.; Dahiru, N.S.; Ruya, W.; Huang, T.; He, H. Halloysite nanotubes in analytical sciences and in drug delivery: A review. Microchim. Acta 2018, 185, 1–33. [Google Scholar] [CrossRef]
- Liu, M.; Fakhrullin, R.; Novikov, A.; Panchal, A.; Lvov, Y. Tubule Nanoclay-Organic Heterostructures for Biomedical Applications. Macromol. Biosci. 2019, 19, 1800419. [Google Scholar] [CrossRef]
- Yuan, P.; Southon, P.D.; Liu, Z.; Green, M.E.; Hook, J.M.; Antill, S.J.; Kepert, C.J. Functionalization of halloysite clay nanotubes by grafting with γ-aminopropyltriethoxysilane. J. Phys. Chem. C 2008, 112, 15742–15751. [Google Scholar] [CrossRef]
- Hamedi, S.; Koosha, M. Designing a pH-responsive drug delivery system for the release of black-carrot anthocyanins loaded in halloysite nanotubes for cancer treatment. Appl. Clay Sci. 2020, 197, 105770. [Google Scholar] [CrossRef]
- Khosravian, P.; Ardestani, M.S.; Khoobi, M.; Ostad, S.N.; Dorkoosh, F.A.; Javar, H.A.; Amanlou, M. Mesoporous silica nanoparticles functionalized with folic acid/methionine for active targeted delivery of docetaxel. OncoTargets Ther. 2016, 9, 7315. [Google Scholar] [CrossRef] [Green Version]
- Massaro, M.; Campofelice, A.; Colletti, C.G.; Lazzara, G.; Noto, R.; Riela, S. Functionalized halloysite nanotubes: Efficient carrier systems for antifungine drugs. Appl. Clay Sci. 2018, 160, 186–192. [Google Scholar] [CrossRef]
- Liu, M.; Cao, X.; Liu, H.; Yang, X.; Zhou, C. Halloysite-Based Polymer Nanocomposites, in Nanomaterials from Clay Minerals; Elsevier: Amsterdam, The Netherlands, 2019; pp. 589–626. [Google Scholar]
- Wang, A.-J.; Feng, J.-J.; Fan, J. Covalent modified hydrophilic polymer brushes onto poly (dimethylsiloxane) microchannel surface for electrophoresis separation of amino acids. J. Chromatogr. A 2008, 1192, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wu, Y.; Shen, Y.; Zhou, C.; Li, Y.-F.; He, R.-R.; Liu, M. Enhanced therapeutic efficacy of doxorubicin for breast cancer using chitosan oligosaccharide-modified halloysite nanotubes. ACS Appl. Mater. Interfaces 2016, 8, 26578–26590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, G. Grafting halloysite nanotubes with amino or carboxyl groups onto carbon fiber surface for excellent interfacial properties of silicone resin composites. Polymers 2018, 10, 1171. [Google Scholar] [CrossRef] [Green Version]
- Cavuoto, P.; Fenech, M.F. A review of methionine dependency and the role of methionine restriction in cancer growth control and life-span extension. Cancer Treat. Rev. 2012, 38, 726–736. [Google Scholar] [CrossRef]
- Jamshidzadeh, F.; Mohebali, A.; Abdouss, M. Three-ply biocompatible pH-responsive nanocarriers based on HNT sandwiched by chitosan/pectin layers for controlled release of phenytoin sodium. Int. J. Biol. Macromol. 2020, 150, 336–343. [Google Scholar] [CrossRef]
- Dai, J.; Wei, X.; Cao, Z.; Zhou, Z.; Yu, P.; Pan, J.; Zou, T.; Li, C.; Yan, Y. Highly-controllable imprinted polymer nanoshell at the surface of magnetic halloysite nanotubes for selective recognition and rapid adsorption of tetracycline. RSC Adv. 2014, 4, 7967–7978. [Google Scholar] [CrossRef]
- Yah, W.O.; Takahara, A.; Lvov, Y.M. Selective modification of halloysite lumen with octadecylphosphonic acid: New inorganic tubular micelle. J. Am. Chem. Soc. 2012, 134, 1853–1859. [Google Scholar] [CrossRef]
- Lun, H.; Ouyang, J.; Yang, H. Natural halloysite nanotubes modified as an aspirin carrier. RSC Adv. 2014, 4, 44197–44202. [Google Scholar] [CrossRef]
- Zeraatpishe, L.; Mohebali, A.; Abdouss, M. Fabrication and characterization of biocompatible pH responsive halloysite nanotubes grafted with sodium alginate for sustained release of phenytoin sodium. New J. Chem. 2019, 43, 10523–10530. [Google Scholar] [CrossRef]
- Asgari, M.; Sundararaj, U. Pre-exfoliated nanoclay through two consecutive reaction systems: Silane functionalization followed by grafting of amino acid monomers. Appl. Clay Sci. 2018, 151, 81–91. [Google Scholar] [CrossRef]
- Liu, M.; Chang, Y.; Yang, J.; You, Y.; He, R.; Chen, T.; Zhou, C. Functionalized halloysite nanotube by chitosan grafting for drug delivery of curcumin to achieve enhanced anticancer efficacy. J. Mater. Chem. B 2016, 4, 2253–2263. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Yuan, P.; Annabi-Bergaya, F.; Liu, D.; Wang, L.; Liu, H.; He, H. Loading and in vitro release of ibuprofen in tubular halloysite. Appl. Clay Sci. 2014, 96, 50–55. [Google Scholar] [CrossRef]
- Mohebali, A.; Abdouss, M. Layered biocompatible pH-responsive antibacterial composite film based on HNT/PLGA/chitosan for controlled release of minocycline as burn wound dressing. Int. J. Biol. Macromol. 2020, 164, 4193–4204. [Google Scholar] [CrossRef] [PubMed]
- Mohebali, A.; Abdouss, M.; Mazinani, S.; Zahedi, P. Synthesis and characterization of poly (methacrylic acid) -based molecularly imprinted polymer nanoparticles for controlled release of trinitroglycerin. Polym. Adv. Technol. 2016, 27, 1164–1171. [Google Scholar] [CrossRef]
- Mohebali, A.; Abdouss, M.; Zahedi, P. Isosorbide dinitrate template-based molecularly imprinted poly (methacrylic acid) nanoparticles: Effect of initiator concentration on morphology and physicochemical properties. Chem. Pap. 2018, 72, 3005–3016. [Google Scholar] [CrossRef]
- Malakinezhad, H.; Kalaee, M.; Abdouss, M.; Mohebali, A.; Hakani, M. Fabrication and Characterization of Biodegradable pH-Responsive Halloysite Poly (lactic-co-glycolic acid) Micro-sphere for Controlled Released of Phenytoin Sodium. J. Inorg. Organomet. Polym. Mater. 2020, 30, 722–730. [Google Scholar] [CrossRef]
- Szczepanik, B.; Słomkiewicz, P.; Garnuszek, M.; Czech, K.; Banaś, D.; Kubala-Kukuś, A.; Stabrawa, I. The effect of chemical modification on the physico-chemical characteristics of halloysite: FTIR, XRF, and XRD studies. J. Mol. Struct. 2015, 1084, 16–22. [Google Scholar] [CrossRef]
- Dash, S.; Murthy, P.N.; Nath, L.; Chowdhury, P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol Pharm. 2010, 67, 217–223. [Google Scholar] [PubMed]
- Arafa, K.; Shamma, R.N.; El-Gazayerly, O.N.; El-Sherbiny, I.M. Facile development, characterization, and optimization of new metformin-loaded nanocarrier system for efficient colon cancer adjunct therapy. Drug Dev. Ind. Pharm. 2018, 44, 1158–1170. [Google Scholar] [CrossRef] [PubMed]




| Sample Name | EE% | LE% |
|---|---|---|
| HNT | 57.5 ± 0.04 | 28.75 ± 0.23 |
| MNT | 43.45 ± 0.07 | 39.20 ± 0.20 |
| MNT-g-Met | 51.22 ± 0.14 | 52.61 ± 0.15 |
| Sample Name | K/h | n | R2 |
|---|---|---|---|
| HNT | 26.57 | 0.47 | 0.9752 |
| HNT-g-Met | 9.22 | 0.5 | 0.9914 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdouss, M.; Radgoudarzi, N.; Mohebali, A.; Kowsari, E.; Koosha, M.; Li, T. Fabrication of Bio-Nanocomposite Based on HNT-Methionine for Controlled Release of Phenytoin. Polymers 2021, 13, 2576. https://doi.org/10.3390/polym13152576
Abdouss M, Radgoudarzi N, Mohebali A, Kowsari E, Koosha M, Li T. Fabrication of Bio-Nanocomposite Based on HNT-Methionine for Controlled Release of Phenytoin. Polymers. 2021; 13(15):2576. https://doi.org/10.3390/polym13152576
Chicago/Turabian StyleAbdouss, Majid, Nastaran Radgoudarzi, Alireza Mohebali, Elaheh Kowsari, Mojtaba Koosha, and Tianduo Li. 2021. "Fabrication of Bio-Nanocomposite Based on HNT-Methionine for Controlled Release of Phenytoin" Polymers 13, no. 15: 2576. https://doi.org/10.3390/polym13152576
APA StyleAbdouss, M., Radgoudarzi, N., Mohebali, A., Kowsari, E., Koosha, M., & Li, T. (2021). Fabrication of Bio-Nanocomposite Based on HNT-Methionine for Controlled Release of Phenytoin. Polymers, 13(15), 2576. https://doi.org/10.3390/polym13152576






