Biomimetic Hierarchical Structuring of PLA by Ultra-Short Laser Pulses for Processing of Tissue Engineered Matrices: Study of Cellular and Antibacterial Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of PLA Samples
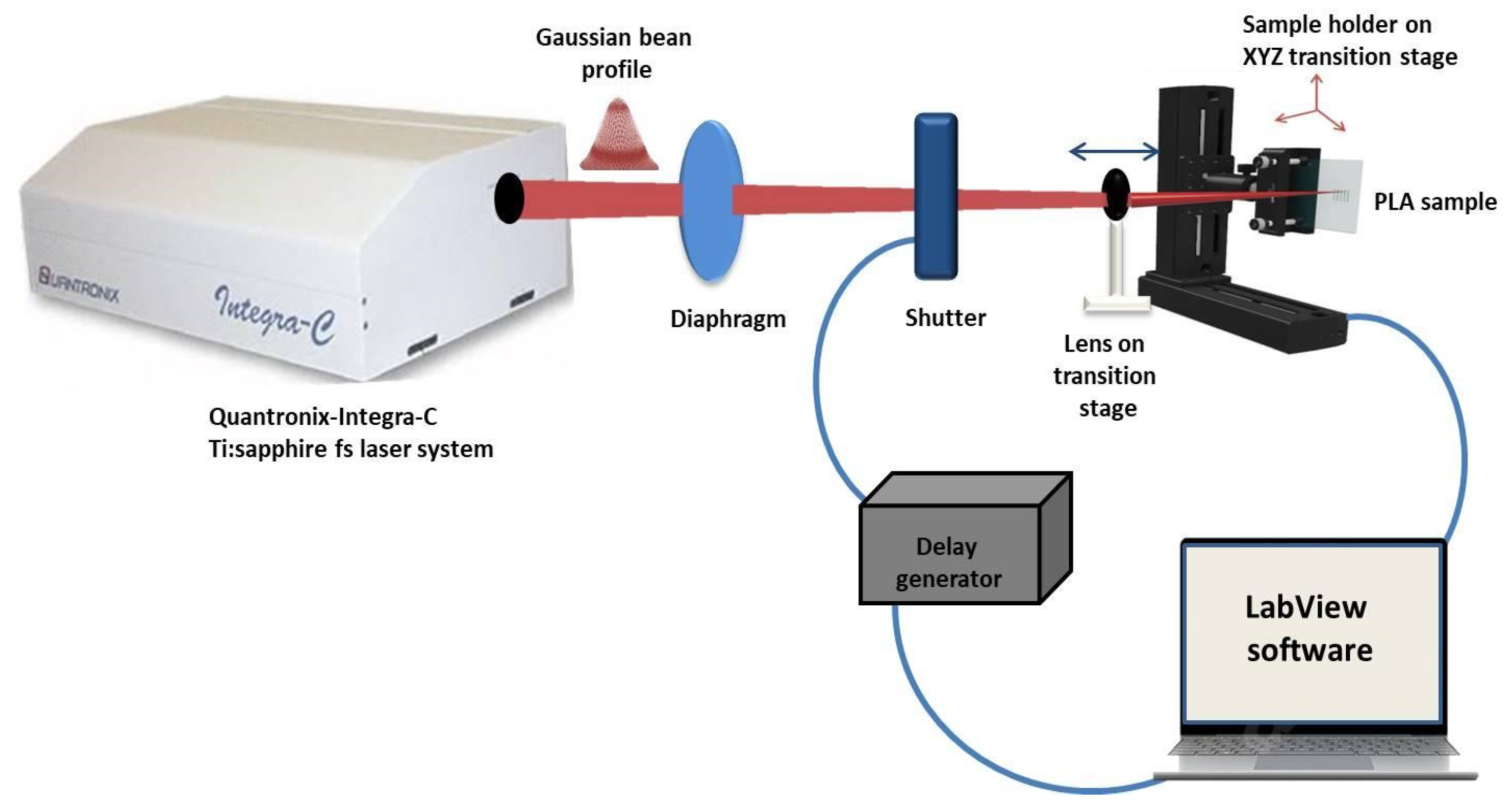
2.2. Fs Laser Experimental SETUP
2.3. Laser-Modified Samples Qualitative Characterization Methods
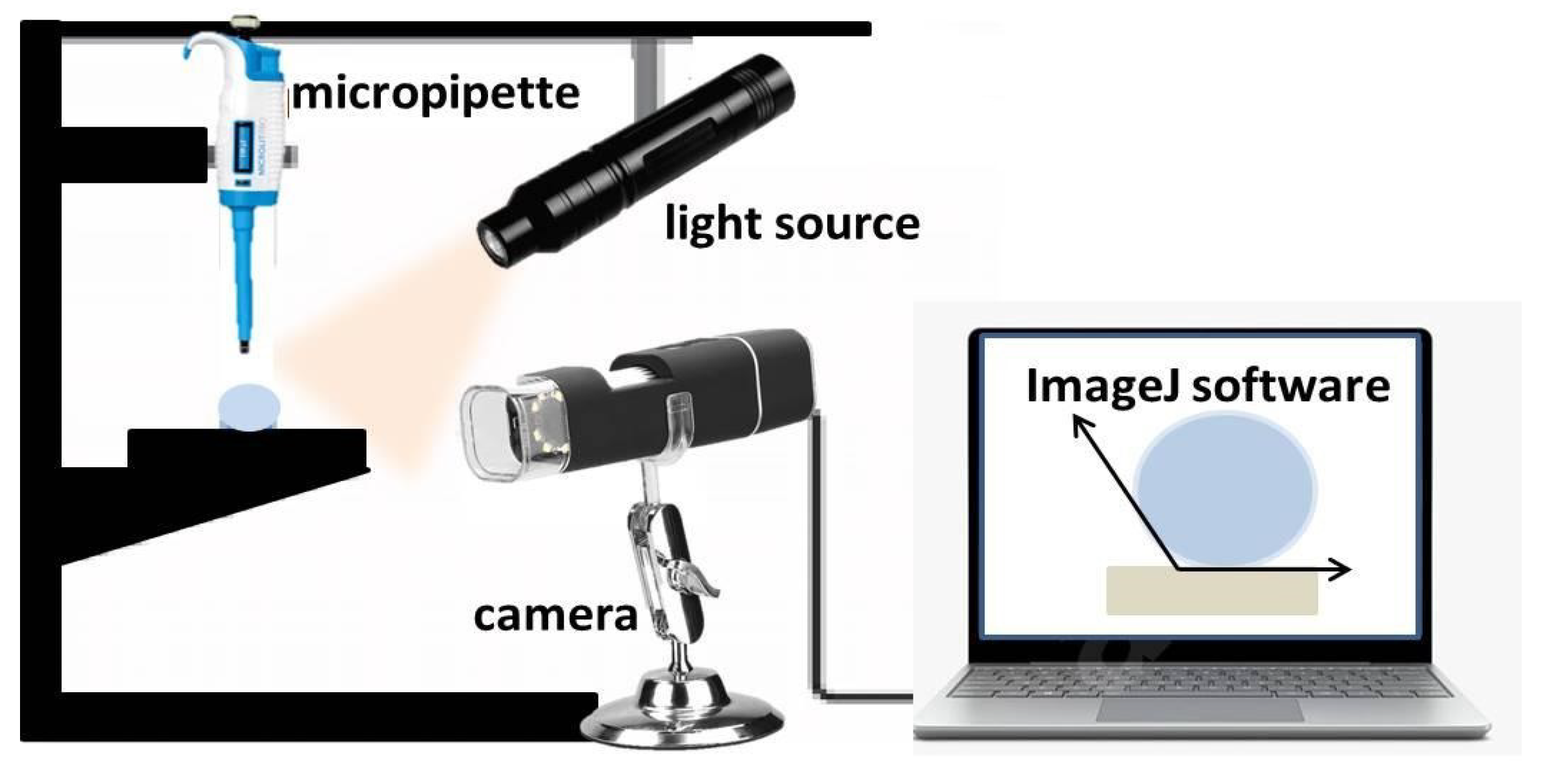
2.3.1. Thickness Measurement
2.3.2. Roughness Measurement
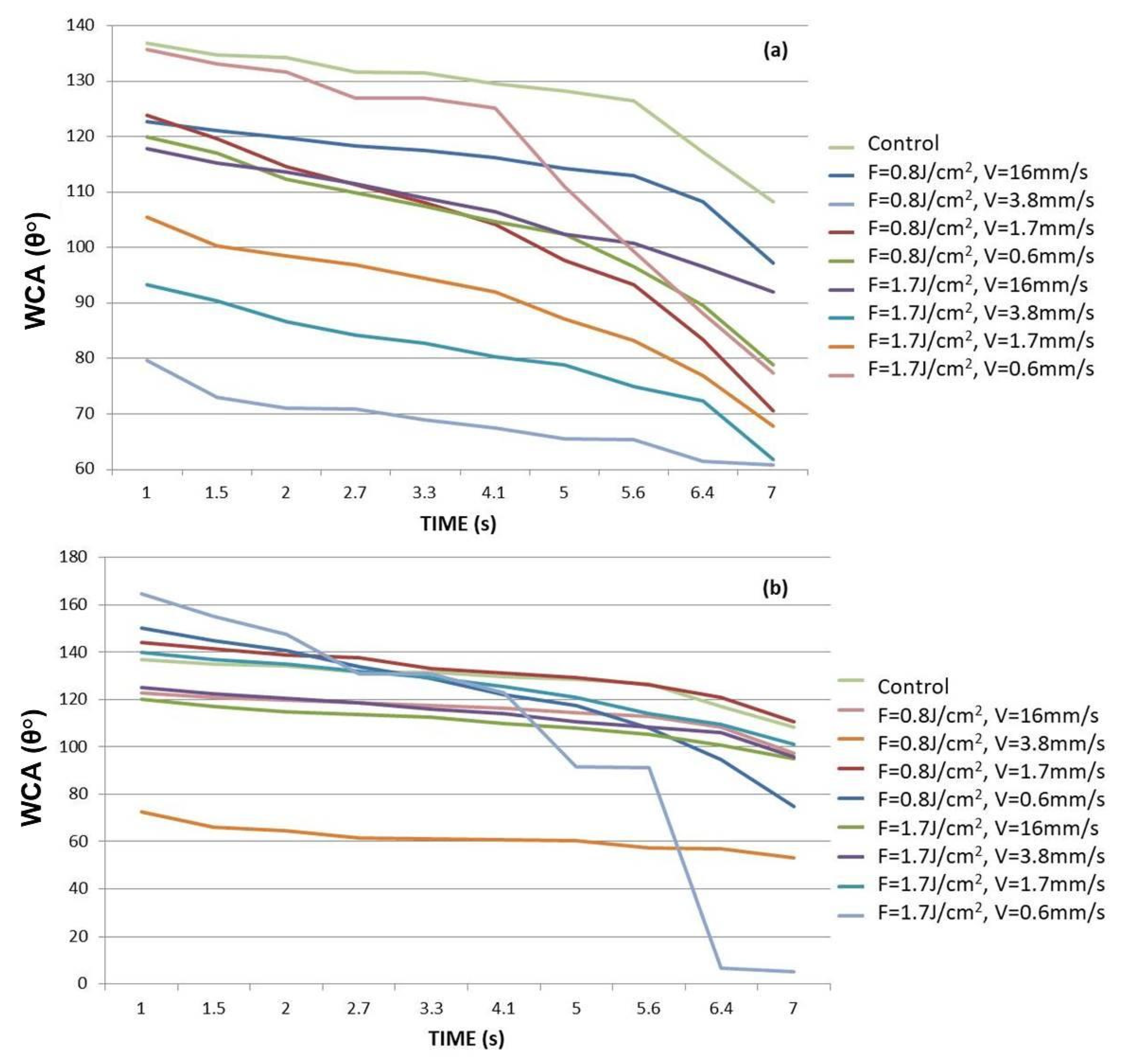
2.3.3. WCA Evaluation
2.3.4. SEM-EDX Analysis
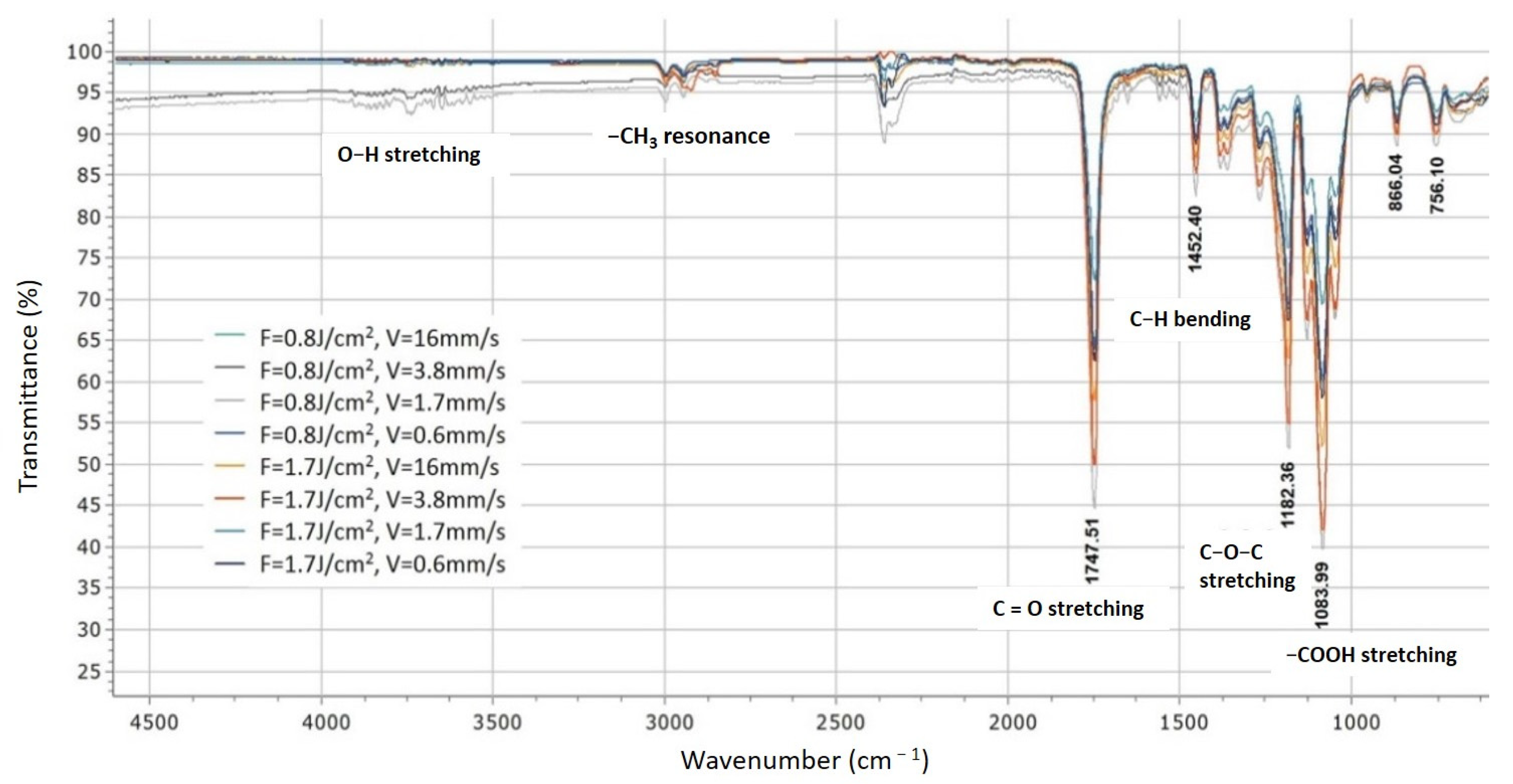
2.3.5. FTIR Analysis
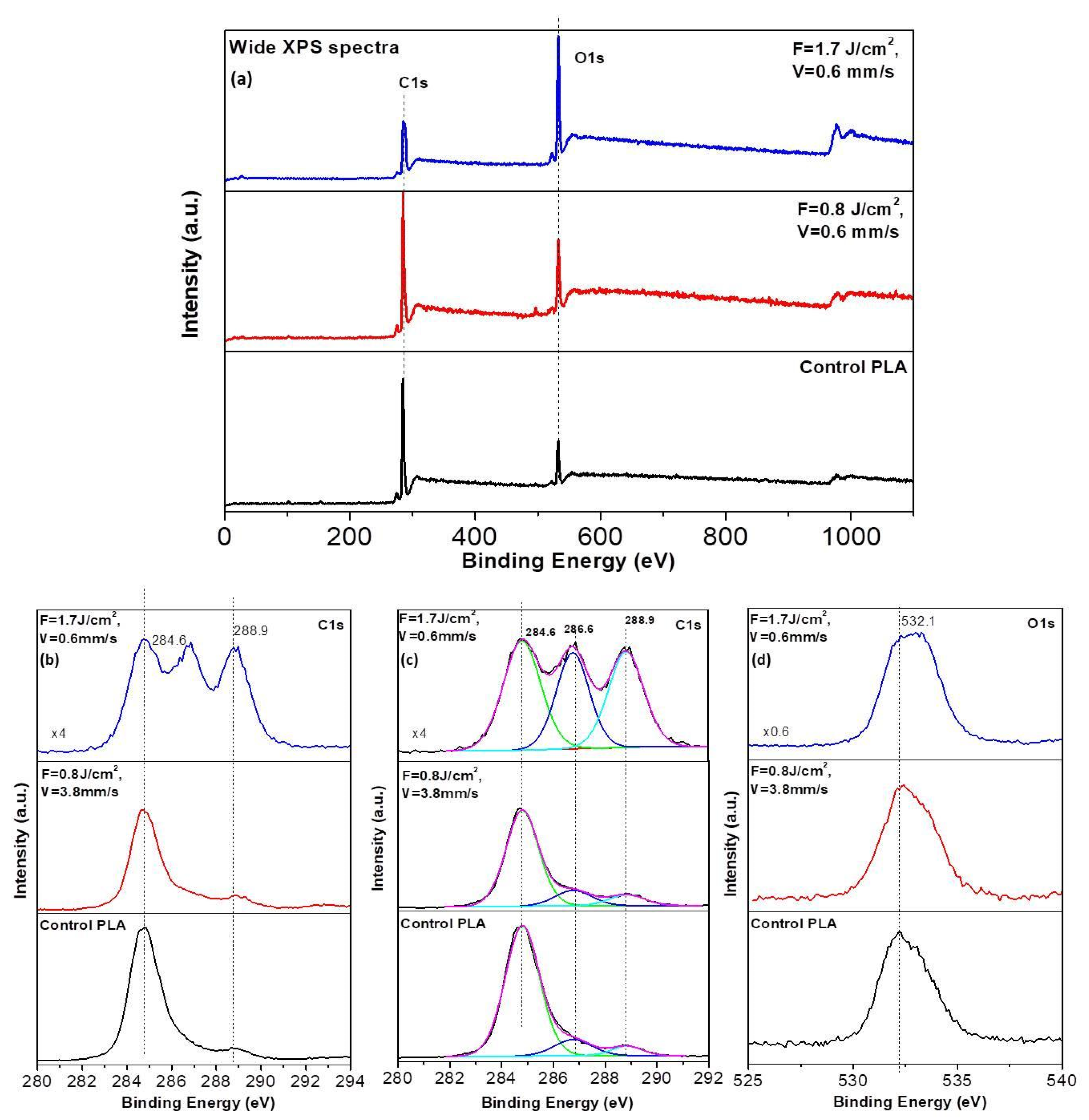
2.3.6. XPS Analysis
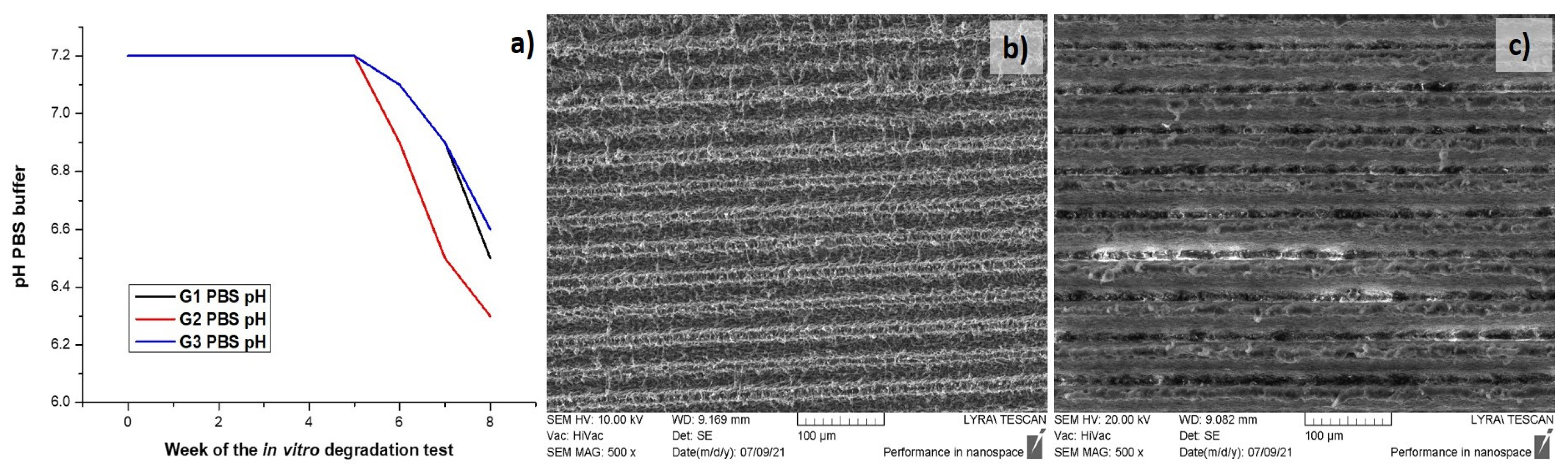
2.3.7. In Vitro Degradation Test
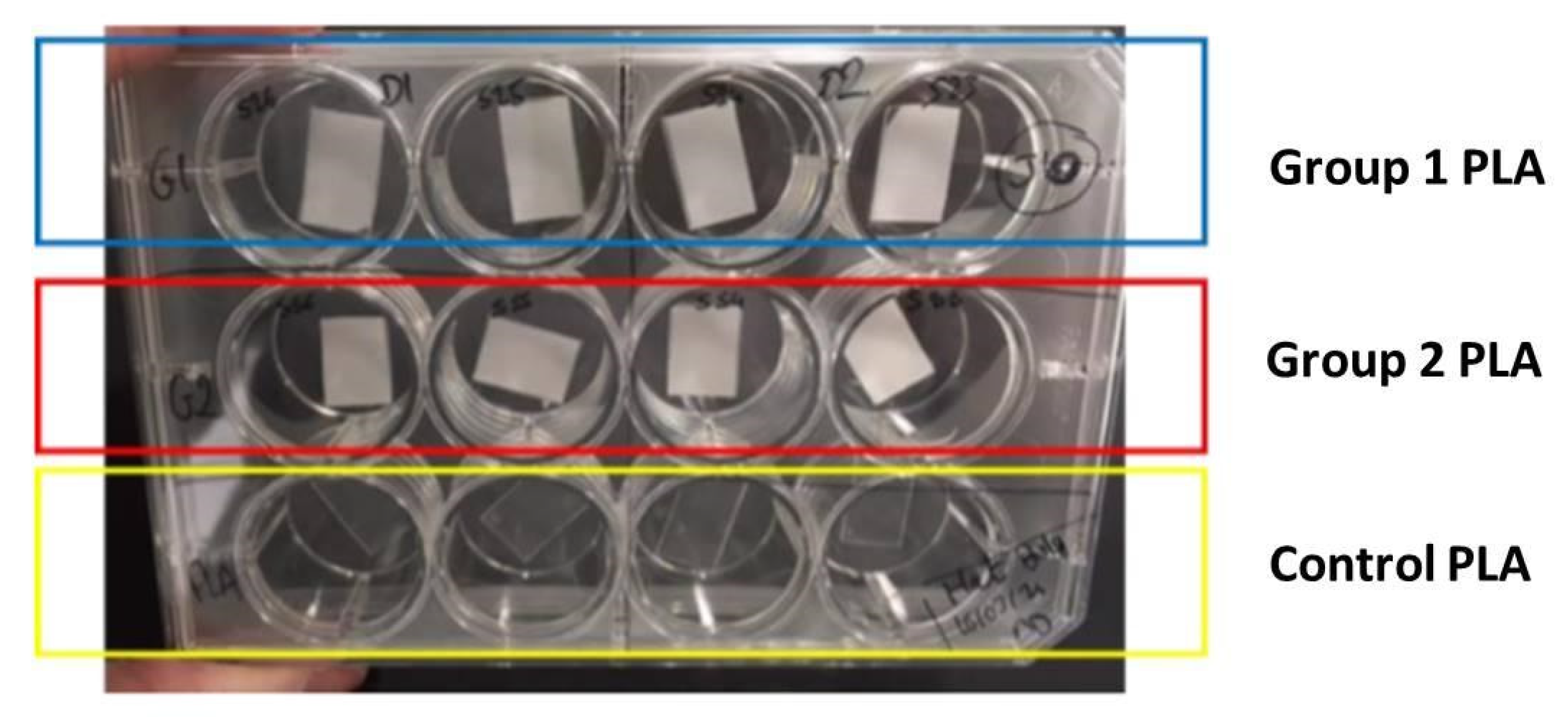
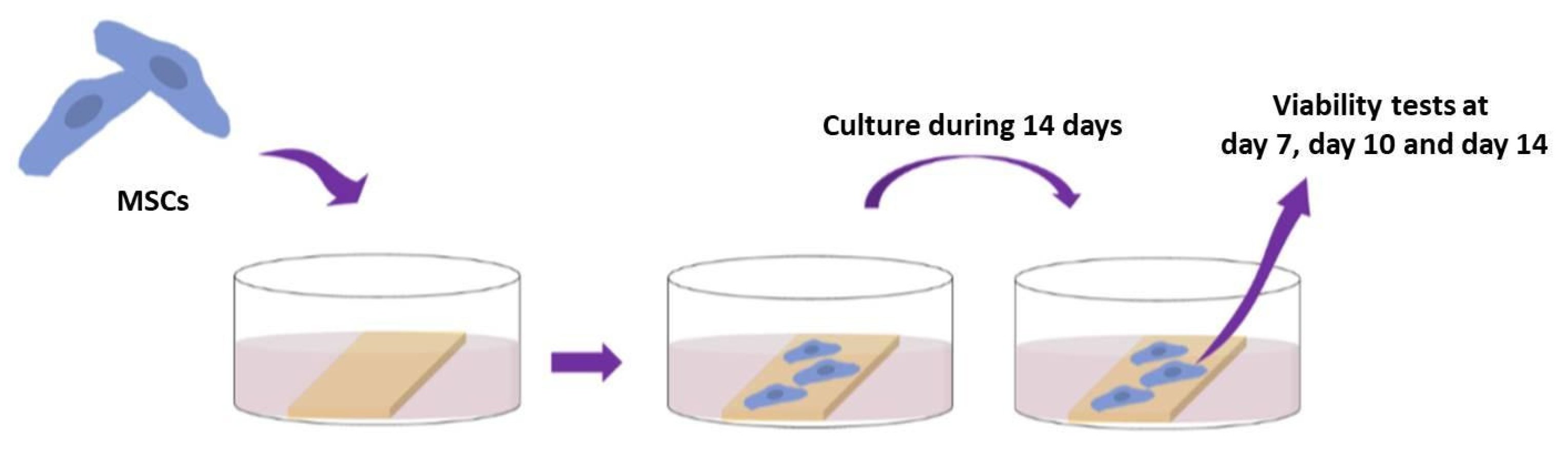
2.4. Cell Behavior on Laser-Textured PLA Matrices
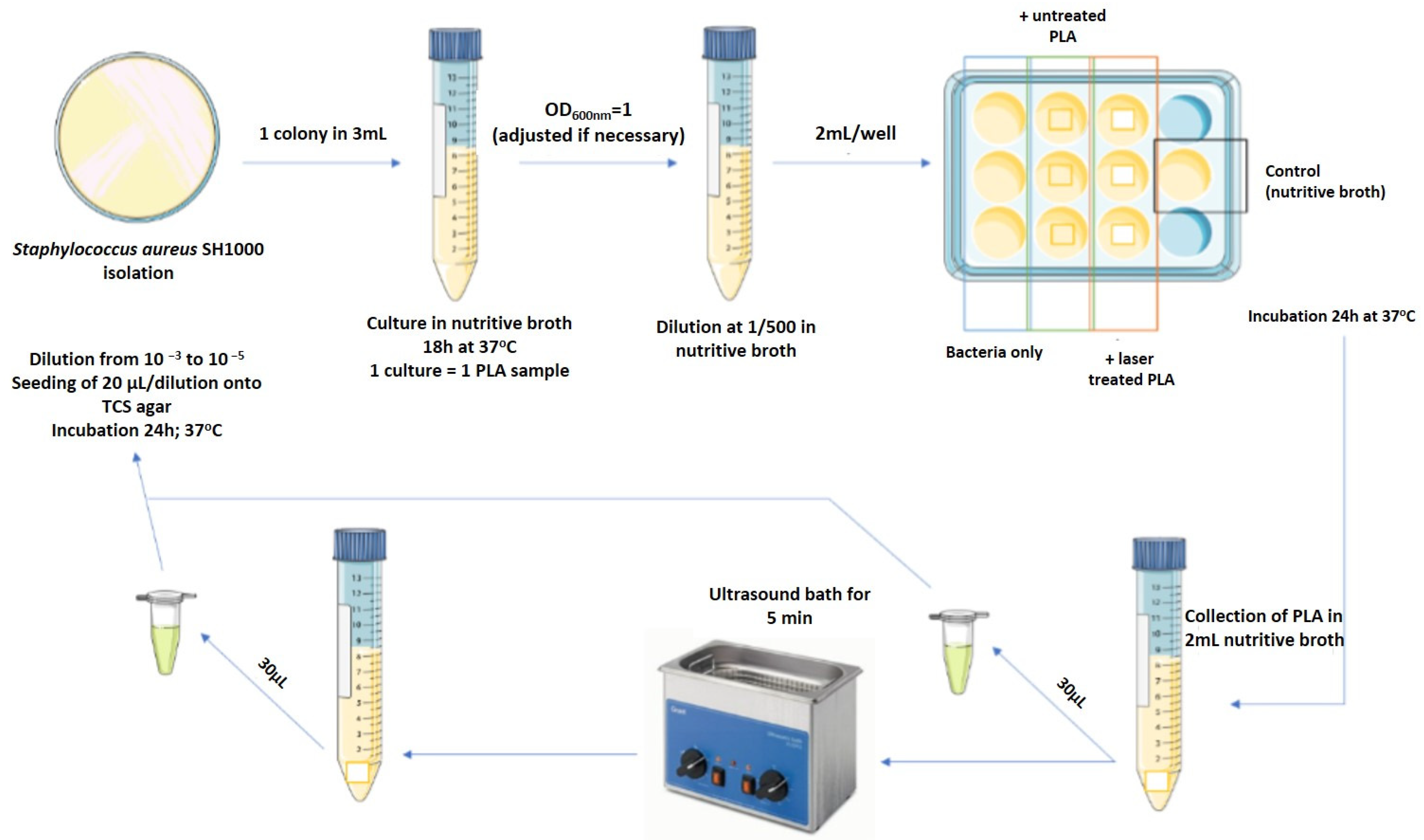
2.5. Microbiology Studies
3. Results and Discussion
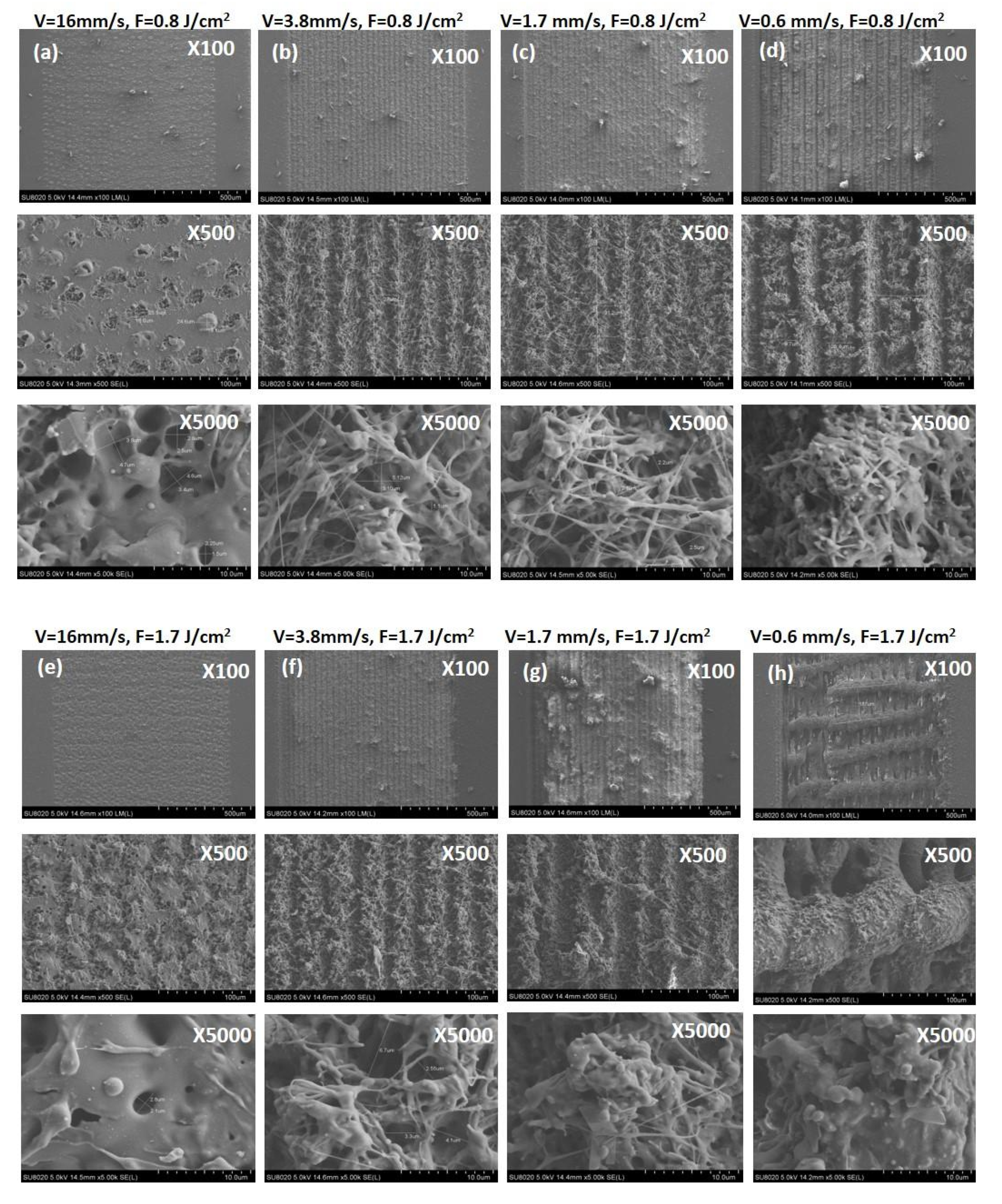
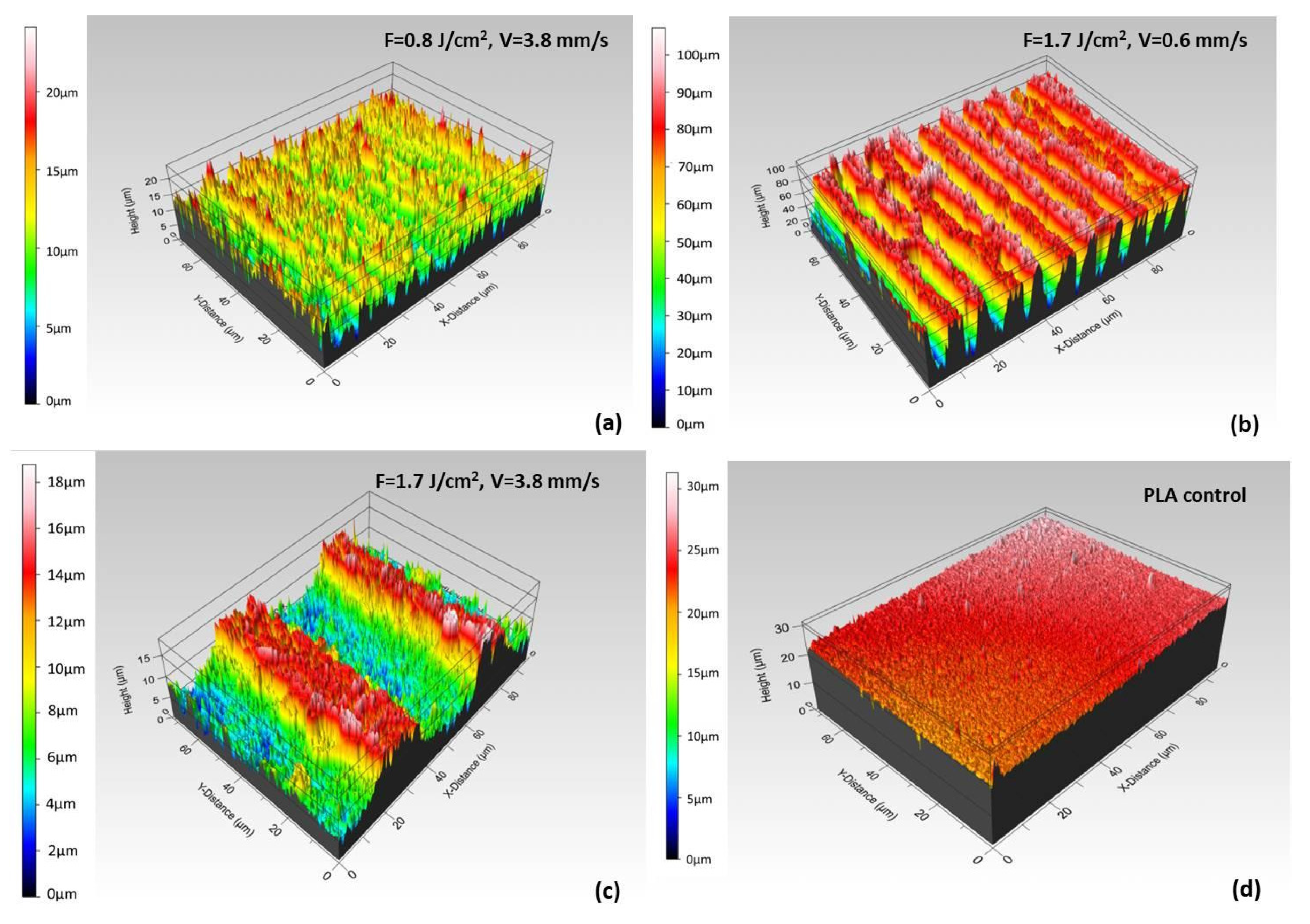
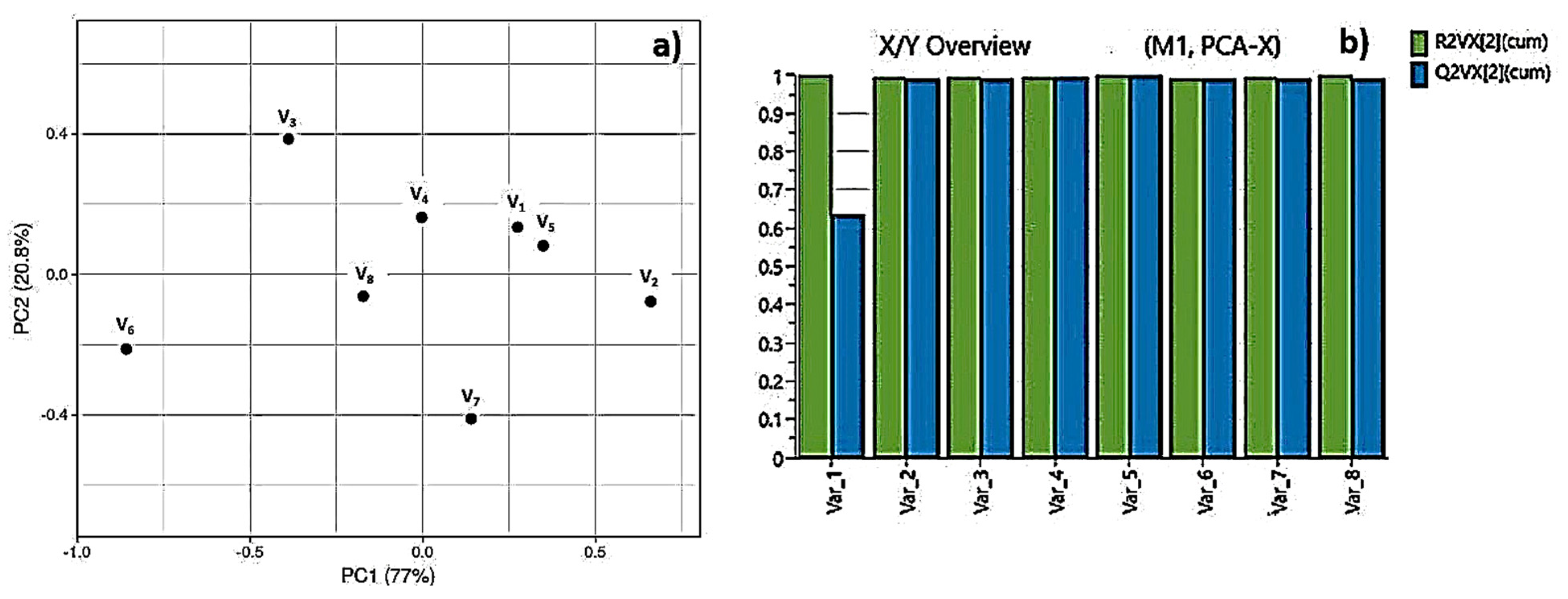
3.1. Morphological, Structural and Chemical Characterization of Fs Modified PLA Specimens
3.2. Microbiology Studies
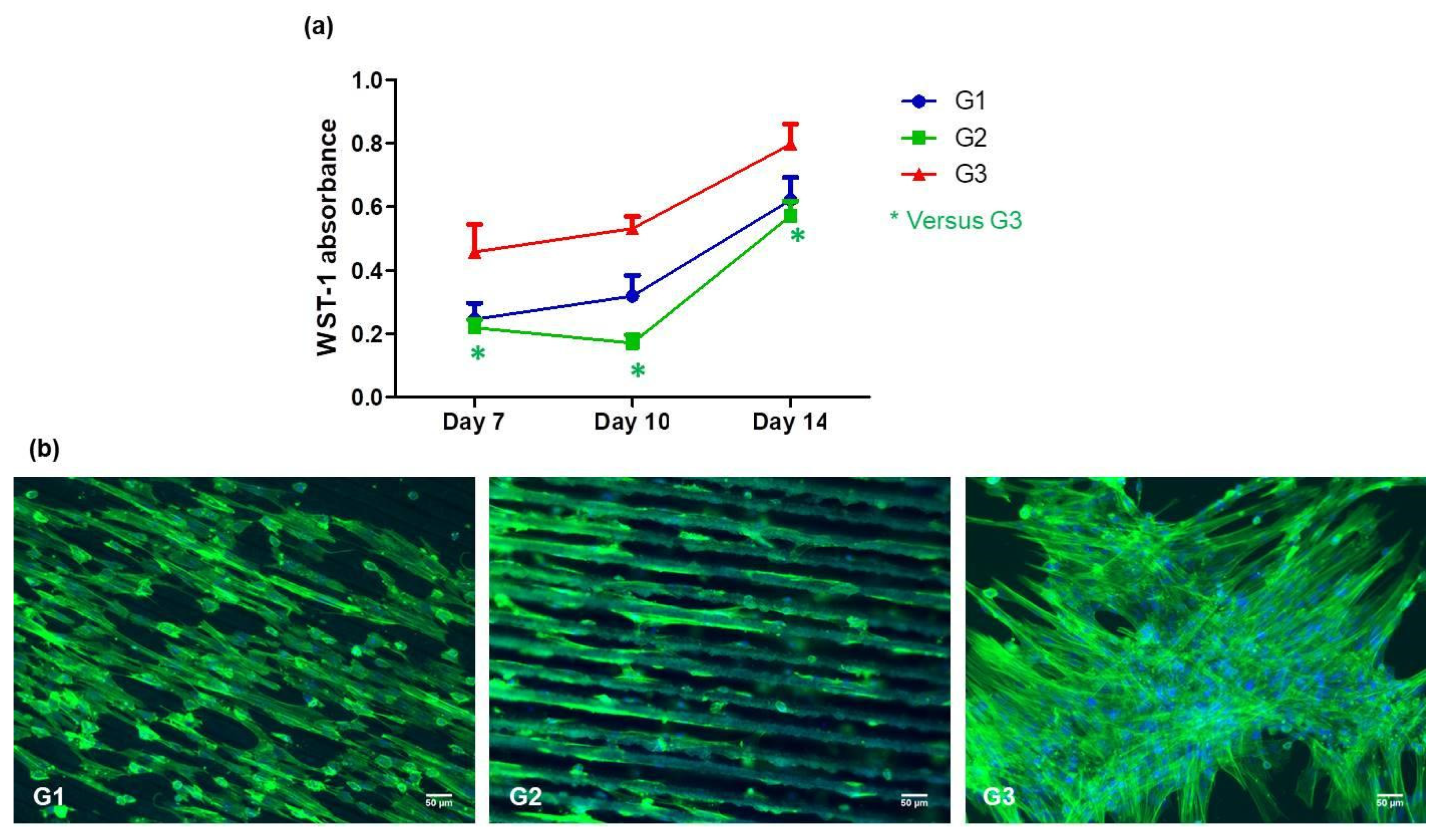
3.3. Mesenchymal Stem Cell Behavior
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- World Population Prospects: The 2019 Revision. United Nations DESA/Population Division. 2019. Available online: https://population.un.org/wpp/ (accessed on 26 March 2021).
- Langer, R. Tissue engineering. Mol. Ther. 2000, 1, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Scheinpflug, J.; Pfeiffenberger, M.; Damerau, A.; Schwarz, F.; Textor, M.; Lang, A.; Schulze, F. Journey into Bone Models: A Review. Genes 2018, 9, 247. [Google Scholar] [CrossRef] [Green Version]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutmacher, D.W.; Schantz, J.T.; Lam, C.X.F.; Tan, K.C.; Lim, T.C. State of the art and future directions of scaffold-based bone engineering from a biomaterials perspective. J. Tissue Eng. Regener. Med. 2007, 1, 245–260. [Google Scholar] [CrossRef]
- Rasal, R.M.; Hirt, D.E. Poly (lactic acid) toughening with a better balance of properties. Macromol. Mater. Eng. 2010, 295, 204–209. [Google Scholar] [CrossRef]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine; Elsevier: San Diego, CA, USA, 2004; ISBN 9780080470368. [Google Scholar]
- Santoro, M.; Shah, S.R.; Walker, J.L.; Mikos, A.G. Poly (lactic acid) nanofibrous scaffolds for tissue engineering. Adv. Drug Deliv. Rev. 2016, 107, 206–212. [Google Scholar] [CrossRef] [Green Version]
- Senatov, F.S.; Niaza, K.V.; Zadorozhnyy, M.Y.; Maksimkin, A.V.; Kaloshkin, S.D.; Estrin, Y.Z. Mechanical properties and shape memory effect of 3D-printed PLA-based porous scaffolds. J. Mech. Behav. Biomed. Mater. 2016, 57, 139–148. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef]
- Carrasco, F.; Page’s, P.; Gamez-Perez, J.; Santana, O.O.; Maspoch, M.L. Processing of poly (lactic acid): Characterization of chemical structure, thermal stability and mechanical properties. Polym. Degrad. Stab. 2010, 95, 116–125. [Google Scholar] [CrossRef]
- Guntillake, P.A.; Adhikari, R. Biodegradable synthetic polymers for tissue engineering. Eur. Cell Mater. 2003, 5, 1–16. [Google Scholar] [CrossRef]
- Barbieri, D.; de Bruijn, J.D.; Luo, X.; Fare, S.; Grijpma, D.W.; Yuan, H. Controlling dynamic mechanical properties and degradation of composites for bone regeneration by means of filler content. J. Mech. Behav. Biomed. Mater. 2013, 20, 162–172. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Leong, K.F.; Du, Z.; Chua, C.K. The design of scaffolds for use in tissue engineering. Part II. Rapid prototyping techniques. Tissue Eng. 2002, 8, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Schneider, G.B.; Zaharias, R.; Seabold, D.; Keller, J.; Stanford, C. Differentiation of preosteoblasts is affected by implant surface microtopographies. J. Biomed. Mater. Res. Part. A. 2004, 69, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Donahue, H.J. Cell sensing and response to micro- and nanostructured surfaces produced by chemical and topographic patterning. Tissue Eng. 2007, 13, 1879–1891. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Q.; Chang, C.C.; Liu, Y.; Yang, Z.; Guo, Y.; Wang, Y.; Galanakis, D.K.; Levon, K.; Rafailovich, M. Potentiometric sensors based on surface molecular imprinting: Detection of cancer biomarkers and viruses. Chemical 2016, 141, 5607–5617. [Google Scholar]
- Guo, Y.; Chang, C.C.; Cuiffo, M.A.; Xue, Y.; Zuo, X.; Pack, S.; Zhang, L.; He, S.; Weil, E.; Rafailovich, M.H. Engineering flame retardant biodegradable polymer nanocomposites and their application in 3D printing. Polym. Degrad. Stab. 2017, 137, 205–215. [Google Scholar] [CrossRef]
- Kim, D.; Kim, J.; Hyun, H.; Kim, K.; Roh, S. A nanoscale ridge/groove pattern arrayed surface enhances adipogenic differentiation of human supernumerary tooth-derived dental pulp stem cells in vitro. Arch. Oral Biol. 2014, 59, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Kolind, K.; Kraft, D.; Bøggild, T.; Duch, M.; Lovmand, J.; Pedersen, F.S.; Bindslev, D.A.; Bünger, C.; Fossand, M.; Besenbacher, F. Control of proliferation and osteogenic differentiation of human dental-pulp-derived stem cells by distinct surface structures. Acta. Biomater. 2014, 10, 641–650. [Google Scholar] [CrossRef]
- Ching, Y.C.; Rosiyah, Y.Y.; Li, G. Preparation and characterization of nano particle reinforced polyactides composite. J. Nano Res. 2013, 25, 128–136. [Google Scholar]
- Conde, C.M.; Demarco, F.F.; Casagrande, L.; Alcazar, J.C.; Nör, J.E.; Tarquinio, S.B.C. Influence of poly-l-lactic acid scaffold’s pore size on the proliferation and differentiation of dental pulp stem cells. Braz. Dent. J. 2015, 26, 93–98. [Google Scholar] [CrossRef]
- Serra, T.; Mateos-Timoneda, M.A.; Planell, J.A.; Navarro, M. 3D printed PLA-based scaffolds. Organogenesis 2013, 9, 239–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Wu, X.; Chen, J.; Lin, K. The development of collagen based composite scaffolds for bone regeneration. Bioact. Mater. 2018, 3, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Ratner, B.D. Surface modification of polymers: Chemical, biological and surface analytical challenges. Biosens. Bioelectron. 1995, 10, 797–804. [Google Scholar] [CrossRef]
- Wakelin, E.A.; Fathi, A.; Kracica, M.; Yeo, G.C.; Wise, S.G.; Weis, A.S.; McCulloch, D.G.; Dehghani, F.; Mckenzie, D.R.; Bilek, M.M. Mechanical properties of plasma immersion ion implanted PEEK for bioactivation of medical devices. ACS Appl. Mater. Interfaces 2015, 7, 23029–23040. [Google Scholar] [CrossRef]
- Terakawa, M. Femtosecond laser processing of biodegradable polymers. Appl. Sci. 2018, 8, 1123. [Google Scholar] [CrossRef] [Green Version]
- Riveiro, A.; Maçon, A.L.B.; del Val, J.; Comesaña, R.; Pou, J. Laser Surface Texturing of Polymers for Biomedical Applications. Front. Phys. 2018, 6, 16. [Google Scholar] [CrossRef]
- Lippert, T. Laser application of polymers. Adv. Polym. Sci. 2004, 168, 51–246. [Google Scholar]
- Bacáková, L.; Filová, E.; Rypácek, F.; Svorcík, V.; Starý, V. Cell adhesion on artificial materials for tissue engineering. Physiol. Res. 2004, 53 (Suppl. 1), S35–S45. [Google Scholar]
- Khalili, A.A.; Ahmad, M.R. A review of cell adhesion studies for biomedical and biological applications. Int. J. Mol. Sci. 2015, 16, 18149–18184. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Wen, F.; Wong, Y.S.; Boey, F.Y.; Subbu, V.S.; Leong, D.T.; Ng, K.W.; Ng, G.K.; Tan, L.P. Direct laser machining-induced topographic pattern promotes up-regulation of myogenic markers in human mesenchymal stem cells. Acta Biomater. 2012, 8, 531–539. [Google Scholar] [CrossRef]
- Mofokeng, J.P.; Luyt, A.S.; Ta’bi, T.; Kova’cs, J. Comparison of injection moulded, natural fibre-reinforced composites with PP and PLA as matrices. J. Thermoplast. Compos. Mater. 2012, 25, 927–948. [Google Scholar] [CrossRef]
- Lee, B.; Jeon, H.; Wang, A.; Yan, Z.; Yu, J.; Grigoropoulos, C.; Li, S. Femtosecond laser ablation enhances cell infiltration into three-dimensional electrospun scaffolds. Acta Biomater. 2012, 8, 2648–2658. [Google Scholar] [CrossRef] [Green Version]
- Yada, S.; Terakawa, M. Femtosecond laser induced periodic surface structure on poly-L-lactic acid. Opt. Express. 2015, 23, 5694–5703. [Google Scholar] [CrossRef]
- Daskalova, A.; Angelova, L.; Carette, X.; Mincheva, R.; Raquez, J.M.; Trifonov, A.; Buchvarov, I. Influence of femtosecond laser processing parameters on surface morphology and wettability properties of polylactic acid (PLA). IOP Mater. Sci. Eng. 2021, 1056, 012001. [Google Scholar]
- Lutey, A.H.A.; Gemin, L.; Romoli, L.; Lazzini, G.; Fuso, F.; Faucon, M.; Kling, R. Towards Laser-Textured Antibacterial Surfaces. Sci. Rep. 2018, 8, 10112. [Google Scholar] [CrossRef] [PubMed]
- Szmukler-Moncler, S.; Perrin, D.; Ahossi, V.; Magnin, G.; Bernard, J.P. Biological properties of acid etched titanium implants: Effect of sandblasting on bone anchorage. J. Biomed. Mater. Res. B. Appl. Biomater. 2004, 68, 149–159. [Google Scholar] [CrossRef]
- Ponsonnet, L.; Reybier, K.; Jaffrezic, N.; Comte, V.; Lagneau, C.; Lissac, M.; Martelet, C. Relationship between surface properties (roughness, wettability) of titanium and titanium alloys and cell behavior. Mater. Sci. Eng. C 2003, 23, 551–560. [Google Scholar] [CrossRef]
- Riveiro, A.; Soto, R.; Comesaña, R.; Boutinguiza, M.; Del Val, J.; Quintero, F.; Lusquiños, F.; Pou, J. Laser surface modification of PEEK. Appl. Surf. Sci. 2012, 258, 9437–9442. [Google Scholar] [CrossRef]
- Gittens, R.A.; Olivares-Navarrete, R.; Schwartz, Z.; Boyan, B.D. Implant osseointegration and the role of microroughness and nanostructures: Lessons for spine implants. Acta Biomater 2014, 10, 3363–3371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dekker, A.; Reitsma, K.; Beugeling, T.; Bantjes, A.; Feijen, J.; Van Aken, W.G. Adhesion of endothelial cells adsorption of serum proteins on gas plasma-treated polytetrafluoroethylene. Biomaterials 1991, 12, 130–138. [Google Scholar] [CrossRef] [Green Version]
- Wan, Y.; Yang, J.; Yang, J.; Bei, J.; Wang, S. Cell adhesion on gaseous plasma modified poly-(L-lactide) surface under shear stress field. Biomaterials 2003, 24, 3757–3764. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546. [Google Scholar] [CrossRef]
- Lasprilla, A.J.R.; Martinez, G.A.R.; Lunelli, B.H.; Jardini, A.L.; Filho, R.M. Poly-lactic acid synthesis for application in biomedical devices—A review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Shah, S.; Mani, G.; Wenke, J.; Agrawal, M. Endothelial cell behavior on gas-plasma-treated PLA surfaces: The roles of surface chemistry and roughness. J. Tissue Eng. Regen Med. 2011, 5, 301–312. [Google Scholar] [CrossRef]
- Wan, Y.; Qu, X.; Zhu, C.; Wan, L.; Yang, J.; Bei, J.; Wang, S. Characterization of surface property of poly (lactide-co-glycolide) after oxygen plasma treatment. Biomaterials 2004, 25, 4777–4783. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, N.; Benning, M.; Ferreira, A.M.; Dixon, L.; Dalgarno, K. Manufacture and Characterisation of Porous PLA Scaffolds. Procedia. CIRP 2016, 49, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Yang, C.; Zhou, Z.; Chen, S.; Li, F. Characterization of biodegradable poly (lactic acid) porous scaffolds prepared using selective enzymatic degradation for tissue engineering. RSC Adv. 2017, 7, 34063. [Google Scholar] [CrossRef] [Green Version]
- Estevam-Alves, R.; Ferreira, P.H.D.; Coatrini, A.C.; Oliveira, O.N.; Fontana, C.R.; Mendonca, C.R. Femtosecond Laser Patterning of the Biopolymer Chitosan for Biofilm Formation. Int. J. Mol. Sci. 2016, 17, 1243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitehead, K.A.; Verran, J. The Effect of Surface Topography on the Retention of Microorganisms. Food Bioprod. Process. 2006, 84, 253–259. [Google Scholar] [CrossRef]
- García, S.; Trueba, A.; Vega, L.M.; Madariaga, E. Impact of the surface roughness of AISI 316L stainless steel on biofilm adhesion in a seawater-cooled tubular heat exchanger-condenser. Biofouling 2016, 32, 1185–1193. [Google Scholar] [CrossRef]
- Dou, X.Q.; Zhang, D.; Feng, C.; Jiang, L. Bioinspired Hierarchical Surface Structures with Tunable Wettability for Regulating Bacteria Adhesion. ACS Nano 2015, 9, 10664–10672. [Google Scholar] [CrossRef]
- Fadeeva, E.; Truong, V.K.; Stiesch, M.; Chichkov, B.N.; Crawford, R.J.; Wang, J.; Ivanova, E.P. Bacterial Retention on Superhydrophobic Titanium Surfaces Fabricated by Femtosecond Laser Ablation. Langmuir 2011, 27, 3012–3019. [Google Scholar] [CrossRef] [PubMed]
- Privett, B.J.; Youn, J.; Hong, S.A.; Lee, J.; Han, J.; Shin, J.H.; Schoenfisch, M.H. Antibacterial Fluorinated Silica Colloid Superhydrophobic Surfaces. Langmuir 2011, 27, 9597–9601. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, R.; Moreno-Flores, S.; Quintana, I.; Vivanco, M.; Sarasua, J.R.; Toca-Herrera, J.L. Ultra-fast laser microprocessing of medical polymers for cell engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 1, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Castillejo, M.; Rebollar, E.; Oujja, M.; Sanz, M.; Selimis, A.; Sigletou, M.; Psycharakis, S.; Ranella, A.; Fotakis, C. Fabrication of porous biopolymer substrates for cell growth by UV laser: The role of pulse duration. Appl. Surf. Sci. 2012, 258, 8919–8927. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Wong, Y.S.; Wen, F.; Ng, K.W.; Ng, G.K.; Venkatraman, S.S.; Boey, F.Y.; Tan, L.P. Human mesenchymal stem-cell behavior on direct laser micropatterned electrospun scaffolds with hierarchical structures. Macromol. Biosci. 2013, 13, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Gaspard, S.; Oujja, M.; de Nalda, R.; Castillejo, M.; Bañares, L.; Lazare, S.; Bonneau, R. Nanofoaming dynamics in biopolymers by femtosecond laser irradiation. Appl. Phys. A. 2008, 93, 209–213. [Google Scholar] [CrossRef] [Green Version]
- Gaspard, S.; Forster, M.; Huber, C.; Zafiu, C.; Trettenhahn, G.; Kautek, W.; Castillejo, M. Femtosecond laser processing of biopolymers at high repetition rate. Phys. Chem. Chem. Phys. 2008, 10, 6174–6181. [Google Scholar] [CrossRef]
- Gaspard, S.; Oujja, M.; de Nalda, R.; Abrusci, C.; Catalina, F.; Bañares, L.; Lazare, S.; Castillejo, M. Nanofoaming in the surface of biopolymers by femtosecond pulsed laser irradiation. Appl. Surf. Sci. 2007, 254, 117–1184. [Google Scholar] [CrossRef]
- Krüger, J.; Kautek, W. Ultrashort Pulse Laser Interaction with Dielectrics and Polymers. In Polymers and Light; Lippert, T., Ed.; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2004; p. 168. [Google Scholar]
- Sima, F.; Sugioka, K.; Vazquez, R.M.; Osellame, R.; Kelemen, L.; Ormos, P. Three-dimensional femtosecond laser processing for lab-on-a-chip applications. Nanophotonics 2018, 7, 613–647. [Google Scholar] [CrossRef]
- Ortiz, R.; Quintana, I.; Etxarri, J.; Lejardi, A.; Sarasua, J.R. Picosecond laser ablation of poly-L-lactide: Effect of crystallinity on the material response. J. Appl. Phys. 2011, 110, 094902. [Google Scholar] [CrossRef]
- Daskalova, A.; Nathala, C.S.R.; Bliznakova, I.; Stoyanova, E.; Zhelyazkova, A.; Ganz, T.; Lueftenegger, S.; Husinsky, W. Controlling the porosity of collagen, gelatin and elastin biomaterials by ultrashort laser pulses. Appl. Surf. Sci. 2014, 292, 367–377. [Google Scholar] [CrossRef]
- Daskalova, A.; Bliznakova, I.; Angelova, L.; Trifonov, A.; Declercq, H.; Buchvarov, I. Femtosecond Laser Fabrication of Engineered Functional Surfaces Based on Biodegradable Polymer and Biopolymer/Ceramic Composite thin films. Polymers 2019, 11, 378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daskalova, A.; Trifonov, A.; Bliznakova, I.; Nathala, C.; Ajami, A.; Husinsky, W.; Declercq, H.; Buchvarov, I. Selective cell response on natural polymer bio-interfaces textured by femtosecond laser. Appl. Phys. A 2018, 124, 207. [Google Scholar] [CrossRef]
- Cordero, D.; López-Álvarez, M.; Rodríguez-Valencia, C.; Serra, J.; Chiussi, S.; González, P. In vitro response of pre-osteoblastic cells to laser microgrooved PEEK. Biomed Mater. 2013, 8, 055006. [Google Scholar] [CrossRef]
- Bačáková, L.; Starý, V.; Kofroňová, O.; Lisá, V. Polishing and coating carbon fiber-reinforced carbon composites with a carbon-titanium layer enhances adhesion and growth of osteoblast-like MG63 cells and vascular smooth muscle cells in vitro. J. Biomed. Mater. Res. 2001, 54, 567–578. [Google Scholar] [CrossRef]
- Bačáková, L.; Stary, V.; Hornik, J.; Glogar, P.; Lisa, V.; Kofronova, O. Osteoblast-like MG63 cells in cultures on carbon fibre-reinforced carbon composites. Eng. Biomater. 2001, 4, 11–12. [Google Scholar]
- Veerachamy, S.; Yarlagadda, T.; Manivasagam, G.; Yarlagadda, P.K.D.V. Bacterial adherence and biofilm formation on medical implants: A review. Proc. Inst. Mech. Eng. Pt. H J. Eng. Med. 2014, 228, 1083–1099. [Google Scholar] [CrossRef] [PubMed]
- Pogodin, S.; Hasan, J.; Baulin, V.A.; Webb, H.K.; Truong, V.K.; Nguyen, T.H.; Boshkovikj, V.; Fluke, C.J.; Watson, G.S.; Watson, J.A.; et al. Biophysical model of bacterial cell interactions with nanopatterned cicada wing surfaces. Biophys. J. 2013, 104, 835–840. [Google Scholar] [CrossRef] [Green Version]
- Hasan, J.; Webb, H.K.; Truong, V.K.; Pogodin, S.; Baulin, V.A.; Watson, G.S.; Watson, J.A.; Crawford, R.J.; Ivanova, E.P. Selective bactericidal activity of nanopatterned superhydrophobic cicada Psaltoda claripennis wing surfaces. Appl. Microbiol. Biotechnol. 2013, 97, 9257–9262. [Google Scholar] [CrossRef]
- Sirdeshmukh, N.; Dongre, G. Laser micro & nano surface texturing for enhancing osseointegration and antimicrobial effect of biomaterials: A review. Mater. Today Proc. 2021, 44, 2348–2355. [Google Scholar]
- Cunha, A.; Elie, A.M.; Plawinski, L.; Serro, A.P.; do Rego, A.M.B.; Almeida, A.; Urdaci, M.C.; Durrieu, M.-C.; Vilar, R. Femtosecond laser surface texturing of titanium as a method to reduce the adhesion of Staphylococcus aureus and biofilm formation. Appl. Surf. Sci. 2016, 360, 485–493. [Google Scholar] [CrossRef]
- Martínez-Calderon, M.; Manso-Silván, M.; Rodríguez, A.; Gómez-Aranzadi, M.; García-Ruiz, J.P.; Olaizola, S.M.; Martín-Palma, R.J. Surface micro- and nano-texturing of stainless steel by femtosecond laser for the control of cell migration. Sci. Rep. 2016, 6, 36296. [Google Scholar] [CrossRef]
- Lazzini, G.; Romoli, L.; Lutey, A.H.A.; Fuso, F. Modelling the interaction between bacterial cells and laser-textured surfaces. Surf. Coat. Technol. 2019, 375, 8–14. [Google Scholar] [CrossRef]
- Jalil, S.A.; Akram, M.; Bhat, J.A.; Hayes, J.J.; Singh, S.C.; Kabbash, M.E.; Guo, C. Creating superhydrophobic and antibacterial surfaces on gold by femtosecond laser pulses. Appl. Surf. Sci. 2020, 506, 144952. [Google Scholar] [CrossRef] [PubMed]
- Jaggessar, A.; Shahali, H.; Mathew, A.; Yarlagadda, P.K.D.V. Bio-mimicking nano and micro-structured surface fabrication for antibacterial properties in medical implants. J. Nanobiotechnol. 2017, 15, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scardino, A.J.; Guenther, J.; de Nys, R. Attachment point theory revisited: The fouling response to a microtextured matrix. Biofouling 2008, 24, 45–53. [Google Scholar] [CrossRef]
- Lorenzetti, M.; Dogša, I.; Stošicki, T.; Stopar, D.; Kalin, M.; Kobe, S.; Novak, S. The influence of surface modification on bacterial adhesion to titanium-based substrates. ACS Appl. Mater. Interfaces 2015, 7, 1644–1651. [Google Scholar] [CrossRef] [PubMed]
- Guenther, D.; Valle, J.; Burgui, S.; Gil, C.; Solano, C.; Arana, A.T.; Helbig, R.; Werner, C.; Lasa, I.; Lasagni, A.F. Direct laser interference patterning for decreased bacterial attachment. Laser-Based Micro Nanoprocess. X 2016, 9736, 11. [Google Scholar]
- Hochbaum, A.; Aizenberg, J. Bacteria pattern spontaneously on periodic nanostructure arrays. Nano Lett. 2010, 10, 3717–3721. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, R.; Kennedy, A.J.; Merritt, M.; Crocker, F.H.; Baney, R.H. Microscale patterned surfaces reduce bacterial fouling microscopic and theoretical analysis. Colloids Surf. B Biointerfaces 2014, 117, 225–232. [Google Scholar] [CrossRef]
- Perera-Costa, D.; Bruque, J.M.; González-Martín, M.L.; Gómez-García, A.C.; Vadillo-Rodriguez, V. Studying the influence of surface topography on bacterial adhesion using spatially organized microtopographic surface patterns. Langmuir 2014, 30, 4633–4641. [Google Scholar] [CrossRef] [PubMed]


| Sample (PLA) | C [wt.%] | O [wt.%] |
|---|---|---|
| Control | 74.27 | 25.73 |
| V = 16 mm/s, F = 0.8 J/cm2 | 68.05 | 31.95 |
| V = 3.8 mm/s, F = 0.8 J/cm2 | 61.51 | 38.49 |
| V = 1.7 mm/s, F = 0.8 J/cm2 | 60.52 | 39.48 |
| V = 0.6 mm/s, F = 0.8 J/cm2 | 58.82 | 41.18 |
| V = 16 mm/s, F = 1.7 J/cm2 | 62.48 | 37.52 |
| V = 3.8 mm/s, F = 1.7 J/cm2 | 60.42 | 39.58 |
| V = 1.7 mm/s, F = 1.7 J/cm2 | 59.09 | 40.91 |
| V = 0.6 mm/s, F = 1.7 J/cm2 | 58.32 | 41.68 |
| Sample (PLA) | Thickness [µm] | Ra [µm] |
|---|---|---|
| Control | 250 | 0.024 |
| V = 16 mm/s, F = 0.8 J/cm2 | 228 | 0.53 |
| V = 3.8 mm/s, F = 0.8 J/cm2 | 215 | 1.92 |
| V = 1.7 mm/s, F = 0.8 J/cm2 | 219 | 2.4 |
| V = 0.6 mm/s, F = 0.8 J/cm2 | 236 | 4.24 |
| V = 16 mm/s, F = 1.7 J/cm2 | 227 | 3.35 |
| V = 3.8 mm/s, F = 1.7 J/cm2 | 223 | 5.8 |
| V = 1.7 mm/s, F = 1.7 J/cm2 | 218 | 4 |
| V = 0.6 mm/s, F = 1.7 J/cm2 | 240 | 5.19 |
| Week | Group 1 PBS pH | Group 2 PBS pH |
|---|---|---|
| 0 | 7.2 | 7.2 |
| 1 | 7.2 | 7.2 |
| 2 | 7.2 | 7.2 |
| 3 | 7.2 | 7.2 |
| 4 | 7.2 | 7.2 |
| 5 | 7.2 | 7.2 |
| 6 | 7.1 | 6.9 |
| 7 | 6.9 | 6.7 |
| 8 | 6.5 | 6.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daskalova, A.; Angelova, L.; Filipov, E.; Aceti, D.; Mincheva, R.; Carrete, X.; Kerdjoudj, H.; Dubus, M.; Chevrier, J.; Trifonov, A.; et al. Biomimetic Hierarchical Structuring of PLA by Ultra-Short Laser Pulses for Processing of Tissue Engineered Matrices: Study of Cellular and Antibacterial Behavior. Polymers 2021, 13, 2577. https://doi.org/10.3390/polym13152577
Daskalova A, Angelova L, Filipov E, Aceti D, Mincheva R, Carrete X, Kerdjoudj H, Dubus M, Chevrier J, Trifonov A, et al. Biomimetic Hierarchical Structuring of PLA by Ultra-Short Laser Pulses for Processing of Tissue Engineered Matrices: Study of Cellular and Antibacterial Behavior. Polymers. 2021; 13(15):2577. https://doi.org/10.3390/polym13152577
Chicago/Turabian StyleDaskalova, Albena, Liliya Angelova, Emil Filipov, Dante Aceti, Rosica Mincheva, Xavier Carrete, Halima Kerdjoudj, Marie Dubus, Julie Chevrier, Anton Trifonov, and et al. 2021. "Biomimetic Hierarchical Structuring of PLA by Ultra-Short Laser Pulses for Processing of Tissue Engineered Matrices: Study of Cellular and Antibacterial Behavior" Polymers 13, no. 15: 2577. https://doi.org/10.3390/polym13152577
APA StyleDaskalova, A., Angelova, L., Filipov, E., Aceti, D., Mincheva, R., Carrete, X., Kerdjoudj, H., Dubus, M., Chevrier, J., Trifonov, A., & Buchvarov, I. (2021). Biomimetic Hierarchical Structuring of PLA by Ultra-Short Laser Pulses for Processing of Tissue Engineered Matrices: Study of Cellular and Antibacterial Behavior. Polymers, 13(15), 2577. https://doi.org/10.3390/polym13152577









