Hybrid Copolymerization of Ethylene Oxide and tert-Butyl Methacrylate with Organocatalyst
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterizations
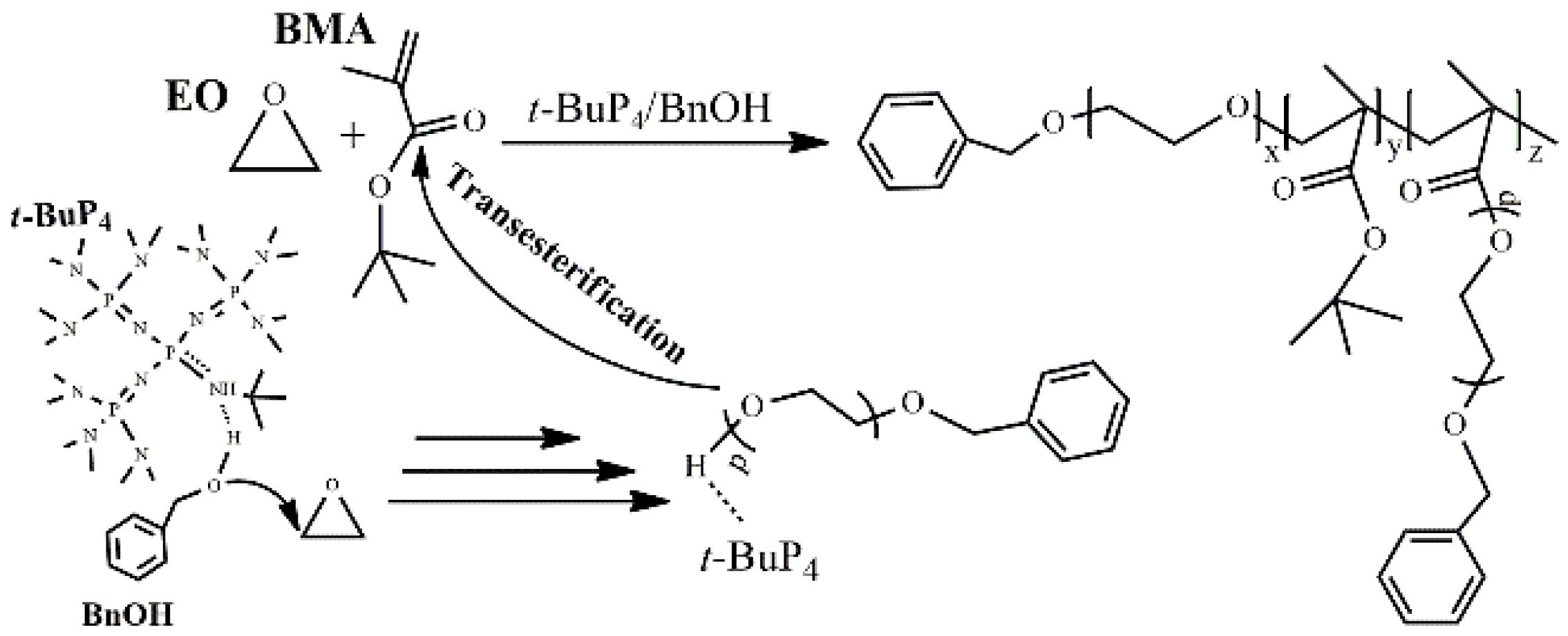
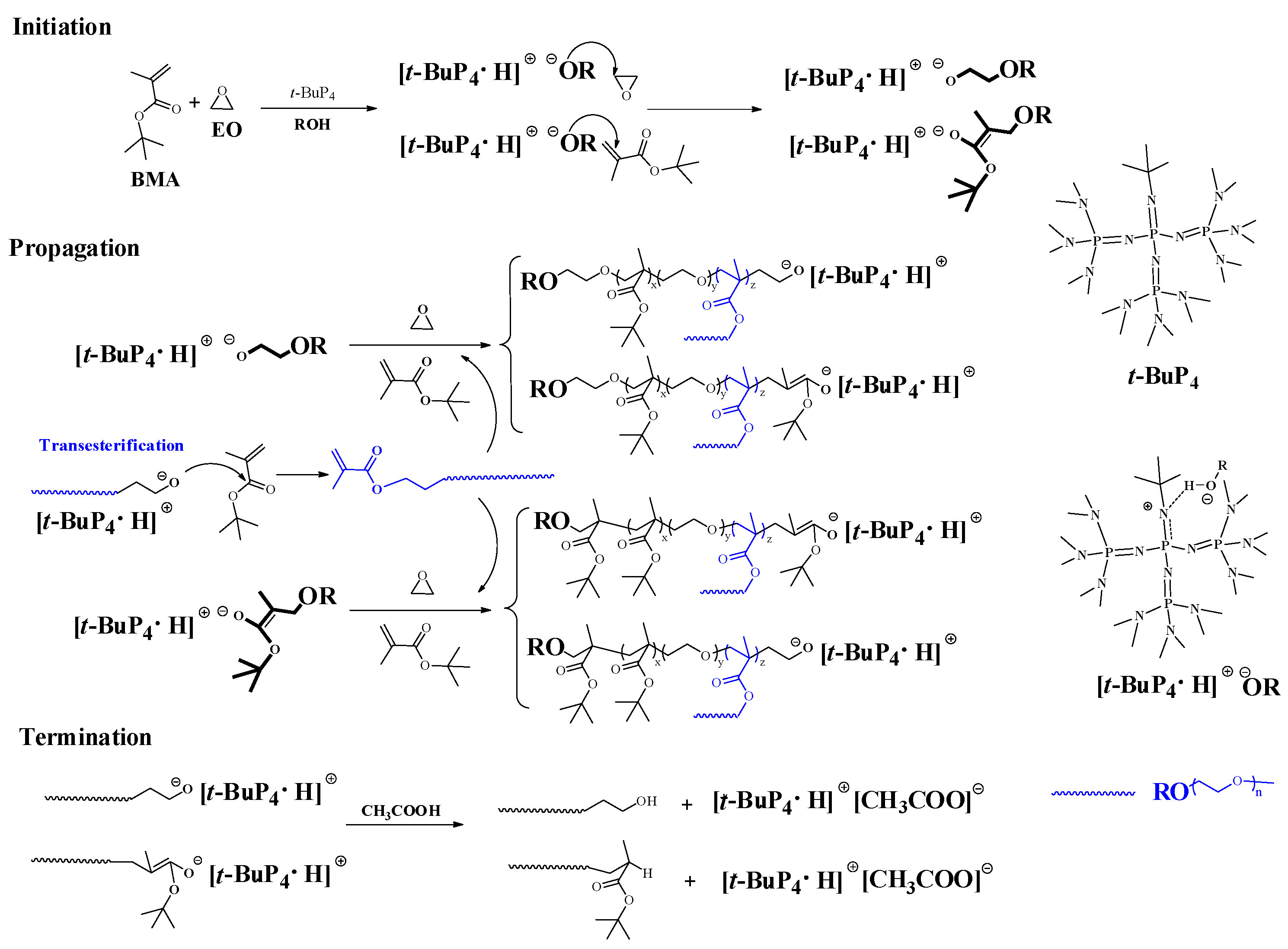
2.3. Hybrid Copolymerization of EO and BMA
2.4. Homopolymerization of BMA
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hadjichristidis, N.; Pitsikalis, M.; Pispas, S.; Iatrou, H. Polymers with complex architecture by living anionic polymerization. Chem. Rev. 2011, 101, 3747–3792. [Google Scholar] [CrossRef]
- Pyun, J. Polymer chemistry: Still in control. Nat. Mater. 2012, 11, 753–754. [Google Scholar] [CrossRef]
- Chen, Y.; Kakuchi, T. Organocatalyzed group transfer polymerization. Chem. Rec. 2016, 16, 2161–2183. [Google Scholar] [CrossRef]
- Perrier, S. 50th Anniversary perspective: RAFT polymerization—A user guide. Macromolecules 2017, 50, 7433–7447. [Google Scholar] [CrossRef]
- Ouchi, M.; Sawamoto, M. A study on physical properties of cyclic poly(vinyl ether)s synthesized via ring-expansion cationic polymerization. Macromolecules 2017, 50, 2603–2614. [Google Scholar] [CrossRef]
- Xia, Y.L.; Zhao, J.P. Macromolecular architectures based on organocatalytic ring-opening (co)polymerization of epoxides. Polymer 2018, 143, 343–361. [Google Scholar] [CrossRef]
- Romain, C.; Zhu, Y.; Dingwall, P.; Paul, S.; Rzepa, H.S.; Buchard, A.; Williams, C.K. Chemoselective polymerizations from mixtures of epoxide, lactone, anhydride, and carbon dioxide. J. Am. Chem. Soc. 2016, 138, 4120–4131. [Google Scholar] [CrossRef] [PubMed]
- Brocas, A.L.; Gervais, M.; Carlotti, S.; Pispas, S. Amphiphilic diblock copolymers based on ethylene oxide and epoxides bearing aliphatic side chains. Polym. Chem. 2012, 3, 2148–2156. [Google Scholar] [CrossRef]
- Sims, M.B.; Lessard, J.J.; Bai, L.; Sumerlin, B.S. Functional diversification of polymethacrylates by dynamic β-ketoester modification. Macromolecules 2018, 51, 6380–6386. [Google Scholar] [CrossRef]
- Sato, M.; Kato, T.; Ohishi, T.; Ishige, R.; Ohta, N.; White, K.L.; Hirai, T.; Takahara, A. Precise synthesis of poly(methyl methacrylate) brush with well-controlled stereoregularity using a surface-initiated living anionic polymerization method. Macromolecules 2016, 49, 2071–2076. [Google Scholar] [CrossRef]
- Yamamoto, S.I.; Sanda, F.; Endo, T. Spontaneous alternating copolymerization of methoxyallene with N-phenylmaleimide. Macromolecules 1999, 32, 5501–5506. [Google Scholar] [CrossRef]
- Kempe, K. Chain and step growth polymerizations of cyclic imino ethers: From poly(2-oxazoline)s to poly(ester amide)s. Macromol. Chem. Phys. 2017, 218, 1700021–1700038. [Google Scholar] [CrossRef]
- Steinkoenig, J.; De Jongh, P.; Haddleton, D.M.; Goldmann, A.S.; Kempe, K. Unraveling the spontaneous zwitterionic copolymerization mechanism of cyclic imino ethers and acrylic acid. Macromolecules 2018, 51, 318–327. [Google Scholar] [CrossRef]
- De Jongh, P.; Haddleton, D.M.; Kempe, K. Spontaneous zwitterionic copolymerisation: An undervalued and efficacious technique for the synthesis of functional degradable oligomers and polymers. Prog. Polym. Sci. 2018, 87, 228–246. [Google Scholar] [CrossRef]
- Yang, H.J.; Xu, J.B.; Pispas, S.; Zhang, G.Z. Hybrid copolymerization of ɛ-caprolactone and methyl methacrylate. Macromolecules 2012, 45, 3312–3317. [Google Scholar] [CrossRef]
- Xu, J.B.; Yang, H.J.; Zhang, G.Z. Synthesis of poly(ɛ-caprolactone-co-methacrylic acid) copolymer via phosphazene-catalyzed hybrid copolymerization. Macromol. Chem. Phys. 2013, 214, 378–385. [Google Scholar] [CrossRef]
- Kanazawa, A.; Aoshima, S. Cationic terpolymerization of vinyl ethers, oxetane, and ketones via concurrent vinyl-addition, ring-opening, and carbonyl-addition mechanisms: Multiblock polymer synthesis and mechanistic investigation. Macromolecules 2017, 50, 6595–6605. [Google Scholar] [CrossRef]
- Kanazawa, A.; Kanaoka, S.; Aoshima, S. Concurrent cationic vinyl-addition and ring-opening copolymerization using B(C6F5)3 as a catalyst: Copolymerization of vinyl ethers and isobutylene oxide via crossover propagation reactions. J. Am. Chem. Soc. 2013, 135, 9330–9333. [Google Scholar] [CrossRef]
- Zhang, Z.; Xia, L.; Zeng, T.Y.; Wu, D.C.; Zhang, W.J.; Hong, C.Y.; You, Y.Z. Hybrid copolymerization via mechanism interconversion between radical vinyl-addition and anion ring-opening polymerization. Polym. Chem. 2019, 10, 2117–2125. [Google Scholar] [CrossRef]
- Wright, P.V. Electrical conductivity in ionic complexes of poly(ethylene oxide). Br. Polym. J. 1975, 7, 319–327. [Google Scholar] [CrossRef]
- Bouchet, R.; Maria, S.; Meziane, R.; Aboulaich, A.; Lienafa, L.; Bonnet, J.P.; Phan, T.N.T.; Bertin, D.; Gigmes, D.; Devaux, D.; et al. Single-ion BAB triblock copolymers as highly efficient electrolytes for lithium-metal batteries. Nat. Mater. 2013, 12, 452–457. [Google Scholar] [CrossRef]
- Xue, Z.; He, D.; Xie, X. Poly(ethylene oxide)-based electrolytes for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 19218–19253. [Google Scholar] [CrossRef]
- Tao, C.; Gao, M.H.; Yin, B.H.; Li, B.; Huang, Y.P.; Xu, G.; Bao, J.J. A promising TPU/PEO blend polymer electrolyte for all-solid-state lithium-ion batteries. Electrochim. Acta 2017, 257, 31–39. [Google Scholar] [CrossRef]
- Shirouchi, T.; Kanazawa, A.; Kanaoka, S.; Aoshima, S. Controlled cationic copolymerization of vinyl monomers and cyclic acetals via concurrent vinyl-addition and ring-opening mechanisms. Macromolecules 2016, 49, 7184–7195. [Google Scholar] [CrossRef]
- Suzuki, T.; Murakami, Y.; Tsuji, Y.; Takegami, Y. Synthesis of poly (ethylene oxide-b-methyl methacrylate). Polym. J. 1976, 14, 675–678. [Google Scholar] [CrossRef]
- Tomoi, M.; Shibayama, Y.; Kakiuchi, H. Polymerization of methyl and ethyl methacrylates initiated with alkali-metal alkoxide derivatives of poly(ethylene oxide). Polym. J. 1976, 8, 190–195. [Google Scholar] [CrossRef][Green Version]
- Suzuki, T.; Murakami, Y.; Takegami, Y. Synthesis and characterization of block copolymers of poly(ethylene oxide) and poly(methyl methacrylate). Polym. J. 1980, 12, 183–192. [Google Scholar] [CrossRef][Green Version]
- Fetters, L.J. Synthesis of block polymers by homogeneous anionic polymerization. J. Polym. Sci. C Polym. Symp. 1969, 26, 1–35. [Google Scholar] [CrossRef]
- Wang, J.; Varshney, S.K.; Jerome, R.; Teyssie, P.; Wang, J.; Varshney, S.K.; Jerome, R.; Teyssie, P. Synthesis of AB(BA), ABA and BAB block copolymers of tert-butyl methacrylate (A) and ethylene oxide (B). J. Polym. Sci. Part A Polym. Chem. 1992, 30, 2251–2261. [Google Scholar] [CrossRef]
- Zhao, J.P.; Pahovnik, D.; Gnanou, Y.; Hadjichristidis, N. A “catalyst switch” strategy for the sequential metal-free polymerization of epoxides and cyclic esters/carbonate. Macromolecules 2014, 47, 3814–3822. [Google Scholar] [CrossRef]
- Zhao, J.P.; Pahovnik, D.; Gnanou, Y.; Hadjichristidis, N. Phosphazene-promoted metal-free ring-opening polymerization of ethylene oxide initiated by carboxylic acid. Macromolecules 2014, 47, 1693–1698. [Google Scholar] [CrossRef]
- Ladelta, V.; Kim, J.D.; Bilalis, P.; Gnanou, Y.; Hadjichristidis, N. Block copolymers of macrolactones/small lactones by a “catalyst-switch” organocatalytic strategy. Thermal properties and phase behavior. Macromolecules 2018, 51, 2428–2436. [Google Scholar] [CrossRef]
- Suzuki, T.; Murakami, Y.; Takegami, Y. Synthesis of block copolymers of poly(methyl methacrylate) and polyoxirane: A new initiator system. J. Polym. Sci. Polym. Lett. Ed. 1979, 17, 241–244. [Google Scholar] [CrossRef]
- Twaik, M.A.; Tahan, M.; Zilkha, A. Grafting of poly(ethylene oxide) on poly(methyl methacrylate) by transesterification. J. Polym. Sci. Part A Polym. Chem. 1969, 7, 2469–2480. [Google Scholar] [CrossRef]
- Xu, J.B.; Fan, X.L.; Yang, J.X.; Ma, C.F.; Ye, X.D.; Zhang, G.Z. Poly(l-lactide-co-2-(2-methoxyethoxy)ethyl methacrylate): A biodegradable polymer with protein resistance. Colloids Surf. B Biointerfaces 2014, 116, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Fox, T.G. Influence of diluent and of copolymer composition on the glass temperature of a polymer system. Bull. Am. Phys. Soc. 1956, 1, 123–124. [Google Scholar]
- Berthier, C.; Gorecki, W.; Minier, M.; Armand, M.B.; Chabagno, J.M.; Rigaud, P. Microscopic investigation of ionic conductivity in alkali metal salts-poly(ethylene oxide) adducts. Solid State Ion. 1983, 11, 91–95. [Google Scholar] [CrossRef]
- Druger, S.D.; Ratner, M.A.; Nitzan, A. Polymeric solid electrolytes: Dynamic bond percolation and free volume models for diffusion. Solid State Ion. 1983, 9, 1115–1120. [Google Scholar] [CrossRef]
- Shi, J.; Yang, Y.; Shao, H. Co-polymerization and blending based PEO/PMMA/P(VDF-HFP) gel polymer electrolyte for rechargeable lithium metal batteries. J. Membr. Sci. 2018, 547, 1–10. [Google Scholar] [CrossRef]
- Porcarelli, L.; Aboudzadeh, M.A.; Rubatat, L.; Nair, J.R.; Shaplov, A.S.; Gerbaldi, C.; Mecerreyes, D. Single-ion triblock copolymer electrolytes based on poly(ethylene oxide) and methacrylic sulfonamide blocks for lithium metal batteries. J. Power Sources 2017, 364, 191–199. [Google Scholar] [CrossRef]
- Genier, F.S.; Burdin, C.V.; Biria, S.; Hosein, I.D. A novel calcium-ion dolid polymer electrolyte based on crosslinked poly(ethylene glycol) diacrylate. J. Power Sources 2019, 414, 302–307. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, Y.; Liang, Y.; Dong, H.; Hao, F.; Wang, A.; Zhu, Y.; Cui, X.; Yao, Y. Hyperbranched PEO-based hyper-star solid polymer electrolytes with simultaneous improvement of ion transport and mechanical strength. ACS Appl. Energy Mater. 2019, 2, 1608–1615. [Google Scholar] [CrossRef]
- Amereller, M.; Schedlbauer, T.; Moosbauer, D.; Schreiner, C.; Stock, C.; Wudy, F.; Zugmann, S.; Hammer, H.; Maurer, A.; Gschwind, R.M.; et al. Electrolytes for lithium and lithium-ion batteries: From synthesis of novel lithium borates and ionic liquids to development of novel measurement methods. Prog. Solid State Chem. 2014, 42, 39–56. [Google Scholar] [CrossRef]



| Samples | fb | Mn × 10−4 (g/mol) c | Ðc | FEOd (mol %) |
|---|---|---|---|---|
| PBMA | 100/0 | 1.38 | 1.26 | 0 |
| PEO | 0/250 | 0.98 | 1.19 | 100 |
| BMA10-co-EO-co-bPEO5 | 30/170 | 1.45 | 1.55 | 85 |
| BMA21-co-EO-co-bPEO9 | 70/130 | 1.83 | 1.78 | 70 |
| BMA38-co-EO-co-bPEO12 | 100/100 | 2.01 | 1.92 | 50 |
| BMA49-co-EO-co-bPEO16 | 130/70 | 2.14 | 2.26 | 35 |
| BMA59-co-EO-co-bPEO16 | 140/60 | 2.25 | 2.32 | 25 |
| Samples | Tm a | Mn × 10−4 (g/mol) b | Ðb | FEO (mol %) c |
|---|---|---|---|---|
| S1 | 42.4 | 0.20 | 1.25 | 10.0 |
| S2 | 44.2 | 0.62 | 1.14 | 9.2 |
| S3 | 44.6 | 1.14 | 1.12 | 9.3 |
| S4 | 44.8 | 1.98 | 1.18 | 9.2 |
| S5 | 45.0 | 3.69 | 1.13 | 9.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, W.; Xu, L.; Liu, P.; Chen, Y.; Zhang, J.; Xu, J. Hybrid Copolymerization of Ethylene Oxide and tert-Butyl Methacrylate with Organocatalyst. Polymers 2021, 13, 2546. https://doi.org/10.3390/polym13152546
Xiao W, Xu L, Liu P, Chen Y, Zhang J, Xu J. Hybrid Copolymerization of Ethylene Oxide and tert-Butyl Methacrylate with Organocatalyst. Polymers. 2021; 13(15):2546. https://doi.org/10.3390/polym13152546
Chicago/Turabian StyleXiao, Wenhao, Liguo Xu, Pan Liu, Yang Chen, Jie Zhang, and Jinbao Xu. 2021. "Hybrid Copolymerization of Ethylene Oxide and tert-Butyl Methacrylate with Organocatalyst" Polymers 13, no. 15: 2546. https://doi.org/10.3390/polym13152546
APA StyleXiao, W., Xu, L., Liu, P., Chen, Y., Zhang, J., & Xu, J. (2021). Hybrid Copolymerization of Ethylene Oxide and tert-Butyl Methacrylate with Organocatalyst. Polymers, 13(15), 2546. https://doi.org/10.3390/polym13152546







