Nanomaterial-Based Dual-Emission Ratiometric Fluorescent Sensors for Biosensing and Cell Imaging
Abstract
:1. Introduction
2. Principles for Designing Ratiometric Fluorescent Nanosensors
2.1. Ratiometric Fluorescence with One Response Signal
2.2. Ratiometric Fluorescence with Two Reversible Signal Changes
3. Nanomaterial-Based Ratiometric Fluorescent Biosensors
3.1. QDs-Based Ratiometric Fluorescent Biosensors
3.2. Silicon Nanomaterial-Based Ratiometric Fluorescent Biosensors
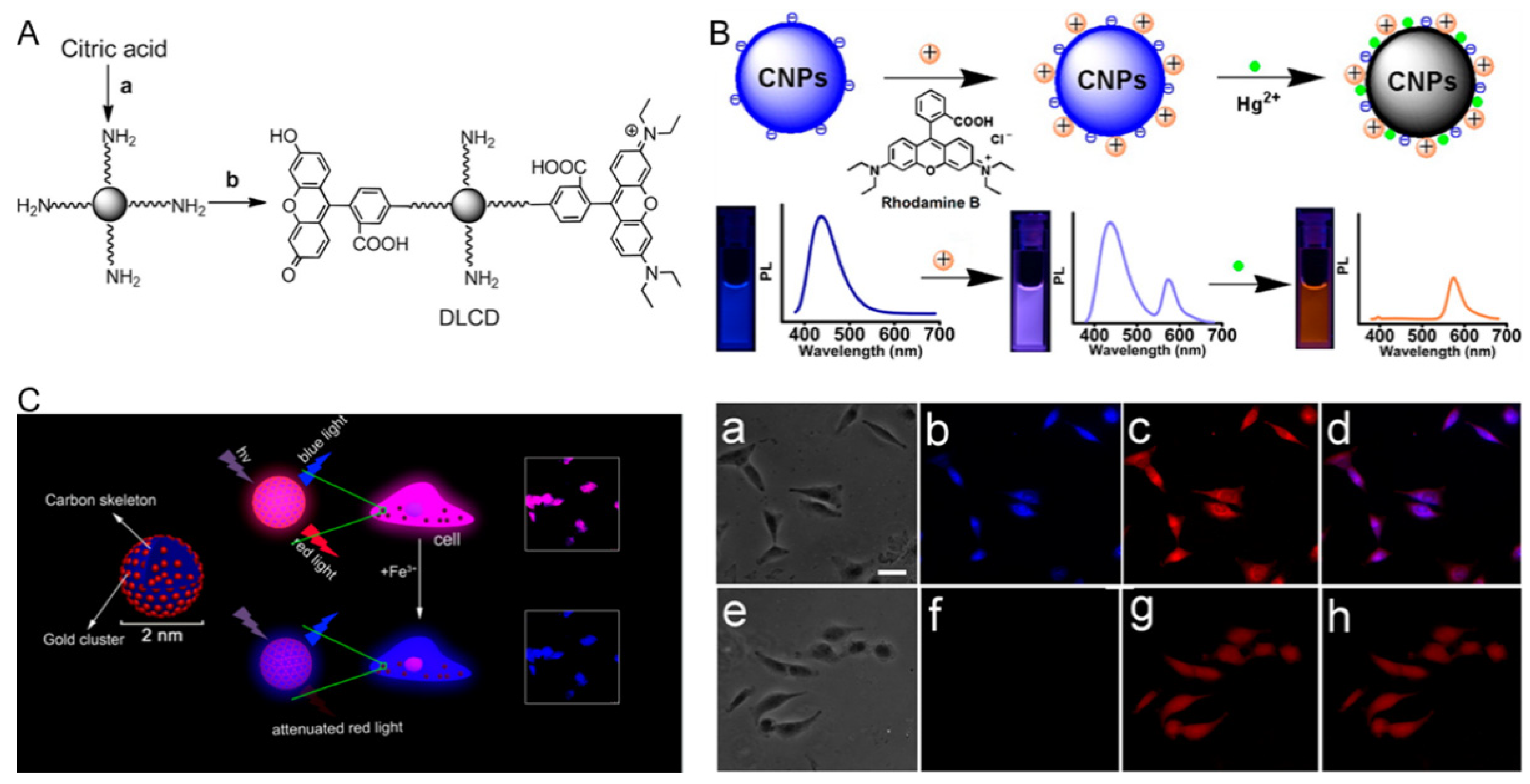
3.3. CDs-Based Ratiometric Fluorescent Biosensors
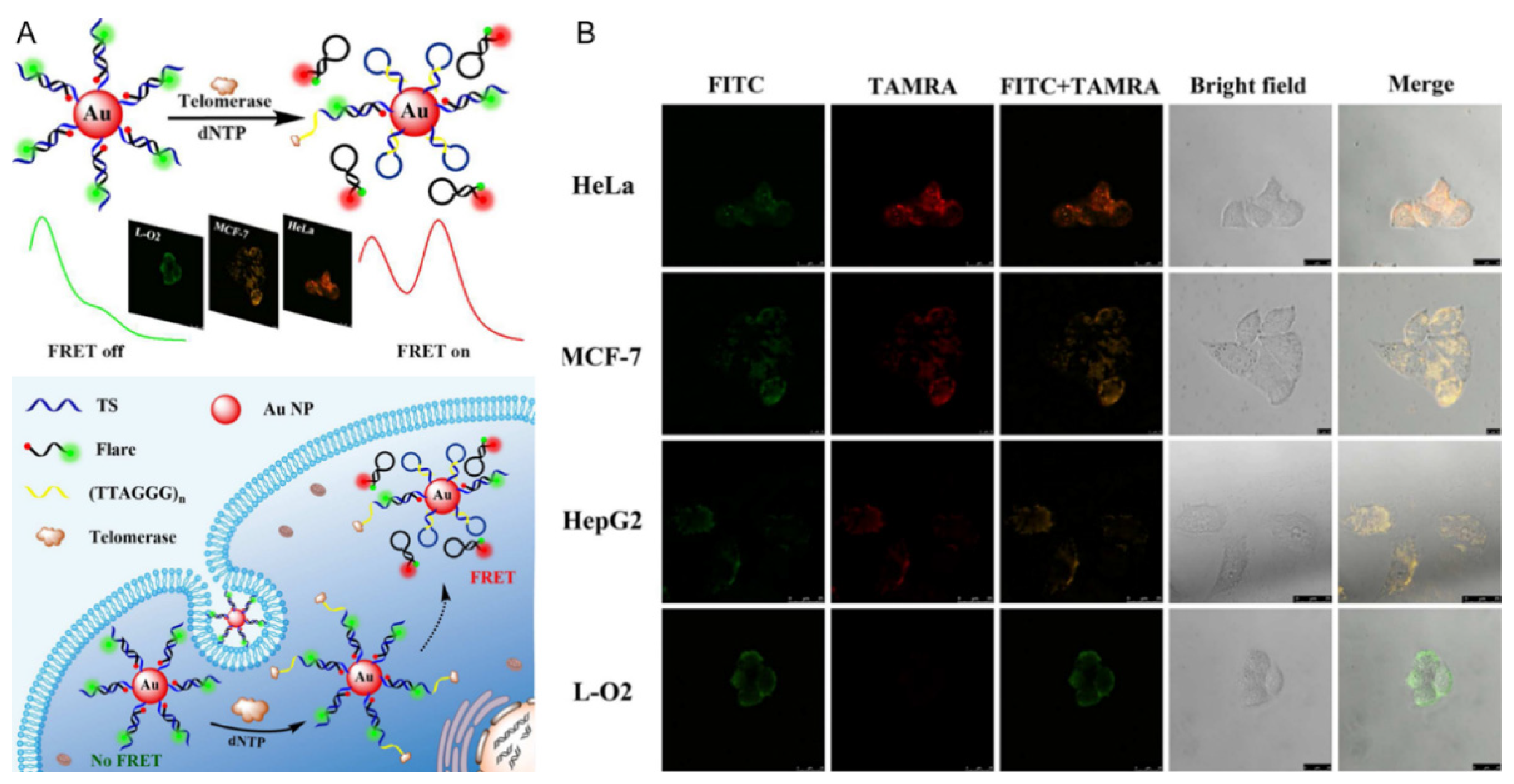
3.4. AuNPs-Based Ratiometric Fluorescent Biosensors
3.5. PNPs-Based Ratiometric Fluorescent Biosensors
4. Conclusions
5. Challenges and Future Outlooks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gui, R.J.; Jin, H.; Bu, X.N.; Fu, Y.X.; Wang, Z.H.; Liu, Q.Y. Recent advances in dual-emission ratiometric fluorescence probes for chemo/biosensing and bioimaging of biomarkers. Coordin. Chem. Rev. 2019, 383, 82–103. [Google Scholar] [CrossRef]
- Huang, X.L.; Song, J.B.; Yung, B.C.; Huang, X.H.; Xiong, Y.H.; Chen, X.Y. Ratiometric optical nanoprobes enable accurate molecular detection and imaging. Chem. Soc. Rev. 2018, 47, 2873–2920. [Google Scholar] [CrossRef]
- Bigdeli, A.; Ghasemi, F.; Abbasi-Moayed, S.; Shahrajabian, M.; Fahimi-Kashani, N.; Jafarinejad, S.; Farahmand Nejad, M.A.; Hormozi-Nezhad, M.R. Ratiometric fluorescent nanoprobes for visual detection: Design principles and recent advances—A review. Anal. Chim. Acta 2019, 1079, 30–58. [Google Scholar] [CrossRef]
- Liu, G.X.; Ma, X.Y.; Tang, Y.G.; Miao, P. Ratiometric fluorescence method for ctDNA analysis based on the construction of a DNA four-way junction. Analyst 2020, 145, 1174–1178. [Google Scholar] [CrossRef]
- Luo, Z.J.; Lv, T.Y.Z.; Zhu, K.N.; Li, Y.; Wang, L.; Gooding, J.J.; Liu, G.Z.; Liu, B. Paper-based ratiometric fluorescence analytical devices towards point-of-care testing of human serum albumin. Angew. Chem. 2020, 59, 3131–3136. [Google Scholar] [CrossRef]
- Na, M.; Zhang, S.P.; Liu, J.J.; Ma, S.D.; Han, Y.X.; Wang, Y.; He, Y.X.; Chen, H.L.; Chen, X.G. Determination of pathogenic bacteria-Bacillus anthrax spores in environmental samples by ratiometric fluorescence and test paper based on dual-emission fluorescent silicon nanoparticles. J. Hazard. Mater. 2020, 386, 121956. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, H.M.; Chudal, L.; Pandey, N.K.; Amador, E.H.; Bui, B.; Wang, L.Y.; Ma, X.D.; Deng, S.P.; Zhu, X.H.; et al. Striking luminescence phenomena of carbon dots and their applications as a double ratiometric fluorescence probes for H2S detection. Mater. Today Phys. 2021, 17, 100328. [Google Scholar] [CrossRef]
- Li, Q.; Liu, L.; Liu, J.W.; Jiang, J.H.; Yu, R.Q.; Chu, X. Nanomaterial-based fluorescent probes for live-cell imaging. Trac-Trend Anal. Chem. 2014, 58, 130–144. [Google Scholar] [CrossRef]
- Li, J.J.; Cheng, F.F.; Huang, H.P.; Li, L.L.; Zhu, J.J. Nanomaterial-based activatable imaging probes: From design to biological applications. Chem. Soc. Rev. 2015, 44, 7855–7880. [Google Scholar] [CrossRef] [PubMed]
- Maity, A.R.; Palmal, S.; Basiruddin, S.K.; Karan, N.S.; Sarkar, S.; Pradhan, N.; Jana, N.R. Doped semiconductor nanocrystal based fluorescent cellular imaging probes. Nanoscale 2013, 5, 5506–5513. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Oh, Y.; Sens, A.; Ataie, N.; Dana, H.; Macklin, J.J.; Laviv, T.; Welf, E.S.; Dean, K.M.; Zhang, F.J.; et al. A bright cyan-excitable orange fluorescent protein facilitates dual-emission microscopy and enhances bioluminescence imaging in vivo. Nat. Biotechnol. 2016, 34, 760–767. [Google Scholar] [CrossRef]
- Hanson, G.T.; McAnaney, T.B.; Park, E.S.; Rendell, M.E.P.; Yarbrough, D.K.; Chu, S.Y.; Xi, L.X.; Boxer, S.G.; Montrose, M.H.; Remington, S.J. Green fluorescent protein variants as ratiometric dual emission pH sensors. 1. structural characterization and preliminary application. Biochemistry 2002, 41, 15477–15488. [Google Scholar] [CrossRef]
- Tang, L.T.; Zhang, S.; Zhao, Y.F.; Rozanov, N.D.; Zhu, L.D.; Wu, J.H.; Campbell, R.E.; Fang, C. Switching between ultrafast pathways enables a green-red emission ratiometric fluorescent-protein-based Ca2+ biosensor. Int. J. Mol. Sci. 2021, 22, 445. [Google Scholar] [CrossRef]
- Yan, F.Y.; Zou, Y.; Wang, M.; Mu, X.L.; Yang, N.; Chen, L. Highly photoluminescent carbon dots-based fluorescent chemosensors for sensitive and selective detection of mercury ions and application of imaging in living cells. Sens. Actuators B 2014, 192, 488–495. [Google Scholar] [CrossRef]
- Ji, X.Y.; Wang, H.Y.; Song, B.; Chu, B.B.; He, Y. Silicon Nanomaterials for Biosensing and Bioimaging Analysis. Front. Chem. 2018, 6, 38. [Google Scholar] [CrossRef] [Green Version]
- Singh, H.; Bamrah, A.; Bhardwaj, S.K.; Deep, A.; Khatri, M.; Kim, K.H.; Bhardwaj, N. Nanomaterial-based fluorescent sensors for the detection of lead ions. J. Hazard. Mater. 2021, 407, 124379. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Pei, H.; Wang, L.H.; Lu, J.X.; Gao, J.M.; Jiang, B.W.; Zhao, X.C.; Fan, C.H. Nanomaterial-based fluorescent DNA analysis: A comparative study of the quenching effects of graphene oxide, carbon nanotubes, and gold nanoparticles. Adv. Funct. Mater. 2013, 23, 4140–4148. [Google Scholar] [CrossRef]
- Chan, Y.H.; Wu, C.; Ye, F.; Jin, Y.; Smith, P.B.; Chiu, D.T. Development of ultrabright semiconducting polymer dots for ratiometric pH sensing. Anal. Chem. 2011, 83, 1448–1455. [Google Scholar] [CrossRef] [Green Version]
- Cai, L.; Chen, Z.Z.; Chen, M.Y.; Tang, H.W.; Pang, D.W. MUC-1 aptamer-conjugated dye-doped silica nanoparticles for MCF-7 cells detection. Biomaterials 2013, 34, 371–381. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Hou, D.J.; Yu, X.L. Facile preparation of FITC-modified silicon nanodots for ratiometric pH sensing and imaging. Spectrochim. Acta Part A 2020, 234, 118276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Guo, S.; Cheng, S.B.; Ji, X.H.; He, Z.K. Label-free silicon nanodots featured ratiometric fluorescent aptasensor for lysosomal imaging and pH measurement. Biosens. Bioelectron. 2017, 94, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Guo, S.; Huang, H.Y.; Mao, G.B.; Ji, X.H.; He, Z.K. Silicon nanodot-based aptasensor for fluorescence turn-on detection of mucin 1 and targeted cancer cell imaging. Anal. Chim. Acta 2018, 1035, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.N.; Guo, S.; Jiang, Z.R.; Mao, G.B.; Ji, X.H.; He, Z.K. Rox-DNA functionalized silicon nanodots for ratiometric detection of mercury ions in live cells. Anal. Chem. 2018, 90, 9796–9804. [Google Scholar] [CrossRef]
- Tao, X.Q.; Peng, Y.Y.; Liu, J.W. Nanomaterial-based fluorescent biosensors for veterinary drug detection in foods. J. Food Drug Anal. 2020, 28, 575–594. [Google Scholar]
- Song, B.; He, Y. Fluorescent silicon nanomaterials: From synthesis to functionalization and application. Nano Today 2019, 26, 149–163. [Google Scholar] [CrossRef]
- Liu, L.; Yang, Q.H.; Lei, J.P.; Xu, N.; Ju, H.X. DNA-regulated silver nanoclusters for label-free ratiometric fluorescence detection of DNA. Chem. Commun. 2014, 50, 13698–13701. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Zhu, G.B.; Cao, W.D.; Liu, Z.J.; Pan, C.G.; Hu, W.J.; Zhao, W.Y.; Sun, J.F. A novel ratiometric fluorescent probe for the detection of uric acid in human blood based on H2O2-mediated fluorescence quenching of gold/silver nanoclusters. Talanta 2019, 191, 46–53. [Google Scholar] [CrossRef] [PubMed]
- He, Y.S.; Pan, C.G.; Cao, H.X.; Yue, M.Z.; Wang, L.; Liang, G.X. Highly sensitive and selective dual-emission ratiometric fluorescence detection of dopamine based on carbon dots-gold nanoclusters hybrid. Sens. Actuators B 2018, 265, 371–377. [Google Scholar] [CrossRef]
- Jin, M.; Mou, Z.L.; Zhang, R.L.; Liang, S.S.; Zhang, Z.Q. An efficient ratiometric fluorescence sensor based on metal-organic frameworks and quantum dots for highly selective detection of 6-mercaptopurine. Biosens. Bioelectron. 2017, 91, 162–168. [Google Scholar] [CrossRef]
- Qi, S.J.; Liu, W.M.; Zhang, P.P.; Wu, J.S.; Zhang, H.Y.; Ren, H.H.; Ge, J.C.; Wang, P.F. A colorimetric and ratiometric fluorescent probe for highly selective detection of glutathione in the mitochondria of living cells. Sens. Actuators B 2018, 270, 459–465. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, F.Y.; Yang, G.C.; Lu, C.F.; Nie, J.Q.; Chen, Z.X.; Ren, J.; Sun, Q.; Zhao, C.C.; Zhu, W.H. A ratiometric fluorescent probe for monitoring leucine aminopeptidase in living cells and zebrafish model. Anal. Chem. 2017, 89, 11576–11582. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Mao, G.B.; Ji, X.H.; He, Z.K. DNA-templated quantum dots and their applications in biosensors, bioimaging, and therapy. J. Mater. Chem. B 2020, 8, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.H.; Jiao, Y.; Du, F.F.; Gao, Y.F.; Lu, W.J.; Shuang, S.M.; Dong, C.; Wang, Y. Facile synthesis of ratiometric fluorescent carbon dots for pH visual sensing and cellular imaging. Talanta 2020, 216, 120943. [Google Scholar] [CrossRef]
- Shi, Y.Q.; Lin, L.; Wei, Y.Z.; Li, W.T.; Nie, P.C.; He, Y.; Feng, X.P. Gold nanoparticles-mediated ratiometric fluorescence aptasensor for ultra-sensitive detection of Abscisic Acid. Biosens. Bioelectron. 2021, 190, 113311. [Google Scholar] [CrossRef]
- Liu, C.; Lu, D.K.; You, X.R.; Shi, G.Y.; Deng, J.J.; Zhou, T.S. Carbon dots sensitized lanthanide infinite coordination polymer nanoparticles: Towards ratiometric fluorescent sensing of cerebrospinal Aβ monomer as a biomarker for Alzheimer’s disease. Anal. Chim. Acta 2020, 1105, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Sheng, E.Z.; Lu, Y.X.; Tan, Y.T.; Xiao, Y.; Li, Z.X.; Dai, Z.H. Ratiometric fluorescent quantum dot-based biosensor for chlorothalonil detection via an inner-filter effect. Anal. Chem. 2020, 92, 4364–4370. [Google Scholar] [CrossRef]
- Chan, W.C.W.; Nie, S.M. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 1998, 281, 2016–2018. [Google Scholar] [CrossRef] [Green Version]
- Oliva-Chatelain, B.L.; Ticich, T.M.; Barron, A.R. Doping silicon nanocrystals and quantum dots. Nanoscale 2016, 8, 1733–1745. [Google Scholar] [CrossRef]
- Ekimov, A.I.; Onushchenko, A.A. Quantum size effect in three-dimensional microscopic semiconductor crystals. Pis’ma Zh. Eksp. Teor. Fiz. 1981, 34, 363–366. [Google Scholar]
- Bruchez, M.; Moronne, M.; Gin, P.; Weiss, S.; Alivisatos, A.P. Semiconductor nanocrystals as fluorescent biological labels. Science 1998, 281, 2013–2016. [Google Scholar] [CrossRef] [Green Version]
- Michalet, X.; Pinaud, F.F.; Bentolila, L.A.; Tsay, J.M.; Doose, S.; Li, J.J.; Sundaresan, G.; Wu, A.M.; Gambhir, S.S.; Weiss, S. Quantum dots for live cells, in vivo imaging, and diagnostics. Science 2005, 307, 538–544. [Google Scholar] [CrossRef] [Green Version]
- Mao, G.B.; Cai, Q.; Wang, F.B.; Luo, C.L.; Ji, X.H.; He, Z.K. One-step synthesis of Rox-DNA functionalized CdZnTeS quantum dots for the visual detection of hydrogen peroxide and blood glucose. Anal. Chem. 2017, 89, 11628–11635. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; He, L.; Long, Y.W.; Pan, S.; Liu, H.; Yang, J.D.; Hu, X.L. S-doped carbon dots capped ZnCdTe quantum dots for ratiometric fluorescence sensing of guanine. Sens. Actuators B 2019, 279, 44–52. [Google Scholar] [CrossRef]
- McVey, B.F.P.; Tilley, R.D. Solution synthesis, optical properties, and bioimaging applications of silicon nanocrystals. Acc. Chem. Res. 2014, 47, 3045–3051. [Google Scholar] [CrossRef]
- Peng, F.; Su, Y.Y.; Zhong, Y.L.; Fan, C.H.; Lee, S.T.; He, Y. Silicon nanomaterials platform for bioimaging, biosensing, and cancer therapy. Acc. Chem. Res. 2014, 47, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, M.; Tan, B.; Venkatakrishnan, K. Multiplex photoluminescent silicon nanoprobe for diagnostic bioimaging and intracellular analysis. Adv. Sci. 2018, 5, 1700548. [Google Scholar] [CrossRef] [Green Version]
- Littau, K.A.; Szajowski, P.J.; Muller, A.J.; Kortan, A.R.; Brus, L.E. A luminescent silicon nanocrystal colloid via a high-temperature aerosol reaction. J. Phys. Chem. 1993, 97, 1224–1230. [Google Scholar] [CrossRef]
- Zhong, Y.L.; Peng, F.; Bao, F.; Wang, S.Y.; Ji, X.Y.; Yang, L.; Su, Y.Y.; Lee, S.T.; He, Y. Large-scale aqueous synthesis of fluorescent and biocompatible silicon nanoparticles and their use as highly photostable biological probes. J. Am. Chem. Soc. 2013, 135, 8350–8356. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.L.; Liu, Y.F.; Su, C.; Ji, X.H.; He, Z.K. New fluorescent pH sensor based on label-free silicon nanodots. Sens. Actuators B 2014, 203, 795–801. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Hou, D.J.; Zhao, B.S.; Li, C.Y.; Wang, X.Y.; Xu, L.Y.; Long, T. Ratiometric fluorescence detection of DNA based on the inner filter effect of Ru(bpy)2(dppx)2+ toward silicon nanodots. ACS Omega 2021, 6, 857–862. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Y.N.; Ji, X.H.; He, Z.K. The ratiometric fluorescent detection of anthrax spore biomarker based on functionalized silicon nanodots. Chem. Pap. 2019, 73, 1753–1759. [Google Scholar] [CrossRef]
- Montalti, M.; Cantelli, A.; Battistelli, G. Nanodiamonds and silicon quantum dots: Ultrastable and biocompatible luminescent nanoprobes for long-term bioimaging. Chem. Soc. Rev. 2015, 44, 4853–4921. [Google Scholar] [CrossRef]
- Dasog, M.; Kehrle, J.; Rieger, B.; Veinot, J.G.C. Silicon Nanocrystals and Silicon-Polymer Hybrids: Synthesis, Surface Engineering, and Applications. Angew. Chem. Int. Ed. 2016, 55, 2322–2339. [Google Scholar] [CrossRef] [PubMed]
- Robidillo, C.J.T.; Wandelt, S.; Dalangin, R.; Zhang, L.J.; Yu, H.Y.; Meldrum, A.; Campbell, R.E.; Veinot, J.G.C. Ratiometric detection of nerve agents by coupling complementary properties of silicon-based quantum dots and green fluorescent protein. ACS Appl. Mater. Inter. 2019, 11, 33478–33488. [Google Scholar] [CrossRef] [PubMed]
- Ru, F.; Du, P.Y.; Lu, X.Q. Efficient ratiometric fluorescence probe utilizing silicon particles/gold nanoclusters nanohybrid for “on-off-on” bifunctional detection and cellular imaging of mercury (II) ions and cysteine. Anal. Chim. Acta 2020, 1105, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Chu, B.B.; Song, B.; Ji, X.Y.; Su, Y.Y.; Wang, H.Y.; He, Y. Fluorescent silicon nanorods-based ratiometric sensors for long-term and real-time measurements of intracellular pH in live cells. Anal. Chem. 2017, 89, 12152–12159. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.J.; Song, Y.B.; Zhao, X.H.; Shao, J.R.; Zhang, J.H.; Yang, B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective. Nano Res. 2015, 8, 355–381. [Google Scholar] [CrossRef]
- Shi, W.; Li, X.H.; Ma, H.M. A tunable ratiometric pH sensor based on carbon nanodots for the quantitative measurement of the intracellular pH of whole cells. Angew. Chem. Int. Edit. 2012, 51, 6432–6435. [Google Scholar] [CrossRef]
- Lan, M.H.; Zhang, J.F.; Chui, Y.S.; Wang, P.F.; Chen, X.F.; Lee, C.S.; Kwong, H.L.; Zhang, W.J. Carbon nanoparticle-based ratiometric fluorescent sensor for detecting mercury ions in aqueous media and living cells. ACS Appl. Mater. Inter. 2014, 6, 21270–21278. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Wang, D.H.; Huang, H.W.; Liu, L.F.; Zhou, Y.; Xia, X.D.; Deng, K.Q.; Liu, X.Y. Preparation of gold–carbon dots and ratiometric fluorescence cellular imaging. ACS Appl. Mater. Inter. 2016, 8, 6646–6655. [Google Scholar] [CrossRef]
- Lee, S.; Cha, E.J.; Park, K.; Lee, S.Y.; Hong, J.K.; Sun, I.C.; Kim, S.Y.; Choi, K.; Kwon, I.C.; Kim, K.; et al. A near-infrared-fluorescence-quenched gold-nanoparticle imaging probe for in vivo drug screening and protease activity determination. Angew. Chem. Int. Ed. 2008, 47, 2804–2807. [Google Scholar] [CrossRef]
- Mayilo, S.; Kloster, M.A.; Wunderlich, M.; Lutich, A.; Klar, T.A.; Nichtl, A.; Kurzinger, K.; Stefani, F.D.; Feldmann, J. Long-range fluorescence quenching by gold nanoparticles in a sandwich immunoassay for cardiac troponin T. Nano Lett. 2009, 9, 4558–4563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, L.H.; Zhang, H.; Boey, F.; Song, S.P.; Fan, C.H. Aptamer-based multicolor fluorescent gold nanoprobes for multiplex detection in homogeneous solution. Small 2010, 6, 201–204. [Google Scholar] [CrossRef]
- Hahn, W.C.; Stewart, S.A.; Brooks, M.W.; York, S.G.; Eaton, E.; Kurachi, A.; Beijersbergen, R.L.; Knoll, J.H.M.; Meyerson, M.; Weinberg, R.A. Inhibition of telomerase limits the growth of human cancer cells. Nat. Med. 1999, 5, 1164–1170. [Google Scholar] [CrossRef]
- Harley, C.B. Telomerase and cancer therapeutics. Nat. Rev. Cancer 2008, 8, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.C.; Goldkorn, A. Telomere and telomerase therapeutics in cancer. Genes 2016, 7, 22. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.J.; Zhang, K.; Zhang, T.T.; Xu, J.J.; Chen, H.Y. Reliable forster resonance energy transfer probe based on structure-switching DNA for ratiometric sensing of telomerase in living cells. Anal. Chem. 2017, 89, 4216–4222. [Google Scholar] [CrossRef]
- Yan, X.; Li, H.X.; Han, X.S.; Su, X.G. A ratiometric fluorescent quantum dots based biosensor for organophosphorus pesticides detection by inner-filter effect. Biosens. Bioelectron. 2015, 74, 277–283. [Google Scholar] [CrossRef]
- Chen, P.P.; Ilyas, I.; He, S.; Xing, Y.C.; Jin, Z.G.; Huang, C.B. Ratiometric pH sensing and imaging in living cells with dual-emission semiconductor polymer dots. Molecules 2019, 24, 2923. [Google Scholar] [CrossRef] [Green Version]
- Bao, Y.Y.; De Keersmaecker, H.; Corneillie, S.; Yu, F.; Mizuno, H.; Zhang, G.F.; Hofkens, J.; Mendrek, B.; Kowalczuk, A.; Smet, M. Tunable ratiometric fluorescence sensing of intracellular pH by aggregation-induced emission-active hyperbranched polymer nanoparticles. Chem. Mater. 2015, 27, 3450–3455. [Google Scholar] [CrossRef]
- Wu, C.F.; Schneider, T.; Zeigler, M.; Yu, J.; Schiro, P.G.; Burnham, D.R.; McNeill, J.D.; Chiu, D.T. Bioconjugation of ultrabright semiconducting polymer dots for specific cellular targeting. J. Am. Chem. Soc. 2010, 132, 15410–15417. [Google Scholar] [CrossRef] [Green Version]
- Verma, M.; Chan, Y.H.; Saha, S.; Liu, M.H. Recent developments in semiconducting polymer dots for analytical detection and NIR-II fluorescence imaging. ACS Appl. Bio Mater. 2021, 4, 2142–2159. [Google Scholar] [CrossRef]
- Men, X.J.; Wang, F.; Chen, H.B.; Liu, Y.B.; Men, X.X.; Yuan, Y.; Zhang, Z.; Gao, D.Y.; Wu, C.F.; Yuan, Z. Ultrasmall semiconducting polymer dots with rapid clearance for second near-infrared photoacoustic imaging and photothermal cancer therapy. Adv. Funct. Mater. 2020, 30, 1909673. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, P.S.; Chen, J.; Li, Y.; Yu, M.L.; Long, Y.F.; Yi, P.G. Polymer nanoparticle-based ratiometric fluorescent probe for imaging Hg2+ ions in living cells. Sens. Actuators B 2017, 242, 818–824. [Google Scholar] [CrossRef]
- Park, E.J.; Brasuel, M.; Behrend, C.; Philbert, M.A.; Kopelman, R. Ratiometric optical PEBBLE nanosensors for real-time magnesium ion concentrations inside viable cells. Anal. Chem. 2003, 75, 3784–3791. [Google Scholar] [CrossRef]
- Sun, H.H.; Scharff-Poulsen, A.M.; Gu, H.; Almdal, K. Synthesis and characterization of ratiometric, pH sensing nanoparticles with covalently attached fluorescent dyes. Chem. Mater. 2006, 18, 3381–3384. [Google Scholar] [CrossRef]
- Liu, X.J.; Zhang, N.; Bing, T.; Shangguan, D.H. Carbon dots based dual-emission silica nanoparticles as a ratiometric nanosensor for Cu2+. Anal. Chem. 2014, 86, 2289–2296. [Google Scholar] [CrossRef]
- Kuo, S.Y.; Li, H.H.; Wu, P.J.; Chen, C.P.; Huang, Y.C.; Chan, Y.H. Dual colorimetric and fluorescent sensor based on semiconducting polymer dots for ratiometric detection of lead ions in living cells. Anal. Chem. 2015, 87, 4765–4771. [Google Scholar] [CrossRef]
- Peng, H.S.; Stolwijk, J.A.; Sun, L.N.; Wegener, J.; Wolfbeis, O.S. A nanogel for ratiometric fluorescent sensing of intracellular pH values. Angew.Chem. Int. Ed. 2010, 49, 4246–4249. [Google Scholar] [CrossRef]
- Mao, G.B.; Ma, Y.X.; Wu, G.Q.; Du, M.Y.; Tian, S.B.; Huang, S.Q.; Ji, X.H.; He, Z.K. Novel method of clickable quantum dot construction for bioorthogonal labeling. Anal. Chem. 2021, 93, 777–783. [Google Scholar] [CrossRef]
- Xia, Q.Y.; Li, H.X.; Xiao, K. Factors affecting the pharmacokinetics, biodistribution and toxicity of gold nanoparticles in drug delivery. Curr. Drug Metab. 2016, 17, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Pompa, P.P.; Vecchio, G.; Galeone, A.; Brunetti, V.; Sabella, S.; Maiorano, G.; Falqui, A.; Bertoni, G.; Cingolani, R. In Vivo toxicity assessment of gold nanoparticles in Drosophila melanogaster. Nano Res. 2011, 4, 405–413. [Google Scholar] [CrossRef]
- Hoan, B.T.; Tam, P.D.; Pham, V.H. Green synthesis of highly luminescent carbon quantum dots from lemon juice. J. Nanotech. 2019, 2019, 2852816. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Hou, D.; Wang, Z.; Cai, N.; Au, C. Nanomaterial-Based Dual-Emission Ratiometric Fluorescent Sensors for Biosensing and Cell Imaging. Polymers 2021, 13, 2540. https://doi.org/10.3390/polym13152540
Zhang Y, Hou D, Wang Z, Cai N, Au C. Nanomaterial-Based Dual-Emission Ratiometric Fluorescent Sensors for Biosensing and Cell Imaging. Polymers. 2021; 13(15):2540. https://doi.org/10.3390/polym13152540
Chicago/Turabian StyleZhang, Yanan, Dajun Hou, Zelong Wang, Ning Cai, and Chaktong Au. 2021. "Nanomaterial-Based Dual-Emission Ratiometric Fluorescent Sensors for Biosensing and Cell Imaging" Polymers 13, no. 15: 2540. https://doi.org/10.3390/polym13152540
APA StyleZhang, Y., Hou, D., Wang, Z., Cai, N., & Au, C. (2021). Nanomaterial-Based Dual-Emission Ratiometric Fluorescent Sensors for Biosensing and Cell Imaging. Polymers, 13(15), 2540. https://doi.org/10.3390/polym13152540





