Recent Advances in Polymer-Inorganic Mixed Matrix Membranes for CO2 Separation
Abstract
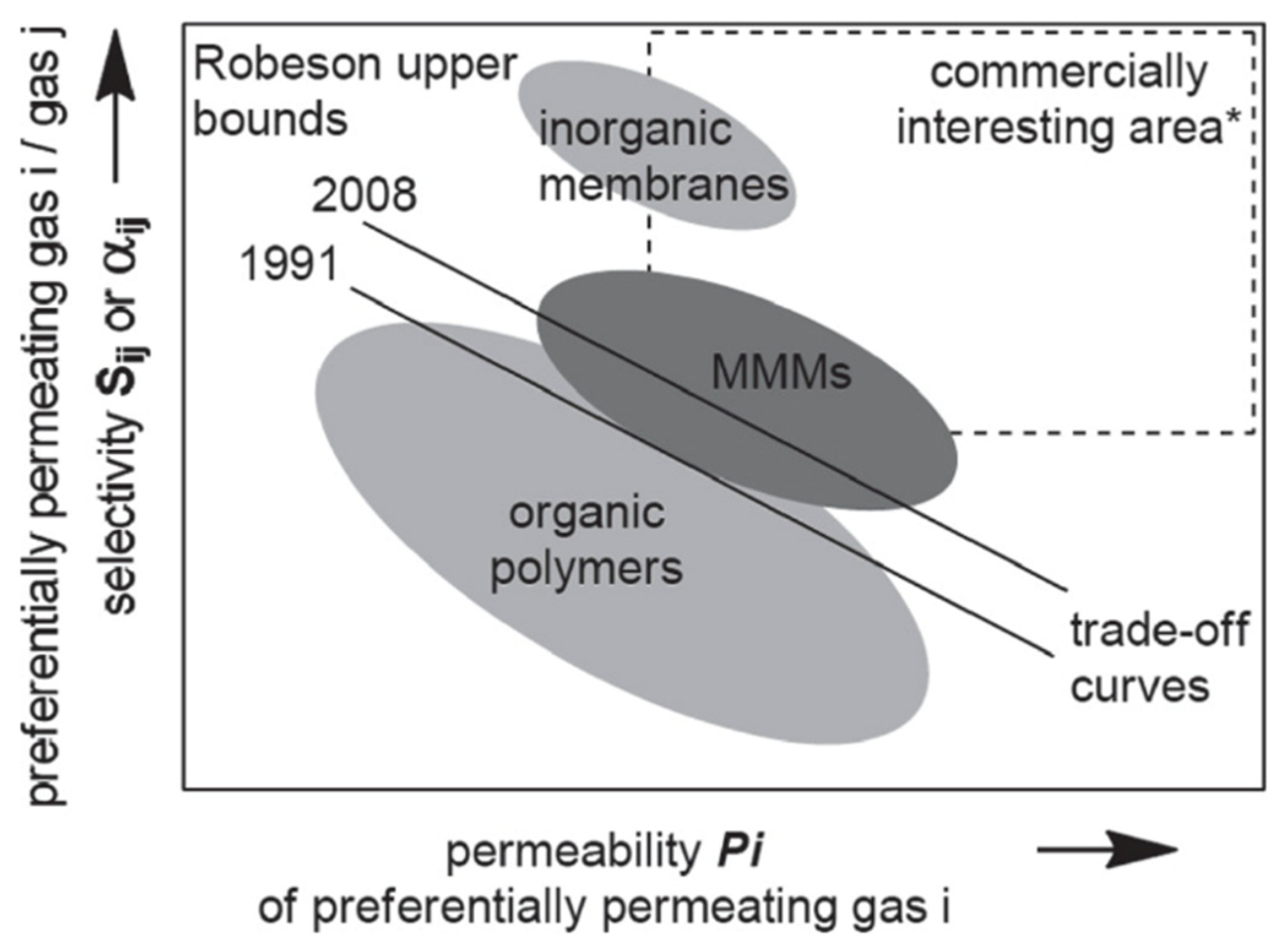
:1. Introduction
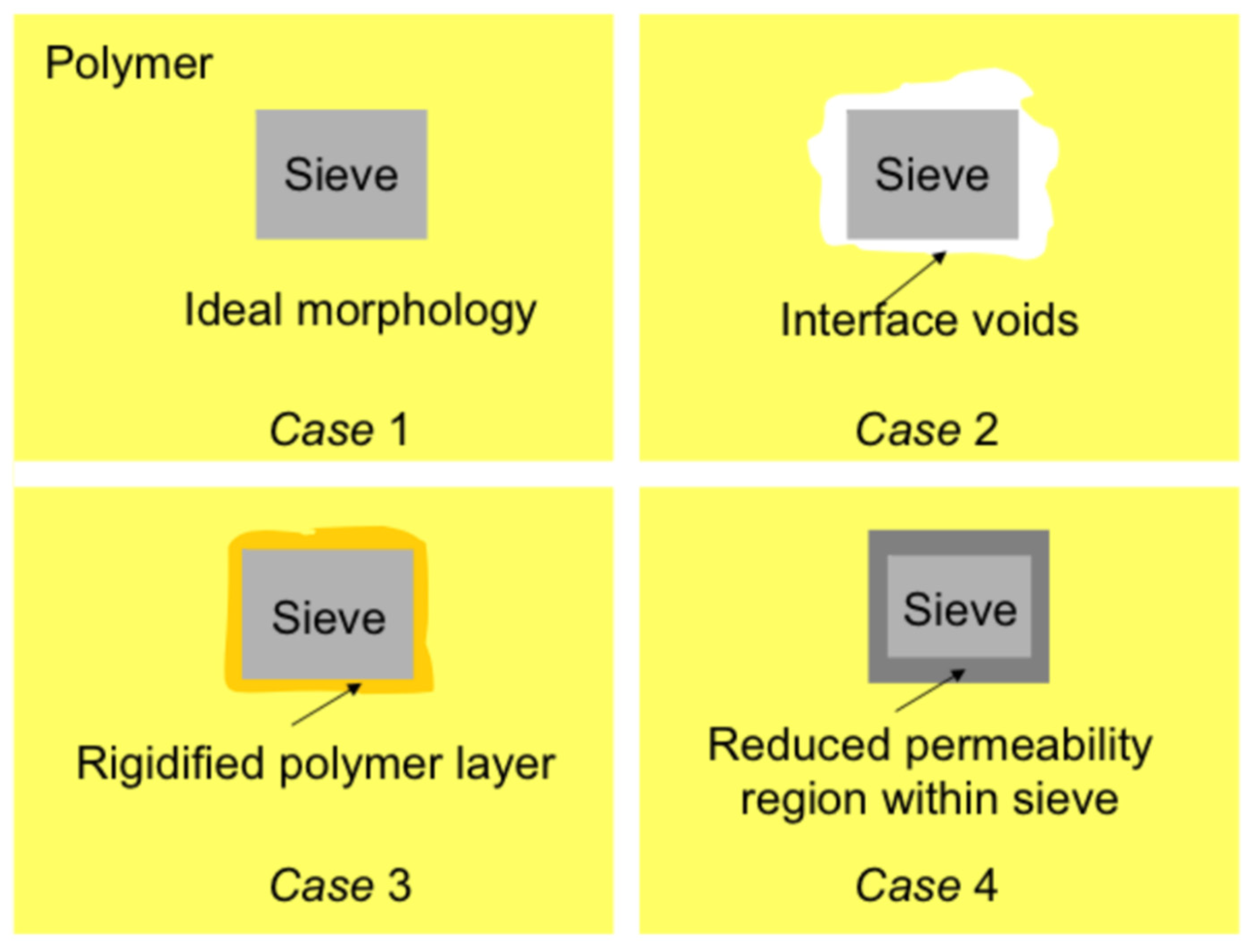
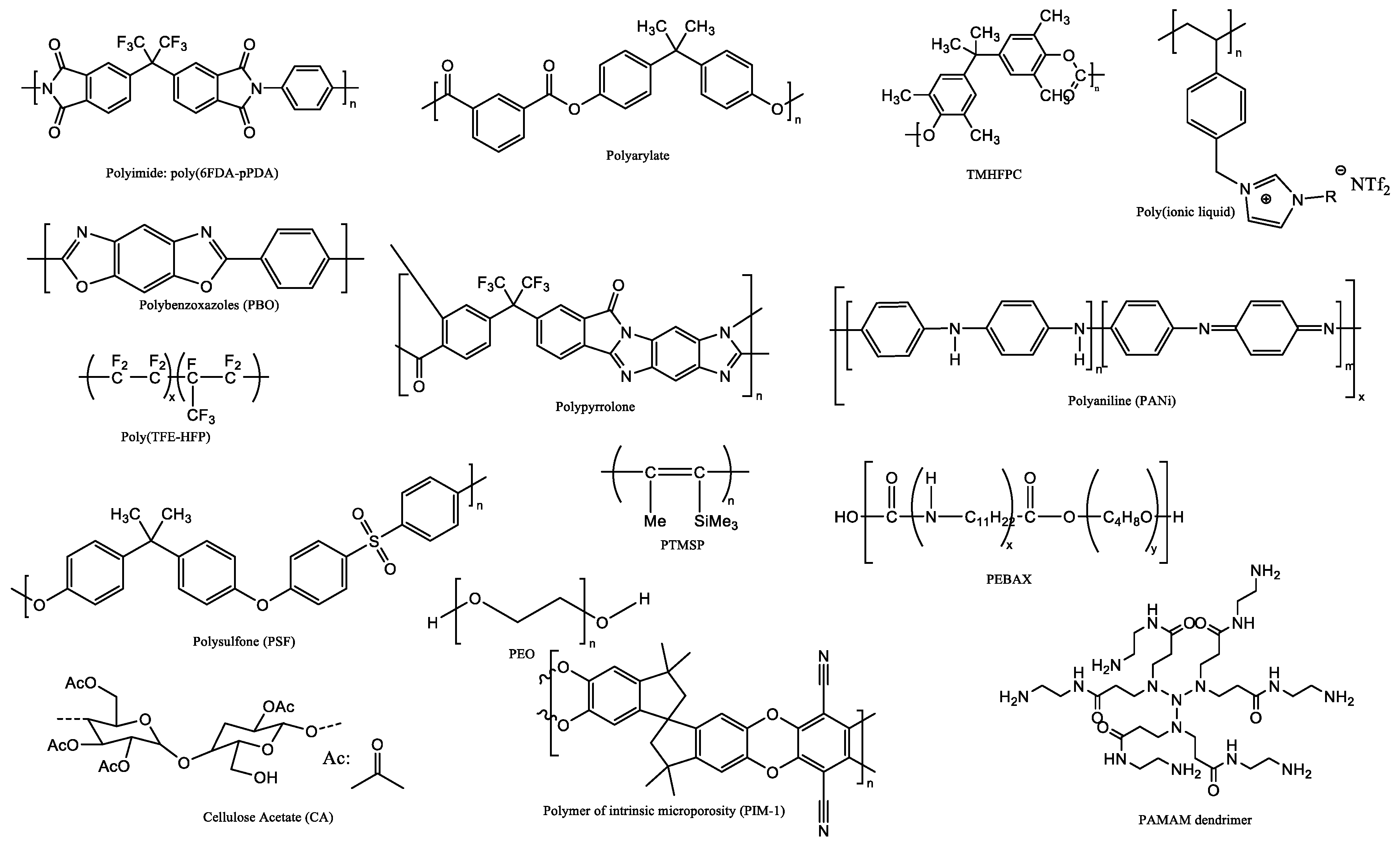
2. Choice of Polymers and Inorganics
3. Polymer/Zeolite Mixed Matrix Membranes
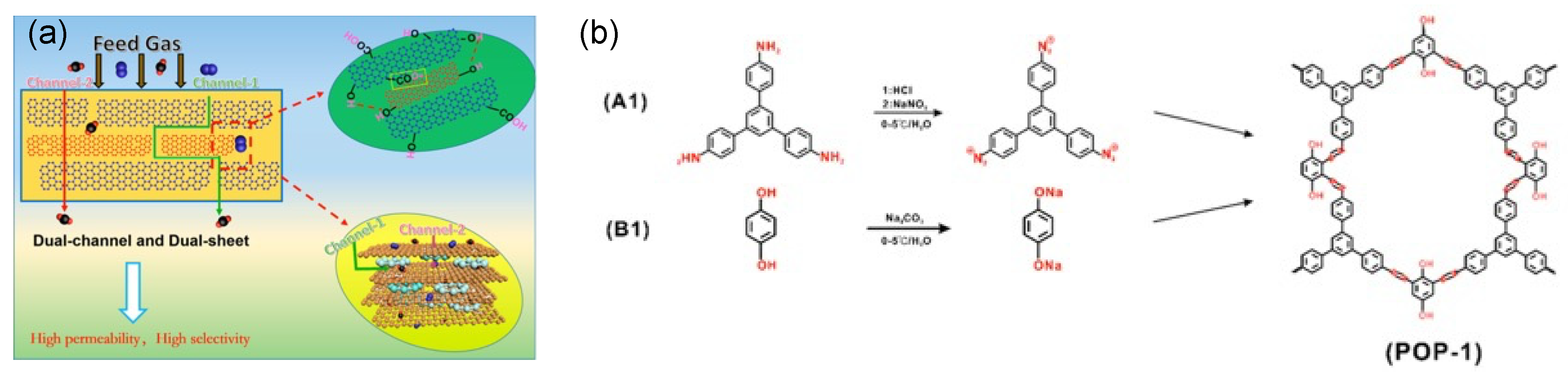
4. Polymer/MOF Mixed Matrix Membranes
5. Polymer/ZIF Mixed Matrix Membranes
6. Polymer/Oxide Nanoparticles Mixed Matrix Membranes
7. Polymer/Nano Carbon Mixed Matrix Membranes
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitsch, W.J.; Bernal, B.; Nahlik, A.M.; Mander, Ü.; Zhang, L.; Anderson, C.J.; Jørgensen, S.E.; Brix, H. Wetlands, carbon, and climate change. Landsc. Ecol. 2013, 28, 583–597. [Google Scholar] [CrossRef]
- Walsh, B.; Ciais, P.; Janssens, I.A.; Peñuelas, J.; Riahi, K.; Rydzak, F.; van Vuuren, D.P.; Obersteiner, M. Pathways for balancing CO2 emissions and sinks. Nat. Commun. 2017, 8, 14856. [Google Scholar] [CrossRef] [Green Version]
- Li, J.-R.; Ma, Y.; McCarthy, M.C.; Sculley, J.; Yu, J.; Jeong, H.-K.; Balbuena, P.B.; Zhou, H.-C. Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks. Coord. Chem. Rev. 2011, 255, 1791–1823. [Google Scholar] [CrossRef]
- Villalobos, L.F.; Hilke, R.; Akhtar, F.H.; Peinemann, K.-V. Fabrication of Polybenzimidazole/Palladium Nanoparticles Hollow Fiber Membranes for Hydrogen Purification. Adv. Energy Mater. 2018, 8, 1701567. [Google Scholar] [CrossRef]
- Sanz-Pérez, E.S.; Murdock, C.R.; Didas, S.A.; Jones, C.W. Direct Capture of CO2 from Ambient Air. Chem. Rev. 2016, 116, 11840–11876. [Google Scholar] [CrossRef]
- Pires, J.C.M.; Martins, F.G.; Alvim-Ferraz, M.C.M.; Simões, M. Recent developments on carbon capture and storage: An overview. Chem. Eng. Res. Des. 2011, 89, 1446–1460. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Pan, T.-H.; Wong, D.S.-H.; Jang, S.-S.; Chi, Y.-W.; Yeh, C.-H. Plantwide Control of CO2 Capture by Absorption and Stripping Using Monoethanolamine Solution. Ind. Eng. Chem. Res. 2011, 50, 1338–1345. [Google Scholar] [CrossRef]
- Salazar Duarte, G.; Schürer, B.; Voss, C.; Bathen, D. Adsorptive Separation of CO2 from Flue Gas by Temperature Swing Adsorption Processes. Chembioeng Rev. 2017, 4, 277–288. [Google Scholar] [CrossRef]
- Mason, E.A. From pig bladders and cracked jars to polysulfones: An historical perspective on membrane transport. J. Membr. Sci. 1991, 60, 125–145. [Google Scholar] [CrossRef]
- Kulkarni, M.G.; Mashelkar, R.A. Diffusion in network polymers: Model development and evaluation. Polymer 1981, 22, 1658–1664. [Google Scholar] [CrossRef]
- Stannett, V. The transport of gases in synthetic polymeric membranes—An historic perspective. J. Membr. Sci. 1978, 3, 97–115. [Google Scholar] [CrossRef]
- Daynes, H.A.; Smith, S.W.J. The process of diffusion through a rubber membrane. Proc. R. Soc. Lond. Ser. A Contain. Pap. Math. Phys. Character 1920, 97, 286–307. [Google Scholar] [CrossRef]
- Barrer, R.M. Diffusivities in glassy polymers for the dual mode sorption model. J. Membr. Sci. 1984, 18, 25–35. [Google Scholar] [CrossRef]
- Bernardo, P.; Drioli, E.; Golemme, G. Membrane Gas Separation: A Review/State of the Art. Ind. Eng. Chem. Res. 2009, 48, 4638–4663. [Google Scholar] [CrossRef]
- Wijmans, J.G.; Baker, R.W. The solution-diffusion model: A review. J. Membr. Sci. 1995, 107, 1–21. [Google Scholar] [CrossRef]
- Ghosal, K.; Freeman, B.D. Gas separation using polymer membranes: An overview. Polym. Adv. Technol. 1994, 5, 673–697. [Google Scholar] [CrossRef]
- Chung, T.-S.; Jiang, L.Y.; Li, Y.; Kulprathipanja, S. Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. Prog. Polym. Sci. 2007, 32, 483–507. [Google Scholar] [CrossRef]
- Galizia, M.; Chi, W.S.; Smith, Z.P.; Merkel, T.C.; Baker, R.W.; Freeman, B.D. 50th Anniversary Perspective: Polymers and Mixed Matrix Membranes for Gas and Vapor Separation: A Review and Prospective Opportunities. Macromolecules 2017, 50, 7809–7843. [Google Scholar] [CrossRef]
- Dai, Z.; Ansaloni, L.; Deng, L. Recent advances in multi-layer composite polymeric membranes for CO2 separation: A review. Green Energy Environ. 2016, 1, 102–128. [Google Scholar] [CrossRef] [Green Version]
- Dechnik, J.; Gascon, J.; Doonan, C.J.; Janiak, C.; Sumby, C.J. Mixed-Matrix Membranes. Angew. Chem. Int. Ed. 2017, 56, 9292–9310. [Google Scholar] [CrossRef] [PubMed]
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Zhao, S.; Wang, J.; Wang, S. Recent advances on mixed matrix membranes for CO2 separation. Chin. J. Chem. Eng. 2017, 25, 1581–1597. [Google Scholar] [CrossRef]
- Vu, D.Q.; Koros, W.J.; Miller, S.J. Mixed matrix membranes using carbon molecular sieves: I. Preparation and experimental results. J. Membr. Sci. 2003, 211, 311–334. [Google Scholar] [CrossRef]
- Torrisi, A.; Mellot-Draznieks, C.; Bell, R.G. Impact of ligands on CO2 adsorption in metal-organic frameworks: First principles study of the interaction of CO2 with functionalized benzenes. II. Effect of polar and acidic substituents. J. Chem. Phys. 2010, 132, 044705. [Google Scholar] [CrossRef]
- Merkel, T.C.; Freeman, B.D.; Spontak, R.J.; He, Z.; Pinnau, I.; Meakin, P.; Hill, A.J. Ultrapermeable, Reverse-Selective Nanocomposite Membranes. Science 2002, 296, 519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, Y.; Lua, A.C. Theoretical and experimental studies on the gas transport properties of mixed matrix membranes based on polyvinylidene fluoride. Aiche J. 2013, 59, 4715–4726. [Google Scholar] [CrossRef]
- Liu, G.; Chernikova, V.; Liu, Y.; Zhang, K.; Belmabkhout, Y.; Shekhah, O.; Zhang, C.; Yi, S.; Eddaoudi, M.; Koros, W.J. Mixed matrix formulations with MOF molecular sieving for key energy-intensive separations. Nat. Mater. 2018, 17, 283–289. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Qiu, W.; Liu, G.; Eddaoudi, M.; Koros, W.J. Penetrant competition and plasticization in membranes: How negatives can be positives in natural gas sweetening. J. Membr. Sci. 2021, 627, 119201. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Z.; Liu, G.; Belmabkhout, Y.; Adil, K.; Eddaoudi, M.; Koros, W. Conformation-Controlled Molecular Sieving Effects for Membrane-Based Propylene/Propane Separation. Adv. Mater. 2019, 31, 1807513. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Ebadi Amooghin, A.; Montazer-Rahmati, M.M.; Ismail, A.F.; Matsuura, T. State-of-the-art membrane based CO2 separation using mixed matrix membranes (MMMs): An overview on current status and future directions. Prog. Polym. Sci. 2014, 39, 817–861. [Google Scholar] [CrossRef]
- Granite, E.J.; Pennline, H.W. Photochemical Removal of Mercury from Flue Gas. Ind. Eng. Chem. Res. 2002, 41, 5470–5476. [Google Scholar] [CrossRef]
- Scholes, C.A.; Stevens, G.W.; Kentish, S.E. Membrane gas separation applications in natural gas processing. Fuel 2012, 96, 15–28. [Google Scholar] [CrossRef]
- Shao, L.; Low, B.T.; Chung, T.-S.; Greenberg, A.R. Polymeric membranes for the hydrogen economy: Contemporary approaches and prospects for the future. J. Membr. Sci. 2009, 327, 18–31. [Google Scholar] [CrossRef]
- Paul, D.R.; Pixton, M.R. Polyarylate gas separation membranes. Macromol. Symp. 1997, 118, 401–406. [Google Scholar] [CrossRef]
- Sanders, D.F.; Smith, Z.P.; Guo, R.; Robeson, L.M.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Energy-efficient polymeric gas separation membranes for a sustainable future: A review. Polymer 2013, 54, 4729–4761. [Google Scholar] [CrossRef] [Green Version]
- de Abajo, J.; de la Campa, J.G.; Lozano, A.E. Designing aromatic polyamides and polyimides for gas separation membranes. Macromol. Symp. 2003, 199, 293–306. [Google Scholar] [CrossRef]
- Zimmerman, C.M.; Koros, W.J. Polypyrrolones for membrane gas separations. I. Structural comparison of gas transport and sorption properties. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 1235–1249. [Google Scholar] [CrossRef]
- Baker, R.W. Future Directions of Membrane Gas Separation Technology. Ind. Eng. Chem. Res. 2002, 41, 1393–1411. [Google Scholar] [CrossRef]
- Illing, G.; Hellgardt, K.; Wakeman, R.J.; Jungbauer, A. Preparation and characterisation of polyaniline based membranes for gas separation. J. Membr. Sci. 2001, 184, 69–78. [Google Scholar] [CrossRef]
- Alexander Stern, S. Polymers for gas separations: The next decade. J. Membr. Sci. 1994, 94, 1–65. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Hu, Y.; Masuda, T. Substituted Polyacetylenes. Membr. Mater. Gas Vap. Sep. 2017, 107–142. [Google Scholar]
- Yoshino, M.; Ito, K.; Kita, H.; Okamoto, K.-I. Effects of hard-segment polymers on CO2/N2 gas-separation properties of poly(ethylene oxide)-segmented copolymers. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 1707–1715. [Google Scholar] [CrossRef]
- Akhtar, F.H.; Kumar, M.; Vovusha, H.; Shevate, R.; Villalobos, L.F.; Schwingenschlögl, U.; Peinemann, K.-V. Scalable Synthesis of Amphiphilic Copolymers for CO2- and Water-Selective Membranes: Effect of Copolymer Composition and Chain Length. Macromolecules 2019, 52, 6213–6226. [Google Scholar] [CrossRef]
- Ioannidi, A.; Vroulias, D.; Kallitsis, J.; Ioannides, T.; Deimede, V. Synthesis and characterization of poly(ethylene oxide) based copolymer membranes for efficient gas/vapor separation: Effect of PEO content and chain length. J. Membr. Sci. 2021, 632, 119353. [Google Scholar] [CrossRef]
- Thankamony, R.L.; Li, X.; Das, S.K.; Ostwal, M.M.; Lai, Z. Porous covalent triazine piperazine polymer (CTPP)/PEBAX mixed matrix membranes for CO2/N2 and CO2/CH4 separations. J. Membr. Sci. 2019, 591, 117348. [Google Scholar] [CrossRef]
- Lee, J.H.; Lim, J.Y.; Park, M.S.; Kim, J.H. Improvement in the CO2 Permeation Properties of High-Molecular-Weight Poly(ethylene oxide): Use of Amine-Branched Poly(amidoamine) Dendrimer. Macromolecules 2018, 51, 8800–8807. [Google Scholar] [CrossRef]
- Achoundong, C.S.K.; Bhuwania, N.; Burgess, S.K.; Karvan, O.; Johnson, J.R.; Koros, W.J. Silane Modification of Cellulose Acetate Dense Films as Materials for Acid Gas Removal. Macromolecules 2013, 46, 5584–5594. [Google Scholar] [CrossRef]
- Nikiforov, R.; Belov, N.; Zharov, A.; Konovalova, I.; Shklyaruk, B.; Yampolskii, Y. Gas permeation and diffusion in copolymers of tetrafluoroethylene and hexafluoropropylene: Effect of annealing. J. Membr. Sci. 2017, 540, 129–135. [Google Scholar] [CrossRef]
- Hu, X.; Lee, W.H.; Bae, J.Y.; Kim, J.S.; Jung, J.T.; Wang, H.H.; Park, H.J.; Lee, Y.M. Thermally rearranged polybenzoxazole copolymers incorporating Tröger’s base for high flux gas separation membranes. J. Membr. Sci. 2020, 612, 118437. [Google Scholar] [CrossRef]
- Tomé, L.C.; Marrucho, I.M. Poly(ionic liquid)s: Designing CO2 Separation Membranes. In Applications of Ionic Liquids in Polymer Science and Technology; Mecerreyes, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 267–295. [Google Scholar]
- Swaidan, R.; Ghanem, B.S.; Litwiller, E.; Pinnau, I. Pure- and mixed-gas CO2/CH4 separation properties of PIM-1 and an amidoxime-functionalized PIM-1. J. Membr. Sci. 2014, 457, 95–102. [Google Scholar] [CrossRef]
- Tul Muntha, S.; Kausar, A.; Siddiq, M. A review on Zeolite-Reinforced Polymeric Membranes: Salient Features and Applications. Polym. Plast. Technol. Eng. 2016, 55, 1971–1987. [Google Scholar] [CrossRef]
- Bastani, D.; Esmaeili, N.; Asadollahi, M. Polymeric mixed matrix membranes containing zeolites as a filler for gas separation applications: A review. J. Ind. Eng. Chem. 2013, 19, 375–393. [Google Scholar] [CrossRef]
- Farha, O.K.; Hupp, J.T. Rational Design, Synthesis, Purification, and Activation of Metal−Organic Framework Materials. Acc. Chem. Res. 2010, 43, 1166–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Feng, X.; Yuan, S.; Zhou, J.; Wang, B. Challenges and recent advances in MOF–polymer composite membranes for gas separation. Inorg. Chem. Front. 2016, 3, 896–909. [Google Scholar] [CrossRef]
- Mirqasemi, M.S.; Homayoonfal, M.; Rezakazemi, M. Zeolitic imidazolate framework membranes for gas and water purification. Environ. Chem. Lett. 2020, 18, 1–52. [Google Scholar] [CrossRef]
- Chew, T.-L.; Ahmad, A.L.; Bhatia, S. Ordered mesoporous silica (OMS) as an adsorbent and membrane for separation of carbon dioxide (CO2). Adv. Colloid Interface Sci. 2010, 153, 43–57. [Google Scholar] [CrossRef]
- Hasebe, S.; Aoyama, S.; Tanaka, M.; Kawakami, H. CO2 separation of polymer membranes containing silica nanoparticles with gas permeable nano-space. J. Membr. Sci. 2017, 536, 148–155. [Google Scholar] [CrossRef]
- Zhang, C.; Li, P.; Cao, B. Electrospun Microfibrous Membranes Based on PIM-1/POSS with High Oil Wettability for Separation of Oil–Water Mixtures and Cleanup of Oil Soluble Contaminants. Ind. Eng. Chem. Res. 2015, 54, 8772–8781. [Google Scholar] [CrossRef]
- Yampolskii, Y.P.; Starannikova, L.E.; Belov, N.A. Hybrid gas separation polymeric membranes containing nanoparticles. Pet. Chem. 2014, 54, 637–651. [Google Scholar] [CrossRef]
- Nguyen, B.H.; Nguyen, V.H. Promising applications of graphene and graphene-based nanostructures. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 023002. [Google Scholar] [CrossRef] [Green Version]
- Wong, K.K.; Jawad, Z.A. A review and future prospect of polymer blend mixed matrix membrane for CO2 separation. J. Polym. Res. 2019, 26, 289. [Google Scholar] [CrossRef]
- Fatemi, S.M.; Foroutan, M. Review on carbon nanotubes and carbon nanotube bundles for gas/ion separation and water purification studied by molecular dynamics simulation. Int. J. Environ. Sci. Technol. 2016, 13, 457–470. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, S.C.; Bose, A. Role of activated carbon pellets in carbon dioxide removal. Energy Convers. Manag. 1997, 38, S105–S110. [Google Scholar] [CrossRef]
- Asghari, M.; Mosadegh, M.; Riasat Harami, H. Supported PEBA-zeolite 13X nano-composite membranes for gas separation: Preparation, characterization and molecular dynamics simulation. Chem. Eng. Sci. 2018, 187, 67–78. [Google Scholar] [CrossRef]
- Jusoh, N.; Yeong, Y.F.; Lau, K.K.; Shariff, A.M. Enhanced gas separation performance using mixed matrix membranes containing zeolite T and 6FDA-durene polyimide. J. Membr. Sci. 2017, 525, 175–186. [Google Scholar] [CrossRef]
- Ahmad, J.; Hägg, M.-B. Preparation and characterization of polyvinyl acetate/zeolite 4A mixed matrix membrane for gas separation. J. Membr. Sci. 2013, 427, 73–84. [Google Scholar] [CrossRef]
- Adams, R.T.; Lee, J.S.; Bae, T.-H.; Ward, J.K.; Johnson, J.R.; Jones, C.W.; Nair, S.; Koros, W.J. CO2–CH4 permeation in high zeolite 4A loading mixed matrix membranes. J. Membr. Sci. 2011, 367, 197–203. [Google Scholar] [CrossRef]
- Zhao, J.; Xie, K.; Liu, L.; Liu, M.; Qiu, W.; Webley, P.A. Enhancing plasticization-resistance of mixed-matrix membranes with exceptionally high CO2/CH4 selectivity through incorporating ZSM-25 zeolite. J. Membr. Sci. 2019, 583, 23–30. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, C.; Zheng, Y.; Wu, Y.; Song, C.; Liu, Q.; Wang, Z. Modification of CO2-selective mixed matrix membranes by a binary composition of poly(ethylene glycol)/NaY zeolite. J. Membr. Sci. 2021, 627, 119239. [Google Scholar] [CrossRef]
- Li, W.; Goh, K.; Chuah, C.Y.; Bae, T.-H. Mixed-matrix carbon molecular sieve membranes using hierarchical zeolite: A simple approach towards high CO2 permeability enhancements. J. Membr. Sci. 2019, 588, 117220. [Google Scholar] [CrossRef]
- Mashhadikhan, S.; Ebadi Amooghin, A.; Moghadassi, A.; Sanaeepur, H. Functionalized filler/synthesized 6FDA-Durene high performance mixed matrix membrane for CO2 separation. J. Ind. Eng. Chem. 2021, 93, 482–494. [Google Scholar] [CrossRef]
- Chen, X.Y.; Nik, O.G.; Rodrigue, D.; Kaliaguine, S. Mixed matrix membranes of aminosilanes grafted FAU/EMT zeolite and cross-linked polyimide for CO2/CH4 separation. Polymer 2012, 53, 3269–3280. [Google Scholar] [CrossRef]
- Ebadi Amooghin, A.; Omidkhah, M.; Kargari, A. The effects of aminosilane grafting on NaY zeolite–Matrimid® 5218 mixed matrix membranes for CO2/CH4 separation. J. Membr. Sci. 2015, 490, 364–379. [Google Scholar] [CrossRef]
- Khan, A.L.; Cano-Odena, A.; Gutiérrez, B.; Minguillón, C.; Vankelecom, I.F.J. Hydrogen separation and purification using polysulfone acrylate–zeolite mixed matrix membranes. J. Membr. Sci. 2010, 350, 340–346. [Google Scholar] [CrossRef]
- Khan, A.L.; Klaysom, C.; Gahlaut, A.; Vankelecom, I.F.J. Polysulfone acrylate membranes containing functionalized mesoporous MCM-41 for CO2 separation. J. Membr. Sci. 2013, 436, 145–153. [Google Scholar] [CrossRef]
- Ahmad, N.N.R.; Leo, C.P.; Ahmad, A.L. Effects of solvent and ionic liquid properties on ionic liquid enhanced polysulfone/SAPO-34 mixed matrix membrane for CO2 removal. Microporous Mesoporous Mater. 2019, 283, 64–72. [Google Scholar] [CrossRef]
- Shindo, R.; Kishida, M.; Sawa, H.; Kidesaki, T.; Sato, S.; Kanehashi, S.; Nagai, K. Characterization and gas permeation properties of polyimide/ZSM-5 zeolite composite membranes containing ionic liquid. J. Membr. Sci. 2014, 454, 330–338. [Google Scholar] [CrossRef]
- Singh, Z.V.; Cowan, M.G.; McDanel, W.M.; Luo, Y.; Zhou, R.; Gin, D.L.; Noble, R.D. Determination and optimization of factors affecting CO2/CH4 separation performance in poly(ionic liquid)-ionic liquid-zeolite mixed-matrix membranes. J. Membr. Sci. 2016, 509, 149–155. [Google Scholar] [CrossRef]
- Dunn, C.A.; Denning, S.; Crawford, J.M.; Zhou, R.; Dwulet, G.E.; Carreon, M.A.; Gin, D.L.; Noble, R.D. CO2/CH4 separation characteristics of poly(RTIL)-RTIL-zeolite mixed-matrix membranes evaluated under binary feeds up to 40 bar and 50 °C. J. Membr. Sci. 2021, 621, 118979. [Google Scholar] [CrossRef]
- Bai, Y.; Dou, Y.; Xie, L.-H.; Rutledge, W.; Li, J.-R.; Zhou, H.-C. Zr-based metal–organic frameworks: Design, synthesis, structure, and applications. Chem. Soc. Rev. 2016, 45, 2327–2367. [Google Scholar] [CrossRef] [PubMed]
- Denny, M.S.; Moreton, J.C.; Benz, L.; Cohen, S.M. Metal-organic frameworks for membrane-based separations. Nat. Rev. Mater. 2016, 1. [Google Scholar] [CrossRef]
- Koros, W.J.; Zhang, C. Materials for next-generation molecularly selective synthetic membranes. Nat. Mater. 2017, 16, 289–297. [Google Scholar] [CrossRef]
- Qian, Q.H.; Asinger, P.A.; Lee, M.J.; Han, G.; Rodriguez, K.M.; Lin, S.; Benedetti, F.M.; Wu, A.X.; Chi, W.S.; Smith, Z.P. MOF-Based Membranes for Gas Separations. Chem. Rev. 2020, 120, 8161–8266. [Google Scholar] [CrossRef]
- Rangaraj, V.M.; Wahab, M.A.; Reddy, K.S.K.; Kakosimos, G.; Abdalla, O.; Favvas, E.P.; Reinalda, D.; Geuzebroek, F.; Abdala, A.; Karanikolos, G.N. Metal Organic Framework—Based Mixed Matrix Membranes for Carbon Dioxide Separation: Recent Advances and Future Directions. Front. Chem. 2020, 8. [Google Scholar] [CrossRef]
- Lin, R.J.; Hernandez, B.V.; Ge, L.; Zhu, Z.H. Metal organic framework based mixed matrix membranes: An overview on filler/polymer interfaces. J. Mater. Chem. A 2018, 6, 293–312. [Google Scholar] [CrossRef]
- Kitao, T.; Zhang, Y.Y.; Kitagawa, S.; Wang, B.; Uemura, T. Hybridization of MOFs and polymers. Chem. Soc. Rev. 2017, 46, 3108–3133. [Google Scholar] [CrossRef] [PubMed]
- Su, N.C.; Sun, D.T.; Beavers, C.M.; Britt, D.K.; Queen, W.L.; Urban, J.J. Enhanced permeation arising from dual transport pathways in hybrid polymer-MOF membranes. Energy Environ. Sci. 2016, 9, 922–931. [Google Scholar] [CrossRef]
- Yu, G.L.; Zou, X.Q.; Sun, L.; Liu, B.S.; Wang, Z.Y.; Zhang, P.P.; Zhu, G.S. Constructing Connected Paths between UiO-66 and PIM-1 to Improve Membrane CO2 Separation with Crystal-Like Gas Selectivity. Adv. Mater. 2019, 31. [Google Scholar] [CrossRef]
- Ashtiani, S.; Khoshnamvand, M.; Bousa, D.; Sturala, J.; Sofer, Z.; Shaliutina-Kolesova, A.; Gardeno, D.; Friess, K. Surface and interface engineering in CO2-philic based UiO-66-NH2-PEI mixed matrix membranes via covalently bridging PVP for effective hydrogen purification. Int. J. Hydrogen Energy 2021, 46, 5449–5458. [Google Scholar] [CrossRef]
- Xiang, F.M.; Marti, A.M.; Hopkinson, D.P. Layer-by-layer assembled polymer/MOF membrane for H-2/CO2 separation. J. Membr. Sci. 2018, 556, 146–153. [Google Scholar] [CrossRef]
- Sutrisna, P.D.; Hou, J.W.; Zulkifli, M.Y.; Li, H.Y.; Zhang, Y.T.; Liang, W.B.; D’Alessandro, D.M.; Chen, V. Surface functionalized UiO-66/Pebax-based ultrathin composite hollow fiber gas separation membranes. J. Mater. Chem. A 2018, 6, 918–931. [Google Scholar] [CrossRef]
- Meshkat, S.; Kaliaguine, S.; Rodrigue, D. Mixed matrix membranes based on amine and non-amine MIL-53(Al) in Pebax (R) MH-1657 for CO2 separation. Sep. Purif. Technol. 2018, 200, 177–190. [Google Scholar] [CrossRef]
- Mubashir, M.; Fong, Y.Y.; Leng, C.T.; Keong, L.K. Optimization of spinning parameters on the fabrication of NH2-MIL-53(Al)/cellulose acetate (CA) hollow fiber mixed matrix membrane for CO2 separation. Sep. Purif. Technol. 2019, 215, 32–43. [Google Scholar] [CrossRef]
- Fan, Y.F.; Yu, H.Y.; Xu, S.; Shen, Q.C.; Ye, H.M.; Li, N.W. Zn(II)-modified imidazole containing polyimide/ZIF-8 mixed matrix membranes for gas separations. J. Membr. Sci. 2020, 597. [Google Scholar] [CrossRef]
- Lai, W.-H.; Zhuang, G.-L.; Tseng, H.-H.; Wey, M.-Y. Creation of tiny defects in ZIF-8 by thermal annealing to improve the CO2/N2 separation of mixed matrix membranes. J. Membr. Sci. 2019, 572, 410–418. [Google Scholar] [CrossRef]
- Wang, B.; Qiao, Z.H.; Xu, J.Y.; Wang, J.X.; Liu, X.L.; Zhao, S.; Wang, Z.; Guiver, M.D. Unobstructed Ultrathin Gas Transport Channels in Composite Membranes by Interfacial Self-Assembly. Adv. Mater. 2020, 32. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, G.P.; Zhang, C.; Qiu, W.L.; Yi, S.L.; Chernikova, V.; Chen, Z.J.; Belmabkhout, Y.; Shekhah, O.; Eddaoudi, M.; et al. Enhanced CO2/CH4 Separation Performance of a Mixed Matrix Membrane Based on Tailored MOF-Polymer Formulations. Adv. Sci. 2018, 5. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.L.; He, S.F.; Qin, X.D.; Li, C.E.; Li, T. Interfacial Engineering in Metal-Organic Framework-Based Mixed Matrix Membranes Using Covalently Grafted Polyimide Brushes. J. Am. Chem. Soc. 2018, 140, 17203–17210. [Google Scholar] [CrossRef]
- Wu, C.H.; Zhang, K.X.; Wang, H.L.; Fan, Y.Q.; Zhang, S.W.; He, S.F.; Wang, F.; Tao, Y.; Zhao, X.W.; Zhang, Y.B.; et al. Enhancing the Gas Separation Selectivity of Mixed-Matrix Membranes Using a Dual-Interfacial Engineering Approach. J. Am. Chem. Soc. 2020, 142, 18503–18512. [Google Scholar] [CrossRef]
- Banerjee, R.; Phan, A.; Wang, B.; Knobler, C.; Furukawa, H.; O’Keeffe, M.; Yaghi, O.M. High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture. Science 2008, 319, 939–943. [Google Scholar] [CrossRef]
- Noh, K.; Lee, J.; Kim, J. Compositions and Structures of Zeolitic Imidazolate Frameworks. Isr. J. Chem. 2018, 58, 1075–1088. [Google Scholar] [CrossRef]
- Tran, N.T.; Trung, L.G.; Nguyen, M.K. The degradation of organic dye contaminants in wastewater and solution from highly visible light responsive ZIF-67 monodisperse photocatalyst. J. Solid State Chem. 2021, 300. [Google Scholar] [CrossRef]
- Yu, S.; Wang, S.; Xie, Z.; Yu, S.; Li, L.; Xiao, H.; Song, Y. Hyaluronic acid coating on the surface of curcumin-loaded ZIF-8 nanoparticles for improved breast cancer therapy: An in vitro and in vivo study. Colloids Surf. B Biointerfaces 2021, 203, 111759. [Google Scholar] [CrossRef]
- Maroofi, S.M.; Mahmoodi, N.M. Zeolitic imidazolate framework-polyvinylpyrrolidone-polyethersulfone composites membranes: From synthesis to the detailed pollutant removal from wastewater using cross flow system. Colloids Surf. A Physicochem. Eng. Asp. 2019, 572, 211–220. [Google Scholar] [CrossRef]
- Basu, S.; Balakrishnan, M. Polyamide thin film composite membranes containing ZIF-8 for the separation of pharmaceutical compounds from aqueous streams. Sep. Purif. Technol. 2017, 179, 118–125. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, X.; Hu, P.; Peng, X. ZIF-8 coated polyvinylidenefluoride (PVDF) hollow fiber for highly efficient separation of small dye molecules. Appl. Mater. Today 2016, 5, 103–110. [Google Scholar] [CrossRef]
- Li, Y.; Lin, Z.; Wang, X.; Duan, Z.; Lu, P.; Li, S.; Ji, D.; Wang, Z.; Li, G.; Yu, D.; et al. High-hydrophobic ZIF-8@PLA composite aerogel and application for oil-water separation. Sep. Purif. Technol. 2021, 270. [Google Scholar] [CrossRef]
- Pan, Y.; Xie, R.; Xu, B.; Chen, C. Determination of sorption and diffusion for ethanol through superhydrophobic ZIF/PDMS mixed matrix membrane. Microporous Mesoporous Mater. 2021, 320. [Google Scholar] [CrossRef]
- Karimi, A.; Khataee, A.; Vatanpour, V.; Safarpour, M. High-flux PVDF mixed matrix membranes embedded with size-controlled ZIF-8 nanoparticles. Sep. Purif. Technol. 2019, 229. [Google Scholar] [CrossRef]
- Şahin, F.; Topuz, B.; Kalıpçılar, H. ZIF filled PDMS mixed matrix membranes for separation of solvent vapors from nitrogen. J. Membr. Sci. 2020, 598. [Google Scholar] [CrossRef]
- Lin, G.-S.; Chen, Y.-R.; Chang, T.-H.; Huang, T.-C.; Zhuang, G.-L.; Huang, W.-Z.; Liu, Y.-C.; Matsuyama, H.; Wu, K.C.W.; Tung, K.-L. A high ZIF-8 loading PVA mixed matrix membrane on alumina hollow fiber with enhanced ethanol dehydration. J. Membr. Sci. 2021, 621. [Google Scholar] [CrossRef]
- Li, G.; Si, Z.; Cai, D.; Wang, Z.; Qin, P.; Tan, T. The in-situ synthesis of a high-flux ZIF-8/polydimethylsiloxane mixed matrix membrane for n-butanol pervaporation. Sep. Purif. Technol. 2020, 236. [Google Scholar] [CrossRef]
- Cheng, N.; Ren, L.; Xu, X.; Du, Y.; Dou, S.X. Recent Development of Zeolitic Imidazolate Frameworks (ZIFs) Derived Porous Carbon Based Materials as Electrocatalysts. Adv. Energy Mater. 2018, 8. [Google Scholar] [CrossRef]
- Karimi, A.; Khataee, A.; Safarpour, M.; Vatanpour, V. Development of mixed matrix ZIF-8/polyvinylidene fluoride membrane with improved performance in solvent resistant nanofiltration. Sep. Purif. Technol. 2020, 237. [Google Scholar] [CrossRef]
- Ying, Q.; Chen, H.; Shao, P.; Zhou, X.; He, X.; Ye, J.; Zhang, S.; Chen, J.; Wang, L. Core-shell magnetic ZIF-8@Fe3O4-carbonic anhydrase biocatalyst for promoting CO2 absorption into MDEA solution. J. CO2 Util. 2021, 49. [Google Scholar] [CrossRef]
- Song, Y.; He, M.; Zhao, J.; Jin, W. Structural manipulation of ZIF-8-based membranes for high-efficiency molecular separation. Sep. Purif. Technol. 2021, 270. [Google Scholar] [CrossRef]
- Guan, W.; Dai, Y.; Dong, C.; Yang, X.; Xi, Y. Zeolite imidazolate framework (ZIF)-based mixed matrix membranes for CO2 separation: A review. J. Appl. Polym. Sci. 2020, 137. [Google Scholar] [CrossRef]
- Abdul Hamid, M.R.; Shean Yaw, T.C.; Mohd Tohir, M.Z.; Wan Abdul Karim Ghani, W.A.; Sutrisna, P.D.; Jeong, H.-K. Zeolitic imidazolate framework membranes for gas separations: Current state-of-the-art, challenges, and opportunities. J. Ind. Eng. Chem. 2021, 98, 17–41. [Google Scholar] [CrossRef]
- Vinoba, M.; Bhagiyalakshmi, M.; Alqaheem, Y.; Alomair, A.A.; Pérez, A.; Rana, M.S. Recent progress of fillers in mixed matrix membranes for CO2 separation: A review. Sep. Purif. Technol. 2017, 188, 431–450. [Google Scholar] [CrossRef]
- Kamble, A.R.; Patel, C.M.; Murthy, Z.V.P. A review on the recent advances in mixed matrix membranes for gas separation processes. Renew. Sustain. Energy Rev. 2021, 145. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, Z.; Zhao, D. Mixed Matrix Membranes for Natural Gas Upgrading: Current Status and Opportunities. Ind. Eng. Chem. Res. 2018, 57, 4139–4169. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, D.; Jin, Y.; Liu, K.; Tao, X.; Zhang, Q.; Zhang, X.; Cui, Y. Transforming from planar to three-dimensional lithium with flowable interphase for solid lithium metal batteries. Sci. Adv. 2017, 3, eaao0713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Yuan, J.; Li, R.; Zhu, H.; Duan, J.; Guo, Y.; Liu, G.; Jin, W. ZIF-301 MOF/6FDA-DAM polyimide mixed-matrix membranes for CO2/CH4 separation. Sep. Purif. Technol. 2021, 264. [Google Scholar] [CrossRef]
- Wang, Q.; Dai, Y.; Ruan, X.; Zheng, W.; Yan, X.; Li, X.; He, G. ZIF-8 hollow nanotubes based mixed matrix membranes with high-speed gas transmission channel to promote CO2/N2 separation. J. Membr. Sci. 2021, 630. [Google Scholar] [CrossRef]
- Sasikumar, B.; Bisht, S.; Arthanareeswaran, G.; Ismail, A.F.; Othman, M.H.D. Performance of polysulfone hollow fiber membranes encompassing ZIF-8, SiO2/ZIF-8, and amine-modified SiO2/ZIF-8 nanofillers for CO2/CH4 and CO2/N2 gas separation. Sep. Purif. Technol. 2021, 264. [Google Scholar] [CrossRef]
- Safak Boroglu, M.; Yumru, A.B. Gas separation performance of 6FDA-DAM-ZIF-11 mixed-matrix membranes for H2/CH4 and CO2/CH4 separation. Sep. Purif. Technol. 2017, 173, 269–279. [Google Scholar] [CrossRef]
- Park, S.; Jeong, H.-K. In-situ linker doping as an effective means to tune zeolitic-imidazolate framework-8 (ZIF-8) fillers in mixed-matrix membranes for propylene/propane separation. J. Membr. Sci. 2020, 596. [Google Scholar] [CrossRef]
- Park, S.; Abdul Hamid, M.R.; Jeong, H.K. Highly Propylene-Selective Mixed-Matrix Membranes by in Situ Metal-Organic Framework Formation Using a Polymer-Modification Strategy. ACS Appl. Mater. Interfaces 2019, 11, 25949–25957. [Google Scholar] [CrossRef]
- Meshkat, S.; Kaliaguine, S.; Rodrigue, D. Comparison between ZIF-67 and ZIF-8 in Pebax® MH-1657 mixed matrix membranes for CO2 separation. Sep. Purif. Technol. 2020, 235. [Google Scholar] [CrossRef]
- Liu, D.; Xiang, L.; Chang, H.; Chen, K.; Wang, C.; Pan, Y.; Li, Y.; Jiang, Z. Rational matching between MOFs and polymers in mixed matrix membranes for propylene/propane separation. Chem. Eng. Sci. 2019, 204, 151–160. [Google Scholar] [CrossRef]
- Lee, C.S.; Kim, N.U.; Park, B.J.; Kim, J.H. In-situ growth of ZIF-8 in amphiphilic graft copolymer for mixed matrix membranes with simultaneous improvement of permeability and selectivity. Sep. Purif. Technol. 2020, 253. [Google Scholar] [CrossRef]
- Jusoh, N.; Yeong, Y.F.; Lau, K.K.; Shariff, A.M. Transport properties of mixed matrix membranes encompassing zeolitic imidazolate framework 8 (ZIF-8) nanofiller and 6FDA-durene polymer: Optimization of process variables for the separation of CO2 from CH4. J. Clean. Prod. 2017, 149, 80–95. [Google Scholar] [CrossRef]
- Japip, S.; Xiao, Y.; Chung, T.-S. Particle-Size Effects on Gas Transport Properties of 6FDA-Durene/ZIF-71 Mixed Matrix Membranes. Ind. Eng. Chem. Res. 2016, 55, 9507–9517. [Google Scholar] [CrossRef]
- Ilicak, I.; Boroglu, M.S.; Durmus, A.; Boz, I. Influence of ZIF-95 on structure and gas separation properties of polyimide-based mixed matrix membranes. J. Nat. Gas Sci. Eng. 2021, 91, 103941. [Google Scholar] [CrossRef]
- Guo, A.; Ban, Y.; Yang, K.; Yang, W. Metal-organic framework-based mixed matrix membranes: Synergetic effect of adsorption and diffusion for CO2/CH4 separation. J. Membr. Sci. 2018, 562, 76–84. [Google Scholar] [CrossRef]
- Ehsani, A.; Pakizeh, M. Synthesis, characterization and gas permeation study of ZIF-11/Pebax® 2533 mixed matrix membranes. J. Taiwan Inst. Chem. Eng. 2016, 66, 414–423. [Google Scholar] [CrossRef]
- Bae, T.-H.; Lee, J.S.; Qiu, W.; Koros, W.; Jones, C.; Nair, S. A high-performance gas-separation membrane containing submicrometer-sized metal-organic framework crystals. Angew. Chem. 2010, 49 51, 9863–9866. [Google Scholar] [CrossRef]
- An, H.; Park, S.; Kwon, H.T.; Jeong, H.-K.; Lee, J.S. A new superior competitor for exceptional propylene/propane separations: ZIF-67 containing mixed matrix membranes. J. Membr. Sci. 2017, 526, 367–376. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, T.; Wang, Y.; Li, J.; Liu, C.; Li, N.; Liao, J. Mixed-matrix membranes based on Zn/Ni-ZIF-8-PEBA for high performance CO2 separation. J. Membr. Sci. 2018, 560, 38–46. [Google Scholar] [CrossRef]
- Yu, S.; Li, S.; Huang, S.; Zeng, Z.; Cui, S.; Liu, Y. Covalently bonded zeolitic imidazolate frameworks and polymers with enhanced compatibility in thin film nanocomposite membranes for gas separation. J. Membr. Sci. 2017, 540, 155–164. [Google Scholar] [CrossRef]
- Xiang, L.; Sheng, L.; Wang, C.; Zhang, L.; Pan, Y.; Li, Y. Amino-Functionalized ZIF-7 Nanocrystals: Improved Intrinsic Separation Ability and Interfacial Compatibility in Mixed-Matrix Membranes for CO2/CH4 Separation. Adv. Mater. 2017, 29, 1606999. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, D.; Zhang, S.; Hu, L.; Jin, J. Interfacial Design of Mixed Matrix Membranes for Improved Gas Separation Performance. Adv. Mater. 2016, 28, 3399–3405. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Zhang, X.; Li, J.; Li, L. Polyvinylamine/ZIF-8-decorated metakaolin composite membranes for CO2/N2 separation. Sep. Purif. Technol. 2021, 270. [Google Scholar] [CrossRef]
- Sarfraz, M.; Ba-Shammakh, M. Pursuit of efficient CO2-capture membranes: Graphene oxide- and MOF-integrated Ultrason® membranes. Polym. Bull. 2018, 75, 5039–5059. [Google Scholar] [CrossRef]
- Sarfraz, M.; Ba-Shammakh, M. Harmonious interaction of incorporating CNTs and zeolitic imidazole frameworks into polysulfone to prepare high performance MMMs for CO2 separation from humidified post combustion gases. Braz. J. Chem. Eng. 2018, 35, 217–228. [Google Scholar] [CrossRef] [Green Version]
- Sarfraz, M.; Ba-Shammakh, M. Synergistic effect of incorporating ZIF-302 and graphene oxide to polysulfone to develop highly selective mixed-matrix membranes for carbon dioxide separation from wet post-combustion flue gases. J. Ind. Eng. Chem. 2016, 36, 154–162. [Google Scholar] [CrossRef]
- Sarfraz, M.; Ba-Shammakh, M. Combined Effect of CNTs with ZIF-302 into Polysulfone to Fabricate MMMs for Enhanced CO2 Separation from Flue Gases. Arab. J. Sci. Eng. 2016, 41, 2573–2582. [Google Scholar] [CrossRef]
- Li, X.; Yu, S.; Li, K.; Ma, C.; Zhang, J.; Li, H.; Chang, X.; Zhu, L.; Xue, Q. Enhanced gas separation performance of Pebax mixed matrix membranes by incorporating ZIF-8 in situ inserted by multiwalled carbon nanotubes. Sep. Purif. Technol. 2020, 248. [Google Scholar] [CrossRef]
- Li, W.; Samarasinghe, S.A.S.C.; Bae, T.-H. Enhancing CO2/CH4 separation performance and mechanical strength of mixed-matrix membrane via combined use of graphene oxide and ZIF-8. J. Ind. Eng. Chem. 2018, 67, 156–163. [Google Scholar] [CrossRef]
- Li, H.; Tuo, L.; Yang, K.; Jeong, H.-K.; Dai, Y.; He, G.; Zhao, W. Simultaneous enhancement of mechanical properties and CO2 selectivity of ZIF-8 mixed matrix membranes: Interfacial toughening effect of ionic liquid. J. Membr. Sci. 2016, 511, 130–142. [Google Scholar] [CrossRef] [Green Version]
- Jeazet, H.; Sorribas, S.; Román-Marín, J.; Zornoza, B.; Téllez, C.; Coronas, J. Increased Selectivity in CO2/CH4 Separation with Mixed-Matrix Membranes of Polysulfone and Mixed-MOFs MIL-101(Cr) and ZIF-8: Increased Selectivity in CO2/CH4 Separation with Mixed-Matrix Membranes of Polysulfone and Mixed-MOFs MIL-101(Cr) and ZIF-8. Eur. J. Inorg. Chem. 2016, 2016. [Google Scholar] [CrossRef]
- Huang, D.; Xin, Q.; Ni, Y.; Shuai, Y.; Wang, S.; Li, Y.; Ye, H.; Lin, L.; Ding, X.; Zhang, Y. Synergistic effects of zeolite imidazole framework@graphene oxide composites in humidified mixed matrix membranes on CO2 separation. RSC Adv. 2018, 8, 6099–6109. [Google Scholar] [CrossRef] [Green Version]
- Hartini Suhaimi, N.; Fong Yeong, Y.; Jusoh, N.; Farid Mohd Asri, M. Amine-Functionalized Metal Organic Framework (MOF)/6FDA-Durene Composite Membranes for CO2 Removal from CH4. Mater. Today Proc. 2019, 19, 1730–1737. [Google Scholar] [CrossRef]
- Gu, Z.; Yang, Z.; Guo, X.; Qiao, Z.; Zhong, C. Vacuum resistance treated ZIF-8 mixed matrix membrane for effective CH4/N2 separation. Sep. Purif. Technol. 2021, 272. [Google Scholar] [CrossRef]
- Gao, J.; Mao, H.; Jin, H.; Chen, C.; Feldhoff, A.; Li, Y. Functionalized ZIF-7/Pebax® 2533 mixed matrix membranes for CO2/N2 separation. Microporous Mesoporous Mater. 2020, 297. [Google Scholar] [CrossRef]
- Dong, L.; Chen, M.; Li, J.; Shi, D.; Dong, W.; Li, X.; Bai, Y. Metal-organic framework-graphene oxide composites: A facile method to highly improve the CO2 separation performance of mixed matrix membranes. J. Membr. Sci. 2016, 520, 801–811. [Google Scholar] [CrossRef]
- Ding, R.; Zheng, W.; Yang, K.; Dai, Y.; Ruan, X.; Yan, X.; He, G. Amino-functional ZIF-8 nanocrystals by microemulsion based mixed linker strategy and the enhanced CO2/N2 separation. Sep. Purif. Technol. 2020, 236. [Google Scholar] [CrossRef]
- Chen, B.; Wan, C.; Kang, X.; Chen, M.; Zhang, C.; Bai, Y.; Dong, L. Enhanced CO2 separation of mixed matrix membranes with ZIF-8@GO composites as fillers: Effect of reaction time of ZIF-8@GO. Sep. Purif. Technol. 2019, 223, 113–122. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Fíla, V.; Martin-Gil, V.; Muller, C. Enhanced CO2 permeability in Matrimid® 5218 mixed matrix membranes for separating binary CO2/CH4 mixtures. Sep. Purif. Technol. 2019, 210, 553–562. [Google Scholar] [CrossRef]
- Chen, R.; Yao, J.; Gu, Q.; Smeets, S.; Baerlocher, C.; Gu, H.; Zhu, D.; Morris, W.; Yaghi, O.M.; Wang, H. A two-dimensional zeolitic imidazolate framework with a cushion-shaped cavity for CO2 adsorption. Chem. Commun. 2013, 49, 9500–9502. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, H.; Yao, J. Synthesis of 2D nanoporous zeolitic imidazolate framework nanosheets for diverse applications. Coord. Chem. Rev. 2021, 431. [Google Scholar] [CrossRef]
- Zhao, P.; Jian, M.; Xu, R.; Zhang, Q.; Xiang, C.; Liu, R.; Zhang, X.; Liu, H. Removal of arsenic(iii) from water by 2D zeolitic imidazolate framework-67 nanosheets. Environ. Sci. Nano 2020, 7, 3616–3626. [Google Scholar] [CrossRef]
- Li, T.; Ren, Y.; Wu, D.; Zhang, W.; Shi, M.; Ji, C.; Lv, L.; Hua, M.; Zhang, W. A novel water-stable two-dimensional zeolitic imidazolate frameworks thin-film composite membrane for enhancements in water permeability and nanofiltration performance. Chemosphere 2020, 261, 127717. [Google Scholar] [CrossRef]
- Zhu, W.; Li, X.; Sun, Y.; Guo, R.; Ding, S. Introducing hydrophilic ultra-thin ZIF-L into mixed matrix membranes for CO2/CH4 separation. RSC Adv. 2019, 9, 23390–23399. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Dou, J.; Zhang, H. Mixed Membranes Comprising Carboxymethyl Cellulose (as Capping Agent and Gas Barrier Matrix) and Nanoporous ZIF-L Nanosheets for Gas Separation Applications. Polymers 2018, 10, 1340. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Shamsaei, E.; Lin, X.; Hu, Y.; Simon, G.P.; Seong, J.G.; Kim, J.S.; Lee, W.H.; Lee, Y.M.; Wang, H. The enhanced hydrogen separation performance of mixed matrix membranes by incorporation of two-dimensional ZIF-L into polyimide containing hydroxyl group. J. Membr. Sci. 2018, 549, 260–266. [Google Scholar] [CrossRef]
- Deng, J.; Dai, Z.; Deng, L. Effects of the Morphology of the ZIF on the CO2 Separation Performance of MMMs. Ind. Eng. Chem. Res. 2020, 59, 14458–14466. [Google Scholar] [CrossRef]
- Tena, A.; Fernández, L.; Sánchez, M.; Palacio, L.; Lozano, A.E.; Hernández, A.; Prádanos, P. Mixed matrix membranes of 6FDA-6FpDA with surface functionalized γ-alumina particles. An analysis of the improvement of permselectivity for several gas pairs. Chem. Eng. Sci. 2010, 65, 2227–2235. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Li, Y.; Chung, T.-S.; Liu, Y. Enhanced gas separation performance of nanocomposite membranes using MgO nanoparticles. J. Membr. Sci. 2007, 302, 207–217. [Google Scholar] [CrossRef]
- Ahn, J.; Chung, W.-J.; Pinnau, I.; Song, J.; Du, N.; Robertson, G.P.; Guiver, M.D. Gas transport behavior of mixed-matrix membranes composed of silica nanoparticles in a polymer of intrinsic microporosity (PIM-1). J. Membr. Sci. 2010, 346, 280–287. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, J.; Hågg, M.B. Polyvinyl acetate/titanium dioxide nanocomposite membranes for gas separation. J. Membr. Sci. 2013, 445, 200–210. [Google Scholar] [CrossRef]
- Farashi, Z.; Azizi, N.; Homayoon, R. Applying Pebax-1657/ZnO mixed matrix membranes for CO2/CH4 separation. Pet. Sci. Technol. 2019, 37, 2412–2419. [Google Scholar] [CrossRef]
- Ameri, E.; Sadeghi, M.; Zarei, N.; Pournaghshband, A. Enhancement of the gas separation properties of polyurethane membranes by alumina nanoparticles. J. Membr. Sci. 2015, 479, 11–19. [Google Scholar] [CrossRef]
- Molki, B.; Aframehr, W.M.; Bagheri, R.; Salimi, J. Mixed matrix membranes of polyurethane with nickel oxide nanoparticles for CO2 gas separation. J. Membr. Sci. 2018, 549, 588–601. [Google Scholar] [CrossRef]
- Su, N.C.; Buss, H.G.; McCloskey, B.D.; Urban, J.J. Enhancing Separation and Mechanical Performance of Hybrid Membranes through Nanoparticle Surface Modification. ACS Macro Lett. 2015, 4, 1239–1243. [Google Scholar] [CrossRef]
- Kudo, Y.; Mikami, H.; Tanaka, M.; Isaji, T.; Odaka, K.; Yamato, M.; Kawakami, H. Mixed matrix membranes comprising a polymer of intrinsic microporosity loaded with surface-modified non-porous pearl-necklace nanoparticles. J. Membr. Sci. 2020, 597, 117627. [Google Scholar] [CrossRef]
- Bilchak, C.R.; Jhalaria, M.; Huang, Y.; Abbas, Z.; Midya, J.; Benedetti, F.M.; Parisi, D.; Egger, W.; Dickmann, M.; Minelli, M.; et al. Tuning Selectivities in Gas Separation Membranes Based on Polymer-Grafted Nanoparticles. ACS Nano 2020, 14, 17174–17183. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, X.; Wu, H.; Wang, S.; Liang, X.; Ma, L.; Ren, Y.; Wu, Y.; Liu, Y.; Sun, M.; et al. Amino-functionalized POSS nanocage intercalated graphene oxide membranes for efficient biogas upgrading. J. Membr. Sci. 2020, 596, 117733. [Google Scholar] [CrossRef]
- Kim, J.H.; Vijayakumar, V.; Kim, D.J.; Nam, S.Y. Preparation and characterization of POSS-PEG high performance membranes for gas separation. J. Membr. Sci. 2020, 606, 118115. [Google Scholar] [CrossRef]
- Ahmad, N.N.R.; Tan, N.I.F.Z.; Leo, C.P.; Ahmad, A.L. Polysulfone-POSS membrane impregnated with ionic liquid for CO2 gas separation. In Proceedings of the 6th International Conference on Environment (Icenv2018): Empowering Environment and Sustainable Engineering Nexus Through Green Technology, Penang, Malaysia, 11–13 December 2018. [Google Scholar]
- Ansaloni, L.; Louradour, E.; Radmanesh, F.; van Veen, H.; Pilz, M.; Simon, C.; Benes, N.E.; Peters, T.A. Upscaling polyPOSS-imide membranes for high temperature H2 upgrading. J. Membr. Sci. 2021, 620. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Zhang, Z.; Hao, Z. Mesoporous KIT-6 silica–polydimethylsiloxane (PDMS) mixed matrix membranes for gas separation. J. Mater. Chem. A 2015, 3, 8650–8658. [Google Scholar] [CrossRef]
- Bilchak, C.R.; Buenning, E.; Asai, M.; Zhang, K.; Durning, C.J.; Kumar, S.K.; Huang, Y.; Benicewicz, B.C.; Gidley, D.W.; Cheng, S.; et al. Polymer-Grafted Nanoparticle Membranes with Controllable Free Volume. Macromolecules 2017, 50, 7111–7120. [Google Scholar] [CrossRef]
- Chen, X.; Dumée, L.F. Polyhedral Oligomeric Silsesquioxane (POSS) Nano-Composite Separation Membranes—A Review. Adv. Eng. Mater. 2019, 21, 1800667. [Google Scholar] [CrossRef]
- Li, Y.; Chung, T.-S. Molecular-level mixed matrix membranes comprising Pebax® and POSS for hydrogen purification via preferential CO2 removal. Int. J. Hydrogen Energy 2010, 35, 10560–10568. [Google Scholar] [CrossRef]
- Shi, F.; Tian, Q.; Wang, J.; Wang, Q.; Shi, F.; Li, Y.; Nunes, S.P. Carbon Quantum Dot-Enabled Tuning of the Microphase Structures of Poly(ether-b-amide) Membrane for CO2 Separation. Ind. Eng. Chem. Res. 2020, 59, 14960–14969. [Google Scholar] [CrossRef]
- Kim, S.; Chen, L.; Johnson, J.K.; Marand, E. Polysulfone and functionalized carbon nanotube mixed matrix membranes for gas separation: Theory and experiment. J. Membr. Sci. 2007, 294, 147–158. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Jawad, Z.A.; Low, S.C.; Zein, S.H.S. A cellulose acetate/multi-walled carbon nanotube mixed matrix membrane for CO2/N2 separation. J. Membr. Sci. 2014, 451, 55–66. [Google Scholar] [CrossRef]
- Zhao, D.; Ren, J.; Li, H.; Li, X.; Deng, M. Gas separation properties of poly(amide-6-b-ethylene oxide)/amino modified multi-walled carbon nanotubes mixed matrix membranes. J. Membr. Sci. 2014, 467, 41–47. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, R.; Hou, J.; Wei, Z.; Li, X. Mixed-Matrix Membranes Containing Carbon Nanotubes Composite with Hydrogel for Efficient CO2 Separation. Acs Appl. Mater. Interfaces 2016, 8, 29044–29051. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.; Park, J.; Ando, S.; Kim, C.B.; Nagai, K.; Freeman, B.D.; Ellison, C.J. Gas permeation and selectivity of poly(dimethylsiloxane)/graphene oxide composite elastomer membranes. J. Membr. Sci. 2016, 518, 131–140. [Google Scholar] [CrossRef]
- Shin, J.E.; Lee, S.K.; Cho, Y.H.; Park, H.B. Effect of PEG-MEA and graphene oxide additives on the performance of Pebax® 1657 mixed matrix membranes for CO2 separation. J. Membr. Sci. 2019, 572, 300–308. [Google Scholar] [CrossRef]
- Zhang, J.; Xin, Q.; Li, X.; Yun, M.; Xu, R.; Wang, S.; Li, Y.; Lin, L.; Ding, X.; Ye, H.; et al. Mixed matrix membranes comprising aminosilane-functionalized graphene oxide for enhanced CO2 separation. J. Membr. Sci. 2019, 570–571, 343–354. [Google Scholar] [CrossRef]
- Dong, G.; Hou, J.; Wang, J.; Zhang, Y.; Chen, V.; Liu, J. Enhanced CO2/N2 separation by porous reduced graphene oxide/Pebax mixed matrix membranes. J. Membr. Sci. 2016, 520, 860–868. [Google Scholar] [CrossRef]
- He, R.; Cong, S.; Wang, J.; Liu, J.; Zhang, Y. Porous Graphene Oxide/Porous Organic Polymer Hybrid Nanosheets Functionalized Mixed Matrix Membrane for Efficient CO2 Capture. ACS Appl. Mater. Interfaces 2019, 11, 4338–4344. [Google Scholar] [CrossRef]
- Li, X.; Ma, L.; Zhang, H.; Wang, S.; Jiang, Z.; Guo, R.; Wu, H.; Cao, X.; Yang, J.; Wang, B. Synergistic effect of combining carbon nanotubes and graphene oxide in mixed matrix membranes for efficient CO2 separation. J. Membr. Sci. 2015, 479, 1–10. [Google Scholar] [CrossRef]
- Anson, M.; Marchese, J.; Garis, E.; Ochoa, N.; Pagliero, C. ABS copolymer-activated carbon mixed matrix membranes for CO2/CH4 separation. J. Membr. Sci. 2004, 243, 19–28. [Google Scholar] [CrossRef]
- Chen, H.; Johnson, J.K.; Sholl, D.S. Transport Diffusion of Gases Is Rapid in Flexible Carbon Nanotubes. J. Phys. Chem. B 2006, 110, 1971–1975. [Google Scholar] [CrossRef]
- Karunakaran, M.; Villalobos, L.F.; Kumar, M.; Shevate, R.; Akhtar, F.H.; Peinemann, K.V. Graphene oxide doped ionic liquid ultrathin composite membranes for efficient CO2 capture. J. Mater. Chem. A 2017, 5, 649–656. [Google Scholar] [CrossRef]
- Luque-Alled, J.M.; Ameen, A.W.; Alberto, M.; Tamaddondar, M.; Foster, A.B.; Budd, P.M.; Vijayaraghavan, A.; Gorgojo, P. Gas separation performance of MMMs containing (PIM-1)-functionalized GO derivatives. J. Membr. Sci. 2021, 623, 118902. [Google Scholar] [CrossRef]

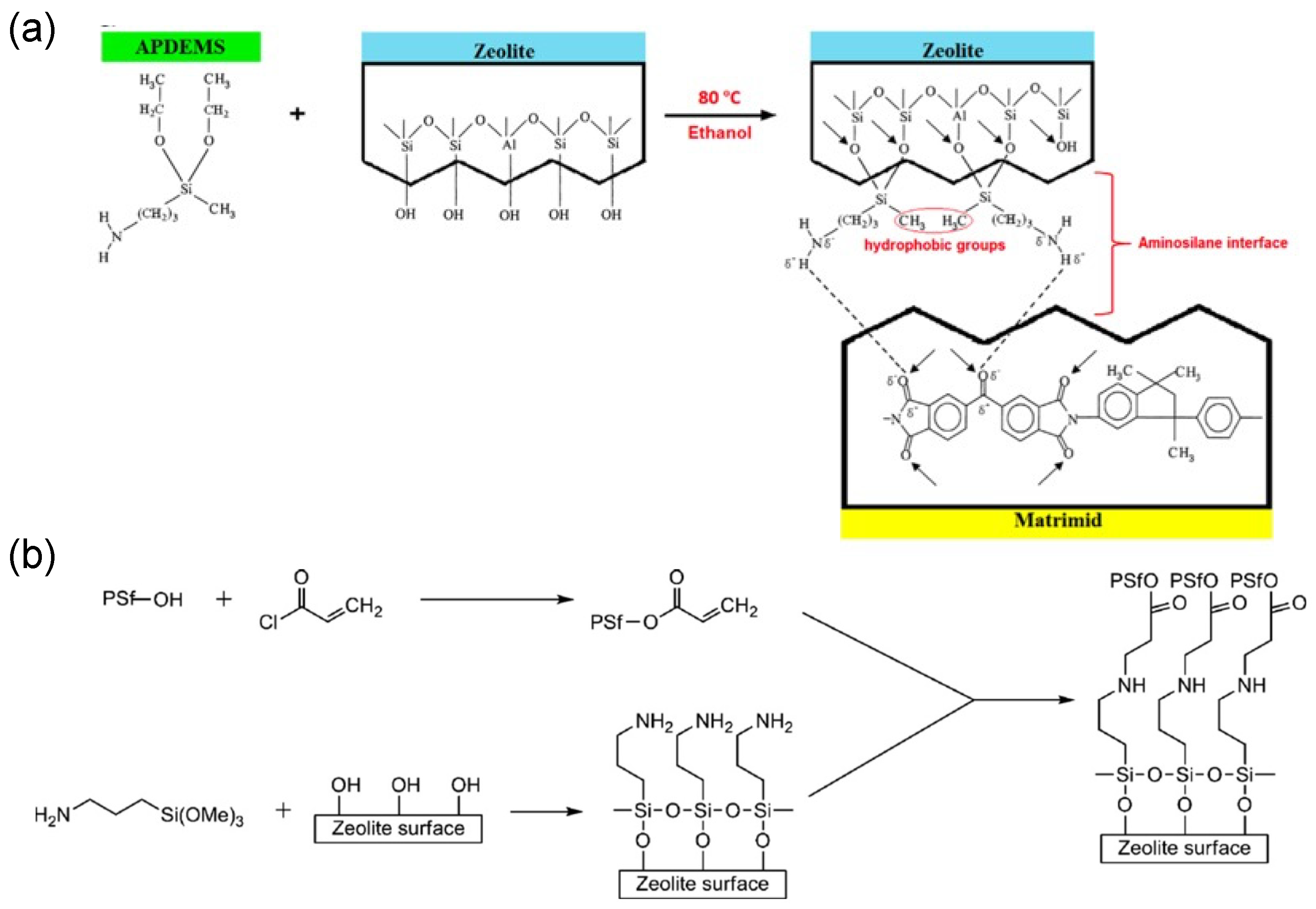

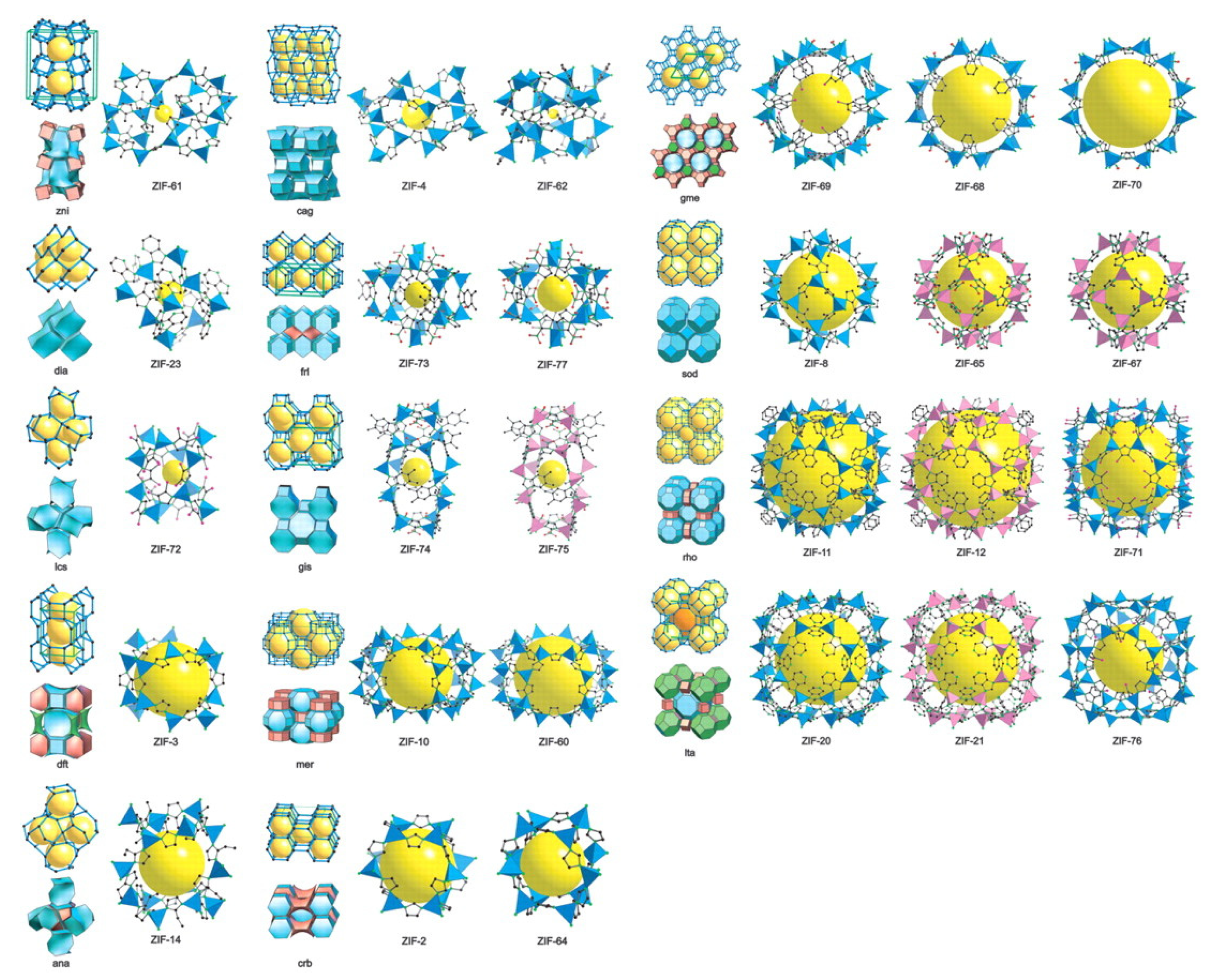




| Zeolite | Polymers | Pressure/Temp | Zeolite Loading | CO2 (Barrer) | CH4 (Barrer) | N2 (Barrer) | H2 (Barrer) | CO2/CH4 | CO2/N2 | H2/CO2 | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zeolite 13X | PEBAX | 14 Bar/25 °C | 1 wt.% | 194.1 | 56 | 56.5 | [65] | ||||
| Zeolite T | 6FDA-durene | 3.5 Bar/30 °C | 843.6 | 44.2 | 19.1 | [66] | |||||
| Zeolite 4A | PVAc | 0.1 MPa/30 °C | 25 wt.% | 2.413 | 0.024 | 3.75 | 100.54 | [67] | |||
| Li/Na-SZM-25 | Matrimid® 5218 | 5 bar/35 °C | 5 wt.% | 12 | 169 | [69] | |||||
| Zeolite NaY | PEBAX/PEG | 0.15 MPa/35 °C | 30 wt.% | 172.6 | 1.6 | 107.9 | [70] | ||||
| Hierarchical Zeolite 5A | Carbonized Matrimid® 5218 | 1 bar/35 °C | 30 wt.% | 2450 | 19.3 | [71] | |||||
| Zeolite 4A | PVAc | 440 psi/35 °C | 50 vol% | 11.4 | 0.457 | 25.0 | [68] | ||||
| Aminosilanized Zeolite 13X | 6FDA-Durene | 0.2 MPa/RT | 15 wt.% | 887 | 25.3 | [72] | |||||
| Aminosilanized Zeolite EMC-2 | 6FDA-ODA | 150 psig/35 °C | 15 wt.% | 40.9 | 0.51 | 80.2 | [73] | ||||
| Aminosilanized NaY zeolite | Matrimid® 5218 | 2 Bar/35 °C | 15 wt.% | 9.7 | 57.1 | [74] | |||||
| Aminosilanized zeolite 3A | PSf | 12 Bar/25 °C | 40 wt.% | 4.22 GPU | 15.1 GPU | 7.12 | [75] | ||||
| Aminosilanized MCM-41 | PSf | 10 Bar/25 °C | 30 wt.% | 9.13 | 31.48 | 32.97 | [76] | ||||
| SAPO-34 with ionic liquid | PSf | 3.5 Bar/30 °C | 5 wt.% | 7.19 GPU | 44.9 | [77] | |||||
| ZSM-5 with ionic liquid | 6FDA-TeMPD | 75 mmHg/35 °C | 15 wt.% | 142 | 9.48 | 5.55 | 32.6 | 15.0 | 25.6 | [78] | |
| SAPO-34 | PIL-RTIL | 2 atm/25 °C | 25 wt.% | 260 | 90 | [79] | |||||
| SAPO-34 | PIL-RTIL | 40 bar/17 °C | 30 wt.% | 202 | 43 (binary feeds) | [80] |
| MOFs | Polymers | Pressure/Temp | MOF Loading % | CO2 (Barrer) | CH4 (Barrer) | N2 (Barrer) | H2 (Barrer) | CO2/CH4 | CO2/N2 | CO2/H2 | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| UiO-66-NH2 | PSf | 3 bar/35 °C | 30 | 46 | ~24–26 | [88] | |||||
| UiO-66-CN | PIM-1 | 1 bar/25 °C | 20 | 12,063.3 | 225.5 | 53.5 | [89] | ||||
| UiO-66-NH2 | PVP_PEI | 1 atm/25 °C | 18 | 394 | 12 | [90] | |||||
| PA-UiO-66 | PVA | >1.3 ka/40 °C | 0.5 | 20.3 | [91] | ||||||
| UiO-66-NH2 | Pebax-1657 | 2–15 bar/25 °C | 50 | 325 GPU | 20 | 56 | [92] | ||||
| NH2–MIL-53(Al) | Pebax | 10 bar/35 °C | 10 | 149 | 23.3 | 59.4 | [93] | ||||
| NH2–MIL-53(Al) | CA | 3 bar/25 °C | 15 | 16 | 12 | [94] | |||||
| ZIF-8 | 6FDA-BI | 4 bar/35 °C | 20 | 20.3 | 0.35 | 0.78 | 78.5 | 58 | 25.9 | ||
| ZIF-8 | Zn2+(0.01 g/mL) | 4 bar/35 °C | 20 | 15.9 | 0.23 | 72.3 | 70.2 | 26.7 | [95] | ||
| ZIF-8 | PS-co-SBC | 2 bar/25 °C | 20 | 40 | 22 | [96] | |||||
| MKP | PVAm/mPSf | 0.5 MPa °C | 44.4 | 164.6 | 242 | [97] | |||||
| Y-fum-fcu-MO | 6FDA-DAM | 3.5 bar/−40–56 °C | 20 | <1000 | 130 | [98] | |||||
| UiO-66-NH2 @PI | ODPA-DAM | 3.1 bar/35 °C | 17 | 103 | 51 | 24 | [99] | ||||
| UiO-66-NH2 @PI | ODPA-DAM | 3.1 bar/35 °C | 27 | 142 | 43 | 27 | [99] | ||||
| 66-NH2@Ni-MOF-74 shell | ODPA-DAM | 3 bar/35 °C | 22 | 90 | 44 | [100] |
| ZIFs | Polymers | Pressure/Temp | ZIF Loading | CO2 (Barrer) | CH4 (Barrer) | N2 (Barrer) | H2 (Barrer) | CO2/CH4 | CO2/N2 | H2/CH4 | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ZIF-8 | 6FDA-Durene | 3.5 bar/30 °C | 10 wt.% | 1426 | 49.71 | 28.7 | [133] | ||||
| ZIF-8 | P84 polyimide | 3 bar/25 °C | 17 wt.% | 6.33 | 0.068 | 93.6 | [136] | ||||
| ZIF-8 | Pebax 1657 | 11 bar/35 °C | 2 wt.% | 118 | 5.5 | 2 | 21.4 | 59 | [130] | ||
| ZIF-8 | PVC-g-POEM | 1 bar/35 °C | 26.8 wt.% | 224 | 15.7 | 5.6 | 14.3 | 40 | [132] | ||
| ZIF-8 | PSF | 4 bar/30 °C | 0.5 wt.% | 3.25 | 0.21 | 0.19 | 15.1 | 17.5 | [126] | ||
| ZIF-8 | Pebax 1657 | 0.5 MPa °C | 5 wt.% | 140 | 67 | [125] | |||||
| ZIF-11 | Pebax 2533 | 2 bar/20 °C | 50 wt.% | 230 | 20 | 5 | 12 | 47 | [137] | ||
| ZIF-11 | 6FDA-DAM | 4 bar/30 °C | 20 wt.% | 257 | 8.3 | 272 | 31 | 32 | [127] | ||
| ZIF-67 | Pebax 1657 | 11 bar/35 °C | 4 wt.% | 16 | 5.8 | 2.2 | 27.6 | 72.7 | [130] | ||
| ZIF-71 | 6FDA-Durene | 3.5 bar/35 °C | 20 wt.% | 2560 | 181 | 186 | 14.2 | 13.8 | [134] | ||
| ZIF-90 | 6FDA-DAM | 4.5 bar/35 °C | 15 wt.% | 720 | 37 | [138] | |||||
| ZIF-90 | Triptycene-PI | 9.8 atm/35 °C | 10 wt.% | 26 | 0.6 | 1.1 | 61 | 42 | 24 | 99 | [123] |
| ZIF-95 | Matrimid | 4 bar/35 °C | 30 wt.% | 23.2 | 0.4 | 76.6 | 58 | 192 | [135] | ||
| ZIF-301 | 6FDA-DAM | 4bar/25°C | 20 wt.% | 891 | 29.3 | [124] |
| ZIFs and Hybrids | Polymers | Pressure/Temp | ZIF Loading | CO2 (Barrer) | CH4 (Barrer) | N2 (Barrer) | H2 (Barrer) | CO2/CH4 | CO2/N2 | H2/CH4 | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Modified ZIFs | |||||||||||
| OH-ZIF-7 | Pebax 2533 | 4.5 bar/20 °C | 14 wt.% | 273 | 38 | [156] | |||||
| ZIF-8@PD | 6FDA-TBDA22 | 1 bar/35 °C | 7 wt.% | 380 | 15 | 20 | 600 | 25 | 19 | 40 | [143] |
| ZIF-8@PEG200 | Matrimid 5218 | 8 bar/25 °C | 30 wt.% | 33.1 | 2.4 | 15.4 | [160] | ||||
| Amino-ZIF-7 | PEG | 5 bar/35 °C | 36 wt.% | 215 | 55 | [142] | |||||
| Amino-ZIF-8 | Pebax 1657 | 0.1 MPa/25 °C | 6 wt.% | 164 | 62 | [158] | |||||
| Amined-ZIF-8 | 6FDA-Durene | 3.5 bar/25 °C | 1 wt.% | 550 | 32.4 | 17 | [154] | ||||
| Amined-ZIF-8 | PSF | 4 bar/30 °C | 0.5 wt.% | 4.2 | 0.19 | 0.15 | 22.3 | 27.8 | [126] | ||
| NH2-ZIF-8 | Polyamine TNF | 1 Mpa/25 °C | 0.5 wt.% | 211GPU | 51 | [141] | |||||
| ZIF-8@VR | PAA | 1.5 bar/35 °C | 66.8 wt.% | 1410 | [155] | ||||||
| ZIF-8@IL | Pebax 1657 | 1 bar/25 °C | 15 wt.% | 104.9 | 34.8 | 83.9 | [151] | ||||
| ZIF-8@Ni | Pebax 2533 | 2 bar/25 °C | 10 wt.% | 321 | 42.8 | [140] | |||||
| ZIF-8@metakaolin | PVAm | 1 bar/25 °C | 3 wt.% | 169 GPU | 86.7 | [144] | |||||
| ZIF-8@Zn(II) | 6FDA-BI | 4 bar/35 °C | 20 wt.% | 20.3 | 0.35 | 0.78 | 78.5 | 57.9 | 25.9 | 223 | [95] |
| Hybrid ZIFs | |||||||||||
| ZIF-301/CNTs | PSF | 2 bar/25 °C | 18 wt.% | 19 | 48 | [146] | |||||
| ZIF-302/CNTs | PSF | 2 bar/25 °C | 18 wt.% | 18 | 35 | [148] | |||||
| ZIF-300/CNTs | Ultrason | 2 bar/25 °C | 30 wt.% | 19.9 | 50.8 | [145] | |||||
| ZIF-302/GO | PSF | 1 bar/25 °C | 18 wt.% | 11.5 | 0.25 | 45.9 | [147] | ||||
| ZIF-8/GO | Matrimid | 1 bar/30 °C | 20 wt.% | 238 | 65 | [153] | |||||
| ZIF-8/GO | PI-ODPA-TMPDA | 1 bar/25 °C | 10 wt.% | 142 | 41 | [150] | |||||
| ZIF-8/GO | Pebax 2533 | 6 bar/25 °C | 6 wt.% | 249 | 47.6 | [157] | |||||
| ZIF-8/GO | XLPEG | 1 bar/25 °C | 6 wt.% | 475 | 58.2 | [159] | |||||
| ZIF-8/MIL-101 (Cr) | PSF | 200 kpa/35 °C | 16 wt.% | 14 | 40 | [152] | |||||
| ZIF-8/MWCN | Pebax 1657 | 0.5 Mpa/35 °C | 8 wt.% | 186 | 61.2 | [149] | |||||
| ZIF-8/SiO2 | PSF | 4 bar/30 °C | 0.5 wt.% | 3.6 | 0.23 | 0.21 | 15 | 16 | [126] |
| Nanoparticles | Polymers | Pressure/Temp | NP Loading | CO2 (Barrer) | CH4 (Barrer) | N2 (Barrer) | H2 (Barrer) | CO2/CH4 | CO2/N2 | H2/CO2 | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MgO-Ag+ | Matrimid® 5218 | 3.5 bar/35C° | 20 wt.% | 4.31 | 0.102 | 0.155 | 22.7 | 42.3 | 5.26 | [170] | |
| SiO2 | PIM | 4 psig/23 °C | 6.7 vol% | 6200 | 460 | 3670 | 15 | [171] | |||
| TiO2 | PVAc | 2 bar/30 °C | 10 wt.% | 5.26 | 0.07 | 9.20 | 74.3 | [172] | |||
| ZnO | PEBAX | 2 bar/30 °C | 10 wt.% | 149.81 | 6.26 | 23.9 | [173] | ||||
| Al2O3 | PU | 76 cmHg/35 °C | 20 wt.% | 74.67 | 3.18 | 1.10 | 23.48 | 67.89 | [174] | ||
| NiO | PU | 1 bar/30 °C | 5 wt.% | 321 | 14.75 | 4.74 | 21.76 | 67.72 | [175] | ||
| Amino SiO2 | XLPEG | NA/35 °C | 2.7 wt.% | 134 | 6.4 | 2.1 | 22 | 62 | [176] | ||
| Dendritic Amino SiO2 | 6FDA-DABA | 76 cmHg/35 °C | 25 wt.% | 1920 | 23 | [58] | |||||
| Dendritic Amino SiO2 | PIM-1 | 76 cmHg/35 °C | 50 wt.% | 15,200 | 1340 | 916 | 11.4 | 16.6 | [177] | ||
| PMA-g-SiO2 | PMA | 24 bar/35 °C Equimolar feed | ~20 wt.% | 42.6 | 1.84 | 23.2 | [178] | ||||
| Amino POSS | GO | 2 bar/25 °C | 5 wt.% | 74.5 | [179] | ||||||
| PEG-POSS | PMHS | 2 bar/25 °C | 80 wt.% | 679 | 38.1 | [180] | |||||
| IL-POSS | PSf | 3.5 bar/25 °C | 5 wt.% | 34.98 GPU | 1.45 GPU | 24.1 | [181] | ||||
| POSS | 6FDA polyimide | 10 bar/35 °C | ~50 wt.% | 424 GPU | 2547 GPU | 6.3 | [182] |
| Nanoparticles | Polymers | Pressure/Temp | NP Loading | CO2 (Barrer) | CH4 (Barrer) | N2 (Barrer) | H2 (Barrer) | CO2/CH4 | CO2/N2 | H2/CH4 | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbon QD | Pebax | 1 bar/25 °C | 0.05 wt.% | 668 | 101 | [187] | |||||
| Alkyl SWCNT | PSf | 4 bar/35 °C | 5 wt.% | 5.12 | 0.27 | 0.23 | 18.82 | [188] | |||
| β-CD-MWCNT | CA | 3 × 105 Pa/35 °C | 0.1 wt.% | 741.67 | 18.46 | 40.17 | [189] | ||||
| NH2-NWCNT | Pebax | 0.7 MPa/35 °C | 33 wt.% | 361 | 16 | 52 | [190] | ||||
| PNIPAM-NWCNT | Pebax | 2 atm/25 °C (Humid state) | 5 wt.% | 567 | 35 | 70 | [191] | ||||
| GO nanosheet | PDMS | 8 wt.% | 10 Bar/35 °C | 27.7 | 24 | [192] | |||||
| GO nanosheet | Pebax/PEG | 1 bar/35 °C | 0.3 wt.% | ~650 | 55.8 | [193] | |||||
| Amino-GO nanosheet | Pebax | 2 bar/35 °C (Humid state) | 0.9 wt.% | 934.3 | 40.9 | 71.1 | [194] | ||||
| Porous reduced GO | Pebax | 0.2 MPa/30 °C | 5 wt.% | 119 | 104 | [195] | |||||
| oPOP-porous GO | Pebax | 1 atm/25 °C | 2 wt.% | 232.7 | 80.7 | [196] | |||||
| MWCNT+GO | Matrimid | 0.2 MPa/30 °C | 5 wt.% each | 38.07 | 0.45 | 0.47 | 84.6 | 81.0 | [197] | ||
| Activated carbon | ABS | 0.2 MPa/20 °C | 40 wt.% | 20.5 | 51 | [198] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Liu, Y.; Wong, D.A.; Yang, J. Recent Advances in Polymer-Inorganic Mixed Matrix Membranes for CO2 Separation. Polymers 2021, 13, 2539. https://doi.org/10.3390/polym13152539
Li S, Liu Y, Wong DA, Yang J. Recent Advances in Polymer-Inorganic Mixed Matrix Membranes for CO2 Separation. Polymers. 2021; 13(15):2539. https://doi.org/10.3390/polym13152539
Chicago/Turabian StyleLi, Sipei, Yang Liu, Dana A. Wong, and John Yang. 2021. "Recent Advances in Polymer-Inorganic Mixed Matrix Membranes for CO2 Separation" Polymers 13, no. 15: 2539. https://doi.org/10.3390/polym13152539
APA StyleLi, S., Liu, Y., Wong, D. A., & Yang, J. (2021). Recent Advances in Polymer-Inorganic Mixed Matrix Membranes for CO2 Separation. Polymers, 13(15), 2539. https://doi.org/10.3390/polym13152539