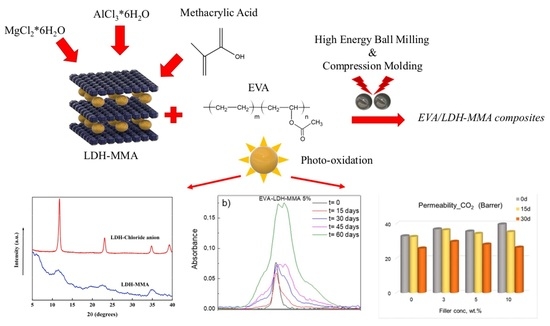
EVA Films Loaded with Layered Double Hydroxide (LDH) Modified with Methacrylic Anion: Effect of the Nanohybrid Filler on the Photodegradation Phenomena
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. MgAl-Methacrylate (LDH-MA) Preparation by Coprecipitation Method
2.3. Preparation of EVA/LDH-MA Composites
2.4. Methods
3. Results
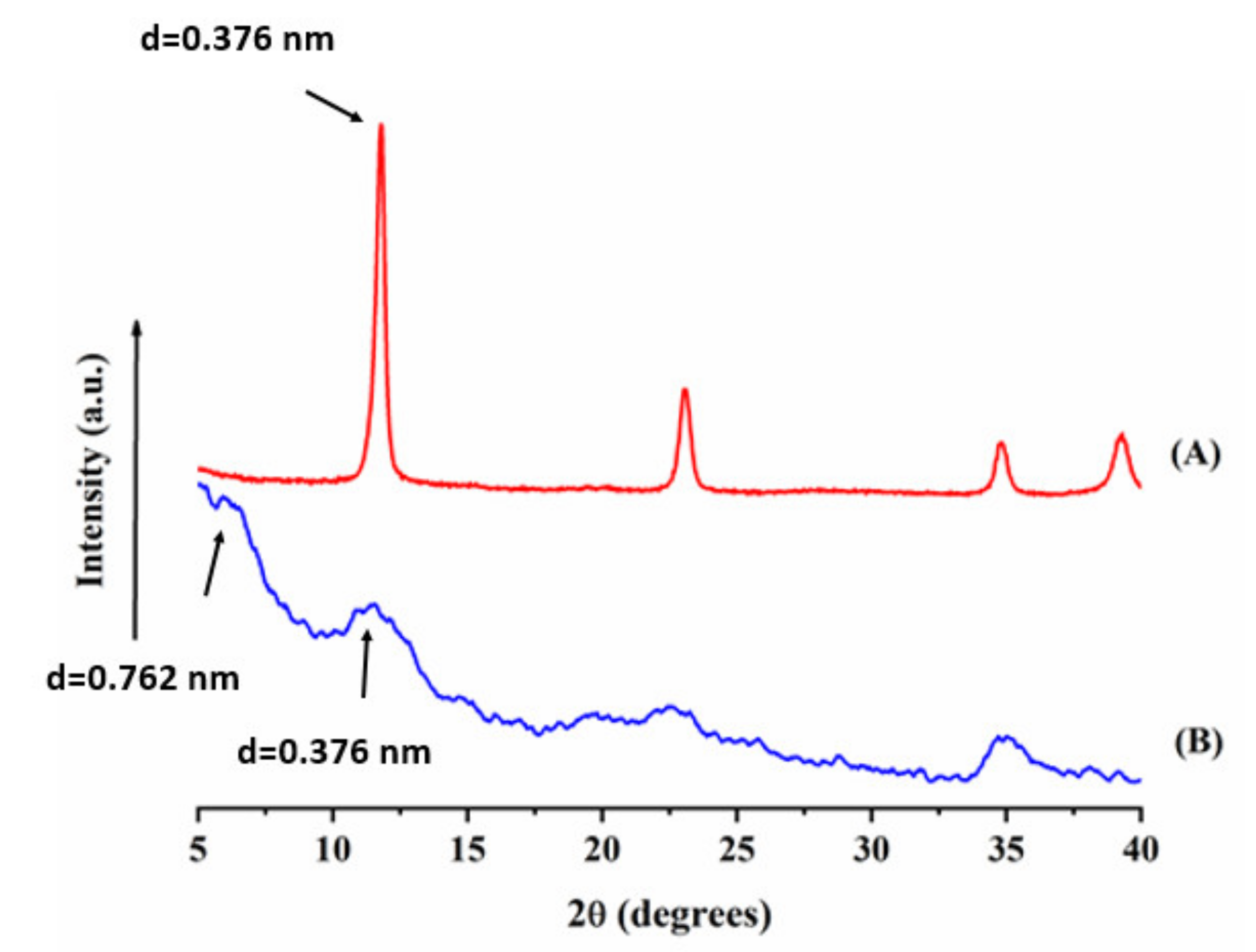
3.1. Materials
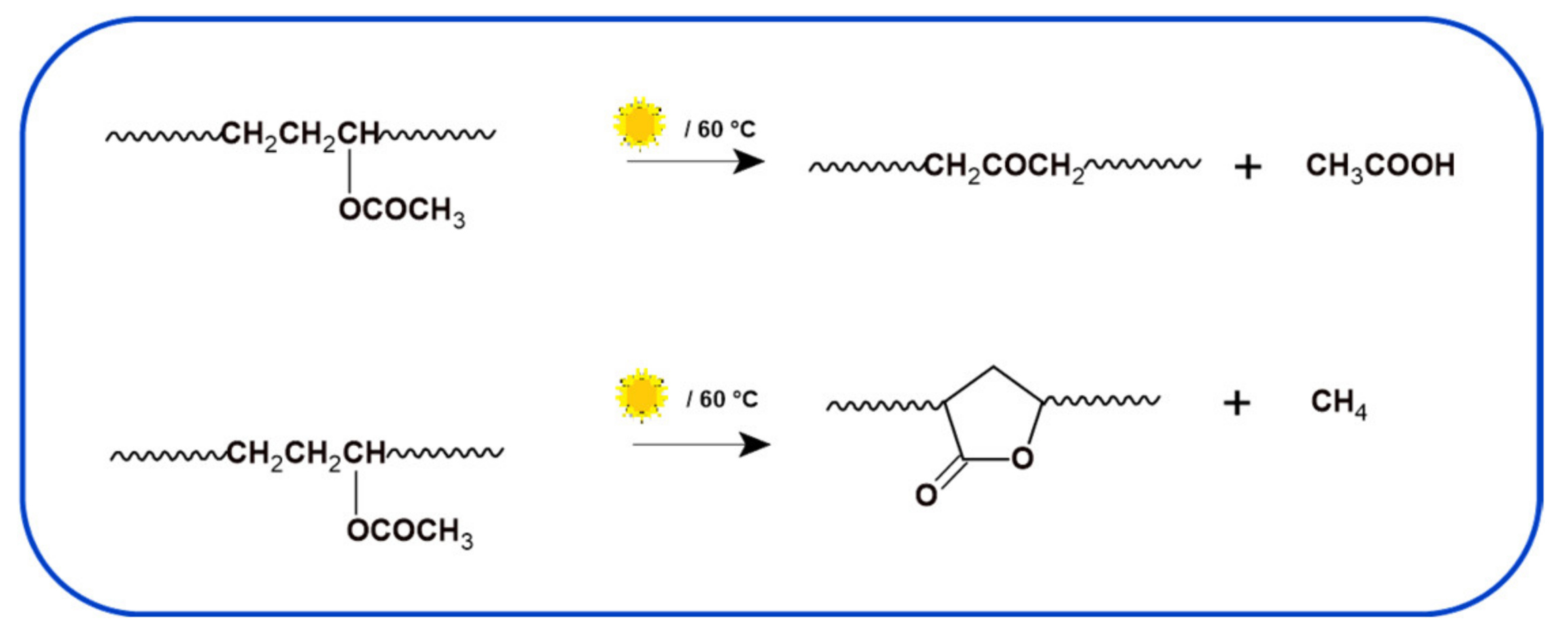
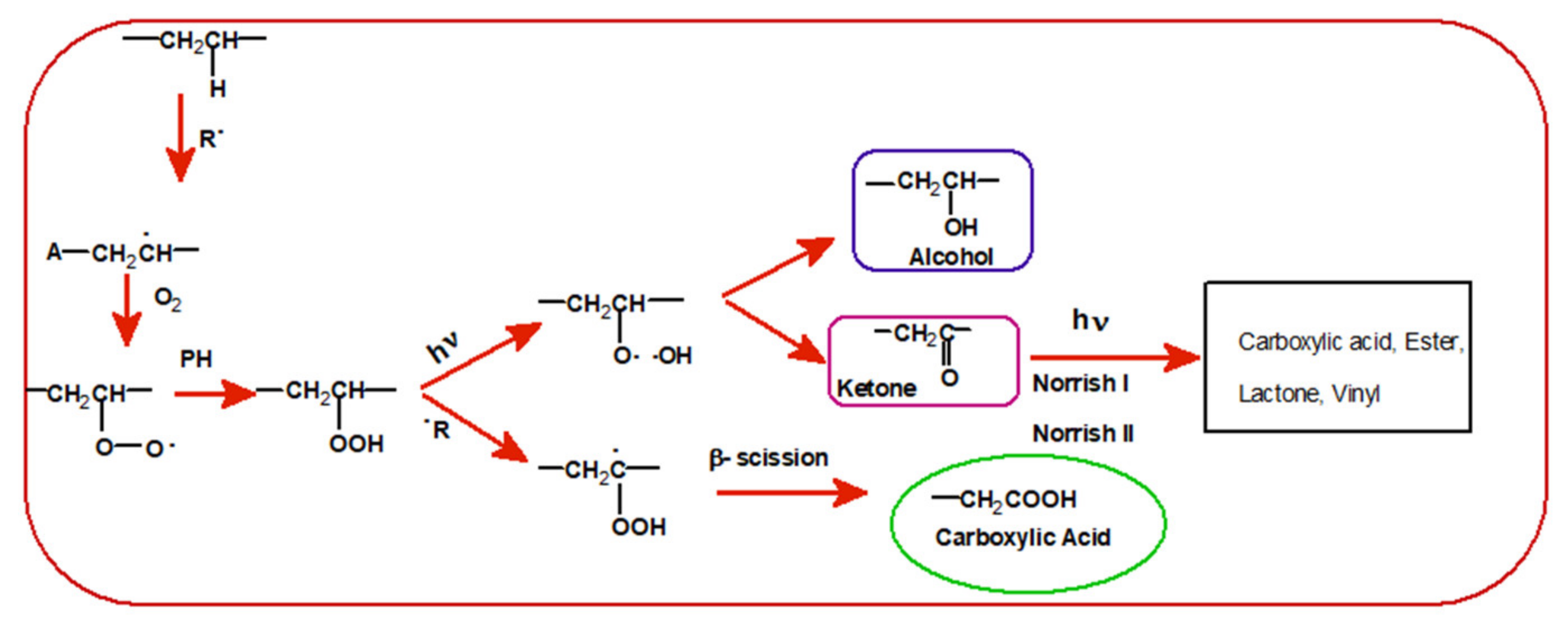
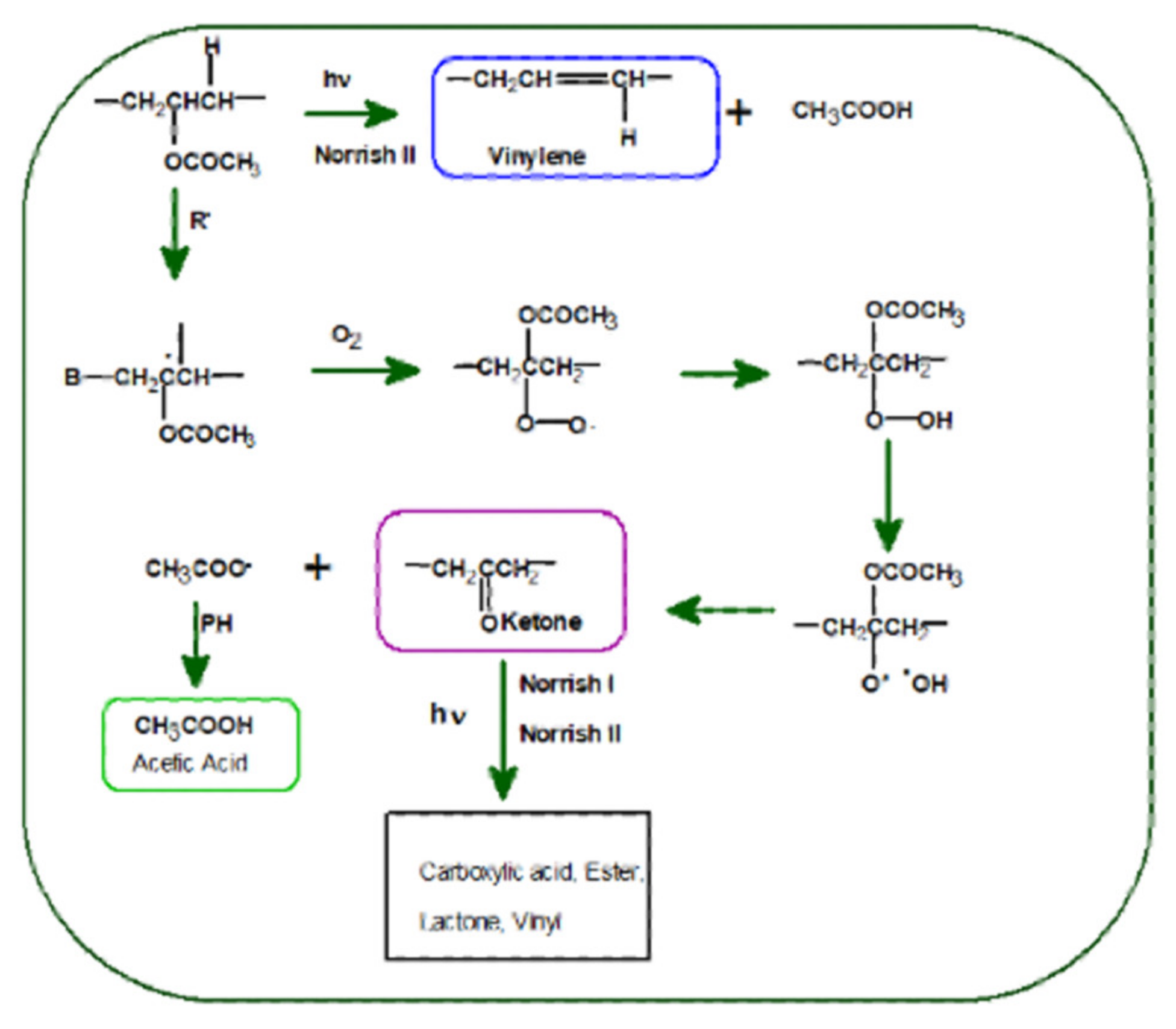
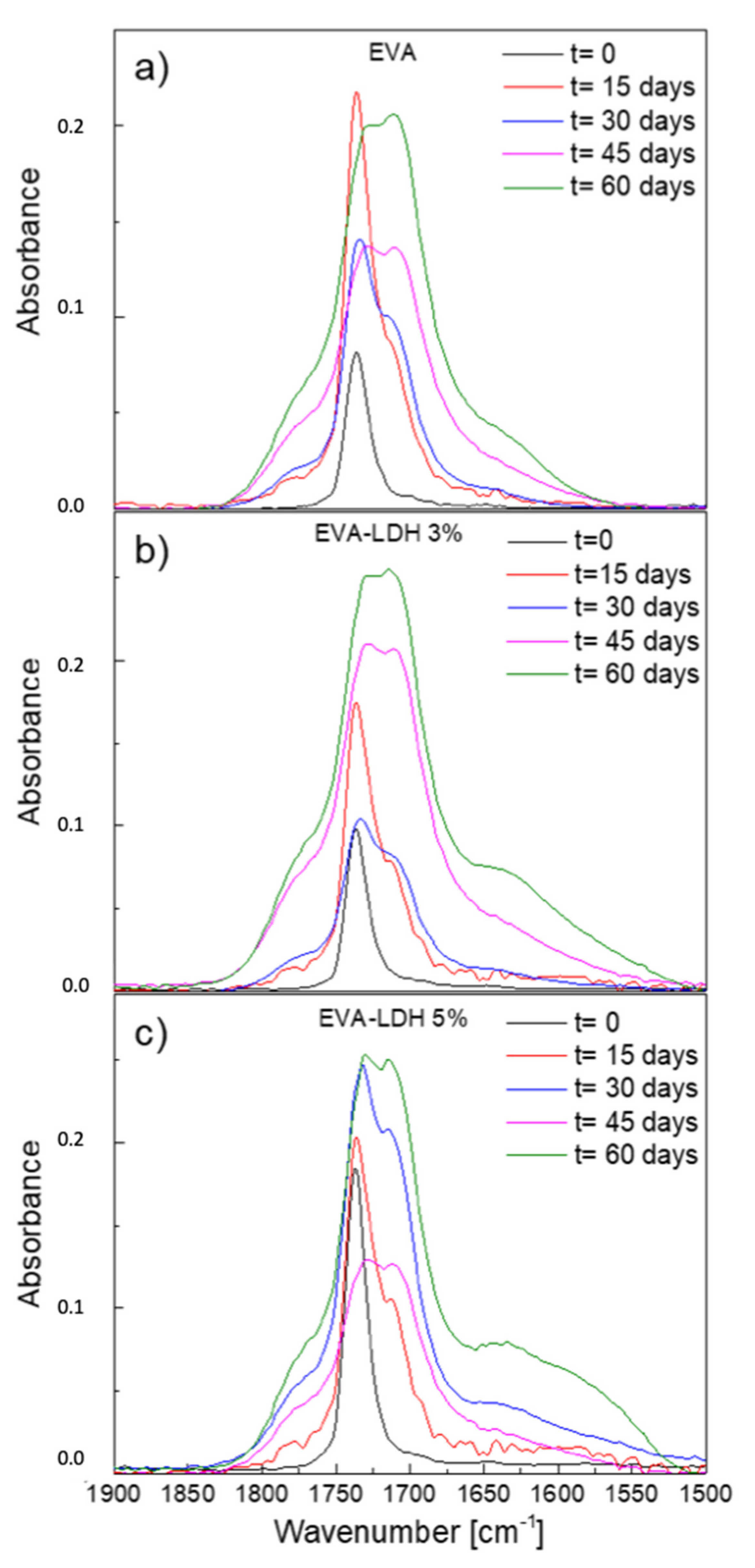
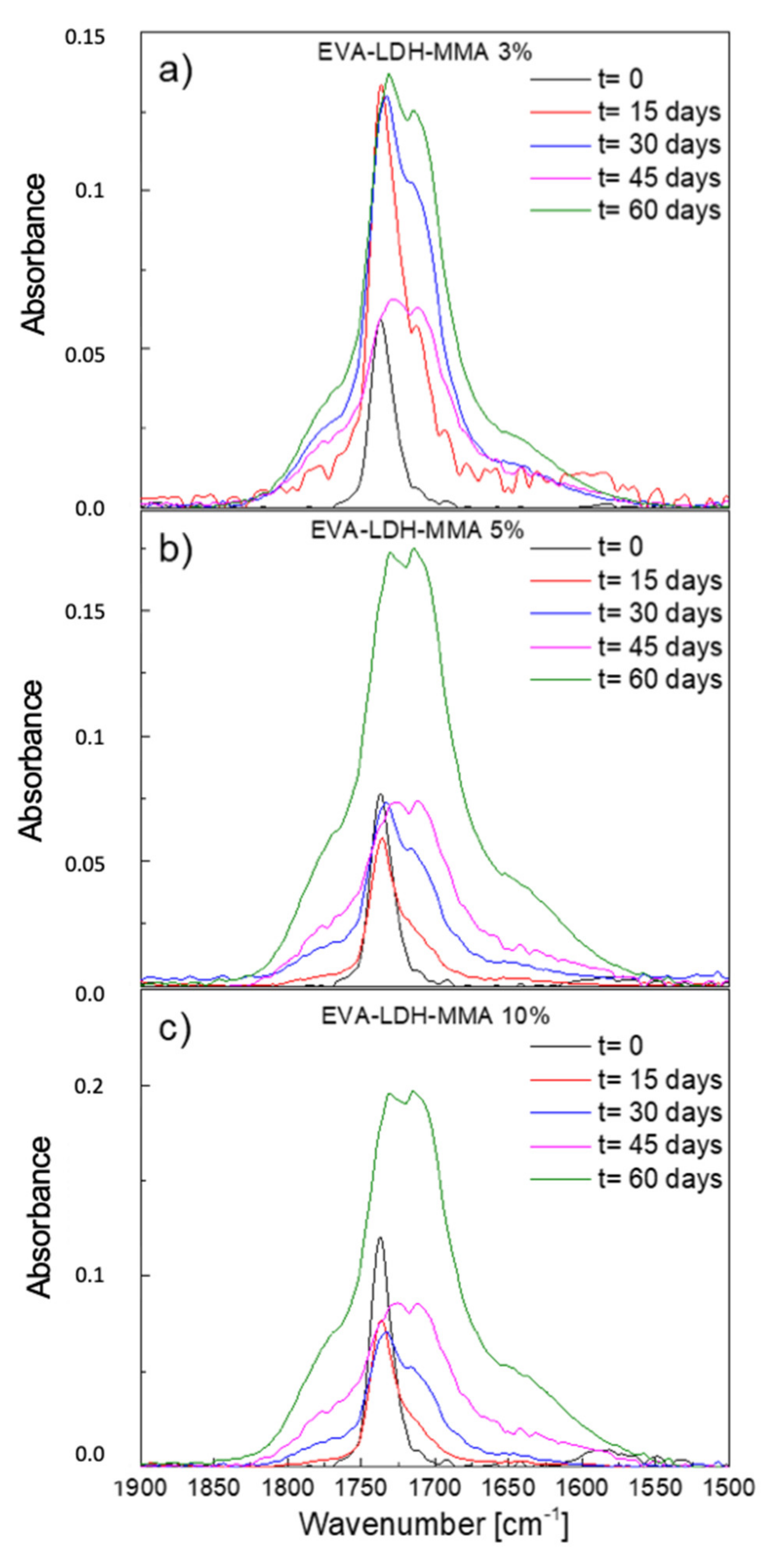
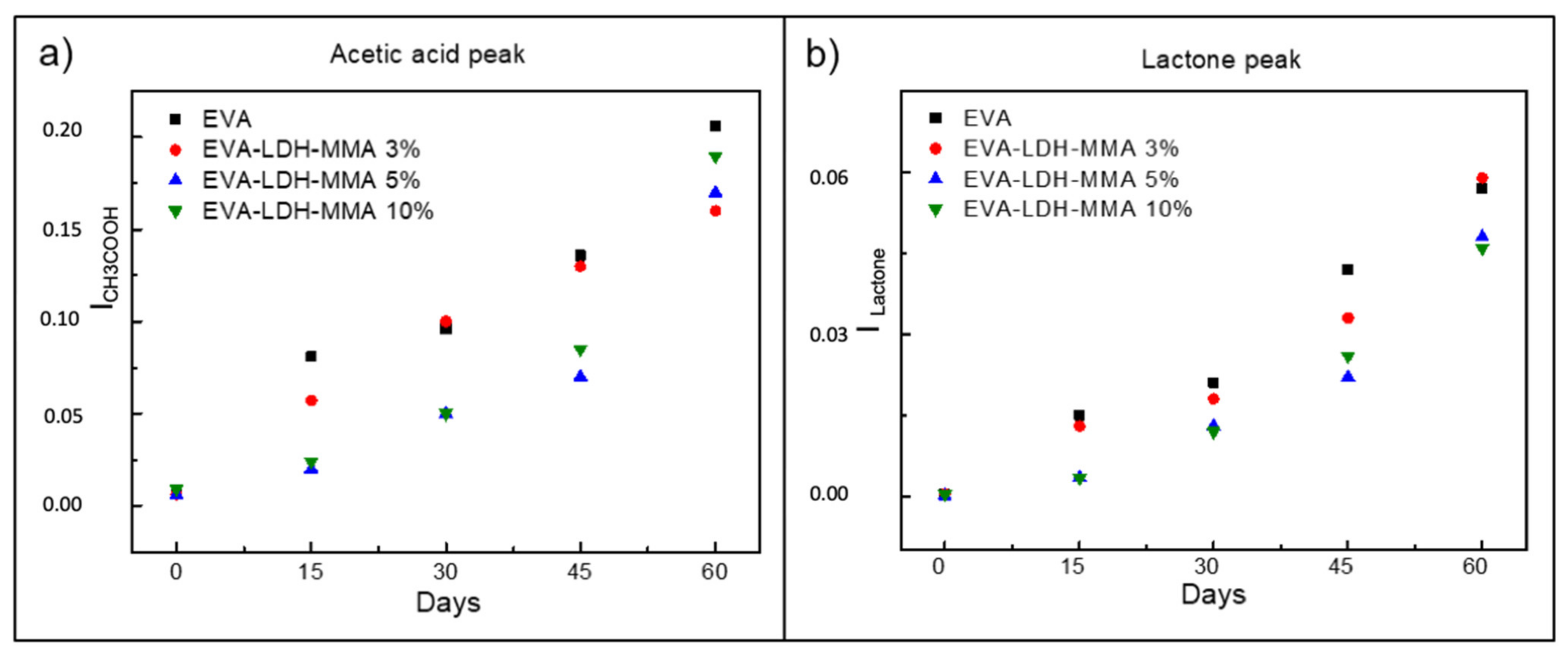
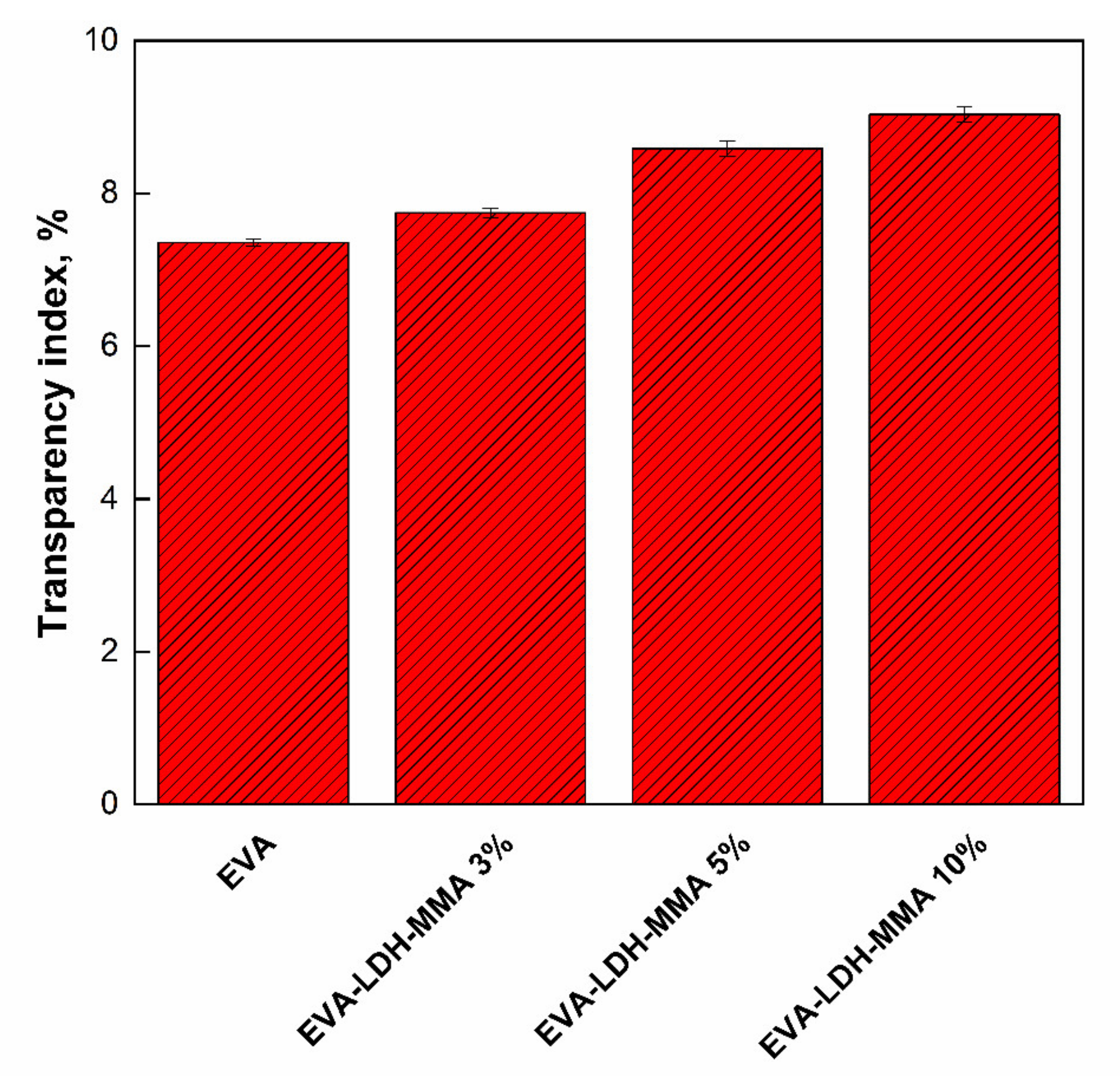
3.2. Photodegradation of EVA and Composites
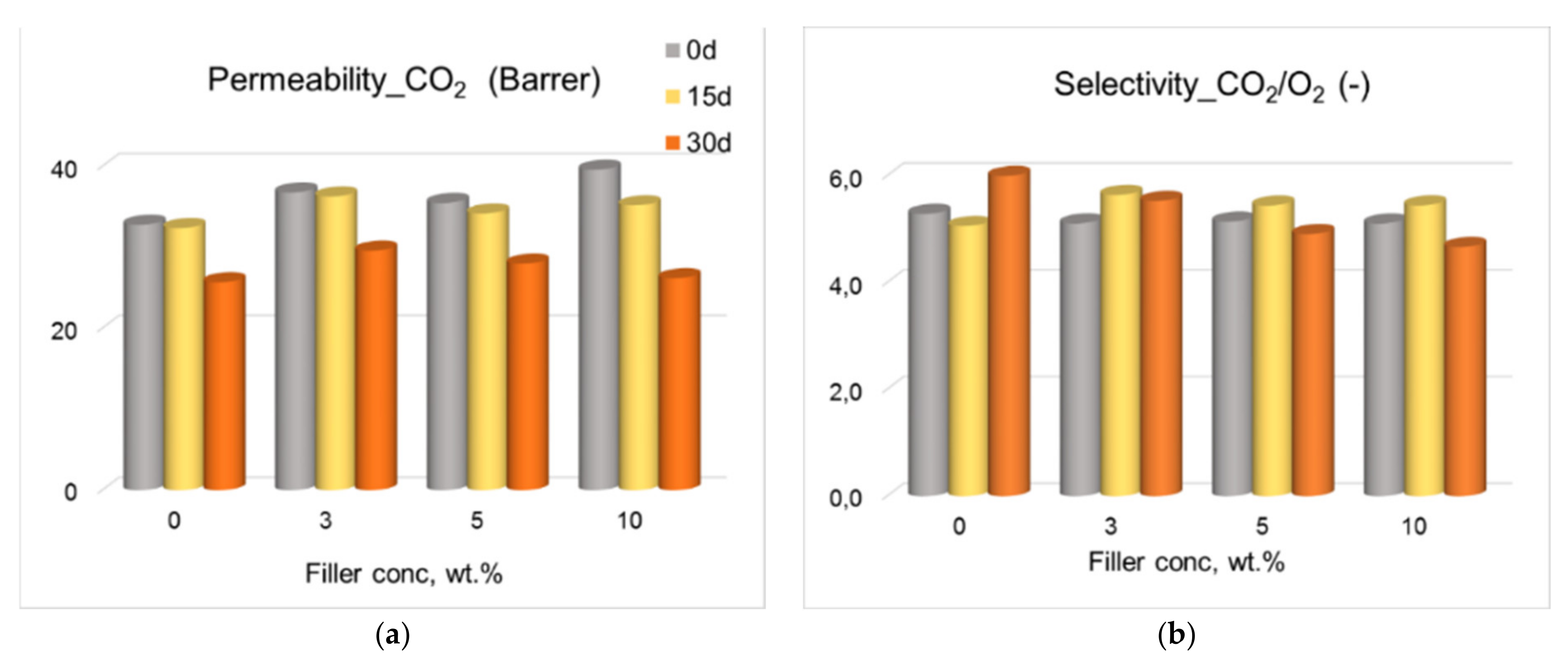
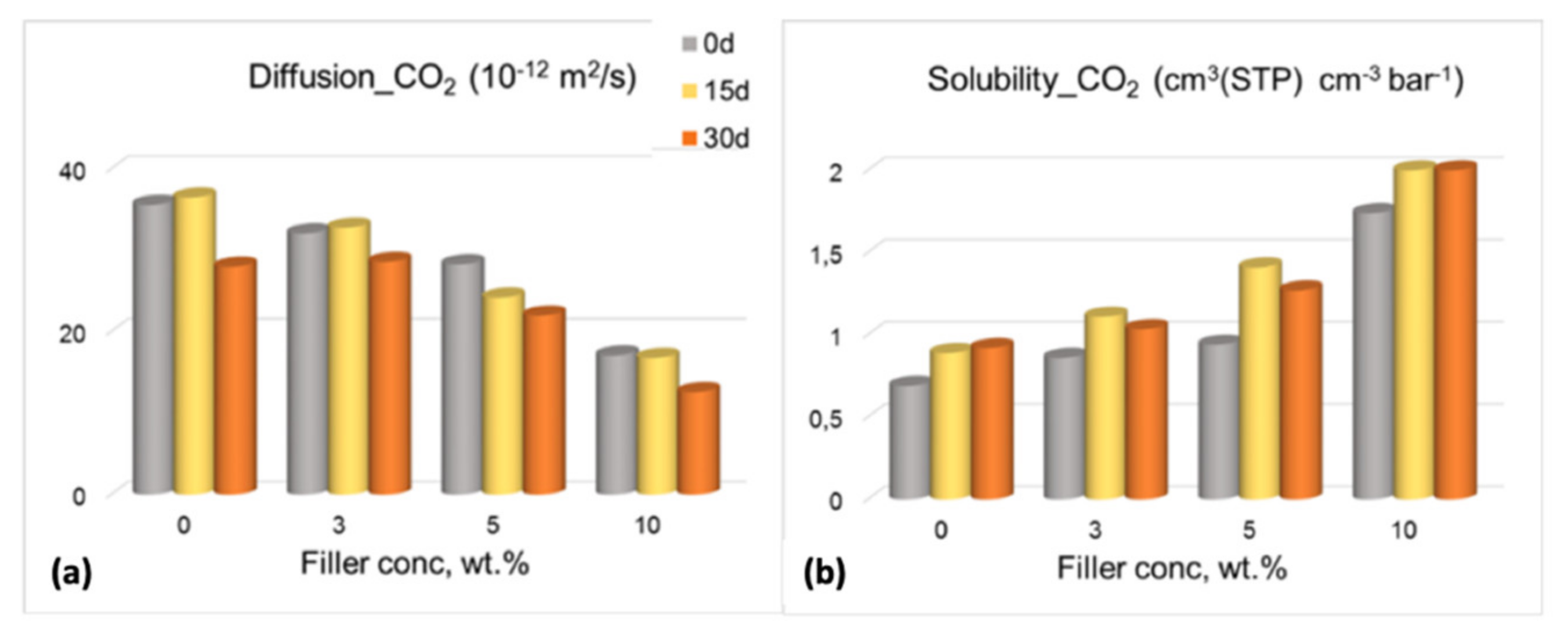
3.3. Gas Permeation Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, V.; Chandel, S.S. Performance and degradation analysis for long term reliability of solar photovoltaic systems: A review. Renew. Sustain. Energy Rev. 2013, 27, 753–767. [Google Scholar] [CrossRef]
- Ottersböck, B.; Oreski, G.; Pinter, G. Comparison of different microclimate effects on the aging behavior of encapsulation materials used in photovoltaic modules. Polym. Degrad. Stab. 2017, 138, 182–191. [Google Scholar] [CrossRef]
- Makrides, G.; Zinsser, B.; Norton, M.; Georghiou, G.E.; Schubert, M.; Werner, J.H. Potential of photovoltaic systems in countries with high solar irradiation. Renew. Sustain. Energy Rev. 2010, 14, 754–762. [Google Scholar] [CrossRef]
- Ndiaye, A.; Charki, A.; Kobi, A.; Kébé, C.M.F.; Ndiaye, P.A.; Sambou, V. Degradations of silicon photovoltaic modules: A literature review. Sol. Energy 2013, 96, 140–151. [Google Scholar] [CrossRef]
- Charki, A. Accelerated degradation testing of a photovoltaic module. J. Photonics Energy 2013, 3, 033099. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, K.; Zhang, H.; Ding, Y.; Yu, Q. Encapsulation of PV Modules Using Ethylene Vinyl Acetate Copolymer as the Encapsulant. Macromol. React. Eng. 2015, 9, 522–529. [Google Scholar] [CrossRef]
- Schneller, E.J.; Brooker, R.P.; Shiradkar, N.S.; Rodgers, M.P.; Dhere, N.G.; Davis, K.O.; Seigneur, H.P.; Mohajeri, N.; Wohlgemuth, J.; Scardera, G.; et al. Manufacturing metrology for c-Si module reliability and durability Part III: Module manufacturing. Renew. Sustain. Energy Rev. 2016, 59, 992–1016. [Google Scholar] [CrossRef] [Green Version]
- De Oliveira, M.C.C.; Diniz Cardoso, A.S.A.; Viana, M.M.; de Lins, V.F.C. The causes and effects of degradation of encapsulant ethylene vinyl acetate copolymer (EVA) in crystalline silicon photovoltaic modules: A review. Renew. Sustain. Energy Rev. 2018, 81, 2299–2317. [Google Scholar] [CrossRef]
- Pern, J. Module Encapsulation Materials, Processing and Testing (Presentation); National Renewable Energy Lab.(NREL): Golden, CO, USA, 2008. [Google Scholar]
- Soheilmoghaddam, M.; Adelnia, H.; Bidsorkhi, H.C.; Sharifzadeh, G.; Wahit, M.U.; Akos, N.I.; Yussuf, A.A. Development of Ethylene-Vinyl Acetate Composites Reinforced with Graphene Platelets. Macromol. Mater. Eng. 2017, 302, 1600260–1600268. [Google Scholar] [CrossRef]
- Ayutthaya, S.I.N.; Wootthikanokkhan, J. Investigation of the photodegradation behaviors of an ethylene/vinyl acetate copolymer solar cell encapsulant and effects of antioxidants on the photostability of the material. J. Appl. Polym. Sci. 2008, 107, 3853–3863. [Google Scholar] [CrossRef]
- Bahmanyar, M.; Sedaghat, S.; Ramazani, S.A.A.; Baniasadi, H. Preparation of Ethylene Vinyl Acetate Copolymer/Graphene Oxide Nanocomposite Films via Solution Casting Method and Determination of the Mechanical Properties. Polym. Plast. Technol. Eng. 2015, 54, 218–222. [Google Scholar] [CrossRef]
- Gorrasi, G.; Sorrentino, A. Photo-oxidative stabilization of carbon nanotubes on polylactic acid. Polym. Degrad. Stab. 2013, 98, 963–971. [Google Scholar] [CrossRef]
- Xue, H.; Xu, Z.; Zhang, M.; Wang, J.; Ruan, W. Ethylene vinyl acetate films filled with ytterbium containing rare earth particles (Y 2 SiO 5: Ce 3+, Yb 3+) which have optical down-conversion capabilities and useful for encapsulating solar cells. J. Plast. Film Sheeting 2015, 31, 233–247. [Google Scholar] [CrossRef]
- Jin, J.; Chen, S.; Zhang, J. UV aging behaviour of ethylene-vinyl acetate copolymers (EVA) with different vinyl acetate contents. Polym. Degrad. Stab. 2010, 95, 725–732. [Google Scholar] [CrossRef]
- Rosu, D.; Visakh, P.M. Photochemical Behavior of Multicomponent Polymeric-Based Materials; Rosu, D., Visakh, P.M., Eds.; Advanced Structured Materials; Springer International Publishing: Cham, Switzerland, 2016; Volume 26, ISBN 978-3-319-25194-3. [Google Scholar]
- Leroux, F.; Meddar, L.; Mailhot, B.; Morlat-Thérias, S.; Gardette, J.L. Characterization and photooxidative behaviour of nanocomposites formed with polystyrene and LDHs organo-modified by monomer surfactant. Polymer 2005, 46, 3571–3578. [Google Scholar] [CrossRef] [Green Version]
- Bocchini, S.; Morlat-Therias, S.; Gardette, J.L.; Camino, G. Influence of nanodispersed hydrotalcite on polypropylene photooxidation. Eur. Polym. J. 2008, 44, 3473–3481. [Google Scholar] [CrossRef]
- Magagula, B.; Nhlapo, N.; Focke, W.W. Mn2Al-LDH- and Co2Al-LDH-stearate as photodegradants for LDPE film. Polym. Degrad. Stab. 2009, 94, 947–954. [Google Scholar] [CrossRef] [Green Version]
- Lonkar, S.P.; Therias, S.; Caperaa, N.; Leroux, F.; Gardette, J.L. Photooxidation of polypropylene/layered double hydroxide nanocomposites: Influence of intralamellar cations. Eur. Polym. J. 2010, 46, 1456–1464. [Google Scholar] [CrossRef]
- Kovanda, F.; Jindová, E.; Lang, K.; Kubát, P.; Sedláková, Z. Preparation of layered double hydroxides intercalated with organic anions and their application in LDH/poly(butyl methacrylate) nanocomposites. Appl. Clay Sci. 2010, 48, 260–270. [Google Scholar] [CrossRef]
- Frunza, M.; Lisa, G.; Popa, M.I.; Miron, N.D.; Nistor, D.I. Thermogravimetric analysis of layered double hydroxides with chloramphenicol and salicylate in the interlayer space. J. Therm. Anal. Calorim. 2008, 93, 373–379. [Google Scholar] [CrossRef]
- Carroccio, S.; Puglisi, C.; Montaudo, G. Photo-oxidation products of polyetherimide ULTEM determined by MALDI-TOF-MS. Kinetics and mechanisms. Polym. Degrad. Stab. 2003, 80, 459–476. [Google Scholar] [CrossRef]
- Clarizia, G.; Bernardo, P.; Gorrasi, G.; Zampino, D.; Carroccio, S. Influence of the Preparation Method and Photo-Oxidation Treatment on the Thermal and Gas Transport Properties of Dense Films Based on a Poly(ether-block-amide) Copolymer. Materials 2018, 11, 1326. [Google Scholar] [CrossRef] [Green Version]
- Crank, J. The Mathematics of Diffusion, 2nd ed.; Clarendon Press: Oxford, UK, 1979; ISBN 3804204422. [Google Scholar]
- Wijmans, J.G.; Baker, R.W. The solution-diffusion model: A review. J. Memb. Sci. 1995, 107, 1–21. [Google Scholar] [CrossRef]
- Pattarasiriroj, K.; Kaewprachu, P.; Rawdkuen, S. Properties of rice flour-gelatine-nanoclay film with catechin-lysozyme and its use for pork belly wrapping. Food Hydrocoll. 2020, 107, 105951–105960. [Google Scholar] [CrossRef]
- Han, J.H.; Floros, J.D. Casting antimicrobial packaging films and measuring their physical properties and antimicrobial activity. J. Plast. Film Sheeting 1997, 13, 287–298. [Google Scholar] [CrossRef]
- Pérez-Mateos, M.; Montero, P.; Gómez-Guillén, M.C. Formulation and stability of biodegradable films made from cod gelatin and sunflower oil blends. Food Hydrocoll. 2009, 23, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Miyata, S. Physico-Chemical Properties of Synthetic Hydrotalcites in Relation to Composition. Clays Clay Miner. 1980, 28, 50–56. [Google Scholar] [CrossRef]
- Gorrasi, G.; Bugatti, V. Mechanical dispersion of layered double hydroxides hosting active molecules in polyethylene: Analysis of structure and physical properties. Appl. Clay Sci. 2016, 132–133, 2–6. [Google Scholar] [CrossRef]
- Helfand, M.A.; Mazzanti, J.B.; Fone, M.; Reamey, R.H.; Lindley, P.M. Effect of acetate distribution on surface segregation in poly(vinyl alcohol-co-vinyl acetate) copolymer films. Langmuir 1996, 12, 1296–1302. [Google Scholar] [CrossRef]
- McEvoy, R.L.; Krause, S.; Peter, W. Surface characterization of ethylene-vinyl acetate (EVA) and ethylene-acrylic acid (EAA) co-polymers using XPS and AFM. Polymer (Guildf) 1998, 39, 5223–5239. [Google Scholar] [CrossRef]
- Rabek, J.F. Photodegradation of Polymers; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Morlat-Therias, S.; Fanton, E.; Gardette, J.L.; Peeterbroeck, S.; Alexandre, M.; Dubois, P. Polymer/carbon nanotube nanocomposites: Influence of carbon nanotubes on EVA photodegradation. Polym. Degrad. Stab. 2007, 92, 1873–1882. [Google Scholar] [CrossRef]
- Nie, B.; Stutzman, J.; Xie, A. A vibrational spectral maker for probing the hydrogen-bonding status of protonated Asp and Glu residues. Biophys. J. 2005, 88, 2833–2847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ussia, M.; Curcuruto, G.; Zampino, D.; Dintcheva, N.T.; Filippone, G.; Mendichi, R.; Carroccio, S.C. Role of organo-modifier and metal impurities of commercial nanoclays in the photo-and thermo-oxidation of polyamide 11 nanocomposites. Polymers 2020, 12, 1034–1045. [Google Scholar] [CrossRef] [PubMed]
- Gorrasi, G.; Bugatti, V.; Ussia, M.; Mendichi, R.; Zampino, D.; Puglisi, C.; Carroccio, S.C. Halloysite nanotubes and thymol as photo protectors of biobased polyamide 11. Polym. Degrad. Stab. 2018, 152, 43–51. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Sadeghi, M.; Motamed-Hashemi, M.M.Y.; Pourafshari Chenar, M.; Roosta-Azad, R.; Sadeghi, M. Study of gas separation properties of ethylene vinyl acetate (EVA) copolymer membranes prepared via phase inversion method. Sep. Purif. Technol. 2008, 62, 642–647. [Google Scholar] [CrossRef]
- Song, Q.; Cao, S.; Zavala-Rivera, P.; Ping Lu, L.; Li, W.; Ji, Y.; Al-Muhtaseb, S.A.; Cheetham, A.K.; Sivaniah, E. Photo-oxidative enhancement of polymeric molecular sieve membranes. Nat. Commun. 2013, 4, 1918. [Google Scholar] [CrossRef] [Green Version]
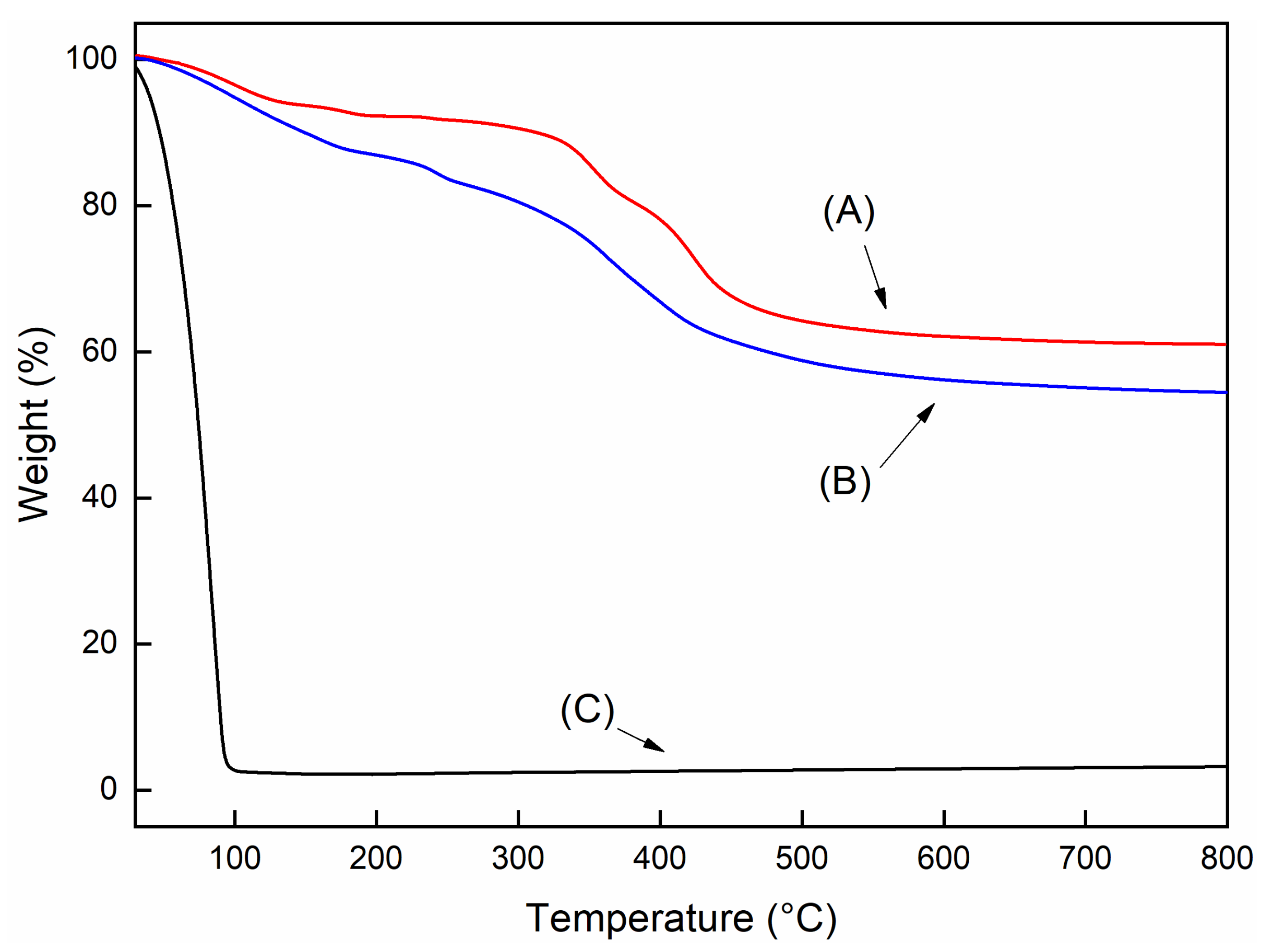
| EVA | T95 (°C) a | Tpeak (°C) b | Residue c |
|---|---|---|---|
| t = 0 | 336 | 471 | - |
| t = 45 | 326 | 471 | - |
| t = 60 | 323 | 472 | - |
| EVA | T95 (°C) a | Tpeak (°C) b | Residue c |
|---|---|---|---|
| t = 0 | 337 | 476 | 0.5 |
| t = 45 | 332 | 473 | 1.6 |
| t = 60 | 328 | 479 | 5.3 |
| EVA | T95 (°C) a | Tpeak (°C) b | Residue c |
|---|---|---|---|
| t = 0 | 336 | 470 | 1.5 |
| t = 45 | 327 | 438 | 3.4 |
| t = 60 | 327 | 465 | 3.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorrasi, G.; Viscusi, G.; Curcuruto, G.; Cantarella, M.; Di Mauro, A.; Bernardo, P.; Clarizia, G.; Scamporrino, A.A.; Carroccio, S. EVA Films Loaded with Layered Double Hydroxide (LDH) Modified with Methacrylic Anion: Effect of the Nanohybrid Filler on the Photodegradation Phenomena. Polymers 2021, 13, 2525. https://doi.org/10.3390/polym13152525
Gorrasi G, Viscusi G, Curcuruto G, Cantarella M, Di Mauro A, Bernardo P, Clarizia G, Scamporrino AA, Carroccio S. EVA Films Loaded with Layered Double Hydroxide (LDH) Modified with Methacrylic Anion: Effect of the Nanohybrid Filler on the Photodegradation Phenomena. Polymers. 2021; 13(15):2525. https://doi.org/10.3390/polym13152525
Chicago/Turabian StyleGorrasi, Giuliana, Gianluca Viscusi, Giusy Curcuruto, Maria Cantarella, Alessandro Di Mauro, Paola Bernardo, Gabriele Clarizia, Andrea A. Scamporrino, and Sabrina Carroccio. 2021. "EVA Films Loaded with Layered Double Hydroxide (LDH) Modified with Methacrylic Anion: Effect of the Nanohybrid Filler on the Photodegradation Phenomena" Polymers 13, no. 15: 2525. https://doi.org/10.3390/polym13152525
APA StyleGorrasi, G., Viscusi, G., Curcuruto, G., Cantarella, M., Di Mauro, A., Bernardo, P., Clarizia, G., Scamporrino, A. A., & Carroccio, S. (2021). EVA Films Loaded with Layered Double Hydroxide (LDH) Modified with Methacrylic Anion: Effect of the Nanohybrid Filler on the Photodegradation Phenomena. Polymers, 13(15), 2525. https://doi.org/10.3390/polym13152525