Nafion/Surface Modified Ceria Hybrid Membranes for Fuel Cell Application
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shaari, N.; Kamarudin, S.K. Recent advances in additive-enhanced polymer electrolyte membrane properties in fuel cell applications: An overview. Int. J. Energy Res. 2019, 43, 2756–2794. [Google Scholar] [CrossRef]
- Ercelik, M.; Ozden, A.; Devrim, Y.; Colpan, C.O. Investigation of Nafion based composite membranes on the performance of DMFCs. Int. J. Hydrogen Energy 2017, 42, 2658–2668. [Google Scholar] [CrossRef]
- Stenina, I.A.; Yaroslavtsev, A.B. Ionic mobility in ion-exchange membranes. Membranes 2021, 11, 198. [Google Scholar] [CrossRef] [PubMed]
- Gashoul, F.; Parnian, M.J.; Rowshanzamir, S. A new study on improving the physicochemical and electrochemical properties of SPEEK nanocomposite membranes for medium temperature proton exchange membrane fuel cells using different loading of zirconium oxide nanoparticles. Int. J. Hydrogen Energy 2017, 42, 590–602. [Google Scholar] [CrossRef]
- Li, J.; Xu, G.; Luo, X.; Xiong, J.; Liu, Z.; Cai, W. Effect of nano-size of functionalized silica on overall performance of swelling-filling modified Nafion membrane for direct methanol fuel cell application. Appl. Energy 2018, 213, 408–414. [Google Scholar] [CrossRef]
- Saccà, A.; Carbone, A.; Gatto, I.; Pedicini, R.; Passalacqua, E. Synthesized yttria stabilised zirconia as filler in proton exchange membranes (PEMs) with enhanced stability. Polym. Test. 2018, 65, 322–330. [Google Scholar] [CrossRef]
- Ketpang, K.; Oh, K.; Lim, S.C.; Shanmugam, S. Nafion-porous cerium oxide nanotubes composite membrane for polymer electrolyte fuel cells operated under dry conditions. J. Power Sources 2016, 329, 441–449. [Google Scholar] [CrossRef]
- Vinothkannan, M.; Ramakrishnan, S.; Kim, A.R.; Lee, H.K.; Yoo, D.J. Ceria stabilized by titanium carbide as a sustainable filler in the Nafion matrix improves the mechanical Integrity, electrochemical durability, and hydrogen impermeability of proton-exchange membrane fuel cells: Effects of the filler content. ACS Appl. Mater. Interfaces 2020, 12, 5704–5716. [Google Scholar] [CrossRef]
- Shin, D.; Han, M.; Shul, Y.G.; Lee, H.; Bae, B. Analysis of cerium-composite polymer-electrolyte membranes during and after accelerated oxidative-stability test. J. Power Sources 2018, 378, 468–474. [Google Scholar] [CrossRef]
- Alavijeh, A.S.; Goulet, M.A.; Khorasany, R.M.; Ghataurah, J.; Lim, C.; Lauritzen, M.; Kjeang, E.; Wang, G.G.; Rajapakse, R.K. Decay in mechanical properties of catalyst coated membranes subjected to combined chemical and mechanical membrane degradation. Fuel Cells 2015, 15, 204–213. [Google Scholar] [CrossRef]
- Zaton, M.; Roziere, J.; Jones, D.J. Current understanding of chemical degradation mechanisms of perfluorosulfonic acid membranes and their mitigation strategies: A review. Sustain. Energy Fuels 2017, 1, 409–438. [Google Scholar] [CrossRef]
- Schlick, S. (Ed.) The Chemistry of Membranes Used in Fuel Cells: Degradation and Stabilization; John Wiley & Sons, Inc.: New York, NY, USA, 2017. [Google Scholar]
- Filippov, S.P.; Yaroslavtsev, A.B. Hydrogen energy: Development prospects and materials. Russ. Chem. Rev. 2021, 90, 627–643. [Google Scholar] [CrossRef]
- Walkey, C.; Das, S.; Seal, S.; Erlichman, J.; Heckman, K.; Ghibelli, L.; Traversa, E.; McGinnis, J.F.; Self, W.T. Catalytic properties and biomedical applications of cerium oxide nanoparticles. Environ. Sci. Nano 2015, 2, 33–53. [Google Scholar] [CrossRef]
- Rui, Z.; Liu, J. Understanding of free radical scavengers used in highly durable proton exchange membranes. Progr. Nat. Sci: Mater. Int. 2020, 30, 732–742. [Google Scholar] [CrossRef]
- Tinh, V.D.; Kim, D. Enhancement of oxidative stability of PEM fuel cell by introduction of HO• radical scavenger in Nafion ionomer. J. Memb. Sci. 2020, 613, 118517. [Google Scholar] [CrossRef]
- Kumar, A.; Hong, J.; Yun, Y.; Bhardwaja, A.; Song, S.J. The role of surface lattice defects of CeO2−δ nanoparticles as a scavenging redox catalyst in polymer electrolyte membrane fuel cells. J. Mater. Chem. A 2020, 8, 26023–26034. [Google Scholar] [CrossRef]
- Coms, F.D.; Liu, H.; Owejan, J.E. Mitigation of perfluorosulfonic acid membrane chemical degradation using cerium and manganese ions. ECS Trans. 2008, 16, 1735–1747. [Google Scholar] [CrossRef]
- Zaton, M.; Prélot, B.; Donzel, N.; Rozière, J.; Jones, D.J. Migration of Ce and Mn ions in PEMFC and its impact on PFSA membrane degradation. J. Electrochem. Soc. 2018, 165, F3281–F3289. [Google Scholar] [CrossRef]
- Pearman, B.P.; Mohajeri, N.; Brooker, R.P.; Rodgers, M.P.; Slattery, D.K.; Hampton, M.D.; Cullen, D.A.; Seal, S. The degradation mitigation effect of cerium oxide in polymer electrolyte membranes in extended fuel cell durability tests. J. Power Sources 2013, 225, 75–83. [Google Scholar] [CrossRef]
- Lim, C.; Alavijeh, A.S.; Lauritzen, M.; Kolodziej, J.; Knights, S.; Kjeang, E. Fuel cell durability enhancement with cerium oxide under combined chemical and mechanical membrane degradation. ECS Electrochem. Lett. 2015, 4, F29–F31. [Google Scholar] [CrossRef]
- Weissbach, T.; Peckham, T.J.; Holdcroft, S. CeO2, ZrO2 and YSZ as mitigating additives against degradation of proton exchange membranes by free radicals. J. Memb. Sci. 2016, 498, 94–104. [Google Scholar] [CrossRef]
- Trogadas, P.; Parrondo, J.; Ramani, V. Degradation mitigation in polymer electrolyte membranes using cerium oxide as a regenerative free-radical scavenger. Electrochem. Solid State Lett. 2008, 11, B113–B116. [Google Scholar] [CrossRef]
- Prabhakaran, V.; Ramani, V. Structurally-tuned nitrogen-doped cerium oxide exhibits exceptional regenerative free radical scavenging activity in polymer electrolytes. J. Electrochem. Soc. 2014, 161, F1–F9. [Google Scholar] [CrossRef]
- Zhao, W.; Haolin, T.; Huijie, Z.; Ming, L.; Rui, C.; Pan, X.; Mu, P. SynthesisofNafion/CeO2 hybrid for chemically durable proton exchange membrane of fuel cell. J. Memb. Sci. 2012, 421–422, 201–210. [Google Scholar]
- Velayutham, P.; Sahu, A.K.; Parthasarathy, S. A Nafion-ceria composite membrane electrolyte for reduced methanol crossover in direct methanol fuel cells. Energies 2017, 10, 259. [Google Scholar] [CrossRef]
- Baker, M.; Williams, S.T.; Mukundan, R.; Spernjak, D.; Advani, S.G.; Prasad, A.K.; Borup, R.L. Zr-doped ceria additives for enhanced PEM fuel cell durability and radical scavenger stability. J. Mater. Chem. A 2017, 5, 15073–15079. [Google Scholar] [CrossRef]
- Akrout, A.; Delrue, A.; Zaton, M.; Duquet, F.; Spanu, F.; Taillades-Jacquin, M.; Cavaliere, S.; Jones, D.; Rozière, J. Immobilisation and release of radical scavengers on nanoclays for chemical reinforcement of proton exchange membranes. Membranes 2020, 10, 208. [Google Scholar] [CrossRef] [PubMed]
- Donnadio, A.; D’Amato, R.; Marmottini, F.; Panzetta, G.; Pica, M.; Battocchio, C.; Capitani, D.; Ziarelli, F.; Casciola, M. On the evolution of proton conductivity of Aquivion membranes loaded with CeO2 based nanofillers: Effect of temperature and relative humidity. J. Memb. Sci. 2019, 574, 17–23. [Google Scholar] [CrossRef]
- Parnian, M.J.; Rowshanzamir, S.; Prasad, A.K.; Advani, S.G. Effect of ceria loading on performance and durability of sulfonated poly (ether ether ketone) nanocomposite membranes for proton exchange membrane fuel cell applications. J. Memb. Sci. 2018, 565, 342–357. [Google Scholar] [CrossRef]
- Golubenko, D.V.; Shaydullin, R.R.; Yaroslavtsev, A.B. Improving the conductivity and permselectivity of ion-exchange membranes by introduction of inorganic oxide nanoparticles: Impact of acid–base properties. Colloid Polym. Sci. 2019, 297, 741–748. [Google Scholar] [CrossRef]
- Bakangura, E.; Wu, L.; Ge, L.; Yang, Z.; Xu, T. Mixed matrix proton exchange membranes for fuel cells: State of the art and perspectives. Progr. Polymer Sci. 2016, 57, 103–152. [Google Scholar] [CrossRef]
- Stenina, I.; Golubenko, D.; Nikonenko, V.; Yaroslavtsev, A. Selectivity of transport processes in ion-exchange membranes: Relationship with the structure and methods for its improvement. Int. J. Mol. Sci. 2020, 21, 5517. [Google Scholar] [CrossRef]
- Apel, P.Y.; Bobreshova, O.V.; Volkov, A.V.; Volkov, V.V.; Nikonenko, V.V.; Stenina, I.A.; Filippov, A.N.; Yampolskii, Y.P.; Yaroslavtsev, A.B. Prospects of membrane science developments. Membr. Membr. Technol. 2019, 1, 45–63. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.Y.; Wong, W.Y.; Ramy, K.; Khalid, M.; Loh, K.S.; Daud, W.R.; Lim, K.L.; Walvekar, R.; Kadhum, A.A. Additives in proton exchange membranes for low- and high-temperature fuel cell applications: A review. Int. J. Hydrogen Energy 2019, 44, 6116–6135. [Google Scholar] [CrossRef]
- Safronova, E.Y.; Stenina, I.A.; Yaroslavtsev, A.B. Synthesis and characterization of MF-4SK+SiO2 hybrid membranes modified with tungstophosphoric heteropolyacid. Russ. J. Inorg. Chem. 2010, 55, 13–17. [Google Scholar] [CrossRef]
- Oh, K.; Kwon, O.; Son, B.; Lee, D.H.; Shanmugam, S. Nafion-sulfonated silica composite membrane for proton exchange membrane fuel cells under operating low humidity condition. J. Memb. Sci. 2019, 583, 103–109. [Google Scholar] [CrossRef]
- Kim, D.J.; Jo, M.J.; Nam, S.Y. A review of polymer-nanocomposite electrolyte membranes for fuel cell application. J. Ind. Eng. Chem. 2015, 21, 36–52. [Google Scholar] [CrossRef]
- Gerasimova, E.; Safronova, E.; Ukshe, A.; Dobrovolsky, Y.; Yaroslavtsev, A. Electrocatalytic and transport properties of hybrid Nafion membranes doped with silica and cesium acid salt of phosphotungstic acid in hydrogen fuel cells. Chem. Eng. J. 2016, 305, 121–128. [Google Scholar] [CrossRef]
- Xu, G.; Wei, Z.; Li, S.; Li, J.; Yang, Z.; Grigoriev, S.A. In-situ sulfonation of targeted silica-filled Nafion for high-temperature PEM fuel cell application. Int. J. Hydrogen Energy 2019, 44, 29711–29716. [Google Scholar] [CrossRef]
- Yurova, P.A.; Tabachkova, N.Y.; Stenina, I.A.; Yaroslavtsev, A.B. Properties of ceria nanoparticles with surface modified by acidic groups. J. Nanopart. Res. 2020, 22, 318. [Google Scholar] [CrossRef]
- Stenina, I.A.; Voropaeva, E.Y.; Brueva, T.R.; Sinel’nikov, A.A.; Drozdova, N.A.; Ievlev, V.M.; Yaroslavtsev, A.B. Heat-treatment induced evolution of the morphology and microstructure of zirconia prepared from chloride solutions during. Russ. J. Inorg. Chem. 2008, 53, 842–848. [Google Scholar] [CrossRef]
- Agafonov, A.V.; Kraev, A.S.; Ivanova, O.S.; Evdokimova, O.L.; Gerasimova, T.V.; Baranchikov, A.E.; Kozik, V.V.; Ivanov, V.K. Comparative study of the electrorheological effect in suspensions of needle-like and isotropic cerium dioxide nanoparticles. Rheol. Acta 2018, 57, 307–315. [Google Scholar] [CrossRef]
- Yurova, P.A.; Stenina, I.A.; Yaroslavtsev, A.B. A comparative study of the transport properties of homogeneous and heterogeneous cation-exchange membranes doped with zirconia modified with phosphoric acid groups. Pet. Chem. 2018, 58, 1144–1153. [Google Scholar] [CrossRef]
- Calaza, F.C.; Chen, T.L.; Mullins, D.R.; Xu, Y.; Overbury, S.H. Reactivity and reaction intermediates for acetic acid adsorbed on CeO2(1 1 1). Catal. Today 2015, 253, 65–76. [Google Scholar] [CrossRef] [Green Version]
- Stenina, I.A.; Yurova, P.A.; Titova, T.S.; Polovkova, M.A.; Korchagin, O.V.; Bogdanovskaya, V.A.; Yaroslavtsev, A.B. The influence of poly(3,4-ethylenedioxythiophene) modification on the transport properties and fuel cell performance of Nafion-117 membranes. J. Appl. Polym. Sci. 2021, 138, 50644. [Google Scholar] [CrossRef]
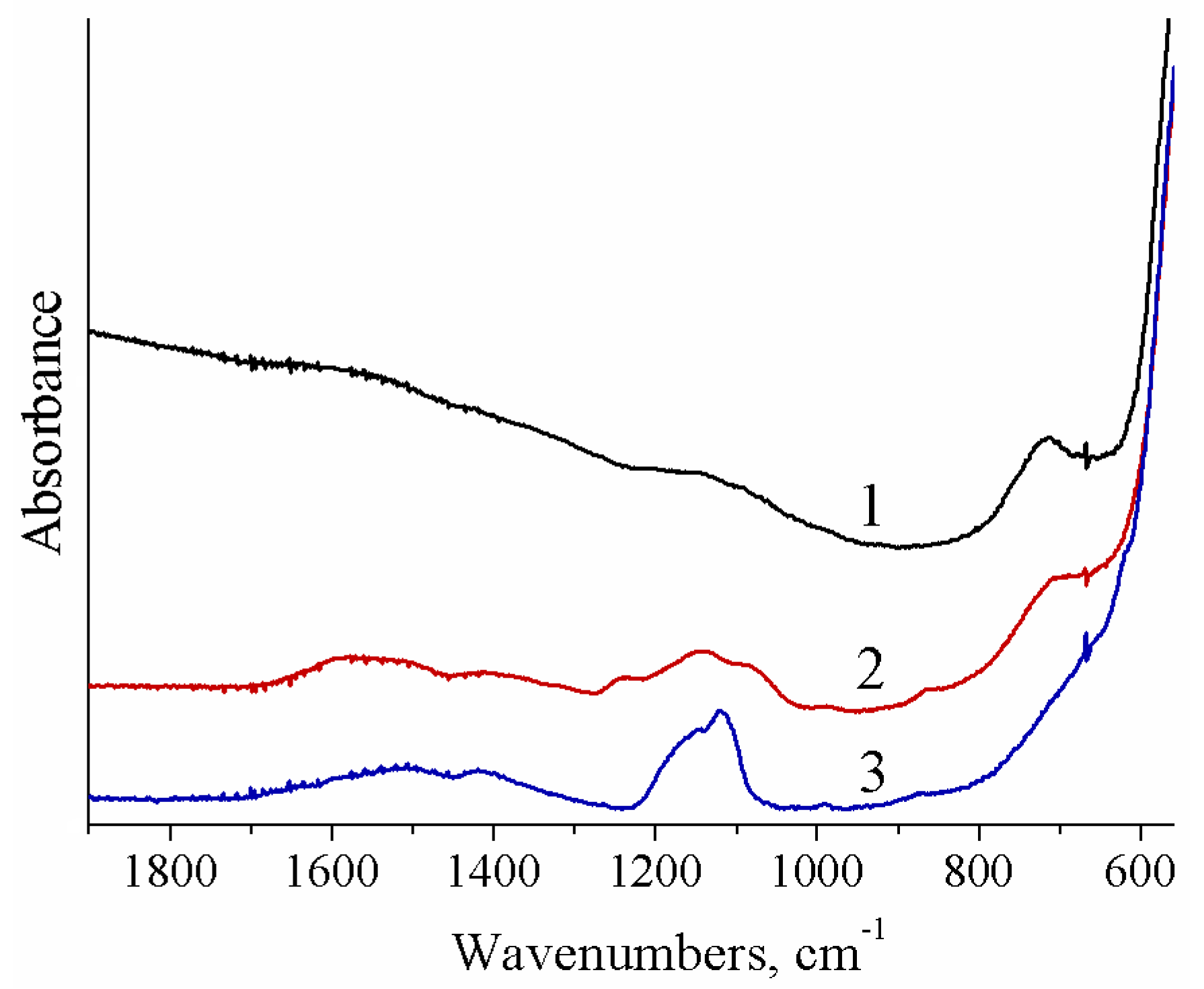
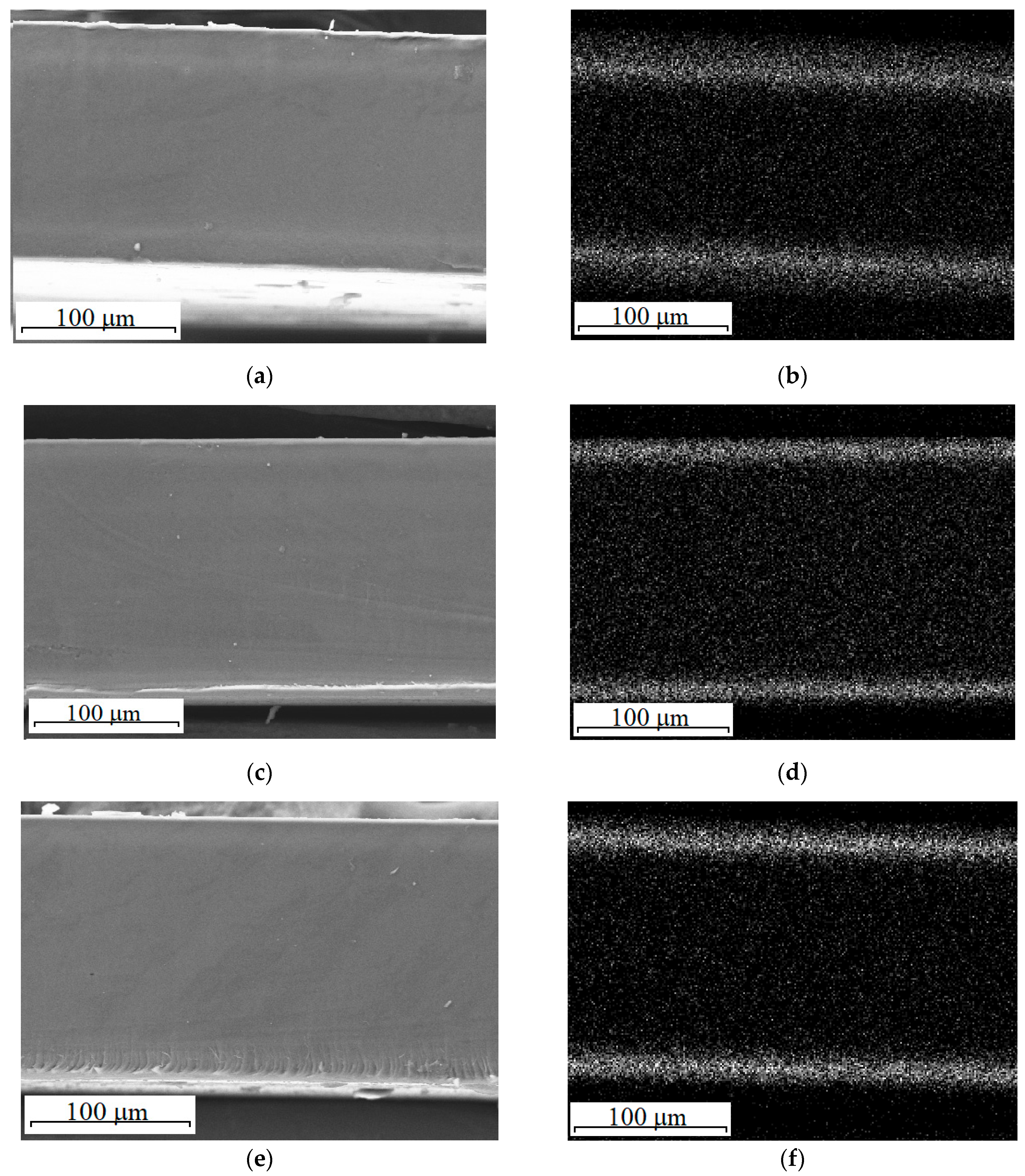

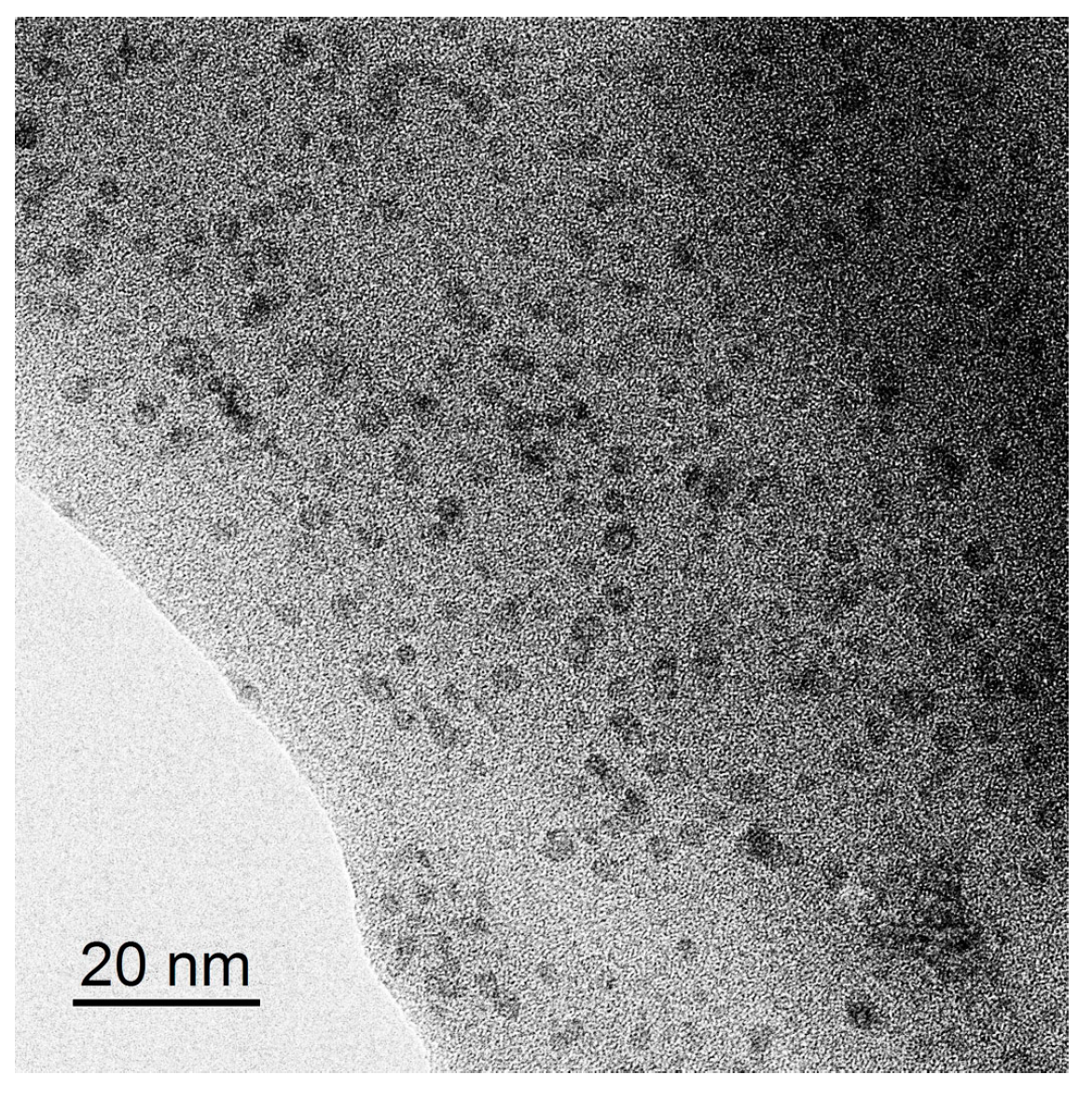




| Sample | Ceria Precursor | Additional Treatment |
|---|---|---|
| Nafion-117 | - | - |
| NC3 | 0.01 M Ce(NO3)3 | - |
| NC3_0.2P | 0.01 M Ce(NO3)3 | 0.2 M H3PO4 |
| NC3_1P | 0.01 M Ce(NO3)3 | 1 M H3PO4 |
| NC3_0.2S | 0.01 M Ce(NO3)3 | 0.2 M H2SO4 |
| NC3_1S | 0.01 M Ce(NO3)3 | 1 M H2SO4 |
| NC4 | 0.3 M (NH4)2Ce(NO3)6 | - |
| NC4_0.2P | 0.3 M (NH4)2Ce(NO3)6 | 0.2 M H3PO4 |
| NC4_1P | 0.3 M (NH4)2Ce(NO3)6 | 1 M H3PO4 |
| NC4_0.2S | 0.3 M (NH4)2Ce(NO3)6 | 0.2 M H2SO4 |
| NC4_1S | 0.3 M (NH4)2Ce(NO3)6 | 1 M H2SO4 |
| NC4(S) | 0.05 M Ce(SO4)2 | - |
| NC4(NS) | 0.05 M (NH4)4Ce(SO4)4 | - |
| Sample | ωdop, % | IEC ± 0.02, mmol/g | ωH2O, % (RH = 95%) | ωH2O, % (RH = 30%) |
|---|---|---|---|---|
| Nafion-117 | - | 0.91 | 23.2 | 5.3 |
| NC3 | 2.2 | 0.80 | 23.7 | 4.7 |
| NC3_0.2P | 2.4 | 0.81 | 23.8 | 4.5 |
| NC3_1P | 1.8 | 0.83 | 23.9 | 4.5 |
| NC3_0.2S | 2.1 | 0.81 | 24.0 | 4.5 |
| NC3_1S | 1.3 | 0.84 | 24.2 | 4.5 |
| NC4 | 0.8 | 0.85 | 26.2 | 4.9 |
| NC4_0.2P | 0.6 | 0.87 | 26.0 | 4.9 |
| NC4_1P | 0.5 | 0.88 | 26.3 | 5.0 |
| NC4_0.2S | 1.0 | 0.85 | 26.0 | 4.5 |
| NC4_1S | 0.9 | 0.87 | 26.9 | 4.4 |
| NC4(S) | 0.4 | 0.89 | 26.4 | 5.5 |
| NC4(NS) | 0.1 | 0.88 | 26.6 | 5.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yurova, P.A.; Malakhova, V.R.; Gerasimova, E.V.; Stenina, I.A.; Yaroslavtsev, A.B. Nafion/Surface Modified Ceria Hybrid Membranes for Fuel Cell Application. Polymers 2021, 13, 2513. https://doi.org/10.3390/polym13152513
Yurova PA, Malakhova VR, Gerasimova EV, Stenina IA, Yaroslavtsev AB. Nafion/Surface Modified Ceria Hybrid Membranes for Fuel Cell Application. Polymers. 2021; 13(15):2513. https://doi.org/10.3390/polym13152513
Chicago/Turabian StyleYurova, Polina A., Viktoria R. Malakhova, Ekaterina V. Gerasimova, Irina A. Stenina, and Andrey B. Yaroslavtsev. 2021. "Nafion/Surface Modified Ceria Hybrid Membranes for Fuel Cell Application" Polymers 13, no. 15: 2513. https://doi.org/10.3390/polym13152513
APA StyleYurova, P. A., Malakhova, V. R., Gerasimova, E. V., Stenina, I. A., & Yaroslavtsev, A. B. (2021). Nafion/Surface Modified Ceria Hybrid Membranes for Fuel Cell Application. Polymers, 13(15), 2513. https://doi.org/10.3390/polym13152513








