Kinetical Study, Thermo-Mechanical Characteristics and Recyclability of Epoxidized Camelina Oil Cured with Antagonist Structure (Aliphatic/Aromatic) or Functionality (Acid/Amine) Hardeners
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
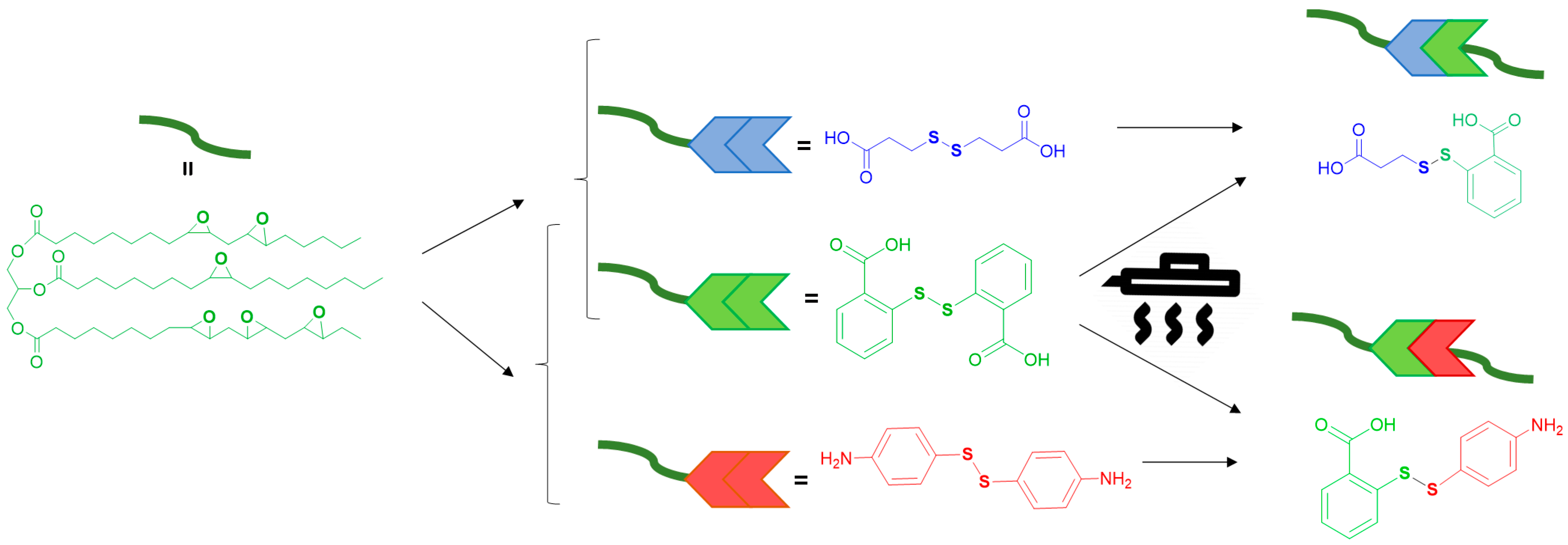
2.2. Preparation of Crosslinked Networks
2.3. Mechanical Reprocessing Procedure
2.4. Chemical Recycling Procedure
2.5. Methods
3. Results and Discussion
3.1. Reactivity Studies on the Function of the Dual-Dynamic Crosslinker Combination
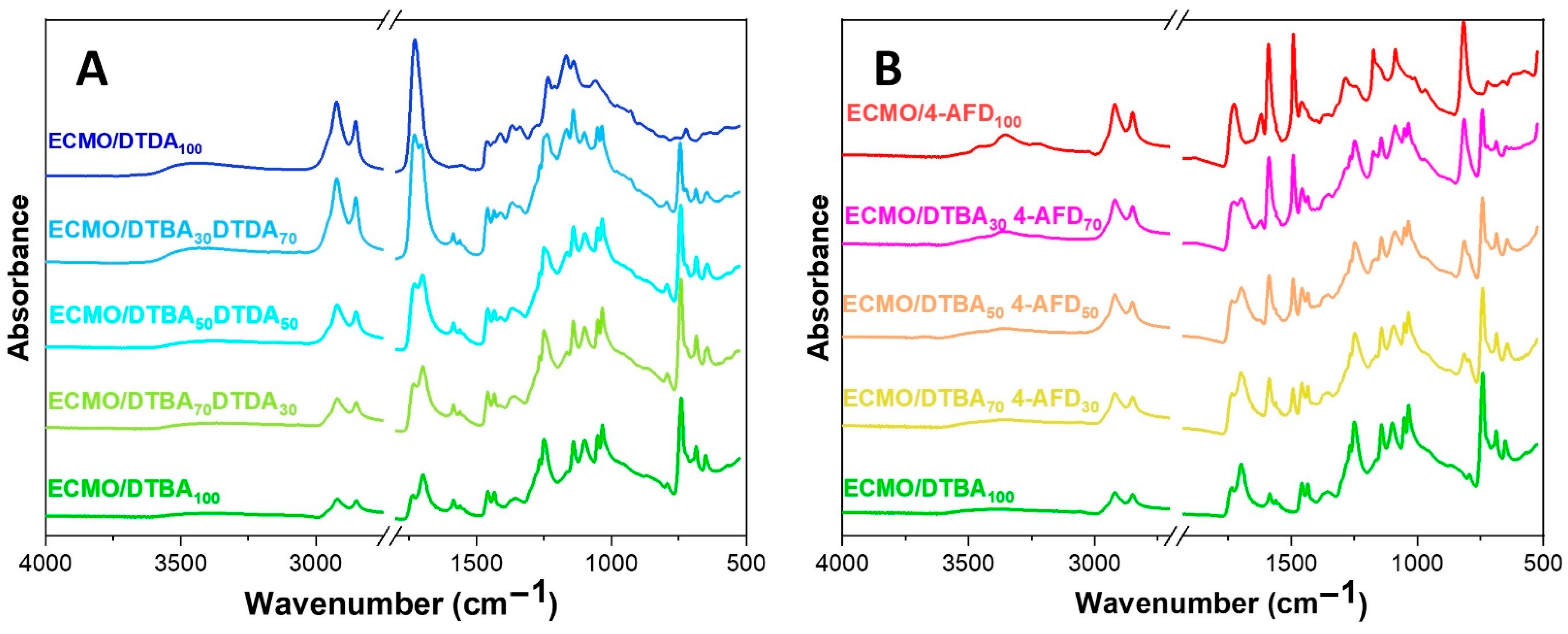
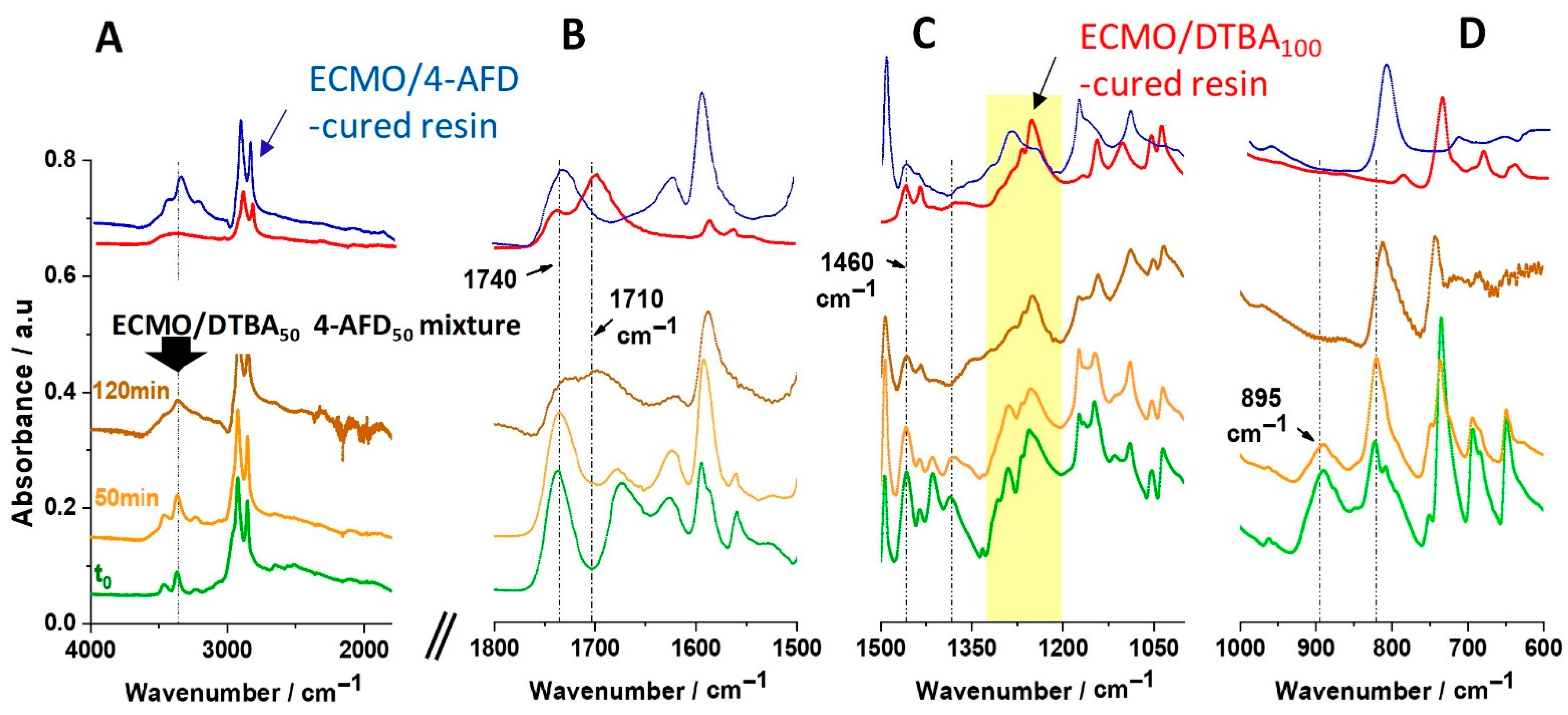
3.2. Structural Evolution by FT-IR Studies
3.3. Thermoset and Recycled Material Characterization
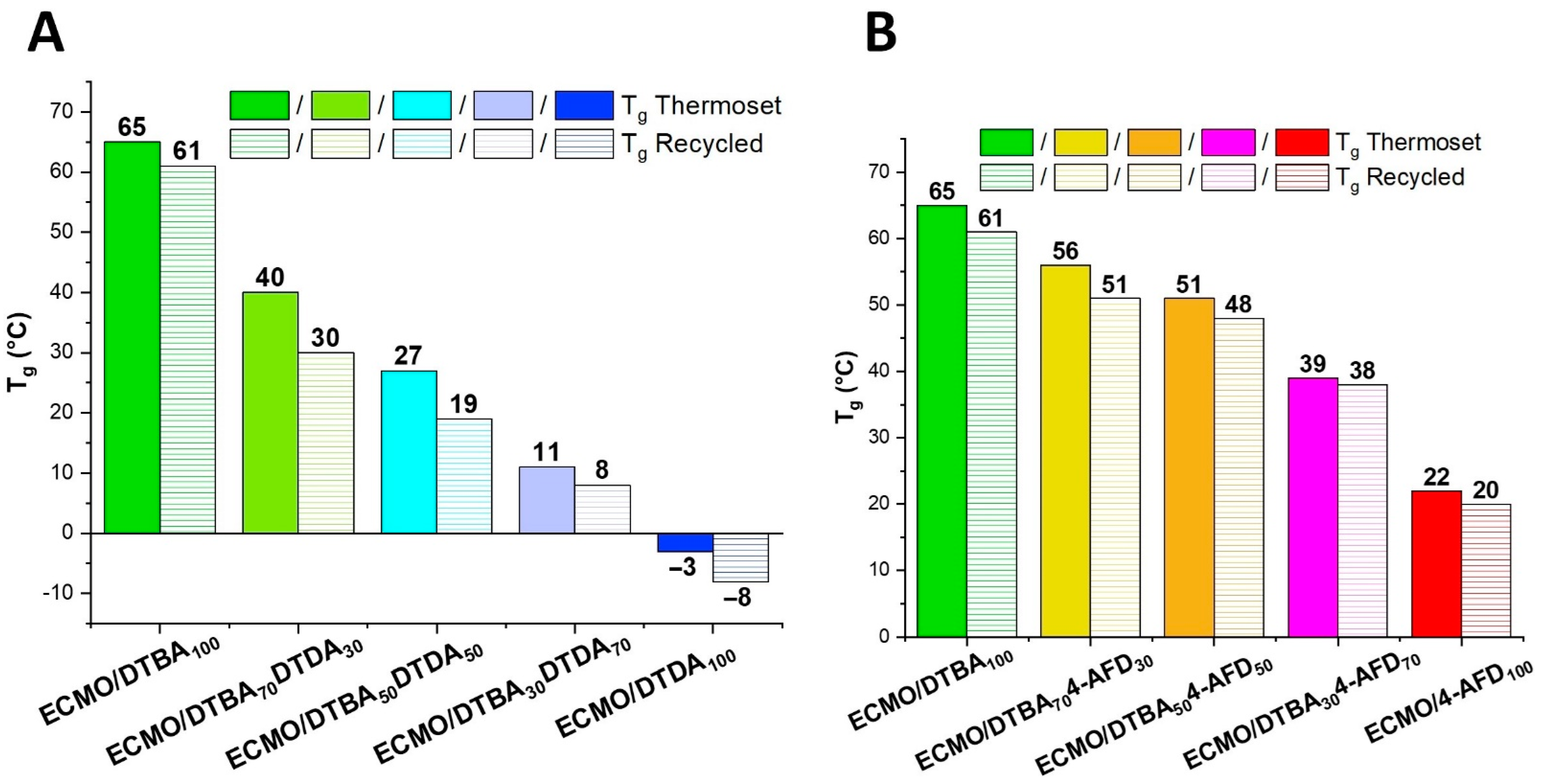
3.3.1. Glass Transition Evaluation by DSC and DMA Analyses
3.3.2. Materials’ Thermal Stabilities
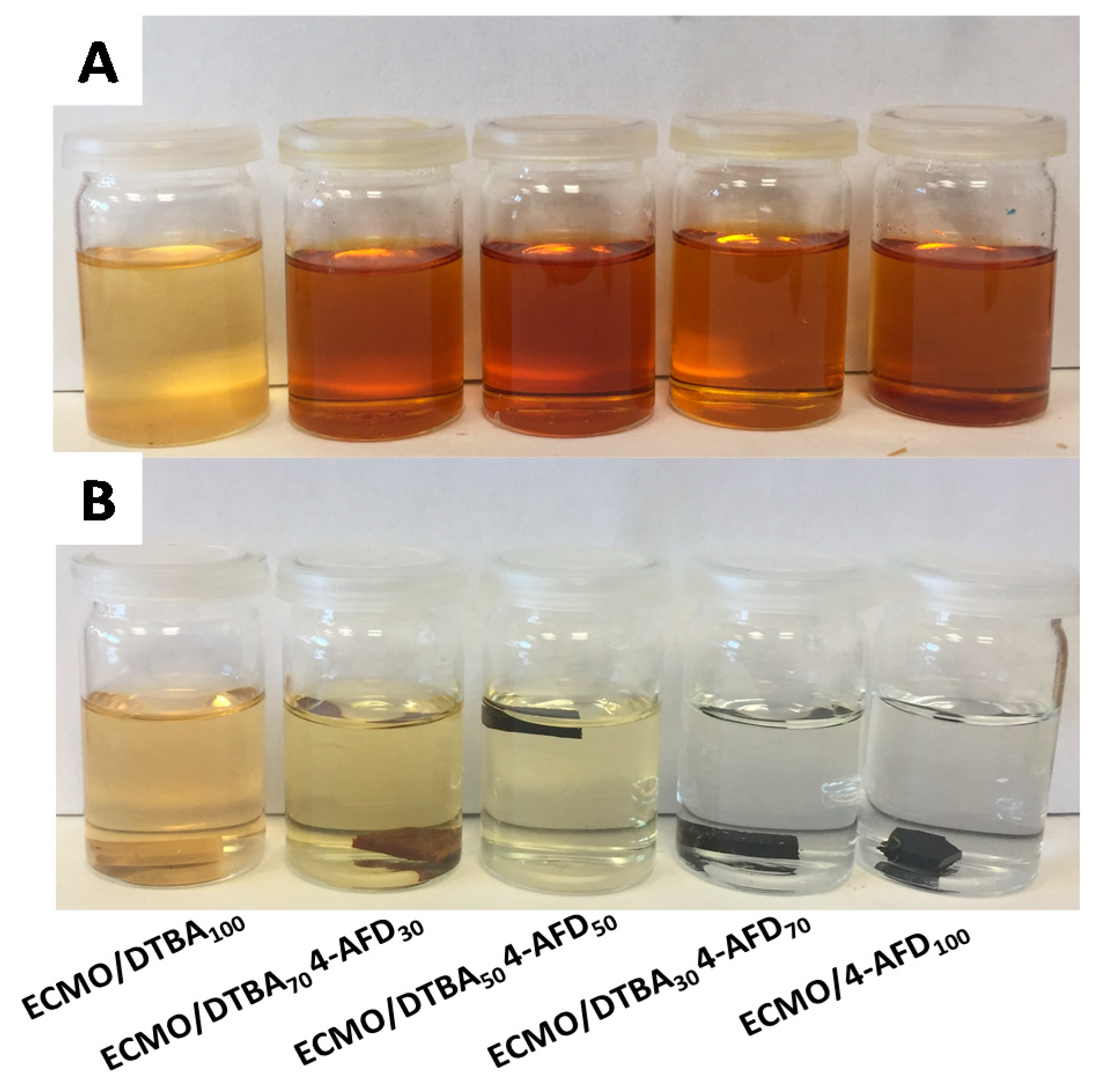
3.4. Solvent Resistance and Chemical Recyclability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Jin, Y.; Yu, C.; Denman, R.J.; Zhang, W. Recent advances in dynamic covalent chemistry. Chem. Soc. Rev. 2013, 42, 6634–6654. [Google Scholar] [CrossRef] [PubMed]
- Capelot, M.; Unterlass, M.M.; Tournilhac, F.; Leibler, L. Catalytic Control of the Vitrimer Glass Transition. ACS Macro Lett. 2012, 1, 789–792. [Google Scholar] [CrossRef]
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. Silica-like malleable materials from permanent organic networks. Science 2011, 334, 965. [Google Scholar] [CrossRef] [PubMed]
- Lehn, J.-M. Dynamic Combinatorial Chemistry and Virtual Combinatorial Libraries. Chem. Eur. J. 1999, 5, 2455–2463. [Google Scholar] [CrossRef]
- Rowan, S.J.; Cantrill, S.J.; Cousins, G.R.L.; Sanders, J.K.M.; Stoddart, J.F. Dynamic Covalent Chemistry. Angew. Chem. Int. Ed. 2002, 41, 898–952. [Google Scholar] [CrossRef]
- Elenguer, A.M.; Friščić, T.; Day, G.M.; Sanders, J.K.M. Solid-state dynamic combinatorial chemistry: Reversibility and thermodynamic product selection in covalent mechanosynthesis. Chem. Sci. 2011, 2, 696–700. [Google Scholar] [CrossRef]
- Lei, Z.Q.; Xiang, H.P.; Yuan, Y.J.; Rong, M.Z.; Zhang, M.Q. Room-Temperature Self-Healable and Remoldable Cross-linked Polymer Based on the Dynamic Exchange of Disulfide Bonds. Chem. Mater. 2014, 26, 2038–2046. [Google Scholar] [CrossRef]
- Rekondo, A.; Martin, R.; Ruiz de Luzuriaga, A.; Cabañero, G.; Grande, H.J.; Odriozola, I. Catalyst-free room-temperature self-healing elastomers based on aromatic disulfide metathesis. Mater. Horiz. 2014, 1, 237–240. [Google Scholar] [CrossRef]
- Chen, J.-H.; Hu, D.-D.; Li, Y.-D.; Meng, F.; Zhu, J.; Zeng, J.-B. Castor oil derived poly(urethane urea) networks with reprocessibility and enhanced mechanical properties. Polymer 2018, 143, 79–86. [Google Scholar] [CrossRef] [Green Version]
- Di Mauro, C.; Tran, T.-N.; Graillot, A.; Mija, A. Enhancing the Recyclability of a Vegetable Oil-Based Epoxy Thermoset through Initiator Influence. ACS Sustain. Chem. Eng. 2020, 8, 7690–7700. [Google Scholar] [CrossRef]
- Tran, T.-N.; Di Mauro, C.; Graillot, A.; Mija, A. Chemical Reactivity and the Influence of Initiators on the Epoxidized Vegetable Oil/Dicarboxylic Acid System. Macromolecules 2020, 53, 2526–2538. [Google Scholar] [CrossRef] [Green Version]
- Di Mauro, C.; Genua, A.; Mija, A. Building thermally and chemically reversible covalent bonds in vegetable oils based epoxy thermosets. Influence of epoxy-hardener ratio to promote recyclability. Mater. Adv. 2020, 1, 1788–1798. [Google Scholar] [CrossRef]
- Tran, T.-N.; Di Mauro, C.; Graillot, A.; Mija, A. Monitoring the structure–reactivity relationship in epoxidized perilla and safflower oil thermosetting resins. Polym. Chem. 2020, 11, 5088–5097. [Google Scholar] [CrossRef]
- Di Mauro, C.; Malburet, S.; Genua, A.; Graillot, A.; Mija, A. Sustainable series of new epoxidized vegetable oils-based thermosets with chemical recycling properties. Biomacromolecules 2020, 21, 3923–3935. [Google Scholar] [CrossRef] [PubMed]
- Fache, M.; Montérémal, C.; Boutevin, B.; Caillol, S. Amine hardeners and epoxy cross-linker from aromatic renewable resources. Eur. Polym. J. 2015, 73, 344–362. [Google Scholar] [CrossRef]
- Frias, C.F.; Serra, A.C.; Ramalho, A.; Coelho, J.F.J.; Fonseca, A.C. Preparation of fully biobased epoxy resins from soybean oil based amine hardeners. Ind. Crops Prod. 2017, 109, 434–444. [Google Scholar] [CrossRef]
- Stemmelen, M.; Lapinte, V.; Habas, J.-P.; Robin, J.-J. Plant oil-based epoxy resins from fatty diamines and epoxidized vegetable oil. Eur. Polym. J. 2015, 68, 536–545. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.-H.; Yuan, W.-Q.; Li, Y.-D.; Weng, Y.-X.; Zeng, J.-B. Malleable and Sustainable Poly(ester amide) Networks Synthesized via Melt Condensation Polymerization. ACS Sustain. Chem. Eng. 2019, 7, 15147–15153. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; He, J.; Li, Y.-D.; Zhao, X.-L.; Zeng, J.-B. Biobased, reprocessable and weldable epoxy vitrimers from epoxidized soybean oil. Ind. Crops Prod. 2020, 153, 112576. [Google Scholar] [CrossRef]
- Kumar, S.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Study of curing kinetics of anhydride cured petroleum-based (DGEBA) epoxy resin and renewable resource based epoxidized soybean oil (ESO) systems catalyzed by 2-methylimidazole. Thermoc. Acta 2017, 654, 112–120. [Google Scholar] [CrossRef]
- Karger-Kocsis, J.; Grishchuk, S.; Sorochynska, L.; Rong, M.Z. Curing, gelling, thermomechanical, and thermal decomposition behaviors of anhydride-cure.d epoxy (DGEBA)/epoxidized soybean oil compositions. Polym. Eng. Sci. 2014, 54, 747–755. [Google Scholar] [CrossRef] [Green Version]
- Altuna, F.I.; Espósito, L.H.; Ruseckaite, R.A.; Stefani, P.M. Thermal and mechanical properties of anhydride-cured epoxy resins with different contents of biobased epoxidized soybean oil. J. Appl. Polym. Sci. 2011, 120, 789–798. [Google Scholar] [CrossRef]
- Ding, C.; Tian, G.; Matharu, A. Adipic acid—Glutaric anhydride—Epoxidised linseed oil biobased thermosets with tunable properties. Mater. Today Commun. 2016, 7, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Altuna, F.I.; Casado, U.; dell’Erba, I.E.; Luna, L.; Hoppe, C.E.; Williams, R.J.J. Epoxy vitrimers incorporating physical crosslinks produced by self-association of alkyl chains. Polym. Chem. 2020, 11, 1337–1347. [Google Scholar] [CrossRef]
- Tran, T.-N.; Di Mauro, C.; Malburet, S.; Graillot, A.; Mija, A. Dual Cross-linking of Epoxidized Linseed Oil with Combined Aliphatic/Aromatic Diacids Containing Dynamic S–S Bonds Generating Recyclable Thermosets. ACS Appl. Bio Mater. 2020, 3, 7550–7561. [Google Scholar] [CrossRef]
- Matxain, J.M.; Asua, J.M.; Ruipérez, F. Design of new disulfide-based organic compounds for the improvement of self-healing materials. Phys. Chem. Chem. Phys. 2016, 18, 1758–1770. [Google Scholar] [CrossRef]
- Matějka, L.; Pokorný, S.; Dušek, K. Acid curing of epoxy resins. A comparison between the polymerization of diepoxide-diacid and monoepoxide-cyclic anhydride systems. Makromol. Chem. Macromol. Chem. Phys. 1985, 186, 2025–2036. [Google Scholar] [CrossRef]
- Kaufman, H.S. Handbook of epoxy resins. Henry Lee and Kris Neville. J. Appl. Polym. Sci. 1970, 14, 253. [Google Scholar] [CrossRef]
- Bershtein, V.A.; Egorov, V.M.; Egorova, L.M.; Ryzhov, V.A. The role of thermal analysis in revealing the common molecular nature of transitions in polymers. Thermochim. Acta 1994, 238, 41–73. [Google Scholar] [CrossRef]
- Zhou, F.; Guo, Z.; Wang, W.; Lei, X.; Zhang, B.; Zhang, H.; Zhang, Q. Preparation of self-healing, recyclable epoxy resins and low-electrical resistance composites based on double-disulfide bond exchange. Compos. Sci. Technol. 2018, 167, 79–85. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, Y.; Zhu, J.; Yu, J.; Hu, Z. Bio-based epoxy vitrimers: Reprocessibility, controllable shape memory, and degradability. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1790–1799. [Google Scholar] [CrossRef]
- Ruiz de Luzuriaga, A.; Martin, R.; Markaide, N.; Rekondo, A.; Cabañero, G.; Rodríguez, J.; Odriozola, I. Epoxy resin with exchangeable disulfide crosslinks to obtain reprocessable, repairable and recyclable fiber-reinforced thermoset composites. Mater. Horiz. 2016, 3, 241–247. [Google Scholar] [CrossRef]
- Imbernon, L.; Oikonomou, E.K.; Norvez, S.; Leibler, L. Chemically crosslinked yet reprocessable epoxidized natural rubber via thermo-activated disulfide rearrangements. Polym. Chem. 2015, 6, 4271–4278. [Google Scholar] [CrossRef]
- Zhou, L.; Chen, M.; Zhao, X. Rapid degradation of disulfide-based thermosets through thiol-disulfide exchange reaction. Polymer 2017, 120, 1–8. [Google Scholar] [CrossRef]



| Thermosets | Tpeak 1 (°C) | Reaction Interval 1 | ΔH1 (J·g−1) | Tpeak 2 (°C) | Reaction Interval 2 | ΔH2 (J·g−1) |
|---|---|---|---|---|---|---|
| ECMO/DTBA100 | 171 | 137–187 | 155 | / | / | / |
| ECMO/DTBA70 4-AFD30 | 145 | 125–175 | 155 ± 1 | 212 | 202–217 | 28 ± 1 |
| ECMO/DTBA50 4-AFD50 | 143 | 118–166 | 151 ± 1 | 211 | 197–221 | 14 ± 1 |
| ECMO/DTBA30 4-AFD70 | 141 | 115–160 | 106 ± 1 | 220 | 214–235 | 50 ± 1 |
| ECMO/4-AFD100 | 248 | 155–/ | / | / | / | / |
| Thermosets | Tan δ, Virgin/Recycled (°C) | Tan δmax, Virgin/Recycled | E’glassy plateau, Virgin/Recycled (MPa) | E’rubbery plateau, Virgin/Recycled (MPa) | Crosslink Density, Virgin/Recycled (mmol/cm3) |
|---|---|---|---|---|---|
| ECMO/DTBA100 | 85/75 ± 1 | 0.9/1.2 | 400/2290 | 2.78/0.83 | 0.71/0.24 |
| ECMO/DTBA70DTDA30 | 50/50 ± 1 | 0.9/1.0 | 1000/1820 | 2.47/3.32 | 0.25/0.34 |
| ECMO/DTBA50DTDA50 | 39/35 ± 1 | 1.0/1.1 | 1100/2030 | 2.60/3.10 | 0.27/0.38 |
| ECMO/DTBA30DTDA70 | 22/22 ± 1 | 1.0/1.1 | 1500/1970 | 2.56/4.57 | 0.27/0.53 |
| ECMO/DTDA100 | 3/2 ± 1 | 1.2/1.3 | 2280/2000 | 2.82/2.87 | 0.35/0.33 |
| ECMO/DTBA70 4-AFD30 | 82/83 ± 1 | 0.79/1.1 | 400/900 | 1.70/0.87 | 0.48/0.18 |
| ECMO/DTBA50 4-AFD50 | 74/81 ± 1 | 1.3/1.53 | 800/720 | 0.74/0.66 | 0.35/0.28 |
| ECMO/DTBA30 4-AFD70 | 65/70 ± 1 | 1.2/1.07 | 400/1460 | 0.91/1.35 | 0.23/0.25 |
| ECMO/4-AFD100 | 36/41 ± 1 | 0.86/1.1 | 1110/1780 | 0.53/0.51 | 0.21/0.17 |
| Thermosets | T5% Virgin/Recycled (°C) | Thermosets | T5% Virgin/Recycled (°C) |
|---|---|---|---|
| ECMO/DTBA100 | 270/265 ± 1 | ||
| ECMO/DTBA70DTDA30 | 255/245 ± 1 | ECMO/DTBA70 4-AFD30 | 260/255 ± 1 |
| ECMO/DTBA50DTDA50 | 250/240 ± 1 | ECMO/DTBA50 4-AFD50 | 260/260 ± 1 |
| ECMO/DTBA30DTDA70 | 250/245 ± 1 | ECMO/DTBA30 4-AFD70 | 265/260 ± 1 |
| ECMO/DTDA100 | 245/225 ± 1 | ECMO/4-AFD100 | 260/260 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Mauro, C.; Genua, A.; Mija, A. Kinetical Study, Thermo-Mechanical Characteristics and Recyclability of Epoxidized Camelina Oil Cured with Antagonist Structure (Aliphatic/Aromatic) or Functionality (Acid/Amine) Hardeners. Polymers 2021, 13, 2503. https://doi.org/10.3390/polym13152503
Di Mauro C, Genua A, Mija A. Kinetical Study, Thermo-Mechanical Characteristics and Recyclability of Epoxidized Camelina Oil Cured with Antagonist Structure (Aliphatic/Aromatic) or Functionality (Acid/Amine) Hardeners. Polymers. 2021; 13(15):2503. https://doi.org/10.3390/polym13152503
Chicago/Turabian StyleDi Mauro, Chiara, Aratz Genua, and Alice Mija. 2021. "Kinetical Study, Thermo-Mechanical Characteristics and Recyclability of Epoxidized Camelina Oil Cured with Antagonist Structure (Aliphatic/Aromatic) or Functionality (Acid/Amine) Hardeners" Polymers 13, no. 15: 2503. https://doi.org/10.3390/polym13152503
APA StyleDi Mauro, C., Genua, A., & Mija, A. (2021). Kinetical Study, Thermo-Mechanical Characteristics and Recyclability of Epoxidized Camelina Oil Cured with Antagonist Structure (Aliphatic/Aromatic) or Functionality (Acid/Amine) Hardeners. Polymers, 13(15), 2503. https://doi.org/10.3390/polym13152503









