A Bacteria and Cell Repellent Zwitterionic Polymer Coating on Titanium Base Substrates towards Smart Implant Devices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Substances, Culture Vessels, and Explant Material
2.2. Coating Procedure
2.3. Structural and Morphological Characterization
2.4. Nanomechanical Characterization of the Adhesion Strength
2.5. Interaction of Substrates with Cell and Bacteria Cultures
2.5.1. Cytocompatibility
2.5.2. Plaque Culture
2.6. Statistical Analysis
3. Results
3.1. Structural and Morphological Characterization
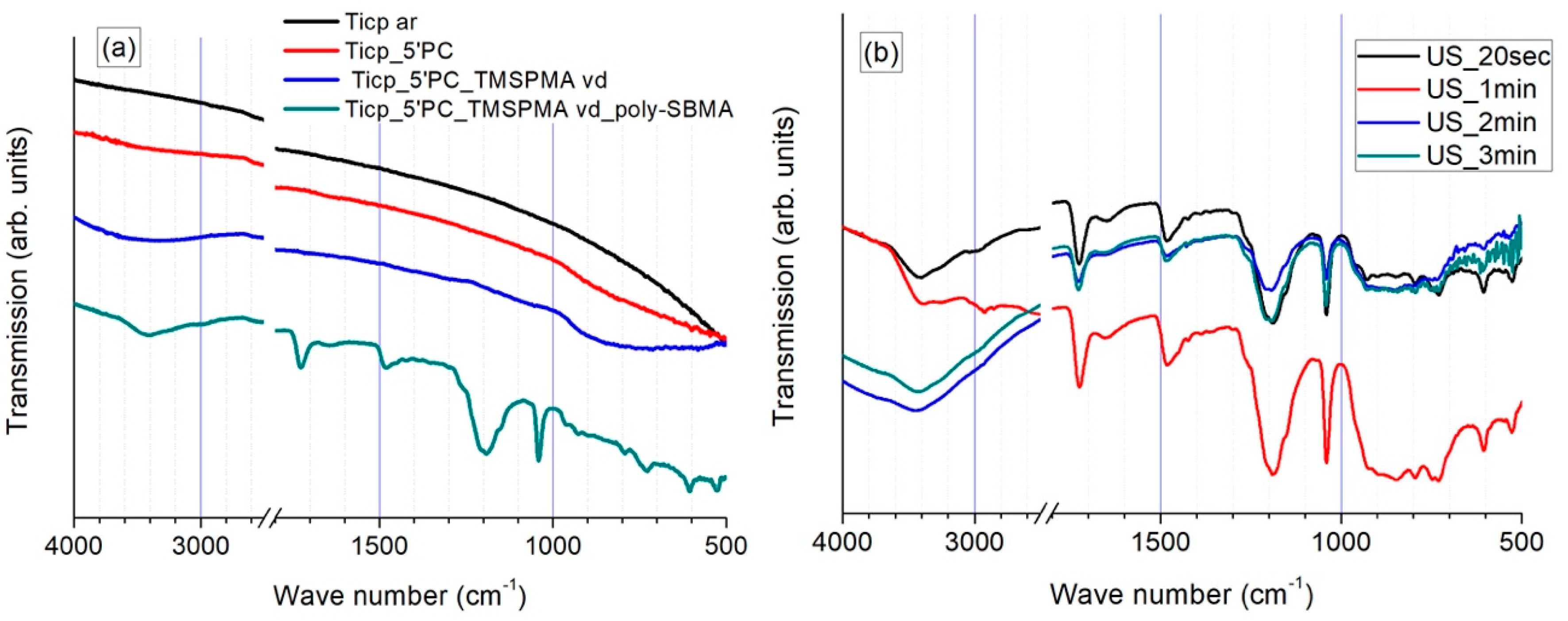
3.1.1. FTIR Investigations
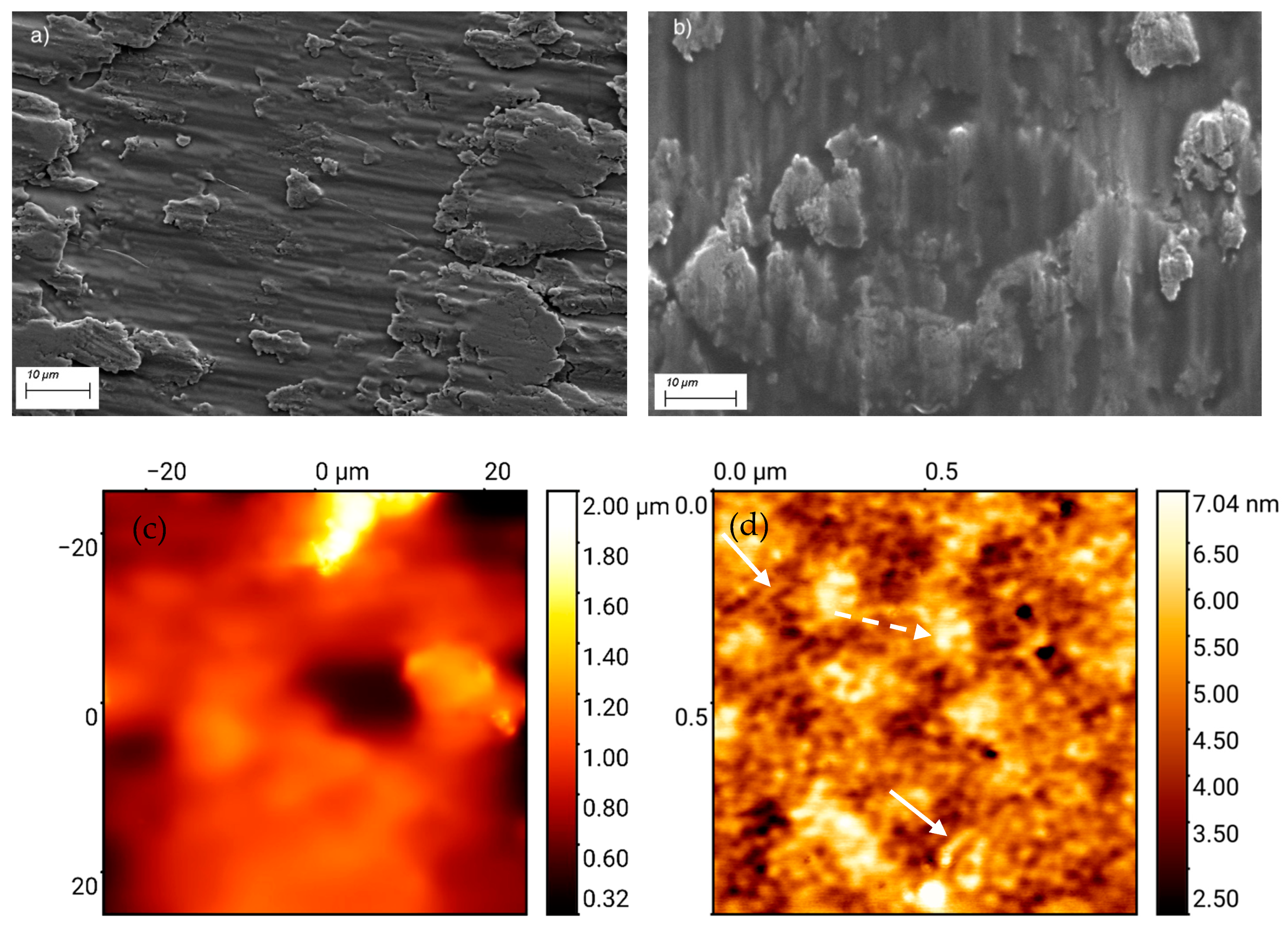
3.1.2. Topography and Microstructure
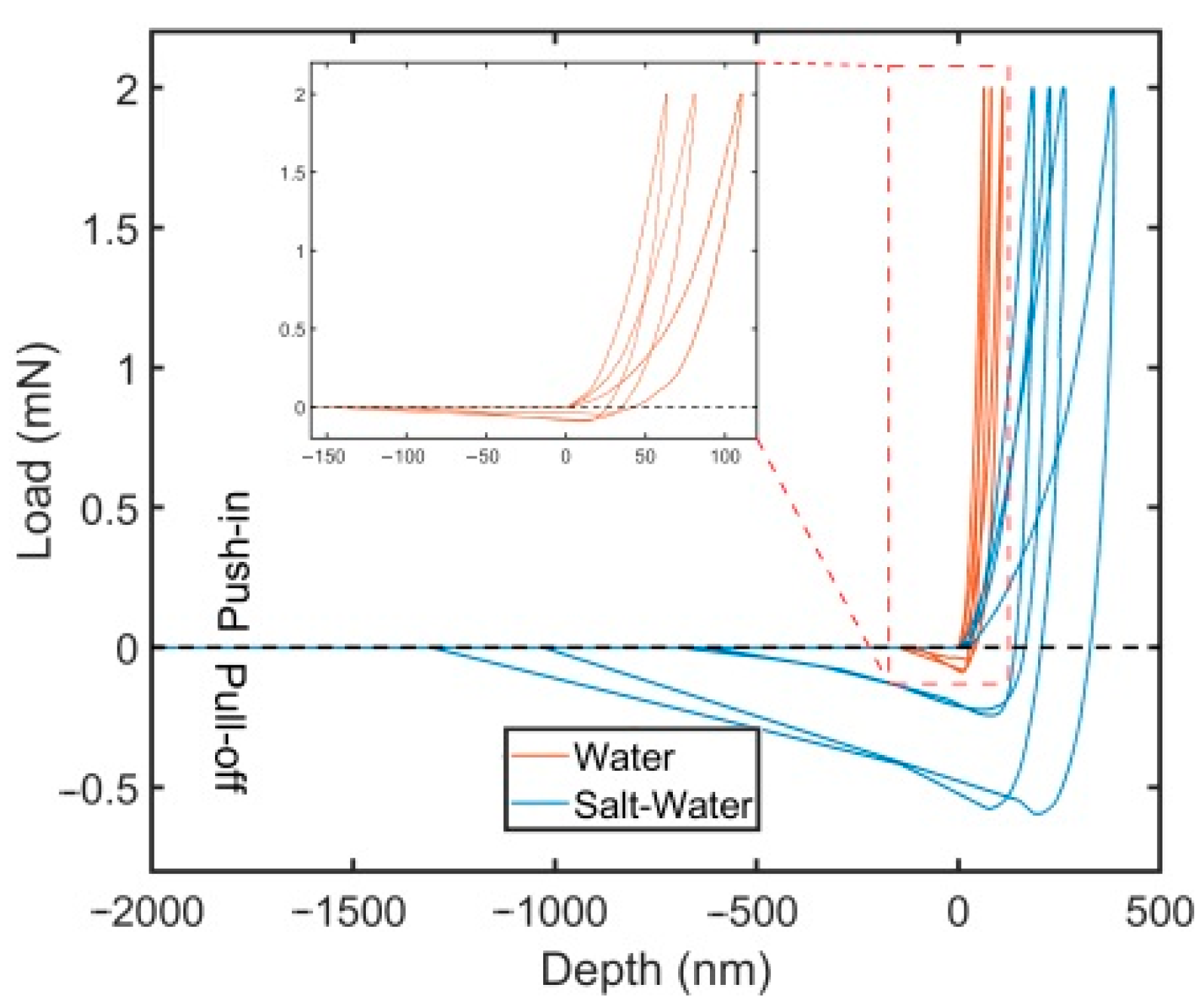
3.2. Nanomechanical Adhesion Strength
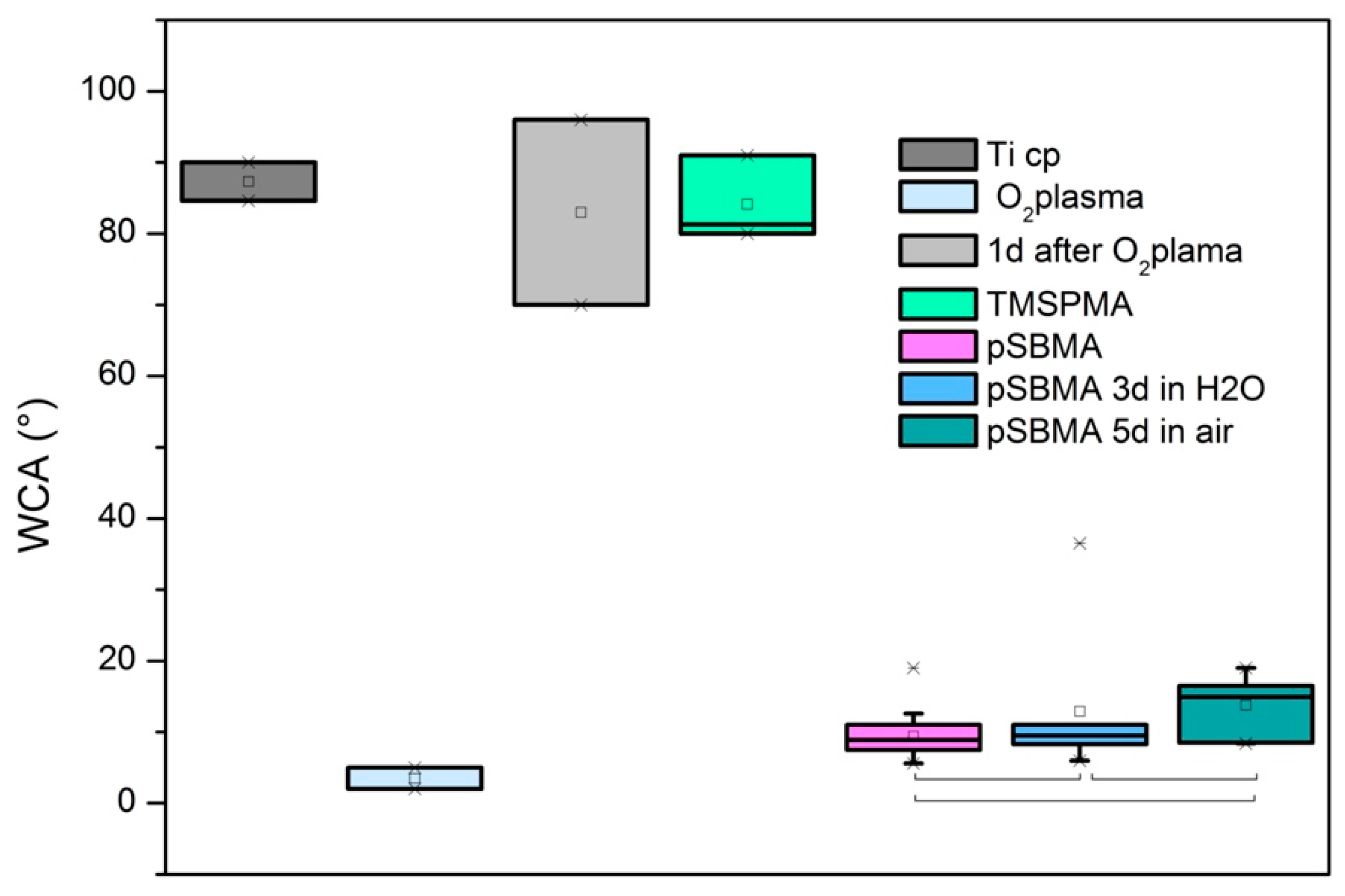
3.3. Wetting Properties
3.4. Cellular Interaction with Poly SBMA Coated TiCP Samples
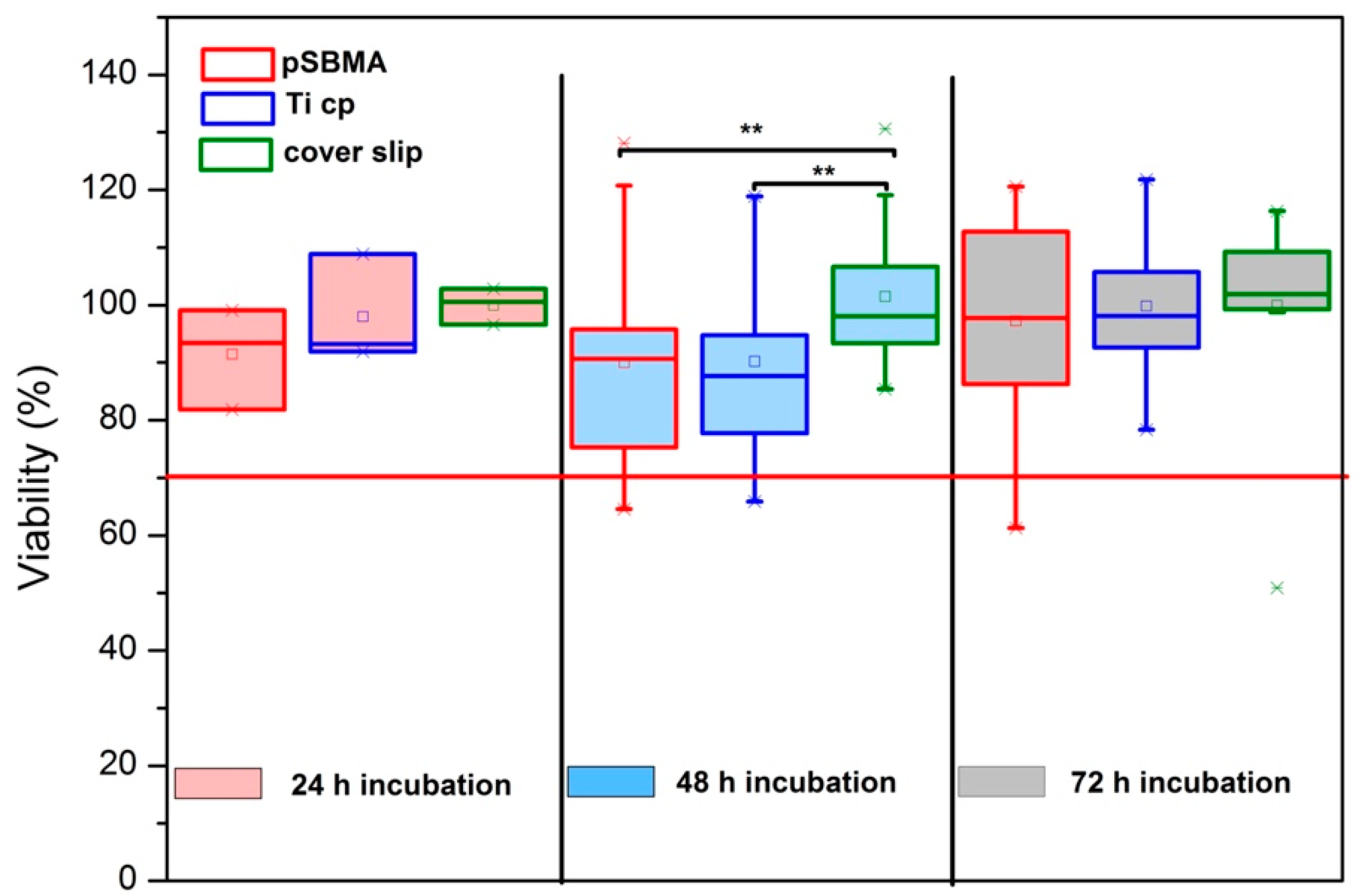
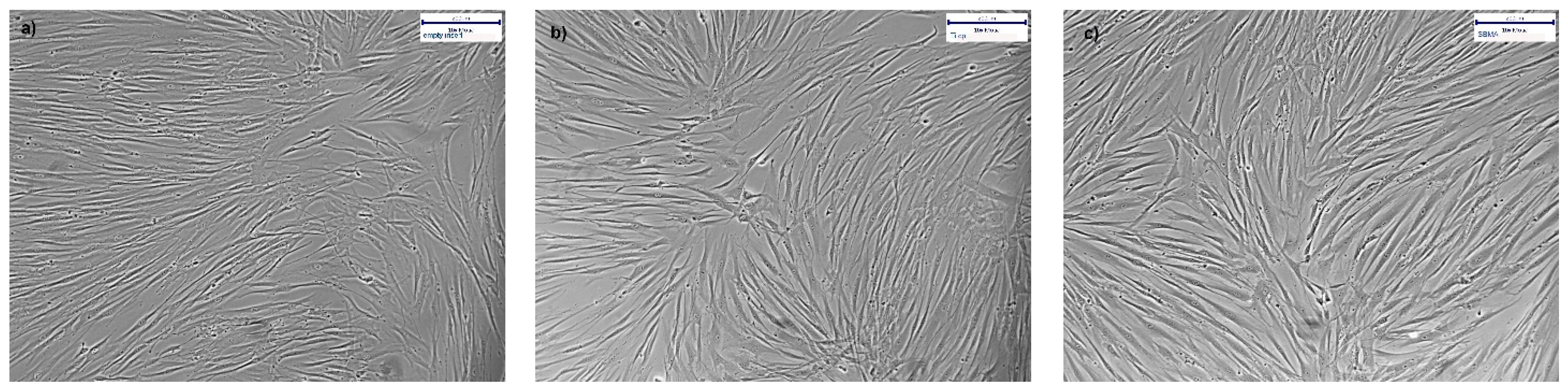
3.4.1. XTT Viability Testing and Microscopy
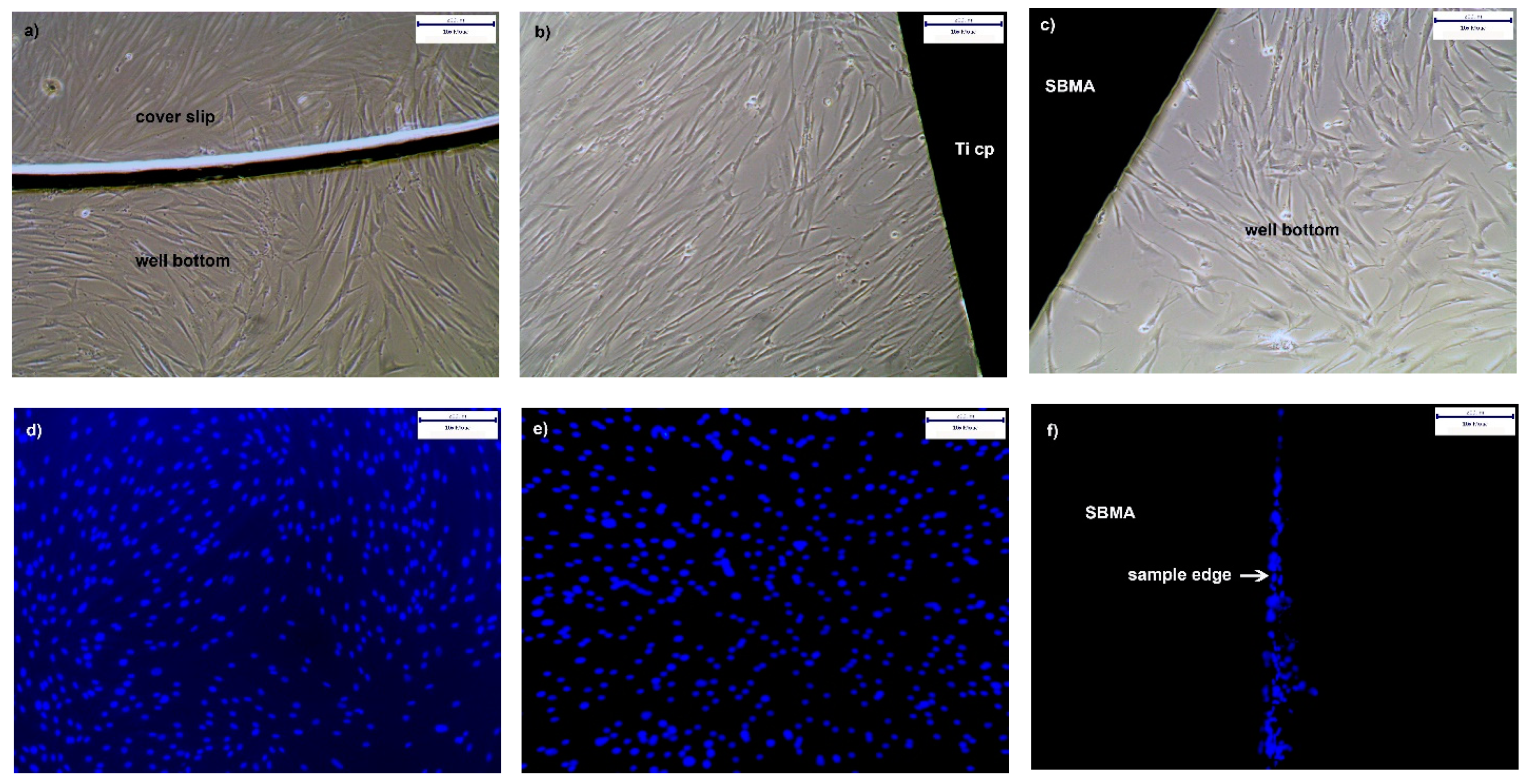
3.4.2. Proliferation of hgF on PolySBMA Coated TiCP
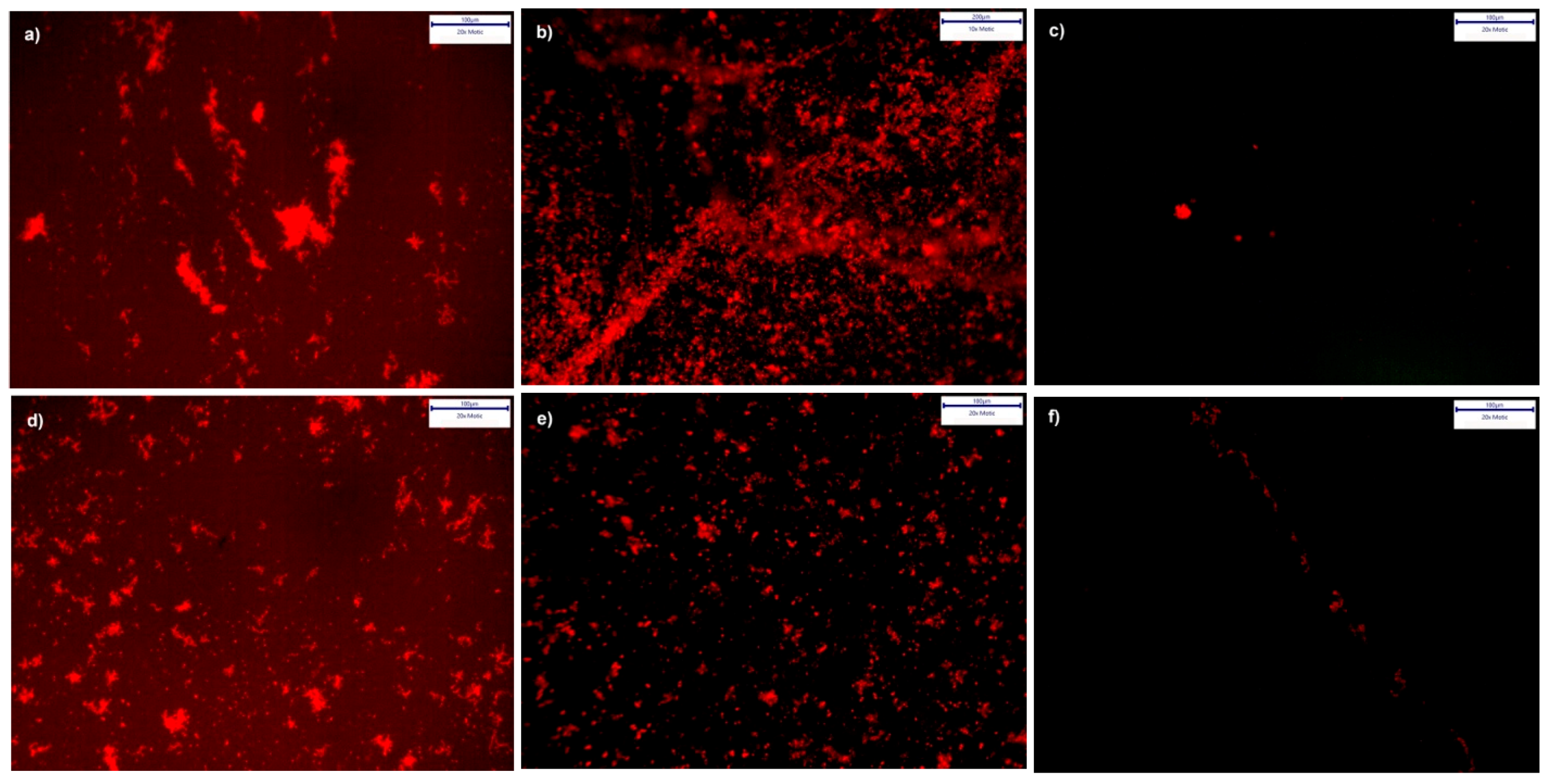
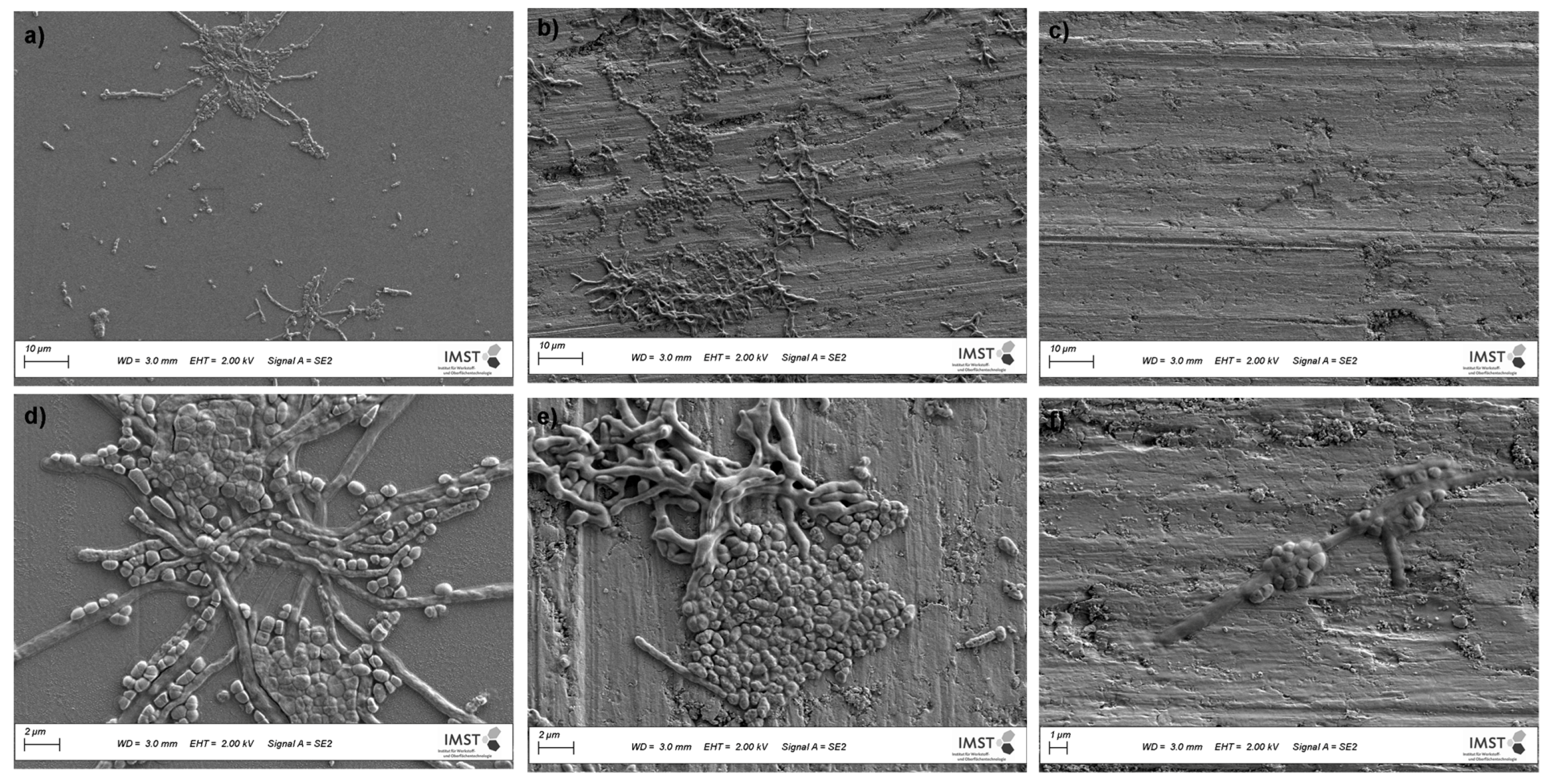
3.4.3. Cultures from Plaque Samples
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Higaki, Y.; Kobayashi, M.; Murakami, D.; Takahara, A. Anti-fouling behavior of polymer brush immobilized surfaces. Polym. J. 2016, 48, 325–331. [Google Scholar] [CrossRef]
- Ngo, B.K.D.; Grunlan, M.A. Protein Resistant Polymeric Biomaterials. ACS Macro Lett. 2017, 6, 992–1000. [Google Scholar] [CrossRef] [Green Version]
- Schlenoff, J.B. Zwitteration: Coating surfaces with zwitterionic functionality to reduce nonspecific adsorption. Langmuir 2014, 30, 9625–9636. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, S.; Chang, Y.; Jiang, S. Surface grafted sulfobetaine polymers via atom transfer radical polymerization as superlow fouling coatings. J. Phys. Chem. B 2006, 110, 10799–10804. [Google Scholar] [CrossRef] [PubMed]
- Mi, L.; Jiang, S. Integrated antimicrobial and nonfouling zwitterionic polymers. Angew. Chem. Int. Ed. Engl. 2014, 53, 1746–1754. [Google Scholar] [CrossRef]
- Zhou, T.; Zhu, Y.; Li, X.; Liu, X.; Yeung, K.W.K.; Wu, S.; Wang, X.; Cui, Z.; Yang, X.; Chu, P.K. Surface functionalization of biomaterials by radical polymerization. Prog. Mater. Sci. 2016, 83, 191–235. [Google Scholar] [CrossRef]
- Estephan, Z.G.; Schlenoff, P.S.; Schlenoff, J.B. Zwitteration as an alternative to PEGylation. Langmuir 2011, 27, 6794–6800. [Google Scholar] [CrossRef] [PubMed]
- Lowe, A.B.; McCormick, C.L. Synthesis and solution properties of zwitterionic polymers. Chem. Rev. 2002, 102, 4177–4189. [Google Scholar] [CrossRef]
- Chen, S.; Li, L.; Zhao, C.; Zheng, J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer 2010, 51, 5283–5293. [Google Scholar] [CrossRef] [Green Version]
- Blake, G.B.; MacFarlane, M.R.; Hinton, J.W. Titanium in reconstructive surgery of the skull and face. Br. J. Plast. Surg. 1990, 43, 528–535. [Google Scholar] [CrossRef]
- Mizrahi, E.; Mizrahi, B. Mini-screw implants (temporary anchorage devices): Orthodontic and pre-prosthetic applications. J. Orthod. 2007, 34, 80–94. [Google Scholar] [CrossRef] [PubMed]
- Gosavi, S.; Gosavi, S.; Alla, R. Titanium: A Miracle Metal in Dentistry. Trends Biomater. Artif. Organs 2013, 27, 42–46. [Google Scholar]
- Bachoura, A.; Yoshida, R.; Lattermann, C.; Kamineni, S. Late removal of titanium hardware from the elbow is problematic. ISRN Orthop. 2012, 2012, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koldsland, O.C.; Aass, A.M. Supportive treatment following peri-implantitis surgery: An RCT using titanium curettes or chitosan brushes. J. Clin. Periodontol. 2020, 47, 1259–1267. [Google Scholar] [CrossRef]
- Yang, W.; Xue, H.; Li, W.; Zhang, J.; Jiang, S. Pursuing “zero” protein adsorption of poly(carboxybetaine) from undiluted blood serum and plasma. Langmuir 2009, 25, 11911–11916. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, L.; Lin, W.; Wang, Z.; Zhang, J.; Ji, F.; Ma, G.; Yuan, Z.; Chen, S. Development of Robust and Recoverable Ultralow-Fouling Coatings Based on Poly(carboxybetaine) Ester Analogue. ACS Appl. Mater. Interfaces 2015, 7, 16938–16945. [Google Scholar] [CrossRef]
- Yeon, D.K.; Ko, S.; Jeong, S.; Hong, S.-P.; Kang, S.M.; Cho, W.K. Oxidation-Mediated, Zwitterionic Polydopamine Coatings for Marine Antifouling Applications. Langmuir 2019, 35, 1227–1234. [Google Scholar] [CrossRef]
- Yao, L.; He, C.; Chen, S.; Zhao, W.; Xie, Y.; Sun, S.; Nie, S.; Zhao, C. Codeposition of Polydopamine and Zwitterionic Polymer on Membrane Surface with Enhanced Stability and Antibiofouling Property. Langmuir 2019, 35, 1430–1439. [Google Scholar] [CrossRef]
- Es-Souni, M.; Wassel, E.; Dietze, M.; Laghrissi, A.; Klöhn, F.; Weyrich, T.; Es-Souni, M. Processing of nanotubes on NiTi-shape memory alloys and their modification with photografted anti-adhesive polymer brushes. Towards smart implant surfaces. Mater. Des. 2019, 182, 108031. [Google Scholar] [CrossRef]
- Bakhti, H.; Laghrissi, A.; Roth, A.; Azrar, L.; Es-Souni, M. Nanomechanical characterization and modeling of anodized porous aluminum oxide thin films with photografted anti-biofouling polymer brushes on their pore walls. Appl. Nanosci. 2020, 10, 2139–2151. [Google Scholar] [CrossRef] [Green Version]
- Es-Souni, M.; Fischer-Brandies, H.; Es-Souni, M. Human gingival fibroblast response to electropolished NiTi surfaces. J. Biomed. Mater. Res. A 2007, 80, 159–166. [Google Scholar] [CrossRef]
- Jhan, Y.-Y.; Tsay, R.-Y. Salt effects on the hydration behavior of zwitterionic poly(sulfobetaine methacrylate) aqueous solutions. J. Taiwan Inst. Chem. Eng. 2014, 45, 3139–3145. [Google Scholar] [CrossRef]
- Sundaram, H.S.; Han, X.; Nowinski, A.K.; Ella-Menye, J.-R.; Wimbish, C.; Marek, P.; Senecal, K.; Jiang, S. One-step dip coating of zwitterionic sulfobetaine polymers on hydrophobic and hydrophilic surfaces. ACS Appl. Mater. Interfaces 2014, 6, 6664–6671. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Quintana, R.; Cirelli, M.; Toa, Z.S.D.; Arjunan Vasantha, V.; Kooij, E.S.; Jańczewski, D.; Vancso, G.J. Brush Swelling and Attachment Strength of Barnacle Adhesion Protein on Zwitterionic Polymer Films as a Function of Macromolecular Structure. Langmuir 2019, 35, 8085–8094. [Google Scholar] [CrossRef] [PubMed]
- Dušek, K.; Choukourov, A.; Dušková-Smrčková, M.; Biederman, H. Constrained Swelling of Polymer Networks: Characterization of Vapor-Deposited Cross-Linked Polymer Thin Films. Macromolecules 2014, 47, 4417–4427. [Google Scholar] [CrossRef]
- Zhang, Q.; Archer, L.A. Interfacial friction and adhesion of cross-linked polymer thin films swollen with linear chains. Langmuir 2007, 23, 7562–7570. [Google Scholar] [CrossRef]
- Yang, Y.; Xiang, Y.; Xu, M. From red to green:The propidium iodide-permeable membrane of Shewanella decolorationis S12 is repairable. Sci. Rep. 2015, 5, 18583. [Google Scholar] [CrossRef] [Green Version]
- Auschill, T.M.; Arweiler, N.B.; Netuschil, L.; Brecx, M.; Reich, E.; Sculean, A.; Artweiler, N.B. Spatial distribution of vital and dead microorganisms in dental biofilms. Arch. Oral Biol. 2001, 46, 471–476. [Google Scholar] [CrossRef]
- Dang, H.; Lovell, C.R. Microbial Surface Colonization and Biofilm Development in Marine Environments. Microbiol. Mol. Biol. Rev. 2016, 80, 91–138. [Google Scholar] [CrossRef] [Green Version]
- Van Wolferen, M.; Orell, A.; Albers, S.-V. Archaeal biofilm formation. Nat. Rev. Microbiol. 2018, 16, 699–713. [Google Scholar] [CrossRef]
- Marsh, P.D. In Sickness and in Health-What Does the Oral Microbiome Mean to Us? An Ecological Perspective. Adv. Dent. Res. 2018, 29, 60–65. [Google Scholar] [CrossRef]
- Marsh, P.D.; Zaura, E. Dental biofilm: Ecological interactions in health and disease. J. Clin. Periodontol. 2017, 44 (Suppl. 18), S12–S22. [Google Scholar] [CrossRef]
- Heitz-Mayfield, L.J.A.; Salvi, G.E. Peri-implant mucositis. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S237–S245. [Google Scholar] [CrossRef] [Green Version]
- Groessner-Schreiber, B.; Hannig, M.; Dück, A.; Griepentrog, M.; Wenderoth, D.F. Do different implant surfaces exposed in the oral cavity of humans show different biofilm compositions and activities? Eur. J. Oral Sci. 2004, 112, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Grössner-Schreiber, B.; Teichmann, J.; Hannig, M.; Dörfer, C.; Wenderoth, D.F.; Ott, S.J. Modified implant surfaces show different biofilm compositions under in vivo conditions. Clin. Oral Implants Res. 2009, 20, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Mabboux, F.; Ponsonnet, L.; Morrier, J.-J.; Jaffrezic, N.; Barsotti, O. Surface free energy and bacterial retention to saliva-coated dental implant materials--an in vitro study. Colloids Surf. B Biointerfaces 2004, 39, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Barkarmo, S.; Longhorn, D.; Leer, K.; Johansson, C.B.; Stenport, V.; Franco-Tabares, S.; Kuehne, S.A.; Sammons, R. Biofilm formation on polyetheretherketone and titanium surfaces. Clin. Exp. Dent. Res. 2019, 5, 427–437. [Google Scholar] [CrossRef]
- Desch, A.; Freifrau von Maltzahn, N.; Stumpp, N.; Dalton, M.; Yang, I.; Stiesch, M. Biofilm formation on zirconia and titanium over time-An in vivo model study. Clin. Oral Implants Res. 2020, 31, 865–880. [Google Scholar] [CrossRef]
- Bixler, G.D.; Bhushan, B. Biofouling: Lessons from nature. Philos. Trans. A Math. Phys. Eng. Sci. 2012, 370, 2381–2417. [Google Scholar] [CrossRef]
- Sotiri, I.; Overton, J.C.; Waterhouse, A.; Howell, C. Immobilized liquid layers: A new approach to anti-adhesion surfaces for medical applications. Exp. Biol. Med. 2016, 241, 909–918. [Google Scholar] [CrossRef]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling coatings: Recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv. Mater. 2011, 23, 690–718. [Google Scholar] [CrossRef]
- Damodaran, V.B.; Murthy, N.S. Bio-inspired strategies for designing antifouling biomaterials. Biomater. Res. 2016, 20, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunrath, M.F.; Diz, F.M.; Magini, R.; Galárraga-Vinueza, M.E. Nanointeraction: The profound influence of nanostructured and nano-drug delivery biomedical implant surfaces on cell behavior. Adv. Colloid Interface Sci. 2020, 284, 102265. [Google Scholar] [CrossRef]
- Nam, G.; Purushothaman, B.; Rangasamy, S.; Song, J.M. Investigating the versatility of multifunctional silver nanoparticles: Preparation and inspection of their potential as wound treatment agents. Int. Nano Lett. 2016, 6, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Stobie, N.; Duffy, B.; Hinder, S.J.; McHale, P.; McCormack, D.E. Silver doped perfluoropolyether-urethane coatings: Antibacterial activity and surface analysis. Colloids Surf. B Biointerfaces 2009, 72, 62–67. [Google Scholar] [CrossRef] [Green Version]
- Es-Souni, M.; Fischer-Brandies, H.; Es-Souni, M. Versatile Nanocomposite Coatings with Tunable Cell Adhesion and Bactericidity. Adv. Funct. Mater. 2008, 18, 3179–3188. [Google Scholar] [CrossRef]
- Hickok, N.J.; Shapiro, I.M.; Chen, A.F. The Impact of Incorporating Antimicrobials into Implant Surfaces. J. Dent. Res. 2018, 97, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Pant, J.; Goudie, M.J.; Chaji, S.M.; Johnson, B.W.; Handa, H. Nitric oxide releasing vascular catheters for eradicating bacterial infection. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 2849–2857. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yang, Y.; Zhang, L.; Xiong, K.; Li, X.; Zhang, F.; Wang, J.; Zhao, X.; Huang, N. Mussel-inspired catalytic selenocystamine-dopamine coatings for long-term generation of therapeutic gas on cardiovascular stents. Biomaterials 2018, 178, 1–10. [Google Scholar] [CrossRef]
- Peng, L.; Chang, L.; Si, M.; Lin, J.; Wei, Y.; Wang, S.; Liu, H.; Han, B.; Jiang, L. Hydrogel-Coated Dental Device with Adhesion-Inhibiting and Colony-Suppressing Properties. ACS Appl. Mater. Interfaces 2020, 12, 9718–9725. [Google Scholar] [CrossRef] [PubMed]
- Wassel, E.; Es-Souni, M.; Dietze, M.; Laghrissi, A.; Es-Souni, M. A non-fouling multilayer structure based on LAPONITE®/PEG-Brushes showing high stiffness and hardness. Prog. Org. Coat. 2019, 132, 108–115. [Google Scholar] [CrossRef]
- Wassel, E.; Es-Souni, M.; Laghrissi, A.; Roth, A.; Dietze, M.; Es-Souni, M. Scratch resistant non-fouling surfaces via grafting non-fouling polymers on the pore walls of supported porous oxide structures. Mater. Des. 2019, 163, 107542. [Google Scholar] [CrossRef]
- Zaugg, L.K.; Astasov-Frauenhoffer, M.; Braissant, O.; Hauser-Gerspach, I.; Waltimo, T.; Zitzmann, N.U. Determinants of biofilm formation and cleanability of titanium surfaces. Clin. Oral Implants Res. 2017, 28, 469–475. [Google Scholar] [CrossRef]
- Bermejo, P.; Sánchez, M.C.; Llama-Palacios, A.; Figuero, E.; Herrera, D.; Sanz Alonso, M. Biofilm formation on dental implants with different surface micro-topography: An in vitro study. Clin. Oral Implants Res. 2019, 30, 725–734. [Google Scholar] [CrossRef]
- Rosenhahn, A.; Schilp, S.; Kreuzer, H.J.; Grunze, M. The role of “inert” surface chemistry in marine biofouling prevention. Phys. Chem. Chem. Phys. 2010, 12, 4275–4286. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, D.; Ren, B.; Gong, X.; Xu, L.; Feng, Z.-Q.; Chang, Y.; He, Y.; Zheng, J. Molecular simulations and understanding of antifouling zwitterionic polymer brushes. J. Mater. Chem. B 2020, 8, 3814–3828. [Google Scholar] [CrossRef]
- Corrigan, N.; Yeow, J.; Judzewitsch, P.; Xu, J.; Boyer, C. Seeing the Light: Advancing Materials Chemistry through Photopolymerization. Angew. Chem. Int. Ed. Engl. 2019, 58, 5170–5189. [Google Scholar] [CrossRef] [PubMed]
- Bearn, D.R.; Alharbi, F. British Orthodontic Society national audit of temporary anchorage devices (TADs): Report of the first thousand TADs placed. J. Orthod. 2015, 42, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Knutson, K.J.; Berzins, D.W. Corrosion of orthodontic temporary anchorage devices. Eur. J. Orthod. 2013, 35, 500–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Sample Types with Cells | No Cells | |||
|---|---|---|---|---|
| Incubation Time (h) | polySBMA Coating | Bare TiCp | Thermanox Control | Empty Insert Blank |
| 24 | 3 | 3 | 3 | 3 |
| 48 | 20 | 20 | 20 | 20 |
| 72 | 21 | 21 | 21 | 21 |
| Ti Specimen | Wave Number (cm−1) | Band Assignment |
|---|---|---|
| As-received TiCP | - | |
| TiCP with O2 plasma treatment | 3400 | O-H from Ti-OH generated species |
| <1000 | Ti-O | |
| TiCP with O2 plasma-treatment and TMSPMA | 3500 | SiO-H |
| 1038 | Si-O-Si | |
| <950 | SiO-H/Si-O-Ti | |
| TiCP with with O2 plasma treatment and TMSPMA-polySBMA | 3400 | O-H from physisorbed water |
| 1725 | C=O | |
| 1640 | O-H from physisorbed water | |
| 1480/1450 | C-H from CH3-N+/CH2 | |
| 1180 | S=O asymmetric | |
| 1040 | S=O symmetric |
| Scheme | Soaking Conditions | Max. Load (mN) | Max. Depth (nm) | St. Dev. | Pull-Off Force (mN) | St. Dev. | Pull-Off Stress (kPa) | St. Dev. |
|---|---|---|---|---|---|---|---|---|
| Ti-polySBMA | Water | 2.0 | 92.2 | 23.8 | 0.1 | 0.0 | 13.0 | 4.0 |
| Saltwater | 2.0 | 266.8 | 86.0 | 0.4 | 0.2 | 52.2 | 26.0 |
| Treatment | Number of Samples | Mean WCA (°) | SD |
|---|---|---|---|
| As-received TiCP | 2 | 87.3 | 3.8 |
| TiCP with O2 plasma treatment | 2 | 3.5 | 2.1 |
| TiCP 24 h after O2 plasma treatment | 2 | 83.0 | 18.4 |
| TMSPMA treatment | 3 | 84.1 | 6.0 |
| PolySBMA coating | 17 | 9.4 | 3.3 |
| PolySBMA stored for 72 h in H2O | 7 | 12.9 | 10.5 |
| PolySBMA stored when dry for 5 days | 8 | 13.8 | 4.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Es-Souni, M.; Es-Souni, M.; Bakhti, H.; Gülses, A.; Fischer-Brandies, H.; Açil, Y.; Wiltfang, J.; Flörke, C. A Bacteria and Cell Repellent Zwitterionic Polymer Coating on Titanium Base Substrates towards Smart Implant Devices. Polymers 2021, 13, 2472. https://doi.org/10.3390/polym13152472
Es-Souni M, Es-Souni M, Bakhti H, Gülses A, Fischer-Brandies H, Açil Y, Wiltfang J, Flörke C. A Bacteria and Cell Repellent Zwitterionic Polymer Coating on Titanium Base Substrates towards Smart Implant Devices. Polymers. 2021; 13(15):2472. https://doi.org/10.3390/polym13152472
Chicago/Turabian StyleEs-Souni, Mona, Martha Es-Souni, Hamzah Bakhti, Aydin Gülses, Helge Fischer-Brandies, Yahya Açil, Jörg Wiltfang, and Christian Flörke. 2021. "A Bacteria and Cell Repellent Zwitterionic Polymer Coating on Titanium Base Substrates towards Smart Implant Devices" Polymers 13, no. 15: 2472. https://doi.org/10.3390/polym13152472
APA StyleEs-Souni, M., Es-Souni, M., Bakhti, H., Gülses, A., Fischer-Brandies, H., Açil, Y., Wiltfang, J., & Flörke, C. (2021). A Bacteria and Cell Repellent Zwitterionic Polymer Coating on Titanium Base Substrates towards Smart Implant Devices. Polymers, 13(15), 2472. https://doi.org/10.3390/polym13152472






