Surface Characterization and Physiochemical Evaluation of P(3HB-co-4HB)-Collagen Peptide Scaffolds with Silver Sulfadiazine as Antimicrobial Agent for Potential Infection-Resistance Biomaterial
Abstract
:1. Introduction
2. Materials and Methods
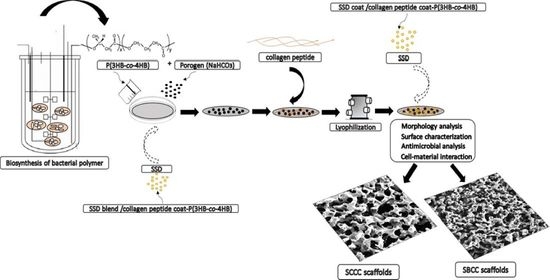
2.1. Biosynthesis of P(3HB-co-95 mol% 4HB) Copolymer
2.2. Surface Functionalization of SSD/Collagen Peptide-P(3HB-co-4HB) Scaffolds
2.3. Characterization of Scaffolds
2.4. Antimicrobial Activity
2.5. Biocompatibility and Cell Proliferation Evaluation
2.6. Statistical Analysis
3. Results and Discussion
3.1. Biosynthesis of P(3HB-co-4HB) via Batch Fermentation
3.2. Fabrication of SBCC and SCCC Scaffolds
3.3. Functional Group Identification Using FTIR Analysis
3.4. Porosity Analysis
3.5. Hydrophilicity of Fabricated Scaffolds
3.6. Evaluation of Cell Proliferation of Fibroblast Cells on Scaffolds
3.7. Antimicrobial Analysis of SCCC and SBCC Scaffolds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding Strategies for Tissue Engineering and Regenerative Medicine Applications. Materials 2019, 12, 1824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdulghani, S.; Mitchell, G.R. Three-dimensional Scaffolds for Tissue Engineering Applications: Role of Porosity and Pore Size. Biomolecules 2019, 9, 750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salim, Y.S.; Sharon, A.; Vigneswari, S.; Mohd Ibrahim, M.N.; Amirul, A.A. Environmental degradation of microbial polyhydroxyalkanoates and oil palm-based composites. Appl. Bichem. Biotechnol. 2012, 167, 314. [Google Scholar] [CrossRef] [PubMed]
- Winnacker, M. Polyhydroxyalkanoates: Recent Advances in Their Synthesis and Applications. Eur. J. Lipid Sci. Technol. 2019, 121, 1900101. [Google Scholar] [CrossRef] [Green Version]
- Trakunjae, C.; Boondaeng, A.; Apiwatanapiwat, W.; Kosugi, A.; Arai, T.; Sudesh, K.; Vaithanomsat, P. Enhanced Polyhydroxybutyrate (PHB) Production by Newly Isolated Rare Actinomycetes Rhodococcus sp. strain BSRT1-1 using Response Surface Methodology. Sci. Rep. 2021, 11, 1896. [Google Scholar] [CrossRef] [PubMed]
- Akinmulewo, A.B.; Nwinyi, O.C. Polyhydroxyalkanoate: A Biodegradable Polymer (a mini review). J. Phys. Conf. Ser. 2019, 1378, 042007. [Google Scholar] [CrossRef]
- Faezah, A.N.; Rahayu, A.; Vigneswari, S.; Majid, M.I.A.; Amirul, A.A. Regulating the molar fraction of 4-hydroxybutyrate in Poly(3-hydroxybutyrate-co-4-hydroxybutyrate) by biological fermentation and enzymatic degradation. World J. Microbiol. Biotechnol. 2011, 27, 2455. [Google Scholar] [CrossRef]
- Vigneswari, S.; Chai, J.M.; Kamarudin, K.H.; Amirul, A.A.; Focarate, M.L.; Ramakrishna, S. Elucidating the Surface Functionality of Biomimetic RGD Peptides Immobilized on Nano-P(3HB-co-4HB) for H9c2 Myoblast Cell Proliferation. Front. Bioeng. Biotechnol. 2020, 8, 567693. [Google Scholar] [CrossRef]
- Vigneswari, S.; Gurusamy, T.P.; Abdul Khalil, H.P.S.; Ramakrishna, S.; Amirul, A.A. Elucidation of Antimicrobial SSD blend/poly (3-hydroxybutyrate-co-4-hydroxybutyrate) Immobilised with Collagen Peptide As Potential Biomaterial. Polymer 2020, 12, 2979. [Google Scholar] [CrossRef]
- Sun, F.; Guo, J.; Liu, Y.; Yu, Y. Preparation and Characterization of Poly (3-hydroxybutyrate-co-4-hydroxybutyrate)/Pullulan-Gelatin Electrospun Nanofibers with Shell-Core Structure. Biomed. Mater. 2020, 15, 045023. [Google Scholar] [CrossRef]
- Lim, Y.S.; Ok, Y.J.; Hwang, S.Y.; Kwak, J.Y.; Yoon, S. Marine Collagen as A Promising Biomaterial for Biomedical Applications. Mar. Drugs 2019, 17, 467. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Gordillo, V.; Chmielewski, J. Mimicking the Extracellular Matrix with Functionalized, Metal-Assembled Collagen Peptide Scaffolds. Biomaterials 2014, 35, 7363–7373. [Google Scholar] [CrossRef]
- Yamada, S.; Yamamoto, K.; Ikeda, T.; Yanagiguchi, K.; Hayashi, Y. Potency of Fish Collagen as a Scaffold for Regenerative Medicine. BioMed Res. Int. 2014, 2014, 302932. [Google Scholar] [CrossRef]
- Xiao, X.; Zhao, W.; Liang, J.; Sauer, K.; Libera, M. Self-Defensive Antimicrobial Biomaterial Surfaces. Colloids Surf. B Biointerfaces 2020, 192, 110989. [Google Scholar] [CrossRef]
- Ilomuanya, M.O.; Adebona, A.C.; Wang, W.; Sowemimo, A.; Eziegbo, C.L.; Silva, B.O.; Adeosun, S.O.; Joubert, E.; De Beer, D. Development and Characterization of Collagen-Based Electrospun Scaffolds Containing Silver Sulphadiazine and Aspalathus linearis Extract for Potential Wound Healing Applications. SN Appl. Sci. 2020, 2, 881. [Google Scholar] [CrossRef] [Green Version]
- Mehta, M.A.; Shah, S.; Ranjan, V.; Sarwade, P.; Philipose, A. Comparative Study of Silver-Sulfadiazine-Impregnated Collagen Dressing Versus Conventional Burn Dressings in Second-Degree Burns. J. Fam. Med. Prim. Care 2019, 8, 215–219. [Google Scholar]
- Banerjee, J.; Seetharaman, S.; Wrice, N.L.; Christy, R.J.; Natesan, S. Delivery of Silver Sulfadiazine and Adipose Derived Stem Cells Using Fibrin Hydrogel Improves Infected Burn Wound Regeneration. PLoS ONE 2019, 14, e0217965. [Google Scholar] [CrossRef]
- Ceresa, C.; Fracchia, L.; Marchetti, A.; Rinaldi, M.; Bosetti, M. Injectable Scaffolds Enriched with Silver to Inhibit Bacterial Invasion in Tissue Regeneration. Materials 2019, 12, 1931. [Google Scholar] [CrossRef] [Green Version]
- Shanmugasundaram, N.; Sundaraseelan, J.; Uma, S.; Selvaraj, D.; Babu, M. Design and Delivery of Silver Sulfadiazine from Alginate Microspheres-Impregnated Collagen Scaffold. J. Biomed. Mater. Res. B Appl. Biomater. 2006, 77, 378–388. [Google Scholar] [CrossRef]
- Nejaddehbashi, F.; Hashemitabar, M.; Bayati, V.; Moghimipour, E.; Movaffagh, J.; Orazizadeh, M.; Abbaspour, M.R. Incorporation of Silver Sulfadiazine into an Electrospun Composite of Polycaprolactone as An Antibacterial Scaffold for Wound Healing in Rats. Cell J. 2020, 21, 379–390. [Google Scholar]
- Mohseni, M.; Shamloo, A.; Aghababaei, Z.; Vossoughi, M.; Moravvej, H. Antimicrobial Wound Dressing Containing Silver Sulfadiazine with High Biocompatibility: In Vitro Study. Artif. Organs 2016, 40, 765–773. [Google Scholar] [CrossRef]
- Malafatti, J.O.D.; Bernardo, M.P.; Moreira, F.K.V.; Ciol, H.; Inada, N.M.; Mattoso, L.H.C.; Paris, E.C. Electrospun Poly (lactic acid) Nanofibers Loaded with Silver Sulfadiazine/[Mg–Al]-Layered Double Hydroxide As An Antimicrobial Wound Dressing. Polym. Adv. Technol. 2020, 31, 1377–1387. [Google Scholar] [CrossRef]
- Huong, K.H.; Azuraini, M.J.; Aziz, N.A.; Amirul, A.A. Pilot scale production of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) biopolymers with high molecular weight and elastomeric properties. J. Biosci. Bioeng. 2017, 124, 76–83. [Google Scholar] [CrossRef]
- Norhafini, H.; Thinagaran, L.; Shantini, K.; Huong, K.H.; Syafiq, I.M.; Bhubalan, K.; Amirul, A.A. Synthesis of Poly (3-hydroxybutyrate-co-4-hydroxybutyrate) with High 4HB Composition and PHA Content Using 1,4-butanediol and 1,6-hexanediol for Medical Application. J. Polym. Res. 2017, 24, 24–189. [Google Scholar] [CrossRef]
- Destaye, A.G.; Lin, C.K.; Lee, C.K. Glutaraldehyde Vapor Cross-Linked Nanofibrous PVA Mat with In Situ Formed Silver Nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 4745–4752. [Google Scholar] [CrossRef]
- Aramwit, P.; Ratanavaraporn, J.; Ekgasit, S.; Tongsakul, D.; Bang, N.A. Green Salt-Leaching Technique to Produce Sericin/ PVA/Glycerin Scaffolds with Distinguished Characteristics for Wound-Dressing Applications. J. Biomed. Mater. Res. B 2015, 103, 915–924. [Google Scholar] [CrossRef]
- Wang, Y.; Ke, Y.; Ren, L.; Wu, G.; Chen, X.; Zhao, Q. Surface engineering of PHBV by covalent collagen immobilization to improve cell compatibility. J. Biomed. Mater. Res. A 2009, 88, 616–627. [Google Scholar] [CrossRef]
- Köse, G.T.; Kenar, H.; Hasirci, N.; Hasirci, V. Macroporous poly (3-hydroxybutyrate-co-3-hydroxyvalerate) matrices for bone tissue engineering. Biomaterials 2003, 24, 1949–1958. [Google Scholar] [CrossRef]
- Ismail, I.; Gurusamy, T.P.; Ramachandran, H.; Amirul, A.A. Enhanced production of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) copolymer and antimicrobial yellow pigmentation from Cupriavidus sp. USMAHM13 with antibiofilm capability. Prep. Biochem. Biotechnol. 2017, 47, 388–396. [Google Scholar] [CrossRef]
- Vigneswari, S.; Murugaiyah, V.; Kaur, G.; Abdul Khalil, H.P.S.; Amirul, A.A. Biomacromolecule Immobilization: Grafting of Fish-Scale Collagen Peptides onto Aminolyzed P(3HB-co-4HB) Scaffolds as A Potential Wound Dressing. Biomed. Mater. 2016, 68, 1927–1934. [Google Scholar] [CrossRef]
- Ahmed, W.; Zhai, Z.; Gao, C. Adaptive Antibacterial Biomaterial Surfaces and Their Applications. Mater. Today Bio 2019, 2, 100017. [Google Scholar] [CrossRef] [PubMed]
- Wieszczycka, K.; Staszak, K.; Woźniak-Budych, M.J.; Litowczenko, J.; Maciejewska, B.M.; Jurga, S. Surface Functionalization–The Way for Advanced Applications of Smart Materials. Coord Chem. Rev. 2021, 436, 213846. [Google Scholar] [CrossRef]
- Zhu, Y.; Ke, J.; Zhang, L. Anti-biofouling and antimicrobial biomaterials for tissue engineering. In Racing for the Surface; Li, B., Moriarty, T., Webster, T., Xing, M., Eds.; Springer: New York, NY, USA, 2020. [Google Scholar]
- Goswami, M.; Rekhi, P.; Debnath, M.; Ramakrishna, S. Microbial Polyhydroxyalkanoates Granules: An Approach Targeting Biopolymer for Medical Applications and Developing Bone Scaffolds. Molecules 2021, 26, 860. [Google Scholar] [CrossRef] [PubMed]
- Bružauskaitė, I.; Bironaitė, D.; Bagdonas, E.; Bernotienė, E. Scaffolds and cells for tissue regeneration: Different scaffold pore sizes—Different cell effects. Cytotechnology 2016, 68, 355–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, C.J.; Chen, C.F.; Chen, J.H.; Chiang, S.F.; Lin, Y.J.; Chang, K.Y. Fabrication of Porous Biodegradable Polymer Scaffolds Using a Solvent Merging/Particulate Leaching Method. J. Biomed. Mater. Res. 2002, 59, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Subia, B.; Kundu, J.C.S. Biomaterial Scaffold Fabrication Techniques for Potential Tissue Engineering Applications. Tissue Eng. 2010, 141, 20–31. [Google Scholar]
- Ho, M.H.; Kuo, P.Y.; Hsieh, H.J.; Hsien, T.Y.; Hou, L.T.; Lai, J.Y.; Wang, D.M. Preparation of Porous Scaffolds by Using Freeze-Extraction and Freeze-Gelation Methods. Biomaterials 2004, 25, 129–138. [Google Scholar] [CrossRef]
- Wang, Z.; Qing, Q.; Chen, X.; Liu, C.; Luo, J.; Hu, J.; Qin, T. Effects of Scaffold Surface Morphology on Cell Adhesion and Survival Rate in Vitreous Cryopreservation of Tenocyte-Scaffold Constructs. Appl. Surf. Sci. 2016, 388, 223–227. [Google Scholar] [CrossRef]
- Zhu, B.; Li, W.; Chi, N.; Lewis, R.V.; Osamor, J.; Wang, R. Optimization of Glutaraldehyde Vapor Treatment for Electrospun Collagen/Silk Tissue Engineering Scaffolds. ACS Omega 2017, 2, 2439–2450. [Google Scholar] [CrossRef] [Green Version]
- Campiglio, C.E.; Negrini, N.C.; Farè, S.; Draghi, L. Cross-linking Strategies for Electrospun Gelatin Scaffolds. Materials 2019, 12, 2476. [Google Scholar] [CrossRef] [Green Version]
- Vashisth, P.; Pruthi, V. Synthesis and Characterization of Crosslinked Gellan/PVA Nanofibers for Tissue Engineering Application. Mater. Sci. Eng. C 2016, 67, 304–312. [Google Scholar] [CrossRef]
- Teixeira, M.A.; Antunes, J.C.; Amorimm, M.T.P.; Felgueiras, H.P. Green Optimization of Glutaraldehyde Vapor-Based Crosslinking on Poly (Vinyl Alcohol)/Cellulose Acetate Electrospun Mats for Applications as Chronic Wound Dressings. Proceedings 2021, 69, 30. [Google Scholar] [CrossRef]
- Bettini, S.; Bonfrate, V.; Syrgiannis, Z.; Sannino, A.; Salvatore, L.; Madaghiele, M.; Valli, L.; Giancane, G. Biocompatible Collagen Paramagnetic Scafold For Controlled Drug Release. Biomacromolecules 2015, 16, 2599–2608. [Google Scholar] [CrossRef]
- Bonfrate, V.; Manno, D.; Serra, A.; Salvatore, L.; Sannino, A.; Buccolieri, A.; Serra, T.; Giancane, G. Enhanced Electrical Conductivity of Collagen Films Through Long-Range Aligned Iron Oxide Nanoparticles. J. Colloid Interface Sci. 2017, 501, 185–191. [Google Scholar] [CrossRef]
- Terzi, A.; Storelli, E.; Bettini, S.; Sibillano, T.; Altamura, D.; Salvatore, L.; Madaghiele, M.; Romano, A.; Siliqi, D.; Ladisa, M.; et al. Effects of Processing on Structural, Mechanical and Biological Properties of Collagen-Based Substrates for Regenerative Medicine. Sci. Rep. 2018, 8, 1429. [Google Scholar] [CrossRef] [Green Version]
- Riaz, T.; Zeeshan, R.; Zarif, F.; Ilyas, K.; Muhammad, N.; Safi, S.Z.; Rahim, A.; Rizvi, S.A.A.; Rehman, I.U. FTIR Analysis of Natural and Synthetic Collagen. Appl. Spectrosc. Rev. 2018, 53, 703–746. [Google Scholar] [CrossRef]
- Sotelo, C.G.; Comesaña, M.B.; Ariza, P.R.; Pérez-Martín, R.I. Characterization of Collagen from Different Discarded Fish Species Of The West Coast of the Iberian Peninsula. J. Aquat. Food Prod. Technol. 2016, 25, 388–399. [Google Scholar] [CrossRef] [Green Version]
- Zhijiang, C.; Qin, Z.; Xianyou, S.; Yuanpei, L. Zein/poly (3-hydroxybutyrate-co-4-hydroxybutyrate) Electrospun Blend Fiber Scaffolds: Preparation, Characterization and Cytocompatibility. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 1, 797–806. [Google Scholar] [CrossRef]
- Bayari, S.; Severcan, F. FTIR Study of Biodegradable Biopolymers: P(3HB), P(3HB-co-4HB) and P(3HB-co-3HV). J. Mol. Strut 2005, 744, 529–534. [Google Scholar] [CrossRef]
- Mighri, N.; Mao, J.; Mighri, F.; Ajji, A.; Rouabhia, M. Chitosan-Coated Collagen Membranes Promote Chondrocyte Adhesion, Growth, and Interleukin-6 Secretion. Materials 2015, 8, 7673–7689. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.C.; Ikoma, T.; Kikuchi, M.; Tanaka, J. Preparation of a porous hydroxyapatite/collagen nanocomposite using glutaraldehyde as a crosslinkage agent. J. Mater. Sci. Lett. 2001, 20, 1199–1201. [Google Scholar] [CrossRef]
- Chen, G.; Ushida, T.; Tateishi, T. Scaffold Design for Tissue Engineering. Macromol Biosci 2002, 2, 67–77. [Google Scholar] [CrossRef]
- Wang, H.M.; Chou, Y.T.; Wen, Z.H.; Wang, Z.R.; Chen, C.H.; Ho, M.L. Novel biodegradable porous scaffold applied to skin regeneration. PLoS ONE 2013, 8, 118–126. [Google Scholar] [CrossRef]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D Biomaterial Scaffolds and Osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Chen, G.; Kawazoe, N. 3.1-Preparation of Polymer Scaffolds by Ice Particulate Method for Tissue Engineering. In Biomaterials Nanoarchitectonics; Ebara, M., Ed.; William Andrew Publishing: New York, NY, USA, 2016; pp. 77–95. [Google Scholar]
- Venkatesan, J.; Kim, S.; Wong, T.W. Chapter 9-Chitosan and Its Application as Tissue Engineering Scaffolds. In Nanotechnology Applications for Tissue Engineering; Thomas, S., Grohens, Y., Ninan, N., Eds.; William Andrew Publishing: New York, NY, USA, 2015; pp. 133–147. [Google Scholar]
- Bartoš, M.; Suchý, T.; Foltán, R. Note on the Use of Different Approaches to Determine the Pore Sizes Of Tissue Engineering Scaffolds: What Do We Measure? BioMed Eng. OnLine 2018, 17, 110. [Google Scholar] [CrossRef] [Green Version]
- Han, F.; Wang, J.; Ding, L.; Hu, Y.; Li, W.; Yuan, Z.; Guo, Q.; Zhu, C.; Yu, L.; Wang, H.; et al. Tissue Engineering and Regenerative Medicine: Achievements, Future, And Sustainability in Asia. Front. Bioeng. Biotechnol. 2020, 8, 83. [Google Scholar] [CrossRef] [Green Version]
- Dzobo, K.; Thomford, N.E.; Senthebane, D.A.; Shipanga, H.; Rowe, A.; Dandara, C.; Pillay, M.; Motaung, K.S.C.M. Advances in Regenerative Medicine and Tissue Engineering: Innovation and Transformation of Medicine. Stem Cells Int. 2018, 2495848. [Google Scholar] [CrossRef] [Green Version]
- Hebbar, R.S.; Isloor, A.M.; Ismail, A.F. Contact angle measurements in membrane characterization. Biomaterials 2017, 131, 167–178. [Google Scholar]
- Wang, W.; Caetano, G.; Ambler, W.S.; Blaker, J.J.; Frade, M.A.; Mandal, P.; Diver, C.; Bártolo, P. Enhancing the Hydrophilicity and Cell Attachment of 3D Printed PCL/Graphene Scaffolds for Bone Tissue Engineering. Materials 2016, 9, 992. [Google Scholar] [CrossRef]
- Remya, K.R.; Chandran, S.; Mani, S.; John, A.; Ramesh, P. Hybrid polycaprolactone/polyethylene oxide scaffolds with tunable fiber surface morphology, improved hydrophilicity and biodegradability for bone tissue engineering applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 12. [Google Scholar] [CrossRef]
- Rouabhia, M.; Mighri, N.; Mao, J.; Park, H.J.; Mighri, F.; Ajji, A.; Zhang, Z. Surface Treatment with Amino Acids of Porous Collagen-Based Scaffolds to Improve Cell Adhesion and Proliferation. Can. J. Chem. Eng. 2018, 39, 875–886. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, X.; Chen, J.; Lin, K. The development of collagen based composite scaffolds for bone regeneration. Bioact. Mater. 2018, 31, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Dan, W.; Xiong, S.; Kang, Y.; Dhinakar, A.; Wu, J.; Gu, Z. Development of Collagen/Polydopamine Complexed Matrix as Mechanically Enhanced and Highly Biocompatible Semi-Natural Tissue Engineering Scaffold. Acta Biomater. 2017, 47, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Tamaddon, M.; Gu, Y.; Yu, J.; Xu, N.; Gang, F.; Sun, X.; Liu, C. Cell Seeding Process Experiment and Simulation on Three-Dimensional Polyhedron and Cross-Link Design Scaffolds. Front. Bioeng. Biotechnol. 2020, 8, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vigneswari, S.; Majid, M.I.A.; Amirul, A.A. Tailoring the Surface Architecture of Poly (3-hydroxybutyrate-co-4-hydroxybutyrate) Scaffolds. J. Appl. Polym. Sci. 2011, 124, 2777–2788. [Google Scholar] [CrossRef]
- Lim, M.M.; Sultana, N. In Vitro Cytotoxicity and Antibacterial Activity of Silver-Coated Electrospun Polycaprolactone/Gelatine Nanofibrous Scaffolds. Biotech 2016, 6, 211. [Google Scholar] [CrossRef] [Green Version]
- Sandri, G.; Bonferoni, M.C.; D’Autilia, F.; Rossi, S.; Ferrari, F.; Grisoli, P.; Caramella, C. Wound Dressings Based on Silver Sulfadiazine Solid Lipid Nanoparticles for Tissue Repairing. Eur. J. Pharm. Biopharm. 2013, 84, 84–90. [Google Scholar] [CrossRef]
- Siedenbiedel, F.; Tiller, J.C. Antimicrobial Polymers in Solution and On Surfaces: Overview and Functional Principles. Polymers 2012, 4, 46–71. [Google Scholar] [CrossRef] [Green Version]
- Heo, D.N.; Yang, D.H.; Lee, J.B.; Bae, M.S.; Kim, J.H.; Moon, S.H.; Chun, H.J.; Kim, C.H.; Lim, H.N.; Kwon, I.K. Burn-Wound Healing Effect of Gelatin/Polyurethane Nanofiber Scaffold Containing Silver-Sulfadiazine. J. Biomed. Nanotechnol. 2013, 9, 511–515. [Google Scholar] [CrossRef]
- Zhong, S.P.; Zhang, Y.Z.; Lim, C.T. Tissue Scaffolds for Skin Wound Healing and Dermal Reconstruction. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 510–525. [Google Scholar] [CrossRef]
- Kalantari, K.; Mostafavi, E.; Afifi, A.M.; Izadiyan, Z.; Jahangirian, H.; Rafiee-Moghaddam, R.; Webster, T.J. Wound Dressings Functionalized with Silver Nanoparticles: Promises and Pitfalls. Nanoscale 2020, 12, 2268–2291. [Google Scholar] [CrossRef]
- Sripriya, R.; Kumar, M.S.; Ahmed, M.R.; Sehgal, P.K. Collagen Bilayer Dressing with Ciprofloxacin, an Effective System for Infected Wound Healing. J. Biomater. Sci. Polym. Ed. 2007, 18, 335–351. [Google Scholar] [CrossRef]
- Timofeeva, L.; Kleshcheva, N. Antimicrobial polymers: Mechanism of action, factors of activity, and applications. Appl. Microbiol. 2011, 89, 475–492. [Google Scholar] [CrossRef]









| Biopolymer/Materials | Fabrication of Scaffolds | Applications | References |
|---|---|---|---|
| Collagen/SSD | Facile blending | Wound dressings | [19] |
| Collagen/SSD | Electrospinning | Wound healing applications | [15] |
| Collagen/SSD | Blending with SSD-loaded alginate microspheres | Conventional burn dressings in second-degree burns | [16] |
| Polycaprolactone (PCL)/SSD | Electrospinning | Antibacterial scaffold | [20] |
| P(3HB-co-4HB)/collagen peptide/SSD | Aminolysis | Potential wound healing | [9] |
| Polycaprolactone (PCL) and Polyvinyl alcohol (PVA)/SSD | Electrospinning | Antimicrobial wound dressing | [21] |
| Poly(lactic acid) (PLA)/SSD | Electrospinning, structural reconstruction | Antimicrobial wound dressing | [22] |
| Copolymer | Tensile Strength | Elongation at Break | Young Modulus | Mw | Mn | PDI b |
|---|---|---|---|---|---|---|
| (MPa) a | (%) a | (Mpa) a | (kDa) b | (kDa) b | ||
| P(3HB-co-95 mol% 4HB) | 23.2 ± 4 | 611.8 ± 1 | 226.6 ± 20 | 585 ± 8 | 132 ± 11 | 3.2 ± 0.5 |
| Collagen Peptide (wt.%) | Types of Scaffolds | |
|---|---|---|
| Coat/Coat | Blend/Coat | |
| 0 |  49.9 ± 2.7 |  73.3 ± 1.4 |
| 2.5 |  32.5 ± 2.8 |  45.2 ± 2.4 |
| 5.0 |  15.3 ± 3.3 |  25 ± 5.4 |
| 7.5 |  8.58 ± 0.8 |  14.81 ± 1.2 |
| 10.0 |  0 |  0 |
| 12.5 |  0 |  0 |
| Time (h) | Inhibition of Microorganisms (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| 6 | 12 | 24 | 48 | |||||
| SCCC | SBCC | SCCC | SBCC | SCCC | SBCC | SCCC | SBCC | |
| Staphylococus aerus ATCC 12600 | 65 ± 5 | 13 ± 1 | 85 ± 3 | 36 ± 5 | 100 ± 0 | 83 ± 8 | NA | 100 ± 0 |
| Escherichia coli ATCC 11303 | 79 ± 8 | 34 ± 5 | 100 ± 0 | 51 ± 9 | 100 ± 0 | 92 ± 6 | NA | 100 ± 0 |
| Pseudomonas aeruginosa ATCC 17588 | 85 ± 7 | 43 ± 9 | 100 ± 0 | 45 ± 5 | 100 ± 0 | 87 ± 12 | NA | 100 ± 0 |
| Bacillus licheniformis | 98 ± 2 | 65 ± 10 | 100 ± 0 | 95 ± 5 | 100 ± 0 | 100 ± 0 | NA | 100 ± 0 |
| Candida albicans | 93 ± 7 | 33 ± 6 | 100 ± 0 | 71 ± 10 | 100 ± 0 | 94 ± 6 | NA | 100 ± 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vigneswari, S.; Gurusamy, T.P.; Khairul, W.M.; H.P.S., A.K.; Ramakrishna, S.; Amirul, A.-A.A. Surface Characterization and Physiochemical Evaluation of P(3HB-co-4HB)-Collagen Peptide Scaffolds with Silver Sulfadiazine as Antimicrobial Agent for Potential Infection-Resistance Biomaterial. Polymers 2021, 13, 2454. https://doi.org/10.3390/polym13152454
Vigneswari S, Gurusamy TP, Khairul WM, H.P.S. AK, Ramakrishna S, Amirul A-AA. Surface Characterization and Physiochemical Evaluation of P(3HB-co-4HB)-Collagen Peptide Scaffolds with Silver Sulfadiazine as Antimicrobial Agent for Potential Infection-Resistance Biomaterial. Polymers. 2021; 13(15):2454. https://doi.org/10.3390/polym13152454
Chicago/Turabian StyleVigneswari, Sevakumaran, Tana Poorani Gurusamy, Wan M. Khairul, Abdul Khalil H.P.S., Seeram Ramakrishna, and Al-Ashraf Abdullah Amirul. 2021. "Surface Characterization and Physiochemical Evaluation of P(3HB-co-4HB)-Collagen Peptide Scaffolds with Silver Sulfadiazine as Antimicrobial Agent for Potential Infection-Resistance Biomaterial" Polymers 13, no. 15: 2454. https://doi.org/10.3390/polym13152454
APA StyleVigneswari, S., Gurusamy, T. P., Khairul, W. M., H.P.S., A. K., Ramakrishna, S., & Amirul, A.-A. A. (2021). Surface Characterization and Physiochemical Evaluation of P(3HB-co-4HB)-Collagen Peptide Scaffolds with Silver Sulfadiazine as Antimicrobial Agent for Potential Infection-Resistance Biomaterial. Polymers, 13(15), 2454. https://doi.org/10.3390/polym13152454