PVDF Fibers Modification by Nitrate Salts Doping
Abstract
:1. Introduction
2. Materials and Methods
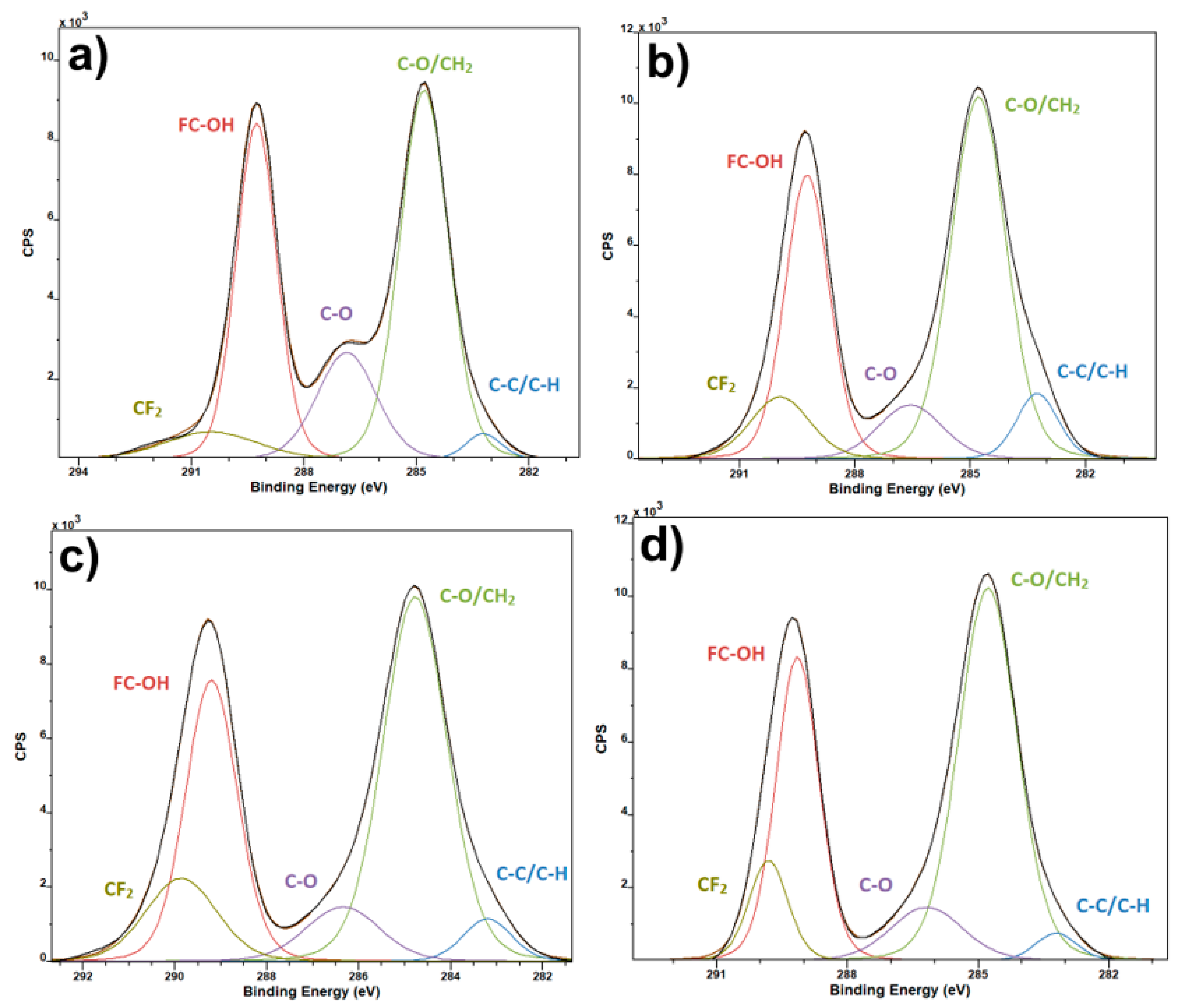
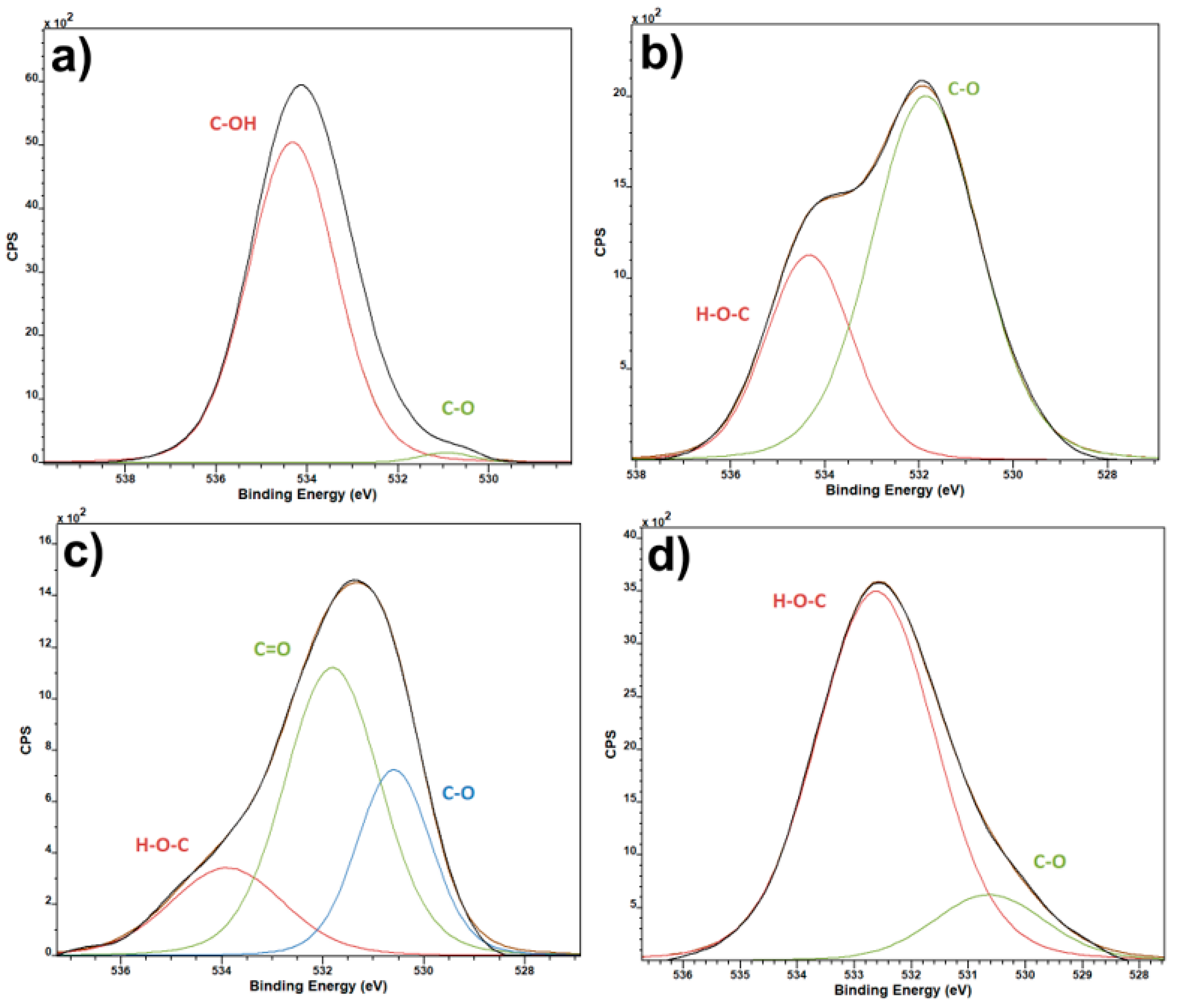
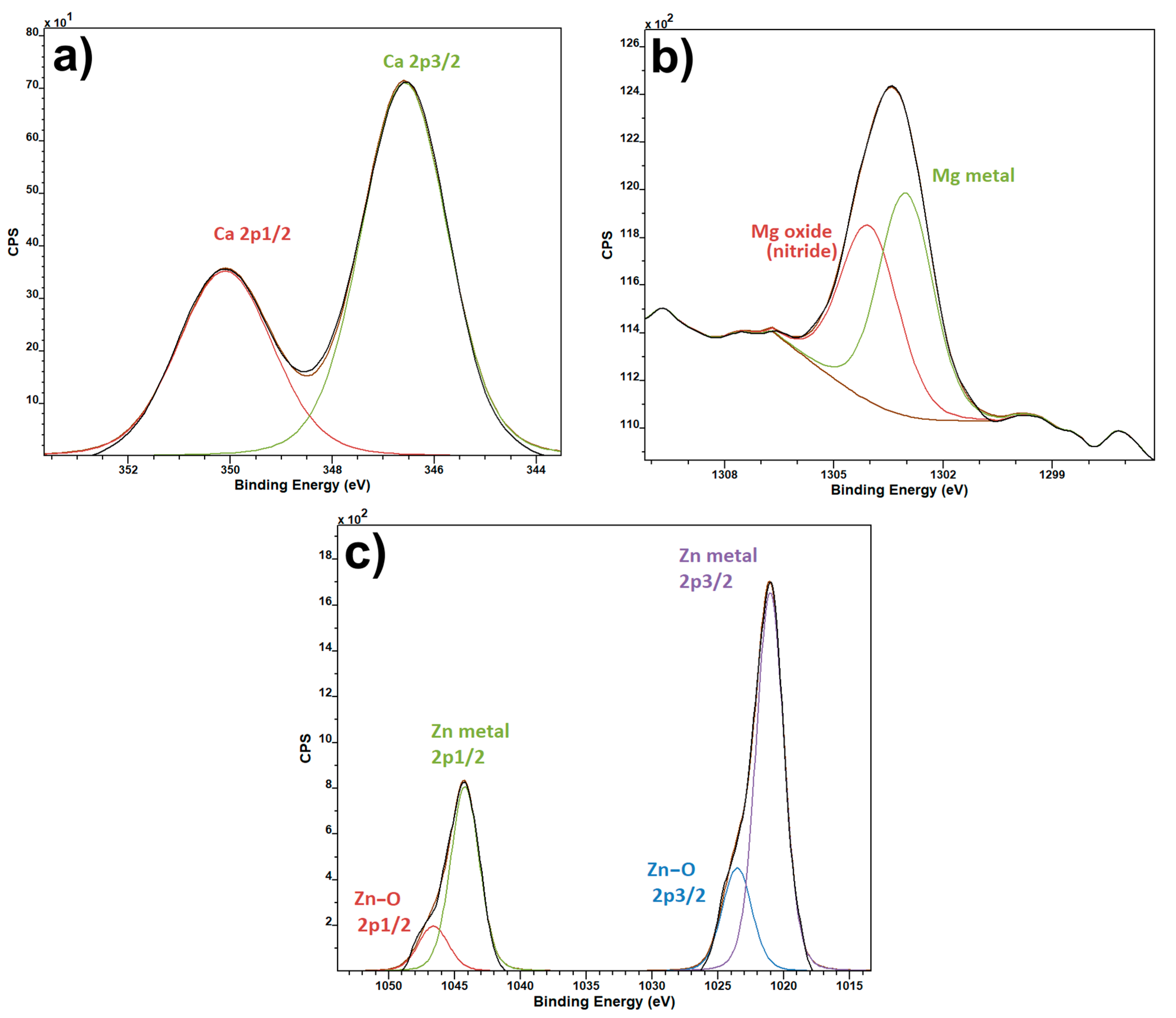
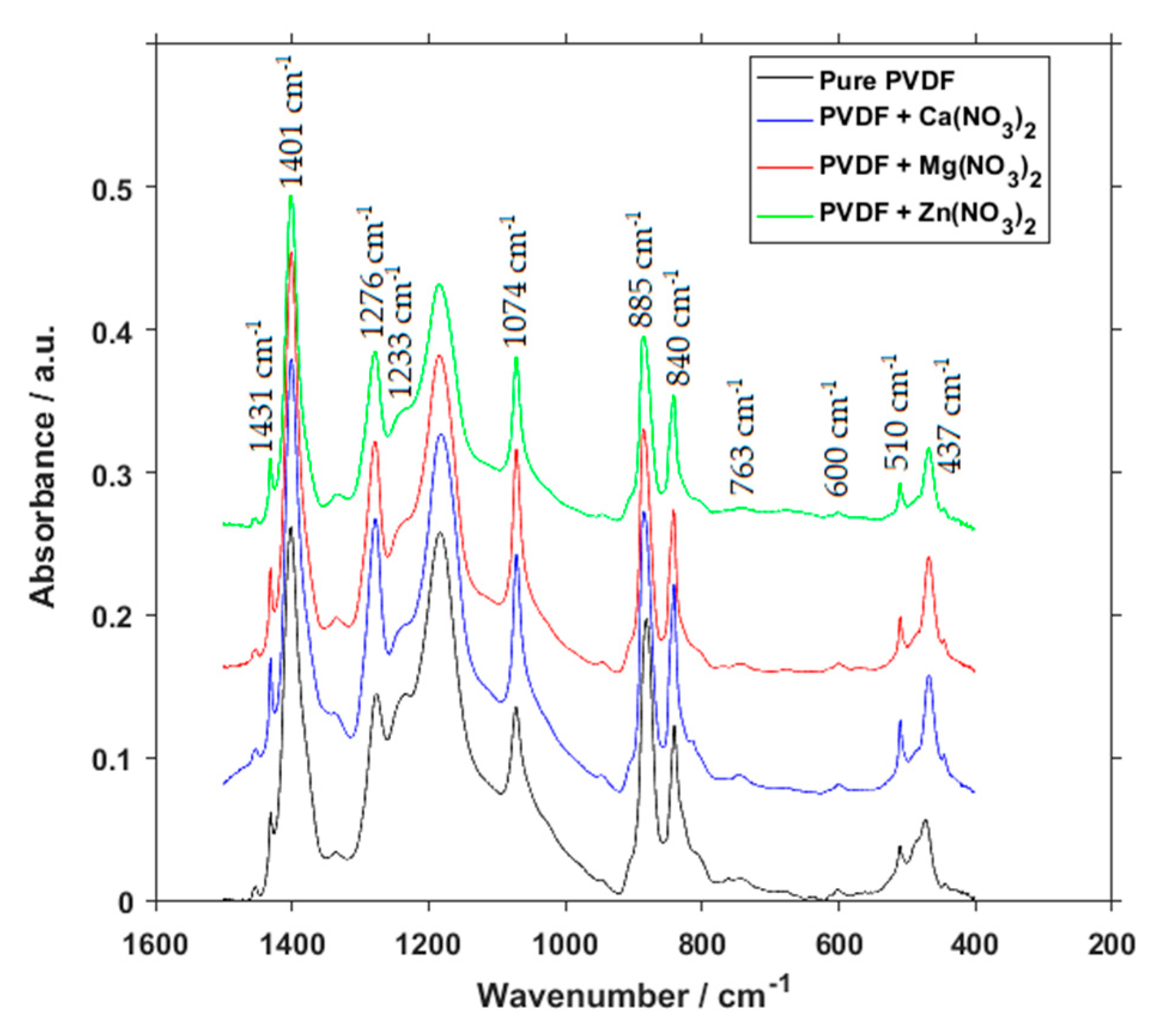
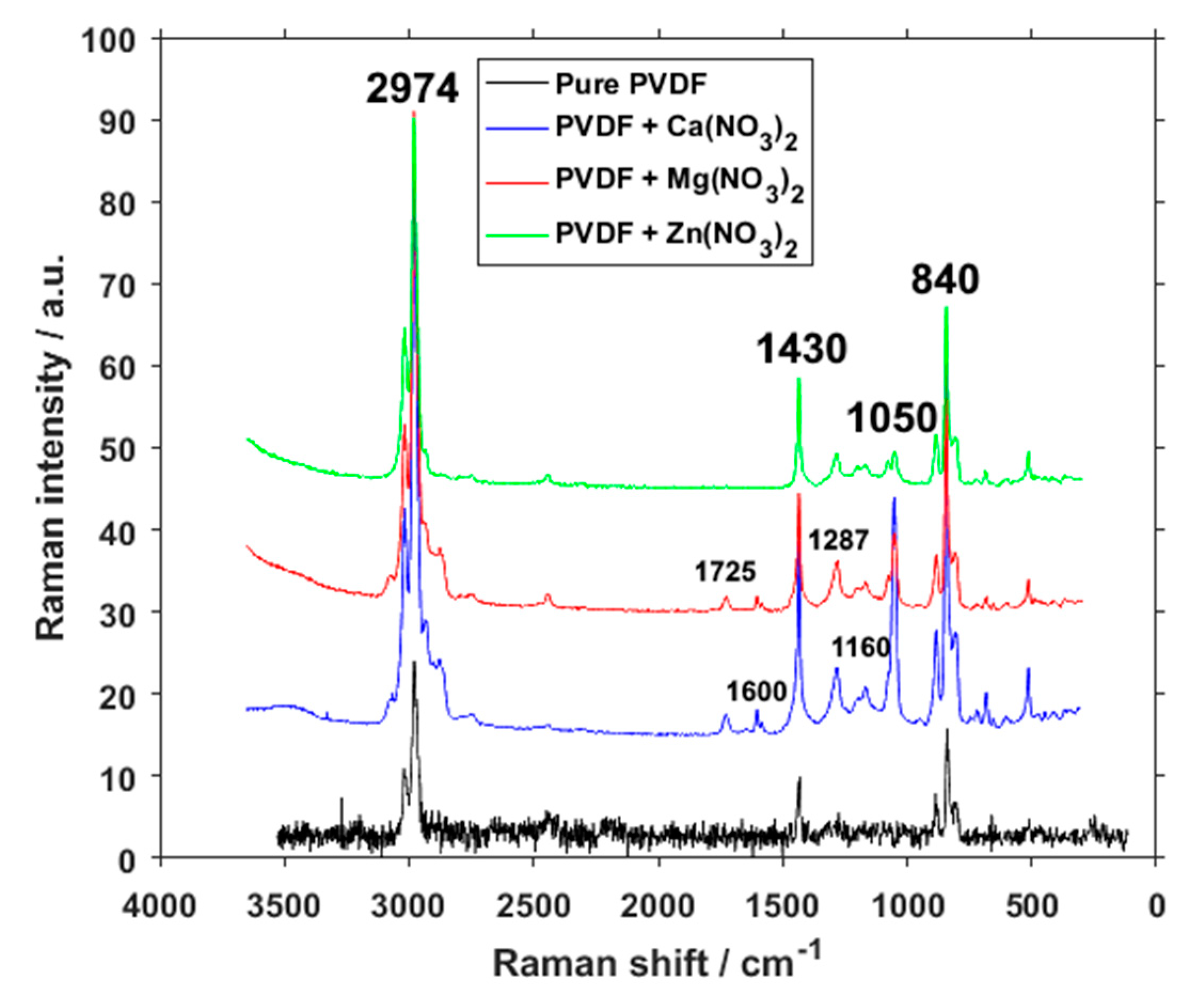
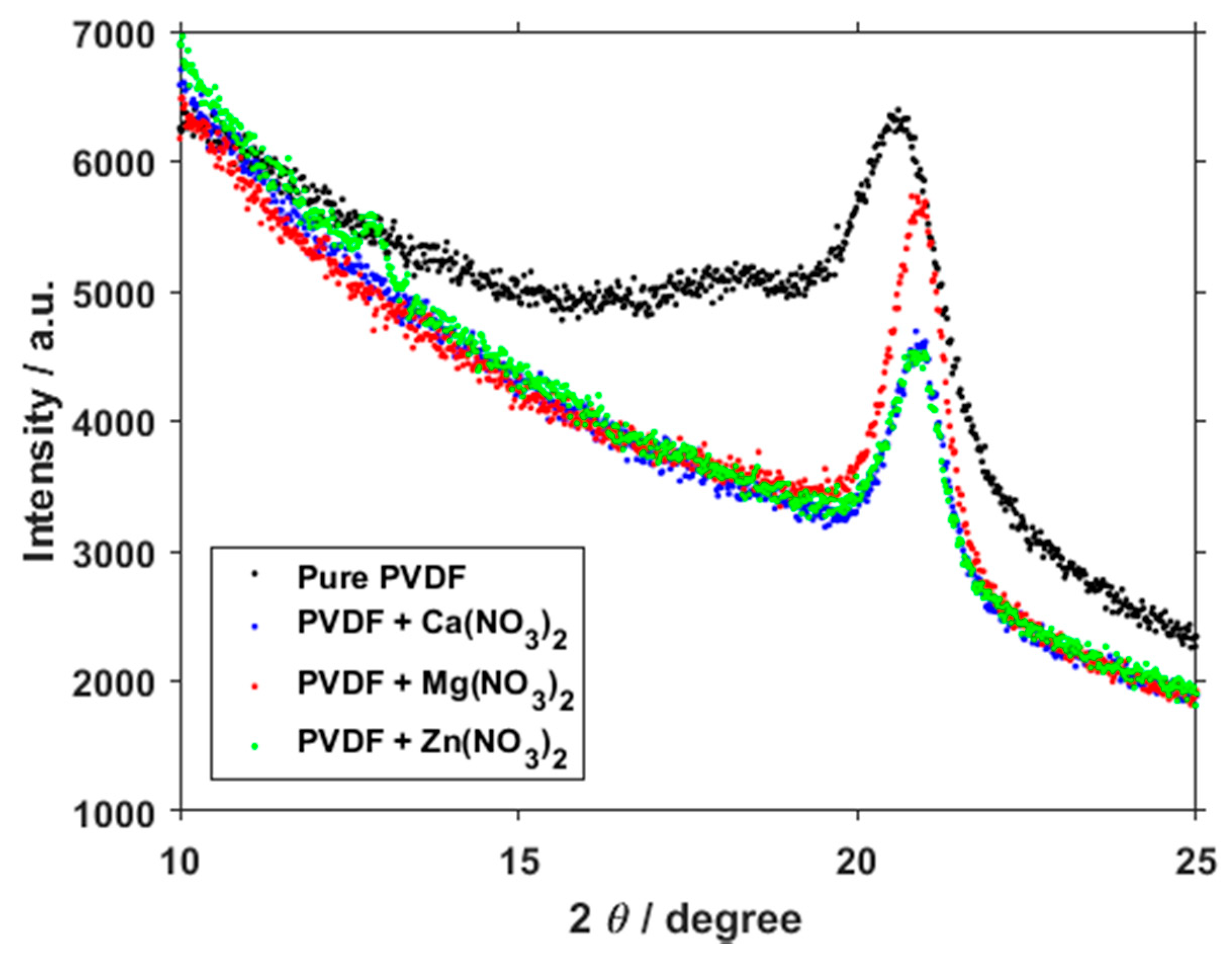
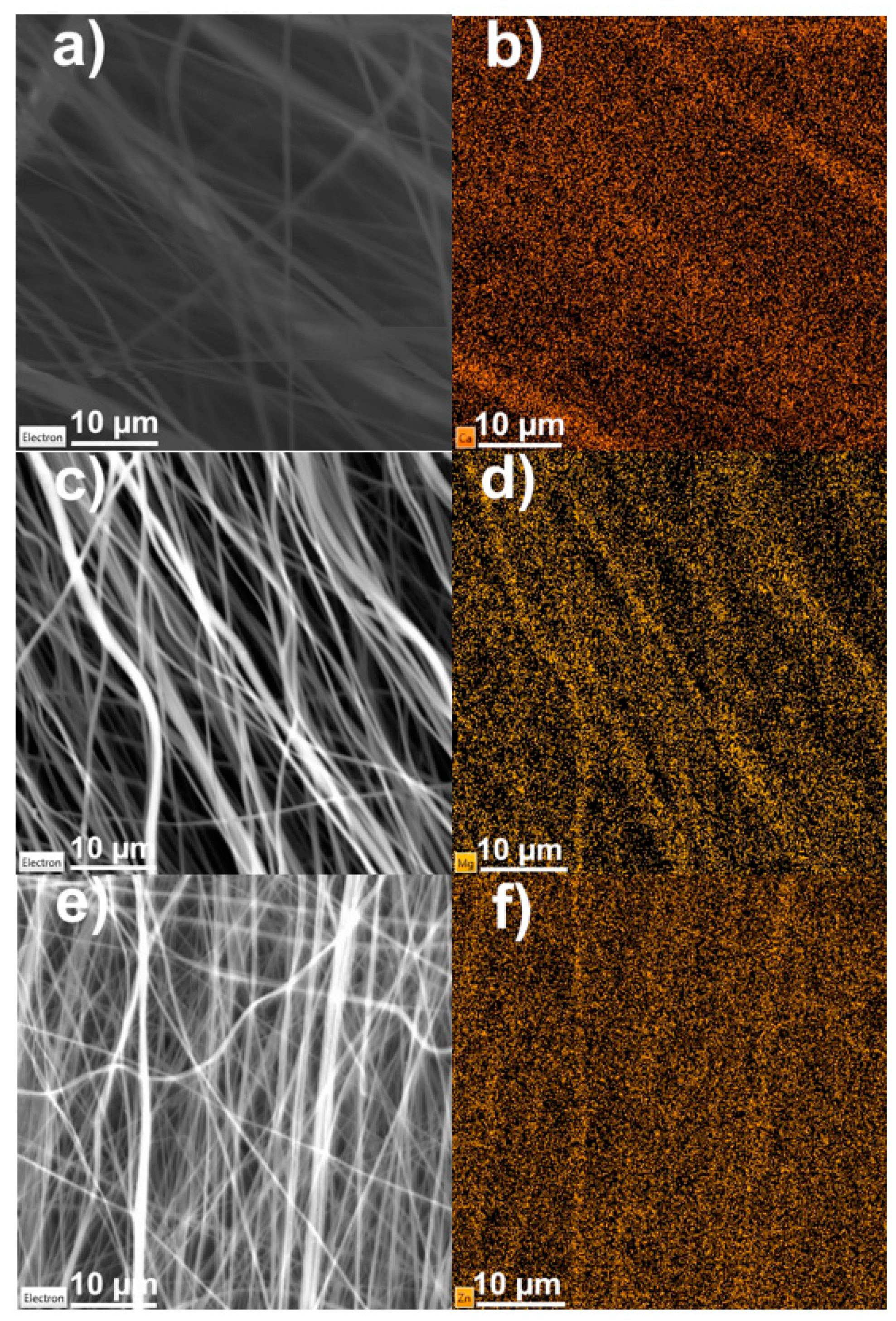
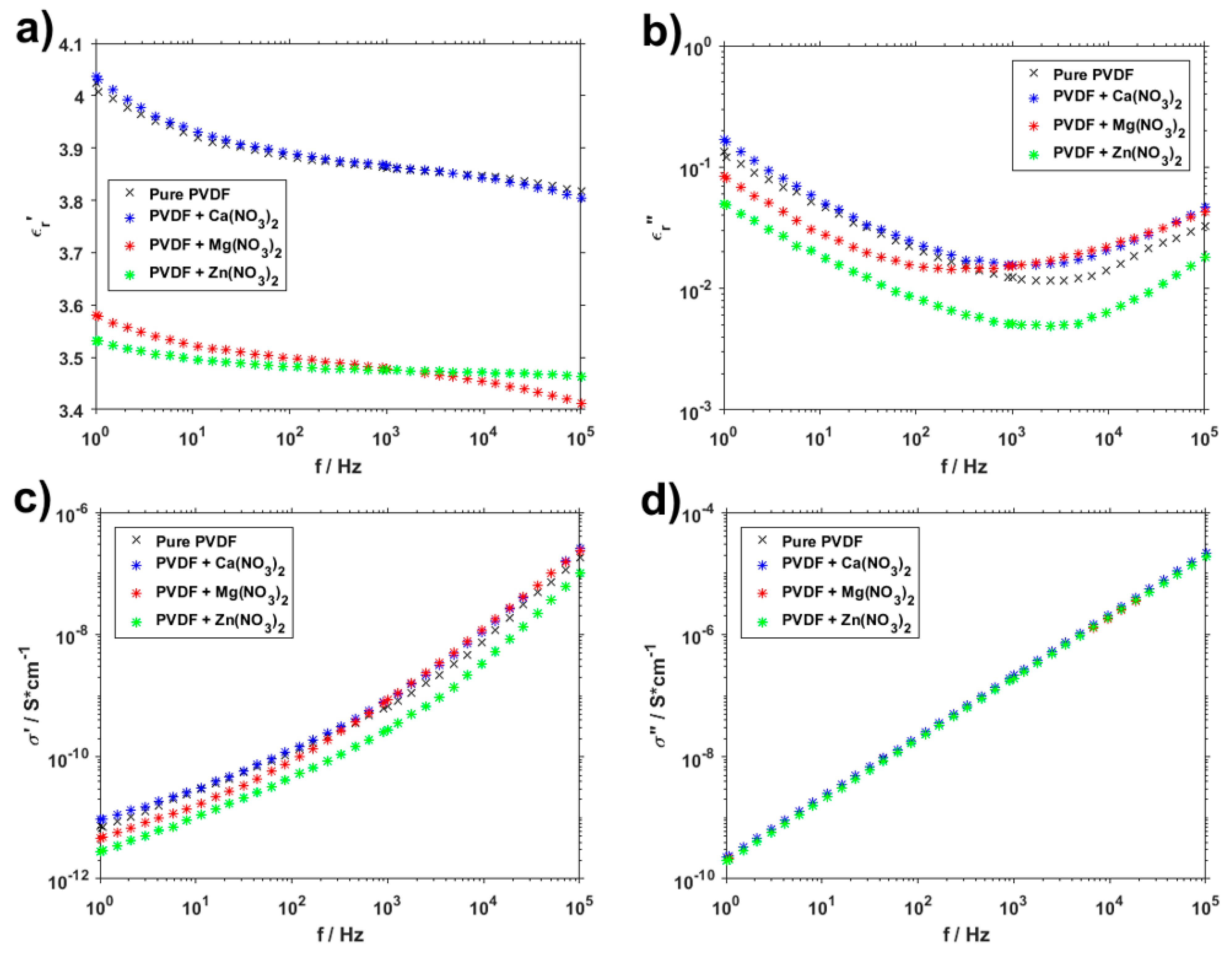
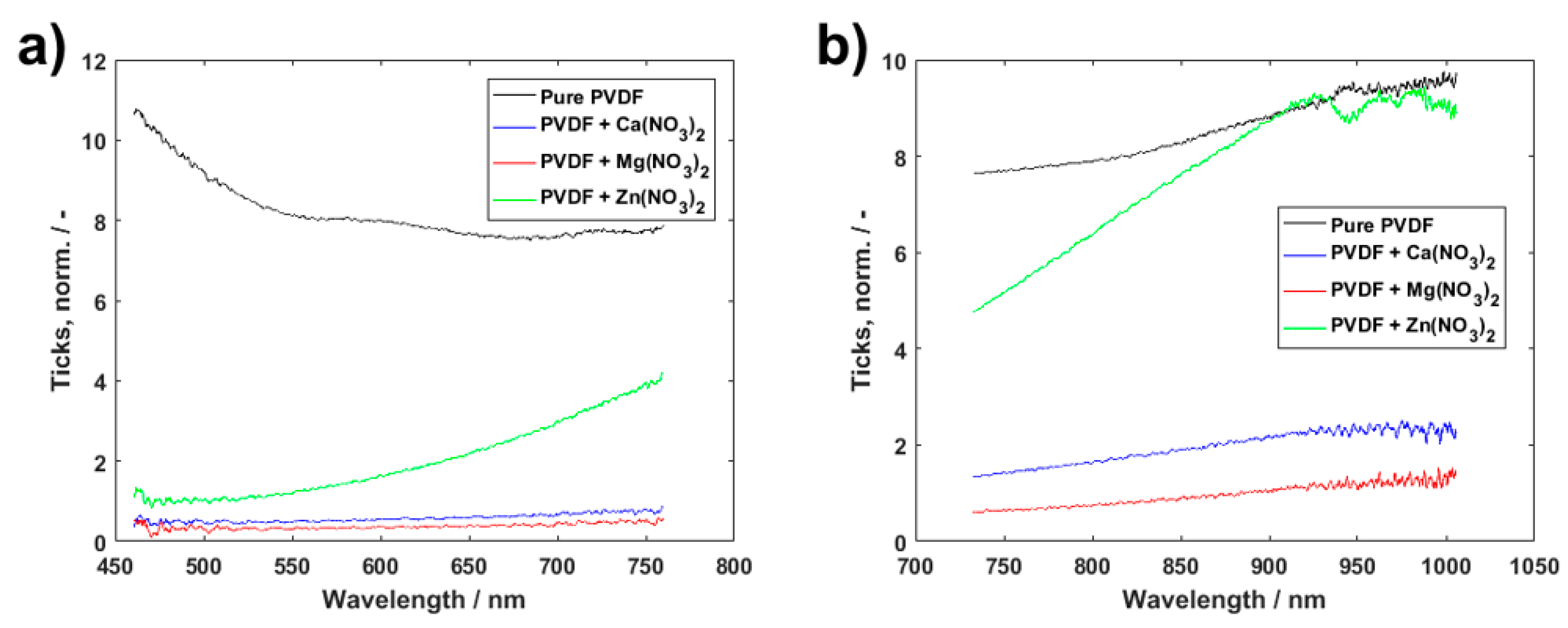
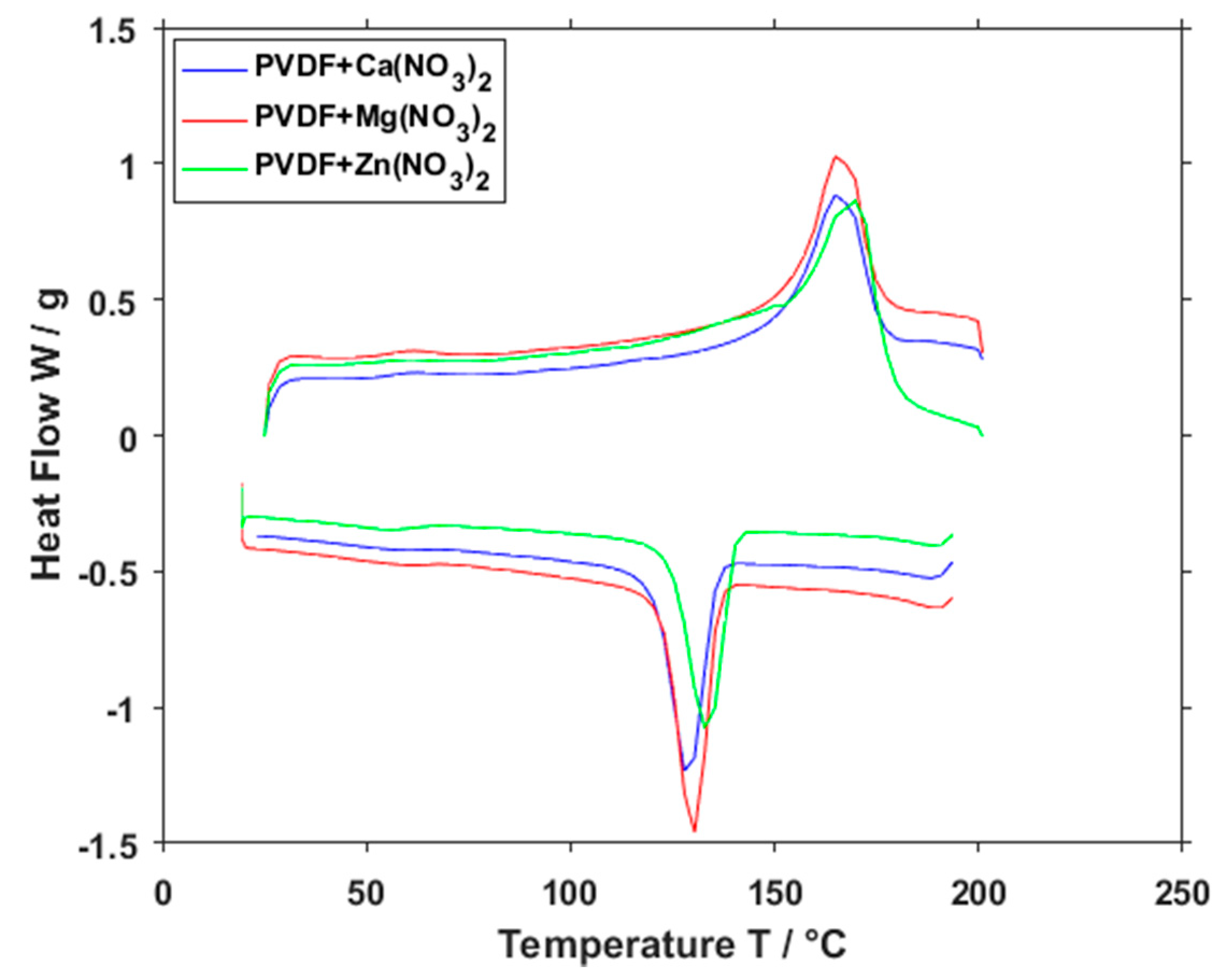
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roopa, T.; Murthy, H.N.; Harish, D.; Jain, A.; Angadi, G. Properties of PVDF films stretched in machine direction. Polym. Polym. Compos. 2021, 29, 198–206. [Google Scholar] [CrossRef]
- Arshad, A.N.; Wahid, M.H.M.; Rusop, M.; Majid, W.H.A.; Subban, R.H.Y.; Rozana, M.D. Dielectric and Structural Properties of Poly(vinylidene fluoride) (PVDF) and Poly(vinylidene fluoride-trifluoroethylene) (PVDF-TrFE) Filled with Magnesium Oxide Nanofillers. J. Nanomater. 2019, 2019, 1–12. [Google Scholar] [CrossRef]
- Ruan, L.; Yao, X.; Chang, Y.; Zhou, L.; Qin, G.; Zhang, X. Properties and Applications of the β Phase Poly(vinylidene fluoride). Polymers 2018, 10, 228. [Google Scholar] [CrossRef] [Green Version]
- McKeen, L.W. Fluorpolymers. In Fatigue and Tribological Properties of Plastics and Elastomers; Elsevier: Kidlington, UK, 2016; pp. 291–315. [Google Scholar]
- Wu, C.-M.; Chou, M.-H.; Zeng, W.-Y. Piezoelectric Response of Aligned Electrospun Polyvinylidene Fluoride/Carbon Nanotube Nanofibrous Membranes. Nanomaterials 2018, 8, 420. [Google Scholar] [CrossRef] [Green Version]
- Castkova, K.; Kastyl, J.; Sobola, D.; Petrus, J.; Stastna, E.; Riha, D.; Tofel, P. Structure–properties relationship of electrospun pvdf fibers. Nanomaterials 2020, 10, 1221. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, F.; Shamshirsaz, M.; Latifi, M. Investigation of β phase formation in piezoelectric response of electrospun polyvinylidene fluoride nanofibers: LiCl additive and increasing fibers tension. Polym. Eng. Sci. 2016, 56, 61–70. [Google Scholar] [CrossRef]
- Mokhtari, F.; Shamshirsaz, M.; Latifi, M.; Asadi, S. Comparative evaluation of piezoelectric response of electrospun PVDF (polyvinilydine fluoride) nanofiber with various additives for energy scavenging application. J. Text. Inst. 2016, 108, 906–914. [Google Scholar] [CrossRef]
- Al Abdullah, K.; Batal, M.A.; Hamdan, R.; Khalil, T.; Zaraket, J.; Aillerie, M.; Salame, C. The Enhancement of PVDF Pyroelectricity (Pyroelectric Coefficient and Dipole Moment) by Inclusions. Energy Procedia 2017, 119, 545–555. [Google Scholar] [CrossRef]
- Kaspar, P.; Sobola, D.; Částková, K.; Knápek, A.; Burda, D.; Orudzhev, F.; Dallaev, R.; Tofel, P.; Trčka, T.; Grmela, L.; et al. Characterization of polyvinylidene fluoride (Pvdf) electrospun fibers doped by carbon flakes. Polymers 2020, 12, 2766. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, P.; Sobola, D.; Částková, K.; Dallaev, R.; Šťastná, E.; Sedlák, P.; Knápek, A.; Trčka, T.; Holcman, V. Case study of polyvinylidene fluoride doping by carbon nanotubes. Materials 2021, 14, 1428. [Google Scholar] [CrossRef]
- Wissbrun, K.F.; Hannon, M.J. Interaction of inorganic salts with polar polymers. II. Infrared studies of polymer–inorganic nitrate systems. In J. Polym. Sci. Polym. Phys. Ed.; 1975; Volume 13, pp. 223–241. [Google Scholar] [CrossRef]
- Prasad, G.; Sathiyanathan, P.; Prabu, A.A.; Kim, K.J. Piezoelectric characteristics of electrospun PVDF as a function of phase-separation temperature and metal salt content. Macromol. Res. 2017, 25, 981–988. [Google Scholar] [CrossRef]
- Akashi, N.; Kuroda, S. Protein immobilization onto poly (vinylidene fluoride) microporous membranes activated by the atmospheric pressure low temperature plasma. Polymer 2014, 55, 2780–2791. [Google Scholar] [CrossRef] [Green Version]
- Fernadéz, V.; Sotiropoulus, T.; Brown, P. Foliar Fertilization: Scientific Principles and Field Practices; Statewide Agricultural Land Use Baseline: Honolulu, HI, USA, 2013; Volume 1, p. 112. [Google Scholar]
- Ghodsi, A.; Fashandi, H.; Zarrebini, M.; Abolhasani, M.M.; Gorji, M. Highly effective CO2 capture using super-fine PVDF hollow fiber membranes with sub-layer large cavities. RSC Adv. 2015, 5, 92234–92253. [Google Scholar] [CrossRef]
- Bormashenko, Y.; Pogreb, R.; Stanevsky, O.; Bormashenko, E. Vibrational spectrum of PVDF and its interpretation. Polym. Test. 2004, 23, 791–796. [Google Scholar] [CrossRef]
- Patro, T.U.; Mhalgi, M.V.; Khakhar, D.V.; Misra, A. Studies on poly(vinylidene fluoride)–clay nanocomposites: Effect of different clay modifiers. Polymer 2008, 49, 3486–3499. [Google Scholar] [CrossRef]
- Baji, A.; Mai, Y.-W.; Li, Q.; Liu, Y. Electrospinning induced ferroelectricity in poly(vinylidene fluoride) fibers. Nanoscale 2011, 3, 3068. [Google Scholar] [CrossRef] [PubMed]
- Thakur, P.; Kool, A.; Bagchi, B.; Hoque, N.A.; Das, S.; Nandy, P. In situ synthesis of Ni(OH) 2 nanobelt modified electroactive poly(vinylidene fluoride) thin films: Remarkable improvement in dielectric properties. Phys. Chem. Chem. Phys. 2015, 17, 13082–13091. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Lei, T.; Sun, D.; Lin, L. A critical analysis of the α, β and γ phases in poly(vinylidene fluoride) using FTIR. RSC Adv. 2017, 7, 15382–15389. [Google Scholar] [CrossRef] [Green Version]
- Gregorio, R. Determination of the α, β, and γ crystalline phases of poly(vinylidene fluoride) films prepared at different conditions. J. Appl. Polym. Sci. 2006, 100, 3272–3279. [Google Scholar] [CrossRef]
- Sarkar, R.; Kundu, T.K. Hydrogen bond interactions of hydrated aluminum nitrate with PVDF, PVDF-TrFE, and PVDF-HFP: A density functional theory-based illustration. Int. J. Quantum Chem. 2020, 120, e26328. [Google Scholar] [CrossRef]
- Constantino, C.J.L.; Job, A.E.; Simões, R.D.; Giacometti, J.A.; Zucolotto, V.; Oliveira, O.N.; Gozzi, G.; Chinaglia, D.L. Phase Transition in Poly(Vinylidene Fluoride) Investigated with Micro-Raman Spectroscopy. Appl. Spectrosc. 2005, 59, 275–279. [Google Scholar] [CrossRef]
- Brooker, M.H. Raman and i.r. spectra of zinc, cadmium and calcium nitrate: A study of the low temperature phase transitions in calcium nitrate. Spectrochim. Acta Part. A Mol. Spectrosc. 1976, 32, 369–377. [Google Scholar] [CrossRef]
- Yaqoob, U.; Uddin, A.S.M.I.; Chung, G.-S. The effect of reduced graphene oxide on the dielectric and ferroelectric properties of PVDF–BaTiO 3 nanocomposites. RSC Adv. 2016, 6, 30747–30754. [Google Scholar] [CrossRef]
- Huang, N.; Short, M.; Zhao, J.; Wang, H.; Lui, H.; Korbelik, M.; Zeng, H. Full range characterization of the Raman spectra of organs in a murine model. Opt. Express 2011, 19, 22892. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lin, Y.; Lee, M.; Malde, C.; Wang, R. Development of low mass-transfer-resistance fluorinated TiO2-SiO2/PVDF composite hollow fiber membrane used for biogas upgrading in gas-liquid membrane contactor. J. Memb. Sci. 2018, 552, 253–264. [Google Scholar] [CrossRef]
- Seki, T.; Chiang, K.-Y.; Yu, C.-C.; Yu, X.; Okuno, M.; Hunger, J.; Nagata, Y.; Bonn, M. The Bending Mode of Water: A Powerful Probe for Hydrogen Bond Structure of Aqueous Systems. J. Phys. Chem. Lett. 2020, 11, 8459–8469. [Google Scholar] [CrossRef]
- Yu, L.; Wang, S.; Li, Y.; Chen, D.; Liang, C.; Lei, T.; Sun, D.; Zhao, Y.; Wang, L. Piezoelectric performance of aligned PVDF nanofibers fabricated by electrospinning and mechanical spinning. In Proceedings of the 2013 13th IEEE International Conference on Nanotechnology (IEEE-NANO 2013), Beijing, China, 5–8 August 2013; IEEE: Beijing, China, 2013; pp. 962–966. [Google Scholar] [CrossRef]
- Fortunato, M.; Chandraiahgari, C.R.; De Bellis, G.; Ballirano, P.; Sarto, F.; Tamburrano, A.; Sarto, M.S. Piezoelectric Effect and Electroactive Phase Nucleation in Self-Standing Films of Unpoled PVDF Nanocomposite Films. Nanomater 2018, 8, 743. [Google Scholar] [CrossRef] [Green Version]
- Francisco, O.A.; Glor, H.M.; Khajehpour, M. Salt Effects on Hydrophobic Solvation: Is the Observed Salt Specificity the Result of Excluded Volume Effects or Water Mediated Ion-Hydrophobe Association? ChemPhysChem 2020, 21, 484–493. [Google Scholar] [CrossRef]
- Merlini, C.; Barra, G.M.O.; Medeiros Araujo, T.; Pegoretti, A. Electrically pressure sensitive poly(vinylidene fluoride)/polypyrrole electrospun mats. RSC Adv. 2014, 4, 15749–15758. [Google Scholar] [CrossRef]
- Dhakras, D.; Borkar, V.; Ogale, S.; Jog, J. Enhanced piezoresponse of electrospun PVDF mats with a touch of nickel chloride hexahydrate salt. Nanoscale 2012, 4, 752–756. [Google Scholar] [CrossRef] [PubMed]

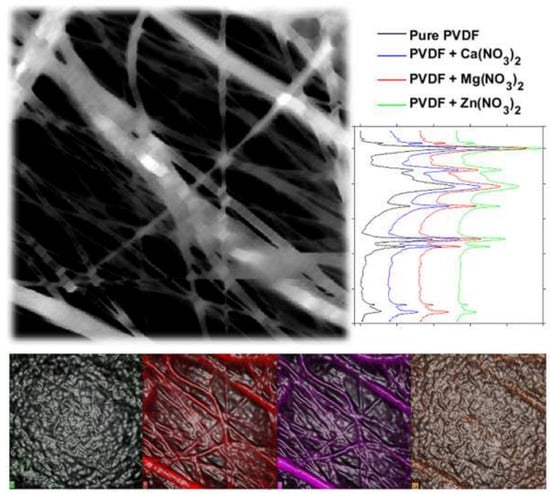
| Average/° | Median/° | Std. Dev./° | Std. Dev./% | |
|---|---|---|---|---|
| PVDF | 127.1 | 127.6 | 7.6 | 6.0 |
| PVDF with Ca(NO3)2 | 124.1 | 125.2 | 11.9 | 9.6 |
| PVDF with Mg(NO3)2 | 131.3 | 131.4 | 4.6 | 3.5 |
| PVDF with Zn(NO3)2 | 135.9 | 135.9 | 7.8 | 5.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobola, D.; Kaspar, P.; Částková, K.; Dallaev, R.; Papež, N.; Sedlák, P.; Trčka, T.; Orudzhev, F.; Kaštyl, J.; Weiser, A.; et al. PVDF Fibers Modification by Nitrate Salts Doping. Polymers 2021, 13, 2439. https://doi.org/10.3390/polym13152439
Sobola D, Kaspar P, Částková K, Dallaev R, Papež N, Sedlák P, Trčka T, Orudzhev F, Kaštyl J, Weiser A, et al. PVDF Fibers Modification by Nitrate Salts Doping. Polymers. 2021; 13(15):2439. https://doi.org/10.3390/polym13152439
Chicago/Turabian StyleSobola, Dinara, Pavel Kaspar, Klára Částková, Rashid Dallaev, Nikola Papež, Petr Sedlák, Tomáš Trčka, Farid Orudzhev, Jaroslav Kaštyl, Adam Weiser, and et al. 2021. "PVDF Fibers Modification by Nitrate Salts Doping" Polymers 13, no. 15: 2439. https://doi.org/10.3390/polym13152439
APA StyleSobola, D., Kaspar, P., Částková, K., Dallaev, R., Papež, N., Sedlák, P., Trčka, T., Orudzhev, F., Kaštyl, J., Weiser, A., Knápek, A., & Holcman, V. (2021). PVDF Fibers Modification by Nitrate Salts Doping. Polymers, 13(15), 2439. https://doi.org/10.3390/polym13152439