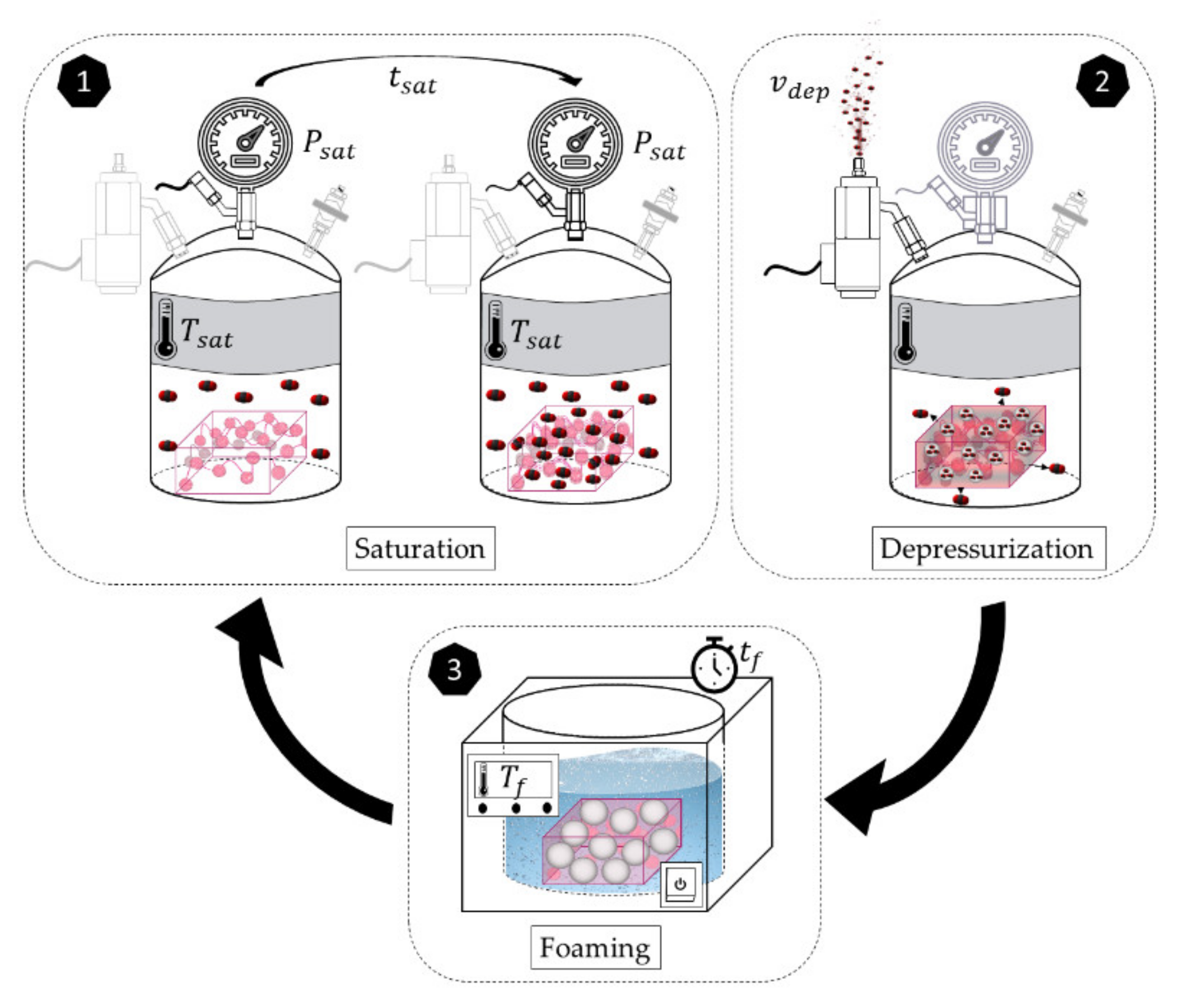
1. Introduction
Nanocellular materials have aroused great attention due to a combination of outstanding properties, such as low thermal conductivity, improved mechanical properties, the possibility of retaining solid polymer transparency after the foaming process, among others [
1,
2,
3,
4]. Industries such as the automotive one or the building sector could improve their current designs by using these materials. However, so as to take advantage of properties, such as the thermal insulation, it is mandatory to reduce the density of the cellular material in addition to the cell size [
5,
6,
7].
Since their discovery, nanocellular materials have been produced using different polymers and through different approaches [
1]. The most common production route is the gas dissolution foaming process using both homogeneous and heterogeneous nucleation mechanisms. Those efforts have led to the production of unique cellular structures [
4,
8,
9]. However, previous studies have demonstrated that there are some limitations regarding the production of these materials. In fact, it is still difficult to produce materials with very small cell sizes and low relative densities [
1,
10,
11].
Polymethylmethacrylate (PMMA) is one of the most common polymers selected for the production of nanocellular polymers. This is due to its great affinity for CO
2, leading to high solubilities [
10,
12,
13]. According to the classical nucleation theory, maximization of the solubility leads to an increase in the number of nucleation points resulting in an easy way to produce cells in the nanometric range by using a homogeneous nucleation approach. Particularly for this polymer, some of the most promising values when the materials are produced by the homogeneous nucleation approach have been presented by Yeh et al. [
14] with cell sizes of 37 nm combined with a relative density of 0.25. However, the reported values were achieved for pellets and not for plates, and therefore the relative density values reported included the solid skin and the transition region with microcells and not only the nanocellular region (these two areas, skin and transition region, are difficult to remove in small pellets). Regarding nanocellular PMMA plates, the better values obtained through homogeneous nucleation have been presented by Martín-de León et al. with a cell size of 74 nm combined with a relative density of 0.24, (values obtained in the nanocellular area of these samples) [
15]. The use of a copolymer (PMMA-co-EMA) allowed Costeux et al. to produce materials with cell sizes of 80 nm with relative densities of 0.17 (values obtained considering skin and transition region) for this particular polymer [
16,
17,
18]. Kumar et al. presented very interesting materials with relative densities of 0.3 and cell sizes of 50 nm, or relative densities of 0.14 with cell sizes of 235 nm, being the reported relative density values with solid skin and transition region [
19].
Regarding heterogeneous nucleation, some interesting results have also been obtained. Pinto et al. produced nanocellular PMMA by adding MAM (block copolymer poly(methyl methacrylate)-poly(butyl acrylate)-poly(methyl methacrylate) with cell sizes around 250 nm and relative densities around 0.5 [
20]. Bernardo et al. reported cell sizes from 200 nm to 300 nm and relative densities of the nanocellular region from 0.23 to 0.47 by adding MAM as nucleating agent [
21]. Wang et al. presented cell sizes of 150 nm with 0.17 of relative density by using TPU as nucleating agent. In this case, the used samples present a cylindrical shape of 5 mm in diameter and 50 mm in length. Density measurements do not include solid skin but they do include the transition region contributing to reduction of the density values [
22]. Costeux et al. reported values of 120 nm-0.15, 99 nm-0.16 and 65 nm-0.26 of cell size-relative density (values including solid skin and transition region) using polyhedral oligomeric silsesquioxane (POSS) as the second phase. However, those works present the main drawback of the necessity of adding this second phase, leading to a more complex process [
23].
On the other hand, when searching for the minimum cell size obtained with this polymer, the smaller values are presented by Martín-de León et al. [
13]. In this work, cell sizes below 50 nm were reported with 39, 24, and 14 nm of cell size combined with relative densities of 0.38, 0.43, and 0.43, respectively, values obtained after removing the skin and transition region.
In summary, plates of nanocellular materials with minimum cell sizes (smaller than 40 nm) have never been reported to have densities below 0.4. While the minimum relative densities reported around 0.15 correspond to materials with cell sizes not smaller than 120 nm.
A new approach for producing nanocellular materials combining small cell sizes with low relative densities is proposed in the present work. This method proposes a cyclic gas dissolution foaming process allowing the production of materials not achievable using the common gas dissolution foaming process. Thus, nanocellular materials have been produced by means of a double saturation process leading to cell sizes as small as 24 nm (very close to the minimum value obtained so far) combined with relative densities of 0.3, a combination of values never reported before.
The influence of the production parameters, for both the first and the second saturation-foaming cycles, on the final cellular structure has been analyzed.
3. Results
3.1. Example of the Nanocellular Materials Obtained When a Double Cycle Is Carried Out
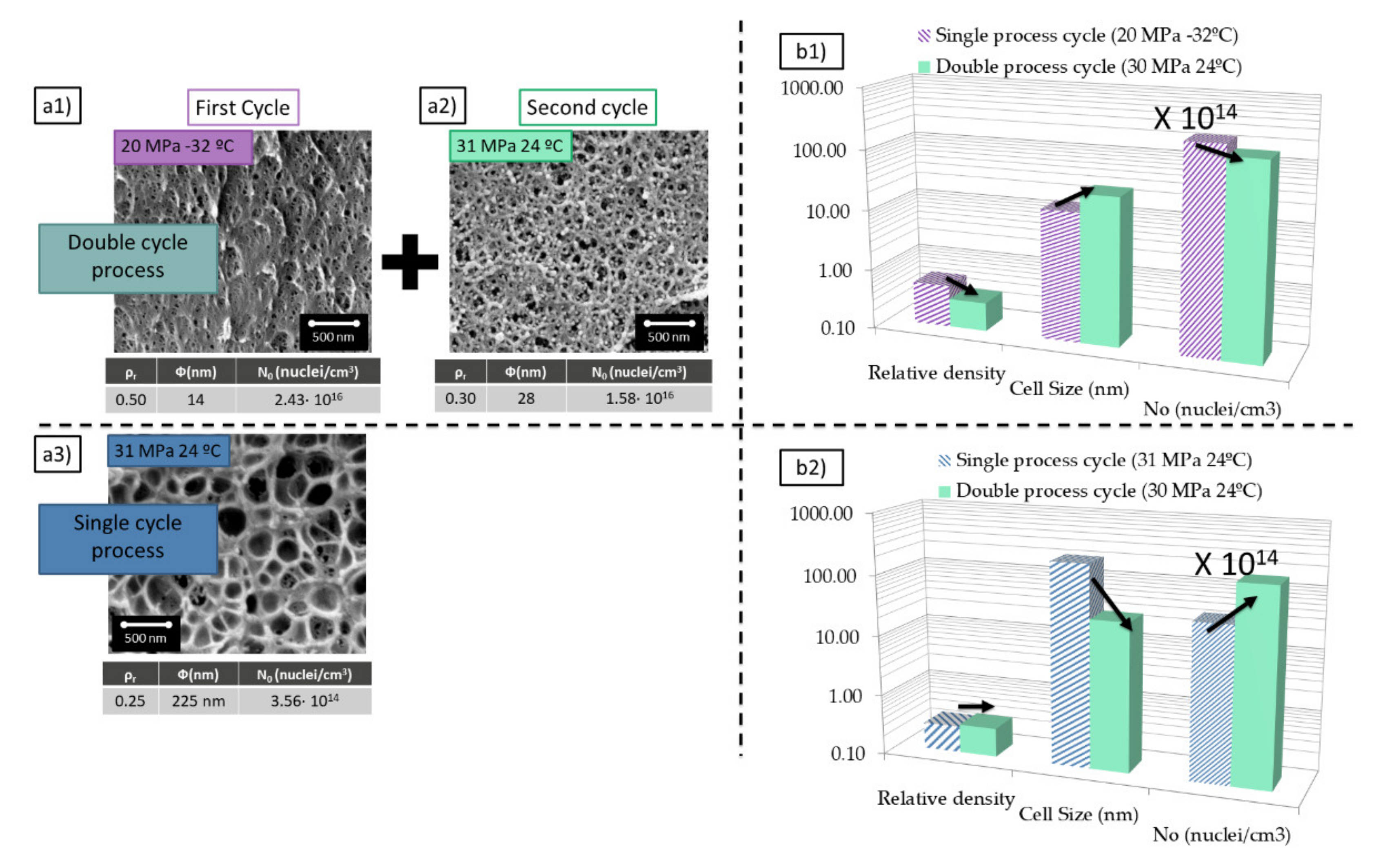
The PMMA grade used in this study shows cellular structures as those shown in the bottom of
Figure 2a.
This material presents cell sizes around 200 nm and relative densities of 0.25 when it is saturated at 31 MPa and 24 °C and foamed at 100 °C for 1 min. These conditions are the ones minimizing the density for this PMMA grade when cell sizes are in the range of 200 nm. The cell nucleation density for these conditions is in the range of 3 × 1014 cells/cm3. The process parameters of this experiment are very the most appropriate to reach low densities at medium cell sizes.
On the other hand, when this material is saturated at 20 MPa and −32 °C presents cell sizes around 14 nm combined with a relative density of 0.5 (foaming at 60 °C for 1 min) (
Figure 2a1). Once again these are the optimum conditions to reduce the density when the cell size is in the range of 14 nm. The cell nucleation density for these particular conditions is in the range of 2.4 × 10
16 cells/cm
3, i.e., two orders of magnitude higher than in the previous conditions. These process parameters minimize cell size but does not allow further reduction of density.
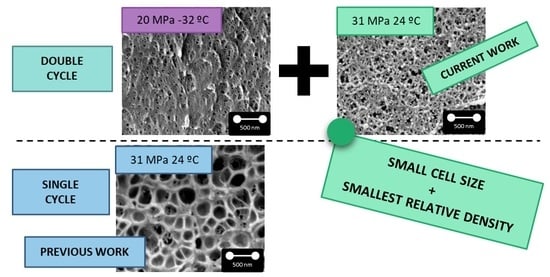
The introduction of a gas dissolution foaming consisting of two cycles that combine the two previous saturation cycles allows obtaining a cellular structure with a combination of characteristics that are not achieved through a single cycle process (
Figure 2a3). Nanocellular PMMA with 28 nm of cell size and a relative density of 0.3 has been produced through a first saturation at 20 MPa and −32 °C and foaming at 60 °C for 1 min and a second saturation at 31 MPa and 24 °C and foaming at 80 °C for 1 min (
Figure 2a2). For these conditions, the cell nucleation density is also very high, with values of 1.6 × 10
16 cells/cm
3. Therefore, using this new approach, it has been possible to keep the cell nucleation density at very high values, but at the same time, it has been possible to reduce the relative density.
The structure obtained in the second cycle compared with the one obtained in the first one presents a significant reduction of the relative density from 0.5 to 0.3 while keeping the cell size and the cell nucleation density in the same order the magnitude (
Figure 2b1).
In comparison with the sample produced with similar conditions in just one cycle (31 MPa 24 °C), the introduction of a double cycle leads to a reduction of one order of magnitude in the cell size and an increase in two orders of magnitude in the cell nucleation density by almost keeping unchanged the relative density (
Figure 2b2).
The achieved nanocellular material produced by the double cycle presents a unique combination of characteristics not reported up to now using a single cycle process.
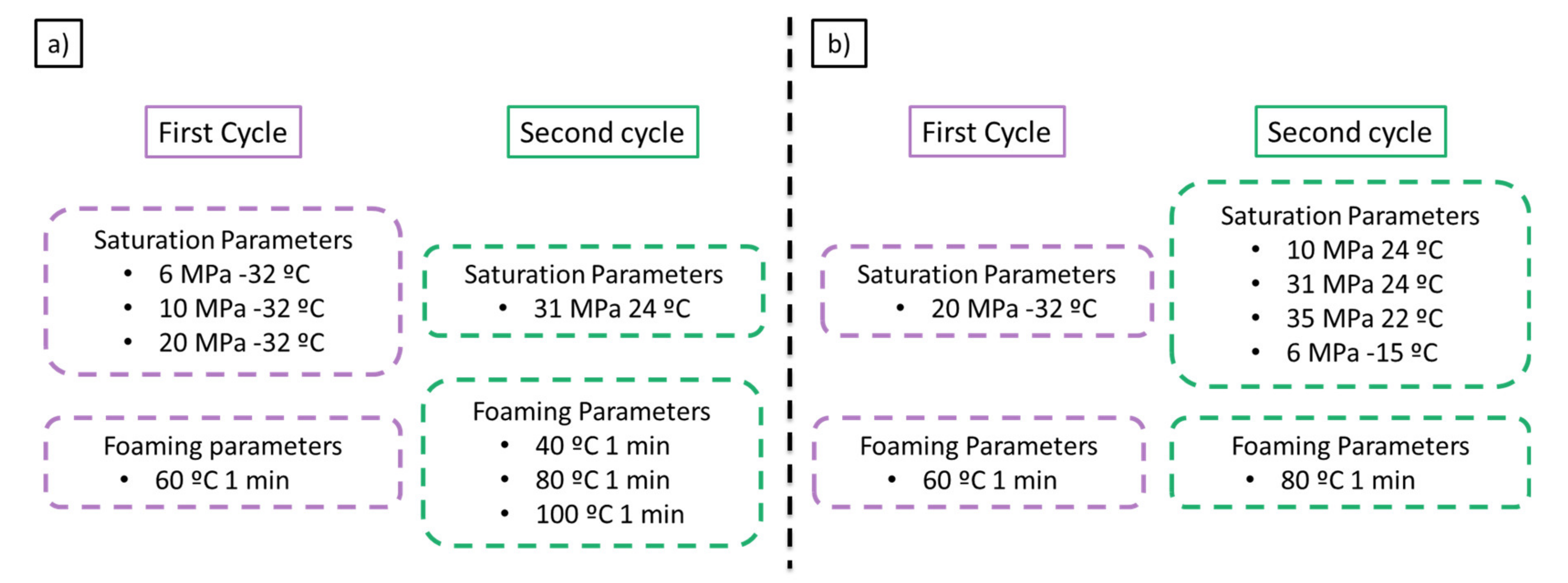
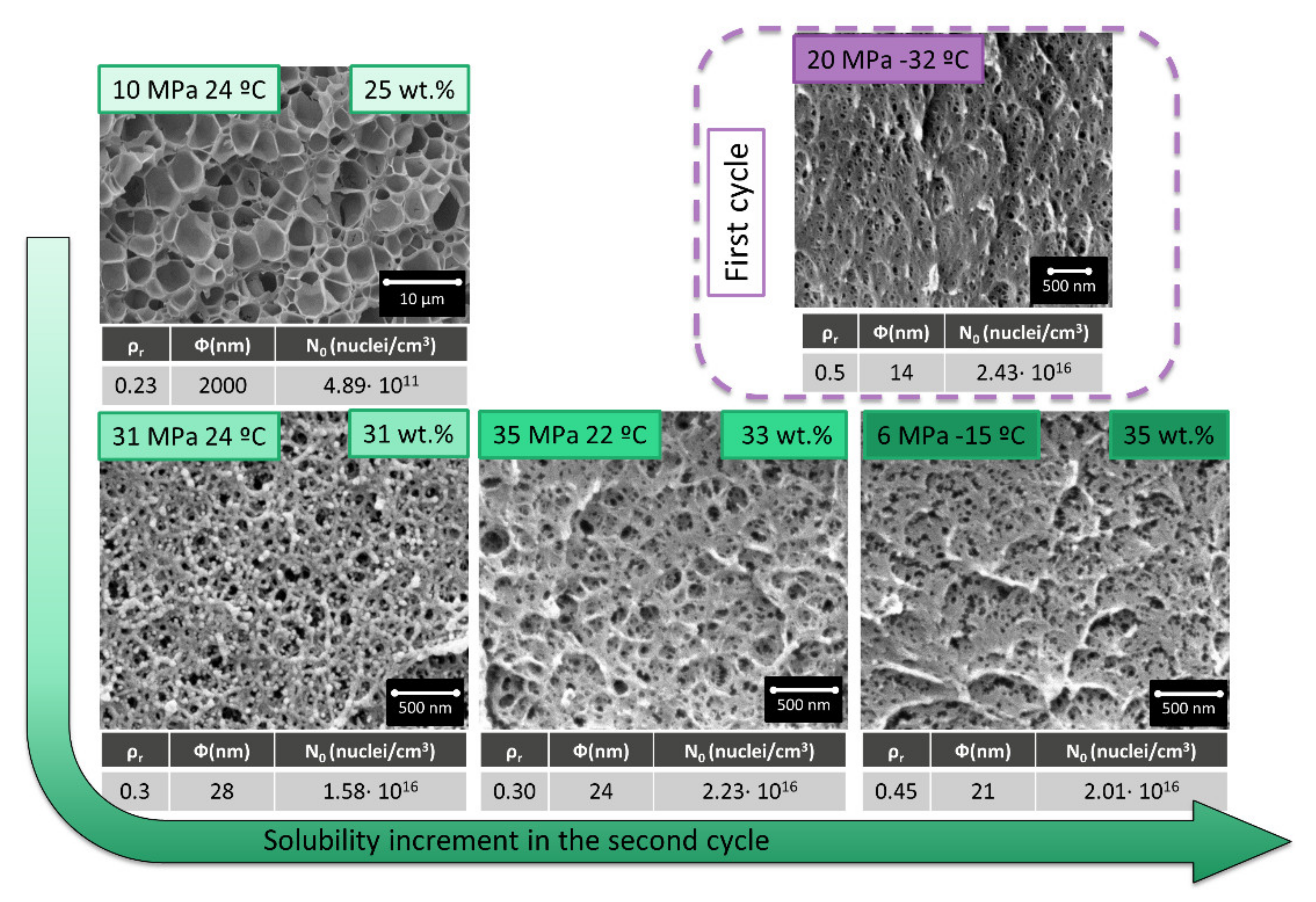
Taking into account the previous promising result a set of experiments were carried out to study the influence of the saturation and foaming parameters on the final cellular structure. Firstly, the effect of the first cycle saturation parameters was studied by changing the saturation pressure while keeping constant the saturation parameters in the second cycle at 31MPa and 24 °C (
Figure 3a). Thus, saturation pressures of 6, 10, and 20 MPa at a saturation temperature of −32 °C were used in the first cycle. The foaming parameters of the second cycle have also been studied, changing the foaming temperature between 40, 80, and 100 °C with a foaming time of 1 min.
Secondly, the influence of the second cycle saturation parameters was studied while keeping constant the first cycle parameters in 20 MPa and −32 °C (
Figure 3b). Saturation conditions allowing an increase in solubility in the second cycle were selected to be 10 MPa and 24 °C, 31 MPa and 24 °C, 35 MPa and 22 °C, and 6 MPa −15 °C. PMMA solubility at those conditions is 24.6 wt.%, 31.3 wt.%, 32.5 wt.%, and 34.8 wt.%, respectively.
The consequences for the cell size, cell nucleation density and relative density of all those modifications are detailed in the following sections.
3.2. Influence of the Parameters of the First Cycle
An increase in the saturation pressure in the first cycle leads to an increase in solubility in the material. Thus 6 MPa leads to 38.6 wt.% of CO
2 uptake, 10 MPa to 41.0 wt.% and 20 MPa to 45.5 wt.%. As expected, smaller cell sizes and higher cell nucleation densities are obtained, as has been previously reported in the literature [
15].
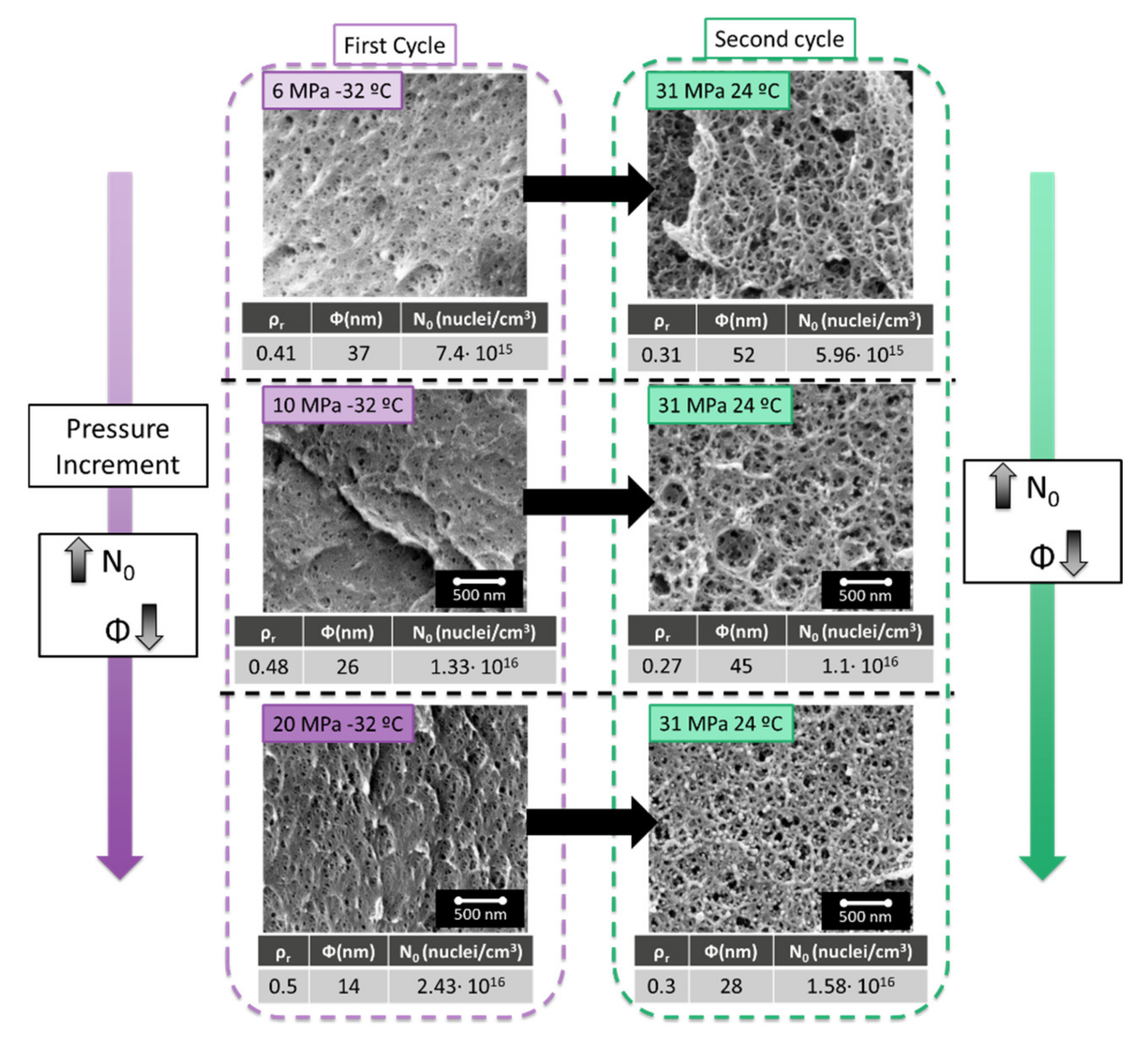
As shown in
Figure 4, the cellular structures obtained after a second cycle performed at 31 MPa and 24 °C present a smaller cell size when the cell size of the starting cellular material obtained after the first cycle is also small. The results are similar for the cell nucleation density. The higher the cell nucleation density in the first cycle, the higher is the one obtained in the second one. The materials produced present interesting characteristics with a cell density over 10
16 cells/cm
3 and low relative densities.
In conclusion, to minimize the cell size and maximize the cell nucleation density after the second cycle, the same should be done in the first cycle. Therefore, it is beneficial to maximize the solubility in the first cycle. On the other hand, the foaming parameters allowing a maximum cell nucleation densities in the first cycle were 60 °C and 1 min for 20 MPa and −32 °C of saturation pressure [
13]. Those conditions have been therefore used in the next section of this paper.
Foaming temperatures in the second cycle have also been evaluated foaming at 40, 80, and 100 °C for 1 min.
Table 1 shows this effect in samples with a fixed first cycle (20 MPa −32 °C foamed at 60 °C for 1 min) and a second cycle performed at 31 MPa 24 °C and foaming at these three temperatures for 1 min. As it can be seen relative densities were minimum through foaming at 80 °C, due to the maximization of the number of nucleation points at this temperature. Thus, for the following sections, these foaming conditions were fixed.
The results obtained in this section can be used to hypothesize about the foaming mechanisms taking place in this cycling gas dissolution foaming. As it is observed in the experimental results the cell nucleation density is almost kept when the second cycle is applied. As this second cycle has been carried out at conditions that typically provide cell nucleation densities in the range of 1014 cells/cm3, the cells in the final materials should come from the first foaming step. Therefore, we can conclude that a very high number of cells is created in the first cycle, and then the second cycle is used mainly for growth of these cells, reducing the relative density.
3.3. Influence of the Saturation Parameters of the Second Cycle
Considering the information extracted from the previous section, the first cycle parameters have been fixed in 20 MPa and −32 °C as saturation parameters and 60 °C and 1 min as foaming parameters. These are the conditions giving the maximum cell nucleation density.
A second cycle of gas dissolution foaming was applied to this material using four different conditions, each of them leading to a higher solubility, as previously indicated.
As it can be seen in
Figure 5, an increase in the solubility in the second cycle leads to a reduction in the cell size and an increase in the cell nucleation density.
Those results can be deeply analyzed by measuring the relative density, cell size, and cell nucleation density (
Figure 6). The dashed line in
Figure 6 indicates the values for a material produced in a single cycle at 20 MPa −32 °C, with a cell size of 14 nm, a cell nucleation density of 2.43 × 10
16 nuclei/cm
3, and a relative density of 0.5. As previously commented, those cell size values are the smallest reported in the literature for PMMA, with a relative density of 0.5.
Cellular structures presented after a second cycle have a decreasing cell size and an increasing cell nucleation density as solubility rises. However, the most remarkable factor is the obtained relative density, which is always smaller than the one presented for the initial material.
Poor solubility in the second cycle of 25 wt.% (obtained at 10 MPa and 24 °C) leads to cells bigger than the micron, while the most extreme conditions of 6 MPa and −15 °C (35 wt.% of gas uptake) leads to a cellular structure very similar in density and with a larger cell size than the initial one. The most remarkable cellular structures are those obtained at intermediate saturation conditions. Saturation parameters of 30 MPa 24 °C and 35 MPa 22 °C, leads to cell sizes clearly below 50 nm and cell nucleation densities that are almost as high as that of the material obtained in the first cycle. However, the relative density of those materials is much smaller (0.3), being this combination of cellular structure parameters unique in the previous literature when only the nanocellular structure is analyzed.
The obtained results can be explained as follows: in the first cycle, a high amount of small cells are created, while in the second cycle, those cells are preferential sites to either nucleate or further grow due to the pre-existing cell decrease the energy barrier for both processes. That means in this process, it can be assumed that nucleation occurs in the first cycle and those cells can further growth in the second one if the conditions are favorable. When saturation parameters are as soft as 10 MPa 24 °C, the solubility is so small that the energy is not enough to take advantage of the previous nucleation points leading to microcellular structures. However, for both intermediate saturation conditions, 14 nm cells act as nucleation points that are further growth. When comparing the cell nucleation densities in the second and in the first cycle, 92% of the nuclei in the first cycle are able to grow in the second one. The initial 14 nm cells grow up to 24 nm leading to the observed reduction in the relative density.
Saturation conditions of 6 MPa and −15 °C lead to such high solubility that the effect is not so notable, although the mechanisms seem to remain the same. All the nucleation points are used, and slightly further growth occurs. The cell size is kept in minimal values, and the reduction in relative density is not so large.
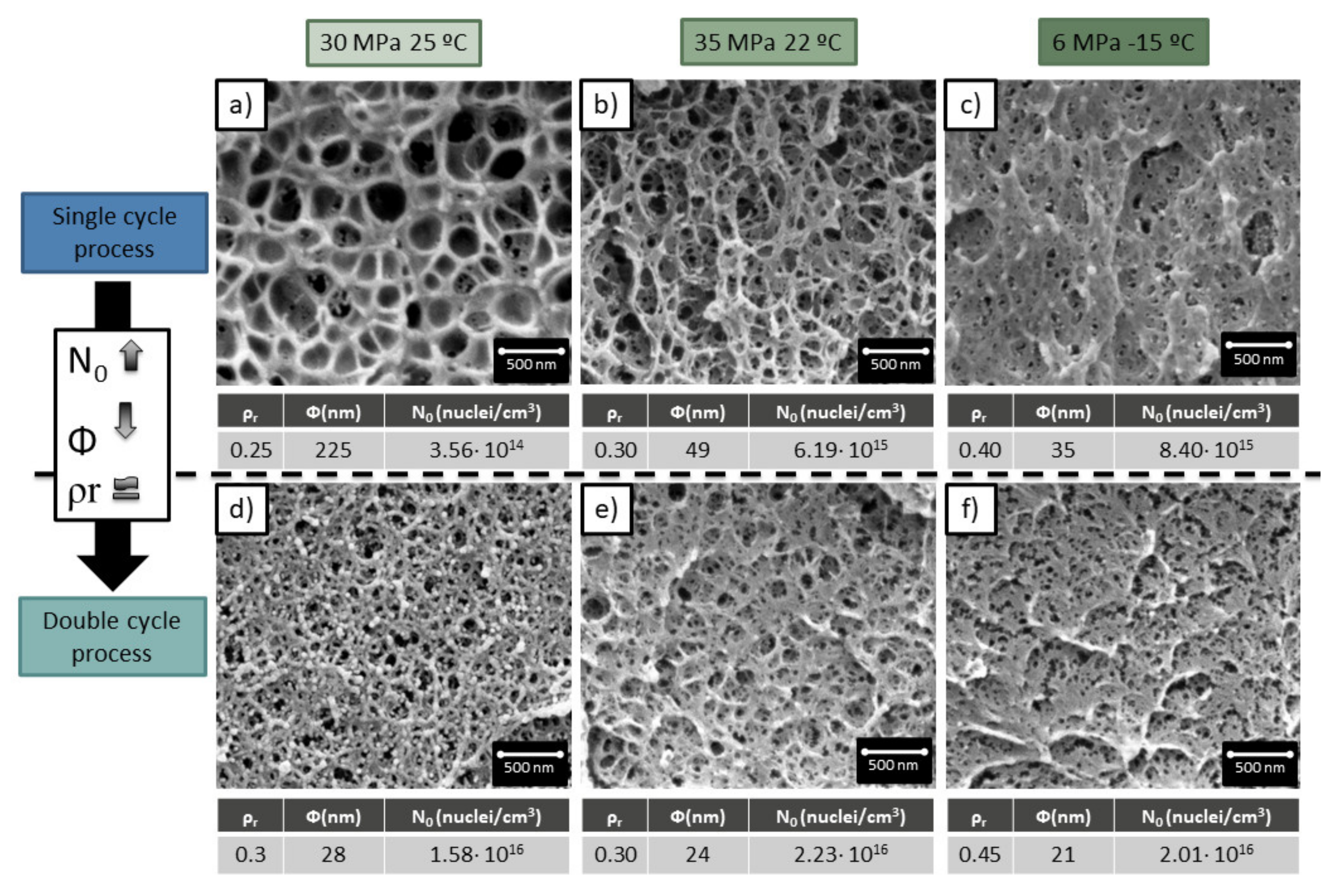
3.4. Single Cycle Process vs. Double Cycle Process
In previous sections, the materials obtained with a double cycle process were compared with the ones obtained in the first cycle.
This last section is dedicated to comparing the materials obtained through a double cycle with samples produced in a single cycle process with the same production conditions of the second cycle of the double cycle samples. That means that a sample produced through a double saturation, first one at 20 MPa −32 °C and second one at 35 MPa 22 °C, will be compared with a sample produced through a single saturation at 35 MPa and 22 °C.
As is shown in
Figure 7, a double cycle process results in smaller cell sizes and higher cell nucleation densities. This confirms the heterogeneous nucleation theory proposed in the previous section.
Although the cell sizes have been strongly reduced with respect to the single cycle process, the significant increment of cell nucleation density, the low relative density is almost maintained.
Therefore, it can be concluded that the newly proposed method leads to cellular materials with characteristics not achievable through the common gas dissolution process. The double cycle process allows producing cell sizes of 24 nm with relative densities of 0.3, being the minimum density reported in the literature of 0.43 for such cell sizes and cell nucleation densities of 2 × 1016 cells/cm3.
4. Conclusions
A double cycle gas dissolution foaming process is proposed in this work for the production of nanocellular PMMA. This process consists of a double saturation and foaming process. The PMMA is firstly saturated and foamed at certain conditions. The obtained nanocellular material is again saturated and foamed.
The influence of the production parameters has been studied. To minimize the cell size of the final material, it has been demonstrated that the cell size should also be minimum in the first cycle. A minimum cell size is reached through a maximum solubility; thus, 20 MPa and −32 °C were selected as the optimum saturation parameters in this work for the first cycle. The foaming parameters in the first cycle have been chosen to be 60 °C and 1 min to maximize the cell nucleation density.
Introducing a second cycle could lead to further growth of the cells created in the first cycle. The influence of the second cycle parameters has been studied, proving that there exists an optimum range of saturation parameters in the second cycle. Thus, a saturation pressure of 35 MPa and a saturation temperature of 22 °C leads to the production of nanocellular PMMA, combining a cell size of 24 nm with a relative density of 0.3. Those values have not been reached through a single gas dissolution foaming process for materials produced with the shape of a plate in which the skin and transition layer have been removed, herein presented for the first time.