Biofabrication of Cell-Laden Gelatin Methacryloyl Hydrogels with Incorporation of Silanized Hydroxyapatite by Visible Light Projection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Gelatin Methacryloyl Hydrogels
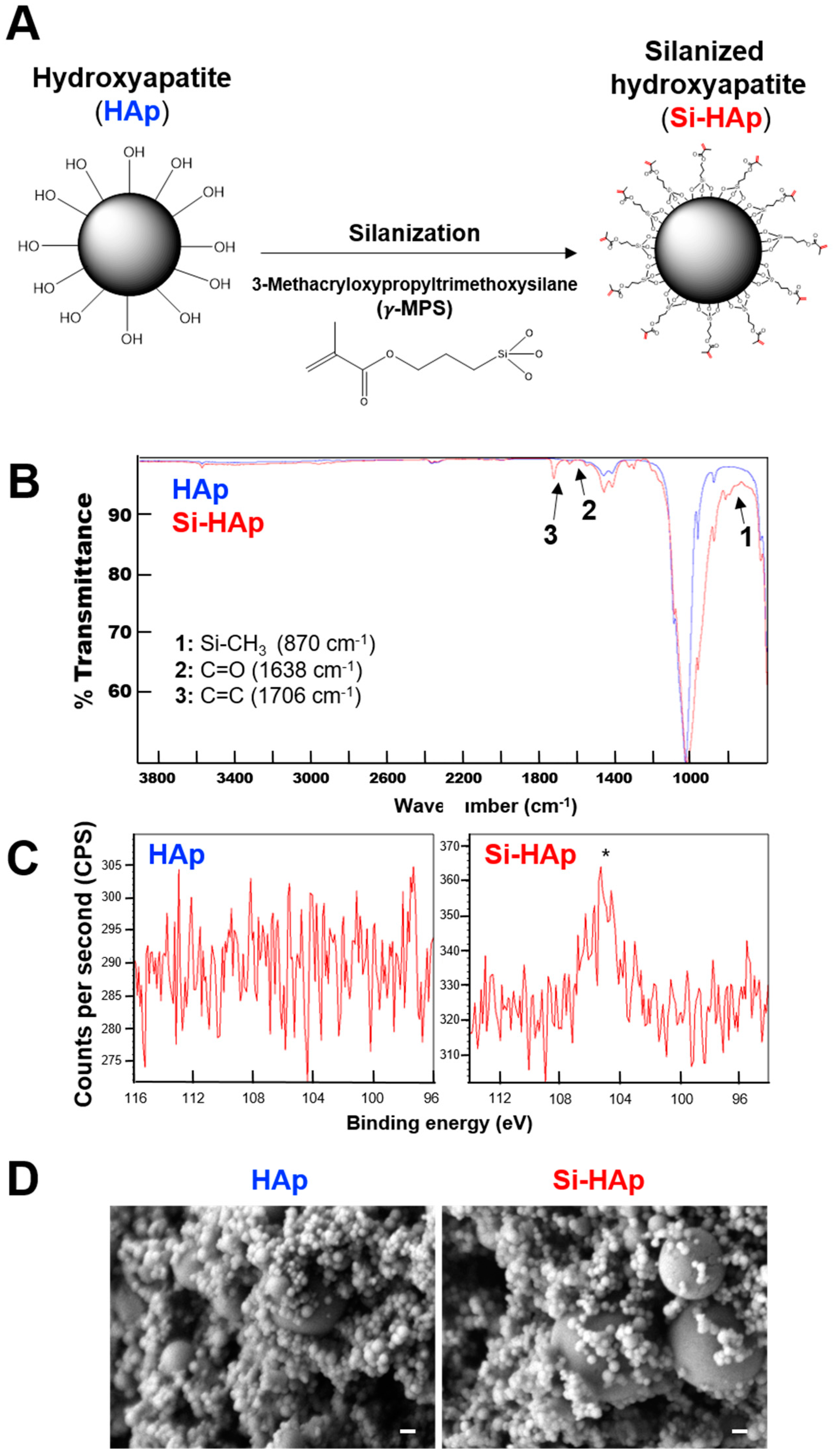
2.2. Silanization of HAp
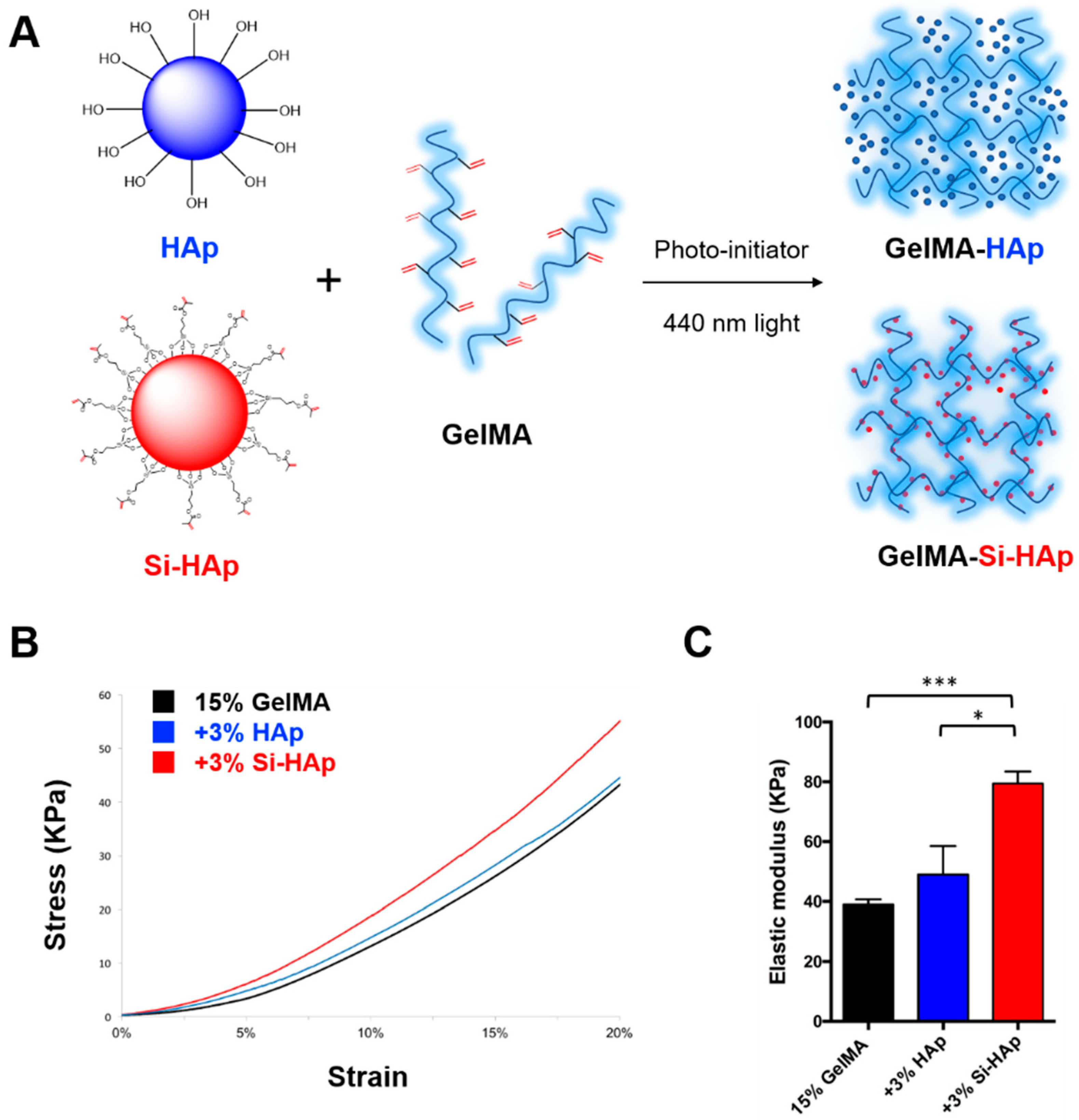
2.3. Preparation of GelMA-HAp Composite Hydrogels
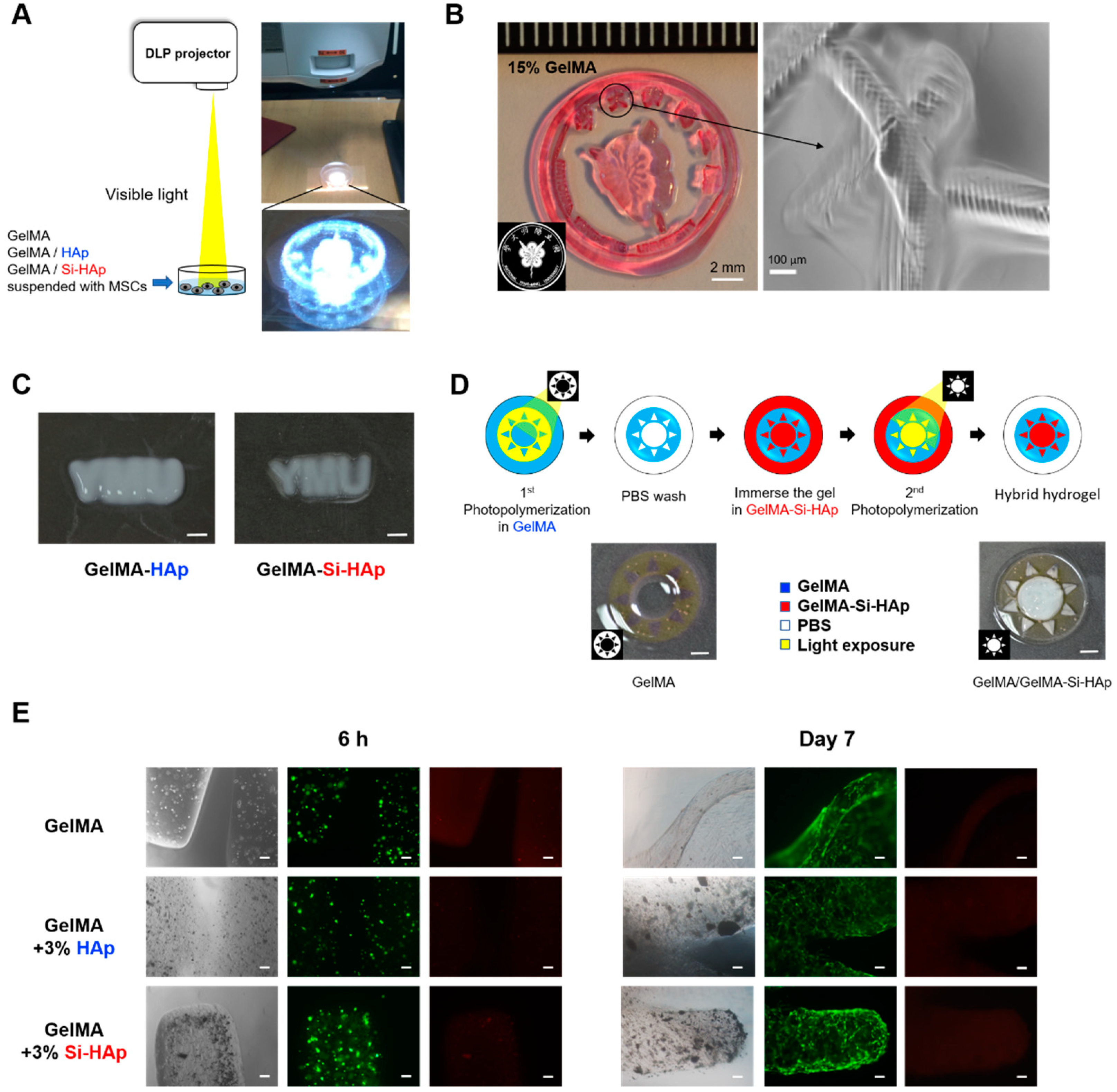
2.4. Projection Photolithography of GelMA Composite Hydrogels Using a DLP-Based Projector
2.5. Characterization of Si-HAp Powders
2.6. Mechanical Properties of GelMA Composites
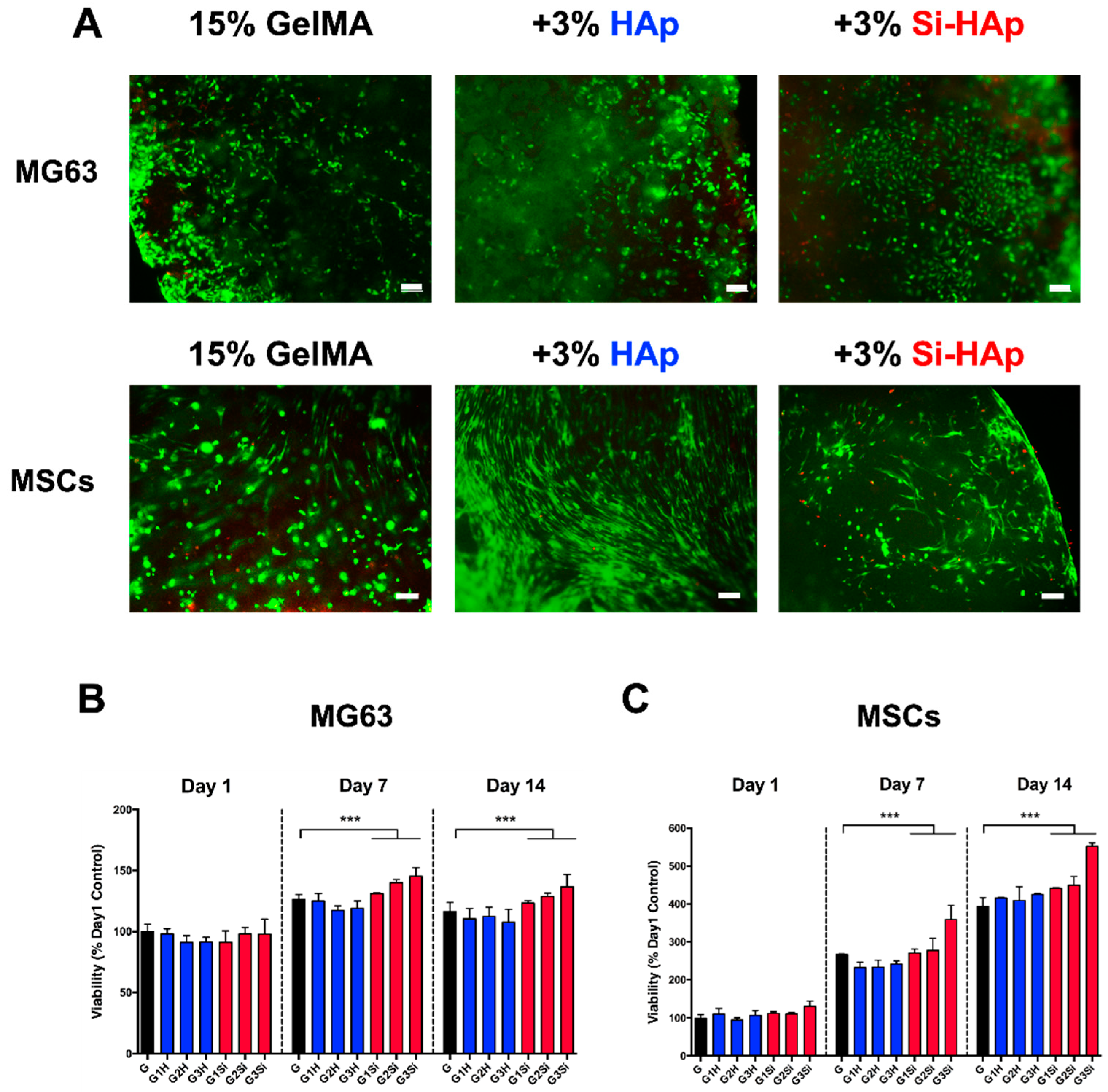
2.7. Culture of Cells
2.8. Viability of MG63 Cells and MSCs within the GelMA Composite
2.9. Cell Proliferation Tests Using MTT Assay
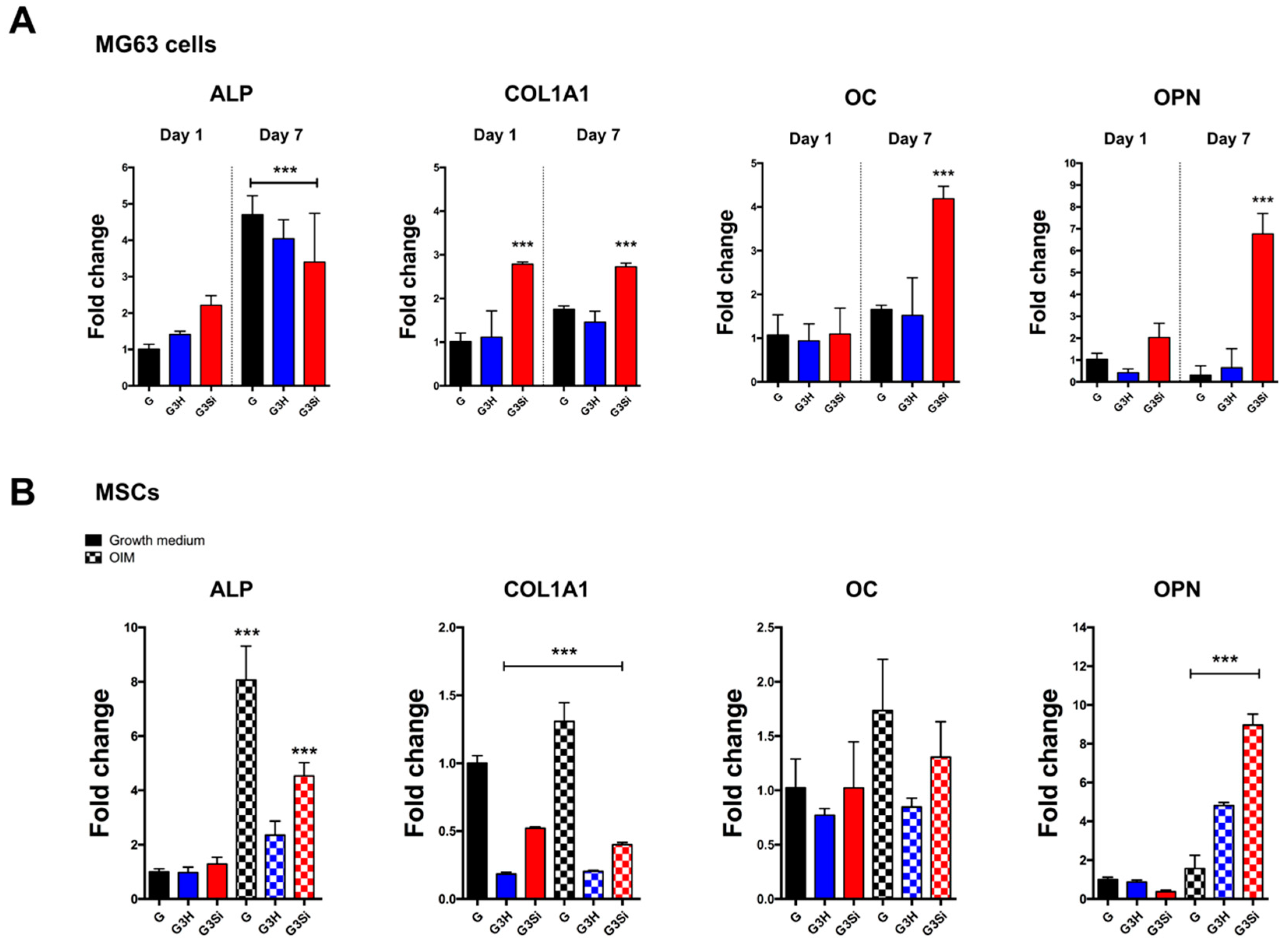
2.10. RNA Isolation and Real-Time Reverse Transcription-PCR
2.11. Statistical Analysis
3. Results and Discussion
3.1. Surface Modification of HAp
3.2. Dispersion of Si-HAp in the GelMA Hydrogel
3.3. Mechanical Properties of Composite Hydrogels
3.4. Cell Encapsulation in the GelMA–Si-HAp Hydrogel
3.5. Osteogenic Differentiation of Encapsulated Cells in the GelMA–Si-HAp Hydrogel
3.6. Photolithography of the GelMA-Si-HAp Composite Hydrogels with Visible Light
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perić Kačarević, Ž.; Rider, P.; Alkildani, S.; Retnasingh, S.; Pejakić, M.; Schnettler, R.; Gosau, M.; Smeets, R.; Jung, O.; Barbeck, M. An introduction to bone tissue engineering. Int. J. Artif. Organs. 2020, 43, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Yue, S.; He, H.; Li, B.; Hou, T. Hydrogel as a biomaterial for bone tissue engineering: A review. Nanomaterials 2020, 10, 1511. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Guillén, M.C.; Giménez, B.; López-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef] [Green Version]
- Qiao, Y.; Liu, X.; Zhou, X.; Zhang, H.; Zhang, W.; Xiao, W.; Pan, G.; Cui, W.; Santos, H.A.; Shi, Q. Gelatin Templated polypeptide co-cross-linked hydrogel for bone regeneration. Adv. Healthc. Mater. 2020, 9, 1901239. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bello, A.B.; Kim, D.; Kim, D.; Park, H.; Lee, S.-H. Engineering and functionalization of gelatin biomaterials: From cell culture to medical applications. Tissue Eng. Part B Rev. 2020, 26, 164–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amirian, J.; Abdi, G.; Shekh, M.I.; Zendehdel, E.A.; Du, B.; Stadler, F.J. Gelatin Based Hydrogels for Tissue Engineering and Drug Delivery Applications. Nanohybrids Future Mater. Biomed. Appl. 2021, 87, 244–270. [Google Scholar]
- Klotz, B.J.; Gawlitta, D.; Rosenberg, A.J.W.P.; Malda, J.; Melchels, F.P.W. Gelatin-Methacryloyl Hydrogels: Towards Biofabrication-Based Tissue Repair. Trends Biotechnol. 2016, 34, 394–407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, M.; Sun, X.; Wang, Z.; Guo, S.; Yu, G.; Yang, H. Synthesis and properties of gelatin methacryloyl (GelMA) hydrogels and their recent applications in load-bearing tissue. Polymers 2018, 10, 1290. [Google Scholar] [CrossRef] [Green Version]
- Adnan, M.; Hani, A.A.; Hussain, M.A.; Musab, A.; Fozia Al, N.; Faten, A.-H.; Rahmi, O.; Ali, K. Hydrogels 2.0: Improved properties with nanomaterial composites for biomedical applications. Biomed. Mater. 2016, 11, 014104. [Google Scholar]
- Zhou, L.; Tan, G.; Tan, Y.; Wang, H.; Liao, J.; Ning, C. Biomimetic mineralization of anionic gelatin hydrogels: Effect of degree of methacrylation. RSC Adv. 2014, 4, 21997–22008. [Google Scholar] [CrossRef]
- Cheng, A.; Schwartz, Z.; Kahn, A.; Li, X.; Shao, Z.; Sun, M.; Ao, Y.; Boyan, B.D.; Chen, H. Advances in porous scaffold design for bone and cartilage tissue engineering and regeneration. Tissue Eng. Part B Rev. 2019, 25, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhou, Y.; Xiao, C.; Zhao, W.; Wu, H.; Tang, J.; Li, Z.; Yu, S.; Li, X.; Min, L.; et al. Application of hydroxyapatite nanoparticles in tumor-associated bone segmental defect. Sci. Adv. 2019, 5, eaax6946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balasundaram, G.; Sato, M.; Webster, T.J. Using hydroxyapatite nanoparticles and decreased crystallinity to promote osteoblast adhesion similar to functionalizing with RGD. Biomaterials 2006, 27, 2798–2805. [Google Scholar] [CrossRef]
- Mondal, S.; Nguyen, T.P.; Hoang, G.; Manivasagan, P.; Kim, M.H.; Nam, S.Y.; Oh, J. Hydroxyapatite nano bioceramics optimized 3D printed poly lactic acid scaffold for bone tissue engineering application. Ceram. Int. 2020, 46, 3443–3455. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; González-Calbet, J.M. Calcium phosphates as substitution of bone tissues. Prog. Solid State Chem. 2004, 32, 1–31. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium orthophosphate cements for biomedical application. J. Mater. Sci. 2008, 43, 3028–3057. [Google Scholar] [CrossRef]
- Johnson, A.J.W.; Herschler, B.A. A review of the mechanical behavior of CaP and CaP/polymer composites for applications in bone replacement and repair. Acta Biomater. 2011, 7, 16–30. [Google Scholar] [CrossRef]
- Guillaume, O.; Geven, M.; Sprecher, C.; Stadelmann, V.; Grijpma, D.; Tang, T.; Qin, L.; Lai, Y.; Alini, M.; De Bruijn, J.; et al. Surface-enrichment with hydroxyapatite nanoparticles in stereolithography-fabricated composite polymer scaffolds promotes bone repair. Acta Biomater. 2017, 54, 386–398. [Google Scholar] [CrossRef]
- Kim, S.-S.; Park, M.S.; Jeon, O.; Choi, C.Y.; Kim, B.-S. Poly (lactide-co-glycolide)/hydroxyapatite composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 1399–1409. [Google Scholar] [CrossRef]
- Zuo, Y.; Liu, X.; Wei, D.; Sun, J.; Xiao, W.; Zhao, H.; Guo, L.; Wei, Q.; Fan, H.; Zhang, X. Photo-cross-linkable methacrylated gelatin and hydroxyapatite hybrid hydrogel for modularly engineering biomimetic osteon. ACS Appl. Mater. Interfaces. 2015, 7, 10386–10394. [Google Scholar] [CrossRef] [PubMed]
- Lung, C.Y.; Sarfraz, Z.; Habib, A.; Khan, A.S.; Matinlinna, J.P. Effect of silanization of hydroxyapatite fillers on physical and mechanical properties of a bis-GMA based resin composite. J. Mech. Behav. Biomed. Mater. 2016, 54, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Siniscalco, D.; Dutreilh-Colas, M.; Hjezi, Z.; Cornette, J.; El Felss, N.; Champion, E.; Damia, C. Functionalization of hydroxyapatite ceramics: Raman mapping investigation of silanization. Ceramics 2019, 2, 372–384. [Google Scholar] [CrossRef] [Green Version]
- Charbord, P. Bone marrow mesenchymal stem cells: Historical overview and concepts. Hum. Gene. Ther. 2010, 21, 1045–1056. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Liu, X.; Zhao, L.; Weir, M.D.; Sun, J.; Chen, W.; Man, Y.; Xu, H.H. Bone tissue engineering via human induced pluripotent, umbilical cord and bone marrow mesenchymal stem cells in rat cranium. Acta Biomater. 2015, 18, 236–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauney, J.R.; Volloch, V.; Kaplan, D.L. Role of adult mesenchymal stem cells in bone tissue engineering applications: Current status and future prospects. Tissue Eng. 2005, 11, 787–802. [Google Scholar] [CrossRef]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [Green Version]
- Khetan, S.; Guvendiren, M.; Legant, W.R.; Cohen, D.M.; Chen, C.S.; Burdick, J.A. Degradation-mediated cellular traction directs stem cell fate in covalently crosslinked three-dimensional hydrogels. Nat. Mater. 2013, 12, 458–465. [Google Scholar] [CrossRef] [Green Version]
- Chaudhuri, O.; Gu, L.; Klumpers, D.; Darnell, M.; Bencherif, S.A.; Weaver, J.C.; Huebsch, N.; Lee, H.-p.; Lippens, E.; Duda, G.N. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 2016, 15, 326–334. [Google Scholar] [CrossRef] [Green Version]
- Bertassoni, L.E.; Cardoso, J.C.; Manoharan, V.; Cristino, A.L.; Bhise, N.S.; Araujo, W.A.; Zorlutuna, P.; Vrana, N.E.; Ghaemmaghami, A.M.; Dokmeci, M.R. Direct-write bioprinting of cell-laden methacrylated gelatin hydrogels. Biofabrication 2014, 6, 024105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, C.-H.; Lin, K.-F.; Mar, K.; Lee, S.-Y.; Lin, Y.-M. Antioxidant N-Acetylcysteine and Glutathione Increase the Viability and Proliferation of MG63 Cells Encapsulated in the Gelatin Methacrylate/VA-086/Blue Light Hydrogel System. Tissue Eng. Part C Methods 2016, 22, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Nichol, J.W.; Koshy, S.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef] [Green Version]
- Sideridou, I.D.; Karabela, M.M. Effect of the amount of 3-methacyloxypropyltrimethoxysilane coupling agent on physical properties of dental resin nanocomposites. Dent. Mater. 2009, 25, 1315–1324. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.X. Effect of a new surface-grafting method for nano-hydroxyapatite on the dispersion and the mechanical enhancement for poly(lactide-co-glycolide). Express Polym. Lett. 2013, 8, 133–141. [Google Scholar] [CrossRef]
- Fedorovich, N.E.; Oudshoorn, M.H.; van Geemen, D.; Hennink, W.E.; Alblas, J.; Dhert, W.J. The effect of photopolymerization on stem cells embedded in hydrogels. Biomaterials 2009, 30, 344–353. [Google Scholar] [CrossRef]
- Brzoska, J.; Azouz, I.B.; Rondelez, F. Silanization of solid substrates: A step toward reproducibility. Langmuir 1994, 10, 4367–4373. [Google Scholar] [CrossRef]
- Ostad-Movahed, S.; Ansar Yasin, K.; Ansarifar, A.; Song, M.; Hameed, S. Comparing effects of silanized silica nanofiller on the crosslinking and mechanical properties of natural rubber and synthetic polyisoprene. J. Appl. Polym. Sci. 2008, 109, 869–881. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, B. Synthesis and properties of silanized waterborne polyurethane/graphene nanocomposites. Colloid Polym. Sci. 2014, 292, 51–58. [Google Scholar] [CrossRef]
- Nakaramontri, Y.; Kummerlöwe, C.; Nakason, C.; Vennemann, N. The effect of surface functionalization of carbon nanotubes on properties of natural rubber/carbon nanotube composites. Polym. Compos. 2015, 36, 2113–2122. [Google Scholar] [CrossRef]
- Rong, M.; Zhang, M.; Ruan, W. Surface modification of nanoscale fillers for improving properties of polymer nanocomposites: A review. Mater. Sci. Technol. 2006, 22, 787–796. [Google Scholar] [CrossRef]
- Cha, C.; Shin, S.R.; Gao, X.; Annabi, N.; Dokmeci, M.R.; Tang, X.S.; Khademhosseini, A. Controlling mechanical properties of cell-laden hydrogels by covalent incorporation of graphene oxide. Small 2014, 10, 514–523. [Google Scholar] [CrossRef] [Green Version]
- Rowlands, A.S.; George, P.A.; Cooper-White, J.J. Directing osteogenic and myogenic differentiation of MSCs: Interplay of stiffness and adhesive ligand presentation. Am. J. Physiol. Cell Physiol. 2008, 295, C1037–C1044. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Xie, J.; Zhong, L.; Li, J.; Rong, D.; Li, X.; Ouyang, J. Biomimetic gelatin methacrylamide hydrogel scaffolds for bone tissue engineering. J. Mater. Chem. B 2016, 4, 1070–1080. [Google Scholar] [CrossRef] [PubMed]
- Celikkin, N.; Mastrogiacomo, S.; Walboomers, X.F.; Swieszkowski, W. Enhancing X-ray attenuation of 3D printed gelatin methacrylate (GelMA) hydrogels utilizing gold nanoparticles for bone tissue engineering applications. Polymers 2019, 11, 367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behnia, H.; Khojasteh, A.; Kiani, M.T.; Khoshzaban, A.; Abbas, F.M.; Bashtar, M.; Dashti, S.G. Bone regeneration with a combination of nanocrystalline hydroxyapatite silica gel, platelet-rich growth factor, and mesenchymal stem cells: A histologic study in rabbit calvaria. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 115, e7–e15. [Google Scholar] [CrossRef] [PubMed]
- Rey, C. Calcium phosphates for medical applications. In Calcium Phosphates in Biological and Industrial Systems; Springer: Berlin/Heidelberg, Germany, 1998; pp. 217–251. [Google Scholar]
- Abouzeid, R.E.; Khiari, R.; Salama, A.; Diab, M.; Beneventi, D.; Dufresne, A. In situ mineralization of nano-hydroxyapatite on bifunctional cellulose nanofiber/polyvinyl alcohol/sodium alginate hydrogel using 3D printing. Int. J. Biol. Macromol. 2020, 160, 538–547. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer Sequence |
|---|---|
| GAPDH | 5′-forward-GGAGCGAGATCCCTCCAAAAT |
| 5′-reverse-GGCTGTTGTCATACTTCTCATGG | |
| COL1A1 | 5′-forward-GAGGGCCAAGACGAAGACATC |
| 5′-reverse-GGCTGTTGTCATACTTCTCATGG | |
| Osteocalcin | 5′-forward-GAGGGCCAAGACGAAGACATC |
| 5′-reverse-CCCTCCTGCTTGGACACAAAG | |
| OPN | 5′-forward-CTCCATTGACTCGAACGACTC |
| 5′-reverse-CAGGTCTGCGAAACTTCTTAGAT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, J.J.-M.; Lin, C.-H.; Chen, H.; Lee, S.-Y.; Lin, Y.-M. Biofabrication of Cell-Laden Gelatin Methacryloyl Hydrogels with Incorporation of Silanized Hydroxyapatite by Visible Light Projection. Polymers 2021, 13, 2354. https://doi.org/10.3390/polym13142354
Su JJ-M, Lin C-H, Chen H, Lee S-Y, Lin Y-M. Biofabrication of Cell-Laden Gelatin Methacryloyl Hydrogels with Incorporation of Silanized Hydroxyapatite by Visible Light Projection. Polymers. 2021; 13(14):2354. https://doi.org/10.3390/polym13142354
Chicago/Turabian StyleSu, Jimmy Jiun-Ming, Chih-Hsin Lin, Hsuan Chen, Shyh-Yuan Lee, and Yuan-Min Lin. 2021. "Biofabrication of Cell-Laden Gelatin Methacryloyl Hydrogels with Incorporation of Silanized Hydroxyapatite by Visible Light Projection" Polymers 13, no. 14: 2354. https://doi.org/10.3390/polym13142354
APA StyleSu, J. J.-M., Lin, C.-H., Chen, H., Lee, S.-Y., & Lin, Y.-M. (2021). Biofabrication of Cell-Laden Gelatin Methacryloyl Hydrogels with Incorporation of Silanized Hydroxyapatite by Visible Light Projection. Polymers, 13(14), 2354. https://doi.org/10.3390/polym13142354






