Effects of Poloxamer Content and Storage Time of Biodegradable Starch-Chitosan Films on Its Thermal, Structural, Mechanical, and Morphological Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Preparation
2.3. Color
2.4. Contact Angle
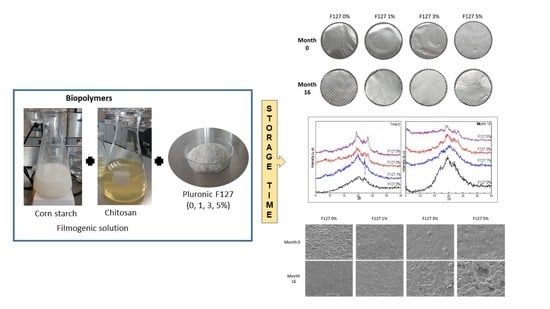
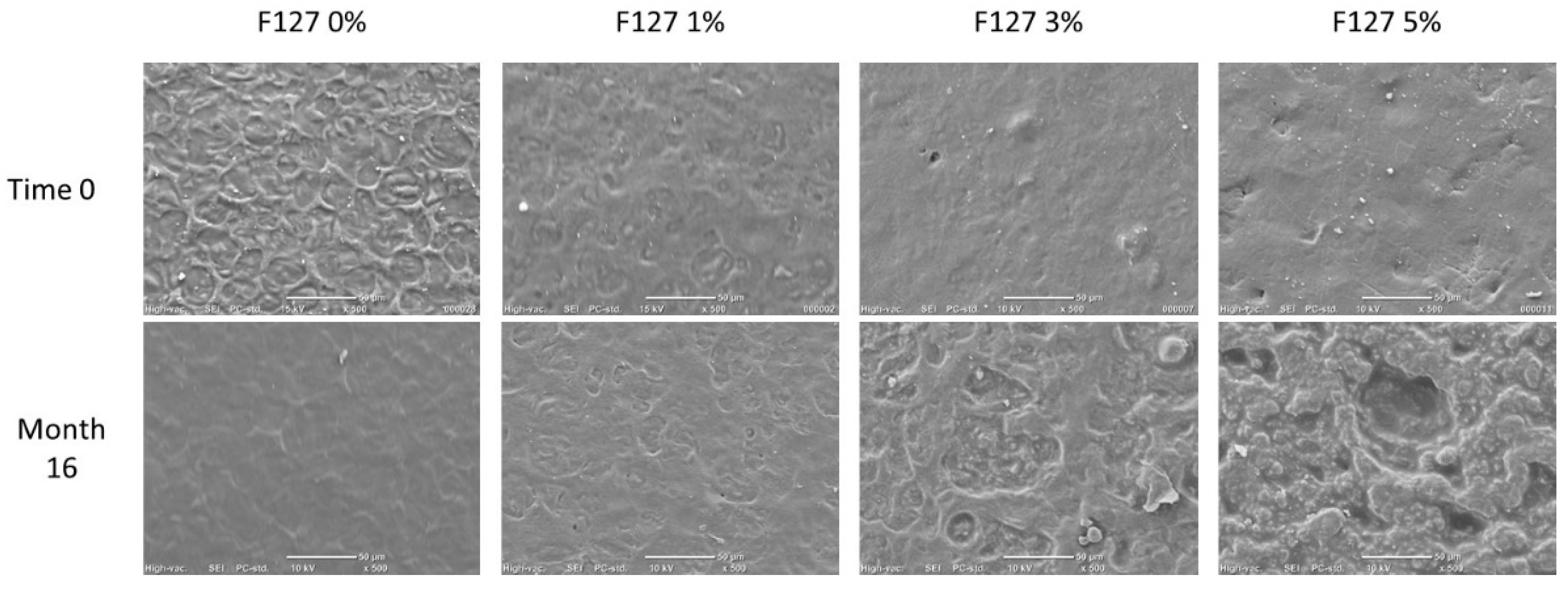
2.5. Scanning Electron Microscopy
2.6. Fourier Transform Infrared Spectroscopy (FTIR)
2.7. X-ray Diffraction (XRD) Analysis
2.8. Differential Scanning Calorimetry
2.9. Water Solubility
2.10. Mechanical Properties
3. Results and Discussion
3.1. Appearance and Color
3.2. Morphology by SEM
3.3. Contact Angle
3.4. FTIR
3.5. X-ray Diffraction
3.6. Thermal Behavior by DSC
3.7. Water Solubility (WS)
3.8. Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gómez-Aldapa, C.A.; Velazquez, G.; Gutierrez, M.C.; Castro-Rosas, J.; Jiménez-Regalado, E.J.; Aguirre-Loredo, R.Y. Characterization of Functional Properties of Biodegradable Films Based on Starches from Different Botanical Sources. Starch Stärke 2020, 72, 1900282. [Google Scholar] [CrossRef]
- Wang, H.; Ding, F.; Ma, L.; Zhang, Y. Edible films from chitosan-gelatin: Physical properties and food packaging application. Food Biosci. 2021, 40, 100871. [Google Scholar] [CrossRef]
- Yadav, M.; Behera, K.; Chang, Y.-H.; Chiu, F.-C. Cellulose Nanocrystal Reinforced Chitosan Based UV Barrier Composite Films for Sustainable Packaging. Polymers 2020, 12, 202. [Google Scholar] [CrossRef] [Green Version]
- Abera, G.; Woldeyes, B.; Demash, H.D.; Miyake, G. The effect of plasticizers on thermoplastic starch films developed from the indigenous Ethiopian tuber crop Anchote (Coccinia abyssinica) starch. Int. J. Biol. Macromol. 2020, 155, 581–587. [Google Scholar] [CrossRef]
- Samsudin, H.; Hani, N.M. Chapter 8—Use of Starch in Food Packaging. In Starch-Based Materials in Food Packaging; Villar, M.A., Barbosa, S.E., García, M.A., Castillo, L.A., López, O.V., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 229–256. [Google Scholar]
- Aguirre-Loredo, R.Y.; Rodriguez-Hernandez, A.I.; Morales-Sánchez, E.; Gomez-Aldapa, C.; Velazquez, G. Effect of equilibrium moisture content on barrier, mechanical and thermal properties of chitosan films. Food Chem. 2016, 196, 560–566. [Google Scholar] [CrossRef]
- Beigmohammadi, F.; Barzoki, Z.M.; Shabaniyan, M. Rye Flour and Cellulose Reinforced Starch Biocomposite: A Green Approach to Improve Water Vapor Permeability and Mechanical Properties. Starch Stärke 2020, 72, 1900169. [Google Scholar] [CrossRef]
- Meng, W.; Shi, J.; Zhang, X.; Lian, H.; Wang, Q.; Peng, Y. Effects of peanut shell and skin extracts on the antioxidant ability, physical and structure properties of starch-chitosan active packaging films. Int. J. Biol. Macromol. 2020, 152, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Aguirre-Loredo, R.Y.; Rodríguez-Hernández, A.I.; Chavarría-Hernández, N. Physical properties of emulsified films based on chitosan and oleic acid. CyTA J. Food 2014, 12, 305–312. [Google Scholar] [CrossRef] [Green Version]
- Caicedo, C.; Loredo, R.Y.A.; García, A.F.; Ossa, O.H.; Arce, A.V.; Pulgarin, H.L.C.; Torres, Y. Ávila Rheological, Thermal, Superficial, and Morphological Properties of Thermoplastic Achira Starch Modified with Lactic Acid and Oleic Acid. Molecules 2019, 24, 4433. [Google Scholar] [CrossRef] [Green Version]
- Fonseca-García, A.; Jiménez-Regalado, E.J.; Aguirre-Loredo, R.Y. Preparation of a novel biodegradable packaging film based on corn starch-chitosan and poloxamers. Carbohydr. Polym. 2021, 251, 117009. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Aldapa, C.A.; Velazquez, G.; Gutiérrez, M.C.; Rangel-Vargas, E.; Castro-Rosas, J.; Aguirre-Loredo, R.Y. Effect of polyvinyl alcohol on the physicochemical properties of biodegradable starch films. Mater. Chem. Phys. 2020, 239, 122027. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Frone, A.N.; Ghiurea, M.; Chiulan, I. Influence of storage conditions on starch/PVA films containing cellulose nanofibers. Ind. Crop. Prod. 2015, 70, 170–177. [Google Scholar] [CrossRef]
- Roman, L.; Yee, J.; Hayes, A.; Hamaker, B.R.; Bertoft, E.; Martinez, M.M. On the role of the internal chain length distribution of amylopectins during retrogradation: Double helix lateral aggregation and slow digestibility. Carbohydr. Polym. 2020, 246, 116633. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Ma, Y.; Sun, D.-W. Impact of amylose content on starch retrogradation and texture of cooked milled rice during storage. J. Cereal Sci. 2009, 50, 139–144. [Google Scholar] [CrossRef]
- Leyva, J.D.H.; Alonso, L.; Rueda-Enciso, J.; Yee-Madeira, H.; Bello-Perez, L.; Alvarez-Ramirez, J. Morphological, physicochemical and functional characteristics of starch from Marantha ruiziana Koern. LWT 2017, 83, 150–156. [Google Scholar] [CrossRef]
- Eliasson, A.C. 10—Gelatinization and Retrogradation of Starch in Foods and Its Implications for Food Quality, in Chemical Deterioration and Physical Instability of Food and Beverages; Skibsted, L.H., Risbo, J., Andersen, M.L., Eds.; Woodhead Publishing: Cambridge, UK, 2010; pp. 296–323. [Google Scholar]
- Gómez-Aldapa, C.A.; Díaz-Cruz, C.A.; Castro-Rosas, J.; Jiménez-Regalado, E.J.; Velazquez, G.; Gutiérrez, M.C.; Aguirre-Loredo, R.Y. Development of Antimicrobial Biodegradable Films Based on Corn Starch with Aqueous Extract of Hibiscus sabdariffa L. Starch Stärke 2021, 73, 2000096. [Google Scholar] [CrossRef]
- ASTM. D3418. Standard Test Method for Transition Temperatures and Enthalpies of Fusion and Crystallization of Polymers by Differential Scanning Calorimetry; ASTM International: Philadelphia, PA, USA, 2015. [Google Scholar]
- Garalde, R.A.; Thipmanee, R.; Jariyasakoolroj, P.; Sane, A. The effects of blend ratio and storage time on thermoplastic starch/poly(butylene adipate-co-terephthalate) films. Heliyon 2019, 5, e01251. [Google Scholar] [CrossRef] [Green Version]
- Rindlava, Å.; Hulleman, S.H.; Gatenholma, P. Formation of starch films with varying crystallinity. Carbohydr. Polym. 1997, 34, 25–30. [Google Scholar] [CrossRef]
- Rubens, P.; Snauwaert, J.; Heremans, K.; Stute, R. In situ observation of pressure-induced gelation of starches studied with FTIR in the diamond anvil cell. Carbohydr. Polym. 1999, 39, 231–235. [Google Scholar] [CrossRef]
- Van Soest, J.J.G.; De Wit, D.; Tournois, H.; Vliegenthart, J.F.G. Retrogradation of Potato Starch as Studied by Fourier Transform Infrared Spectroscopy. Starch Stärke 1994, 46, 453–457. [Google Scholar] [CrossRef] [Green Version]
- Casu, B.; Reggiani, M. Infrared spectra of amylose and its oligomers. In Journal of Polymer Science Part C: Polymer Symposia; Wiley Subscription Services, Inc., A Wiley Company: New York, NY, USA, 1964. [Google Scholar]
- Kahvand, F.; Fasihi, M. Plasticizing and anti-plasticizing effects of polyvinyl alcohol in blend with thermoplastic starch. Int. J. Biol. Macromol. 2019, 140, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, A.; Mucha, M. Thermogravimetric and FTIR studies of chitosan blends. Thermochim. Acta 2003, 396, 153–166. [Google Scholar] [CrossRef]
- Hernandez, J.H.M. Effect of the Incorporation of Polycaprolactone (PCL) on the Retrogradation of Binary Blends with Cassava Thermoplastic Starch (TPS). Polymers 2020, 13, 38. [Google Scholar] [CrossRef]
- Vamadevan, V.; Bertoft, E. Impact of different structural types of amylopectin on retrogradation. Food Hydrocoll. 2018, 80, 88–96. [Google Scholar] [CrossRef]
- Li, C.; Hamaker, B.R. Effects of different storage temperatures on the intra- and intermolecular retrogradation and digestibility of sago starch. Int. J. Biol. Macromol. 2021, 182, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Shaker, M.A.; Elbadawy, H.M.; Shaker, M.A. Improved solubility, dissolution, and oral bioavailability for atorvastatin-Pluronic® solid dispersions. Int. J. Pharm. 2020, 574, 118891. [Google Scholar] [CrossRef]



| Content of F127 | Water Solubility (%S) | Tensile Strength (MPa) | Elongation at Break (%) | Hunter Color Values 1 | Contact Angle (°) | ||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | Ѳ | ||||
| Time 0 months | |||||||
| F127 0% | 42.58 § | 4.24 a,§ | 149.05 c,§ | 93.74 | 2.29 | −11.50 | 99.93 |
| F127 1% | 7.81 § | 6.49 a,b,§ | 53.30 a,§ | 93.52 | 1.87 | −9.31 | 33.22 |
| F127 3% | 4.02 § | 4.96 a,§ | 95.20 b,§ | 94.22 | 2.31 | −11.56 | 43.88 |
| F127 5% | 3.34 § | 3.68 a,§ | 84.96 b,§ | 94.53 | 2.10 | −10.54 | 46.35 |
| Time 16 months | |||||||
| F127 0% | 24.40 | 5.18 b | 68.53 c | 90.88 | 1.53 | −8.05 | 67.66 |
| F127 1% | 17.66 | 7.01 c | 60.60 c | 90.81 | 1.47 | −7.75 | 31.89 |
| F127 3% | 24.17 | 5.96 b | 30.67 b | 91.09 | 1.41 | −7.79 | 46.09 |
| F127 5% | 56.98 | 3.27 a | 12.40 a | 89.53 | 1.30 | −6.02 | 50.74 |
| Film Sample | Tp (°C) | Tm1 (°C) | Tm2 (°C) | ∆Hm2 (J g−1) | |||
|---|---|---|---|---|---|---|---|
| Month 0 | Month 0 | Month 16 | Month 0 | Month 16 | Month 0 | Month 16 | |
| Corn starch | 63.13 | -- | -- | 141.03 | 117.75 | 6.0 | 18.7 |
| Chitosan | -- | -- | -- | 104.68 | 119.47 | 28.0 | 19.6 |
| F127 | 56.10 | 56.10 | |||||
| F127 0% | 62.06 | -- | -- | 138.96 | 106.63 | 6.4 | 10.3 |
| F127 1% | -- | 45.52 | 51.52 | 137.63 | 112.94 | 8.4 | 9.1 |
| F127 3% | -- | 51.95 | 51.68 | -- | 108.05 | -- | 6.3 |
| F127 5% | -- | 47.84 | 51.46 | 114.36 | 115.22 | 8.5 | 4.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonseca-García, A.; Caicedo, C.; Jiménez-Regalado, E.J.; Morales, G.; Aguirre-Loredo, R.Y. Effects of Poloxamer Content and Storage Time of Biodegradable Starch-Chitosan Films on Its Thermal, Structural, Mechanical, and Morphological Properties. Polymers 2021, 13, 2341. https://doi.org/10.3390/polym13142341
Fonseca-García A, Caicedo C, Jiménez-Regalado EJ, Morales G, Aguirre-Loredo RY. Effects of Poloxamer Content and Storage Time of Biodegradable Starch-Chitosan Films on Its Thermal, Structural, Mechanical, and Morphological Properties. Polymers. 2021; 13(14):2341. https://doi.org/10.3390/polym13142341
Chicago/Turabian StyleFonseca-García, Abril, Carolina Caicedo, Enrique Javier Jiménez-Regalado, Graciela Morales, and Rocio Yaneli Aguirre-Loredo. 2021. "Effects of Poloxamer Content and Storage Time of Biodegradable Starch-Chitosan Films on Its Thermal, Structural, Mechanical, and Morphological Properties" Polymers 13, no. 14: 2341. https://doi.org/10.3390/polym13142341
APA StyleFonseca-García, A., Caicedo, C., Jiménez-Regalado, E. J., Morales, G., & Aguirre-Loredo, R. Y. (2021). Effects of Poloxamer Content and Storage Time of Biodegradable Starch-Chitosan Films on Its Thermal, Structural, Mechanical, and Morphological Properties. Polymers, 13(14), 2341. https://doi.org/10.3390/polym13142341