Preparation, Characterization, and Evaluation of Macrocrystalline and Nanocrystalline Cellulose as Potential Corrosion Inhibitors for SS316 Alloy during Acid Pickling Process: Experimental and Computational Methods
Abstract
:1. Introduction
2. Experimental Part
2.1. Materials
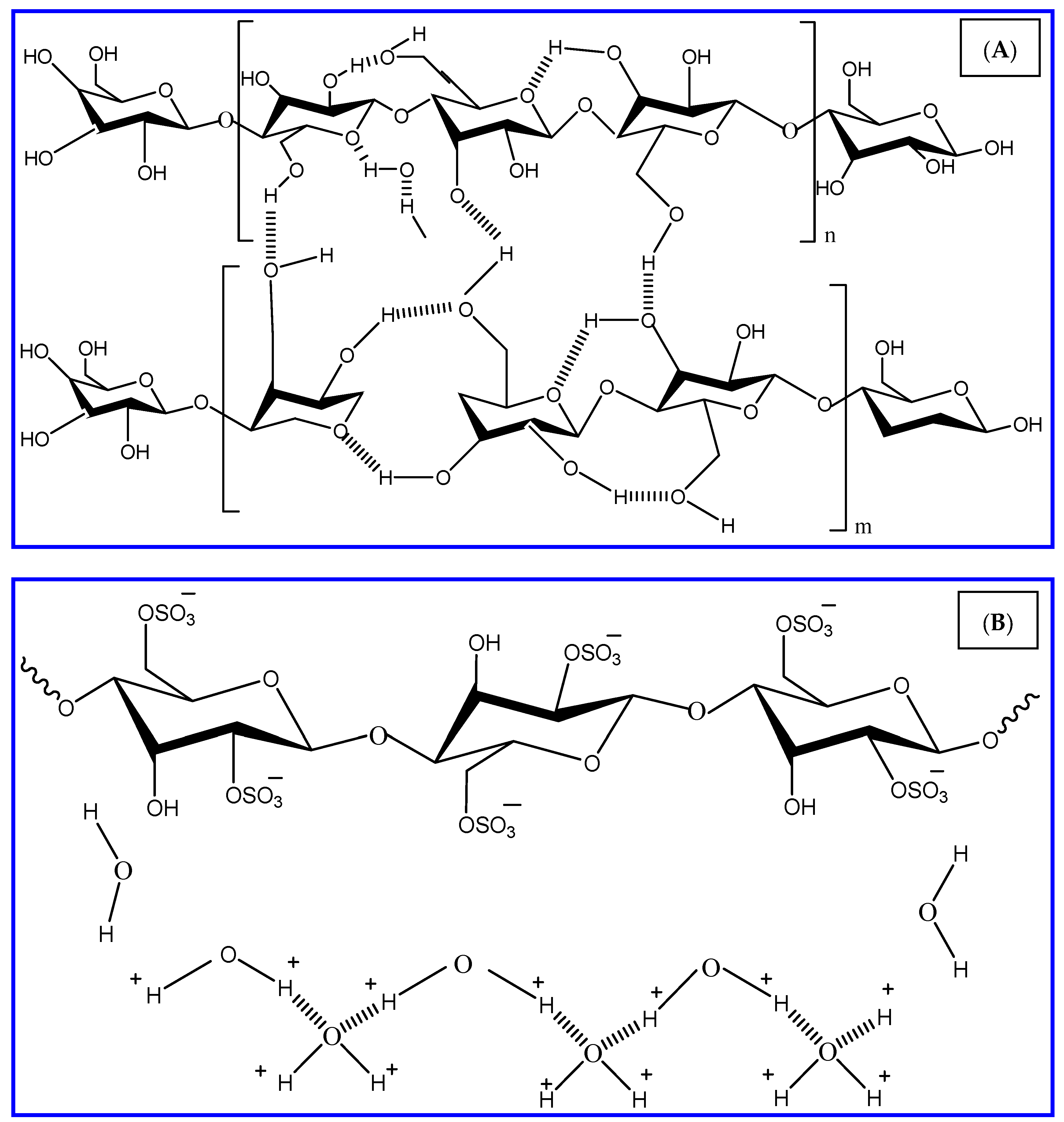
2.2. Synthesis of Nanocrystalline Cellulose (NCC)
2.3. Characterization
2.4. Preparation of Electrode Coupons and the Inhibitor Solutions
2.5. Electrochemical and Weight Loss Measurements
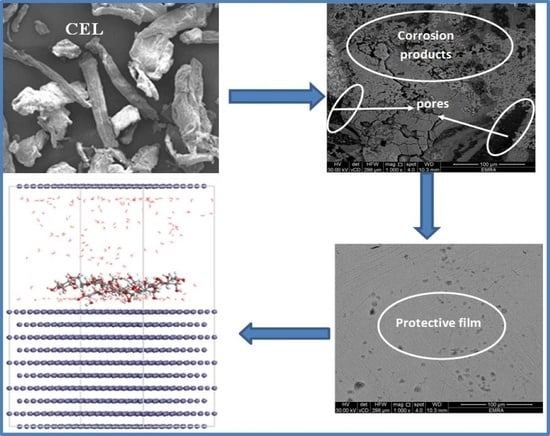
2.6. Surface Characterization
2.7. DFT Calculations and MC Simulations
3. Results and Discussions
3.1. Spectroscopic and Microscopic Analysis
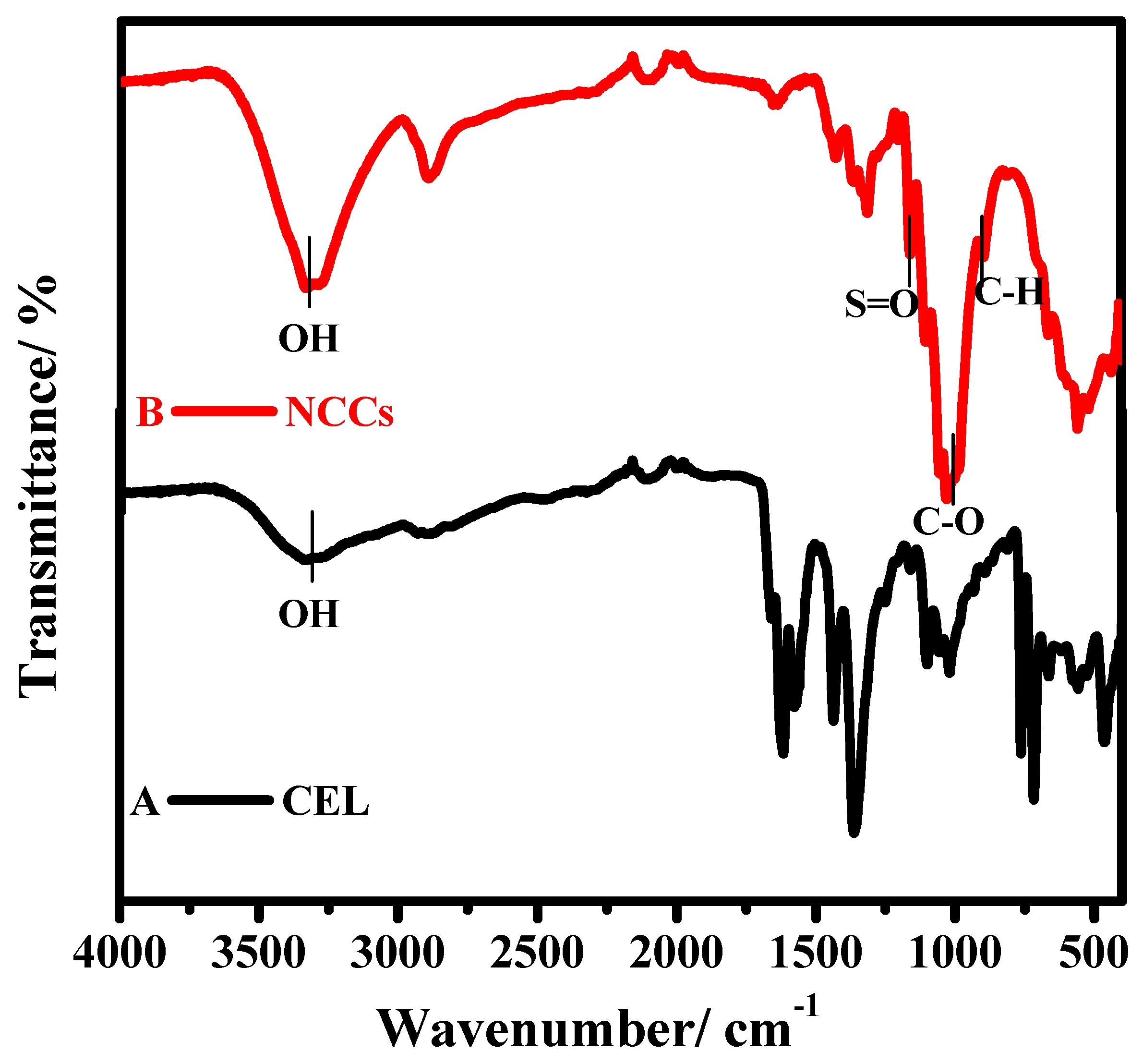
3.1.1. FT-IR
3.1.2. Raman Analysis
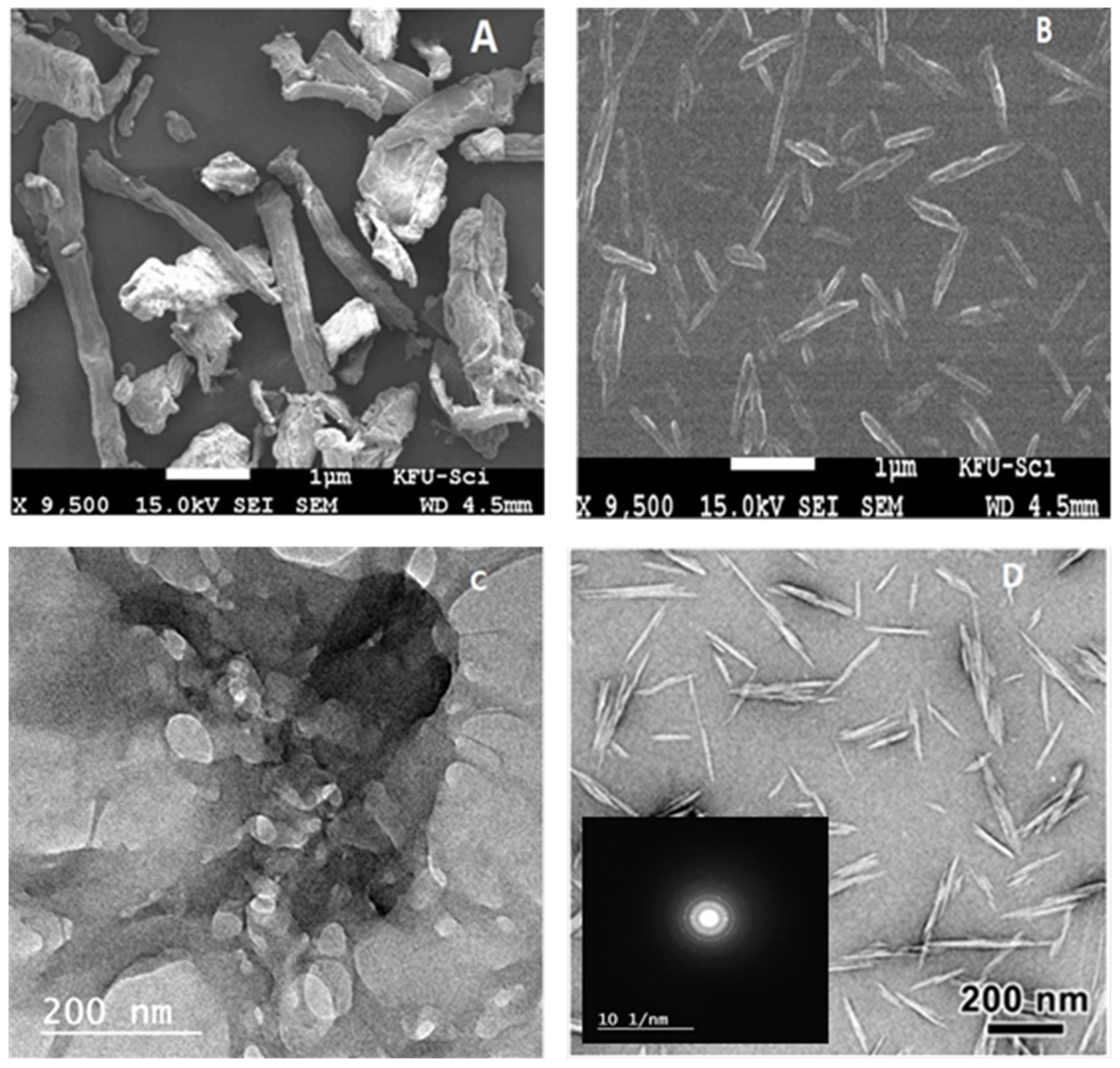
3.1.3. FE-SEM and TEM
3.1.4. BET Analysis
3.2. Corrosion Inhibition Data
3.2.1. Weight Loss Analysis
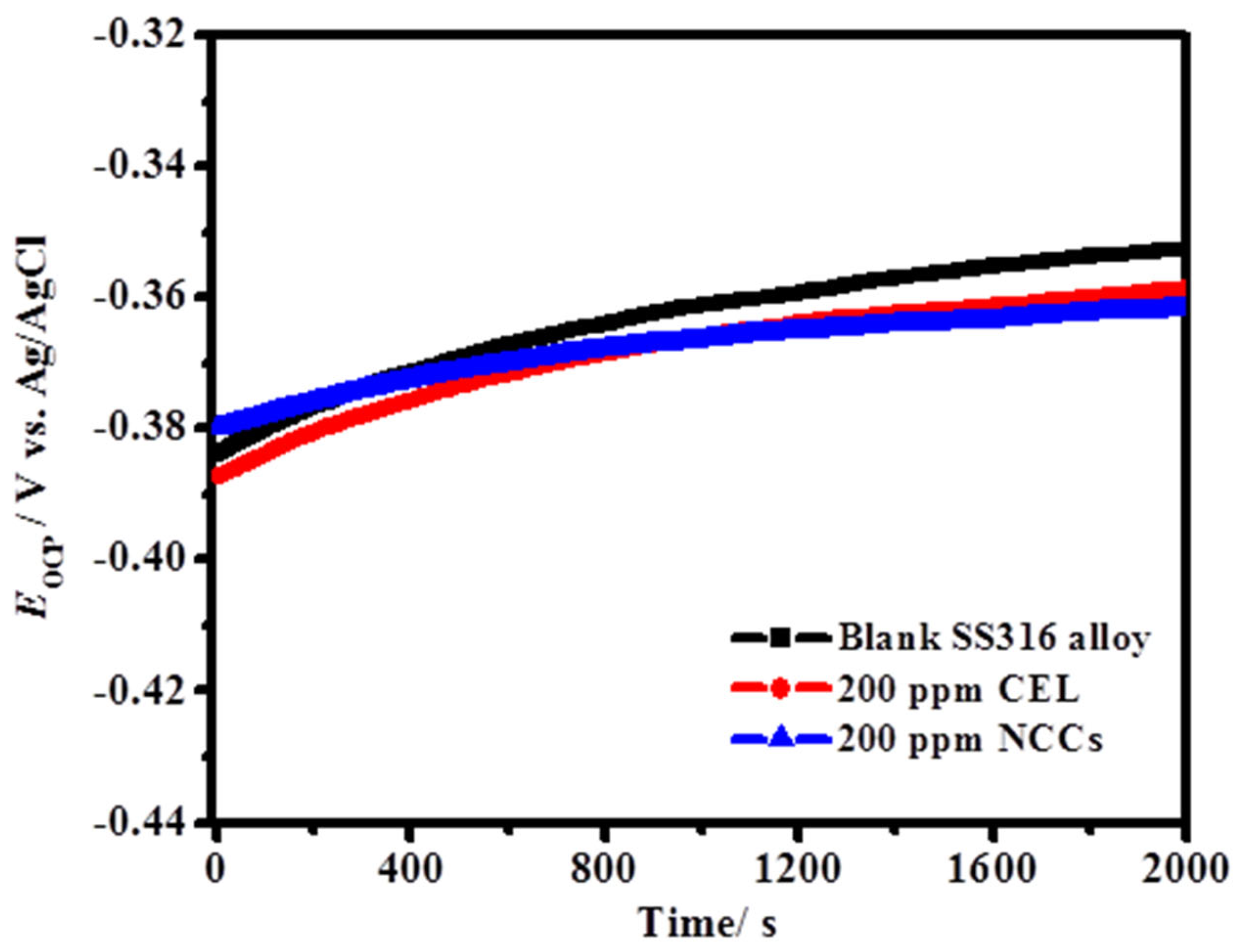
3.2.2. Open Circuit Potential
3.2.3. Tafel Plots
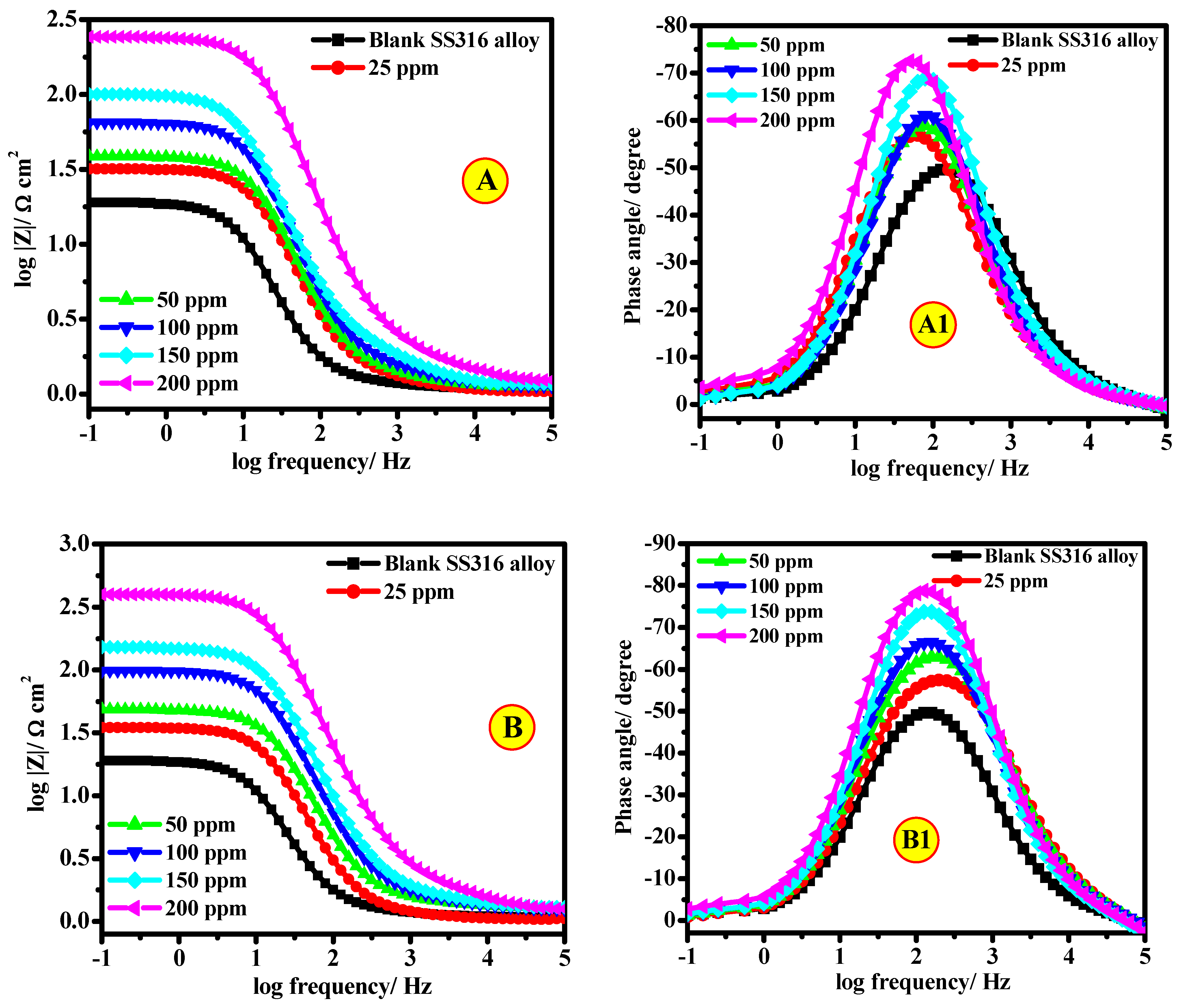
3.2.4. EIS Studies
3.2.5. Adsorption Isotherm Analysis
3.2.6. Surface Analysis and Characterization
3.3. Theoretical Approaches
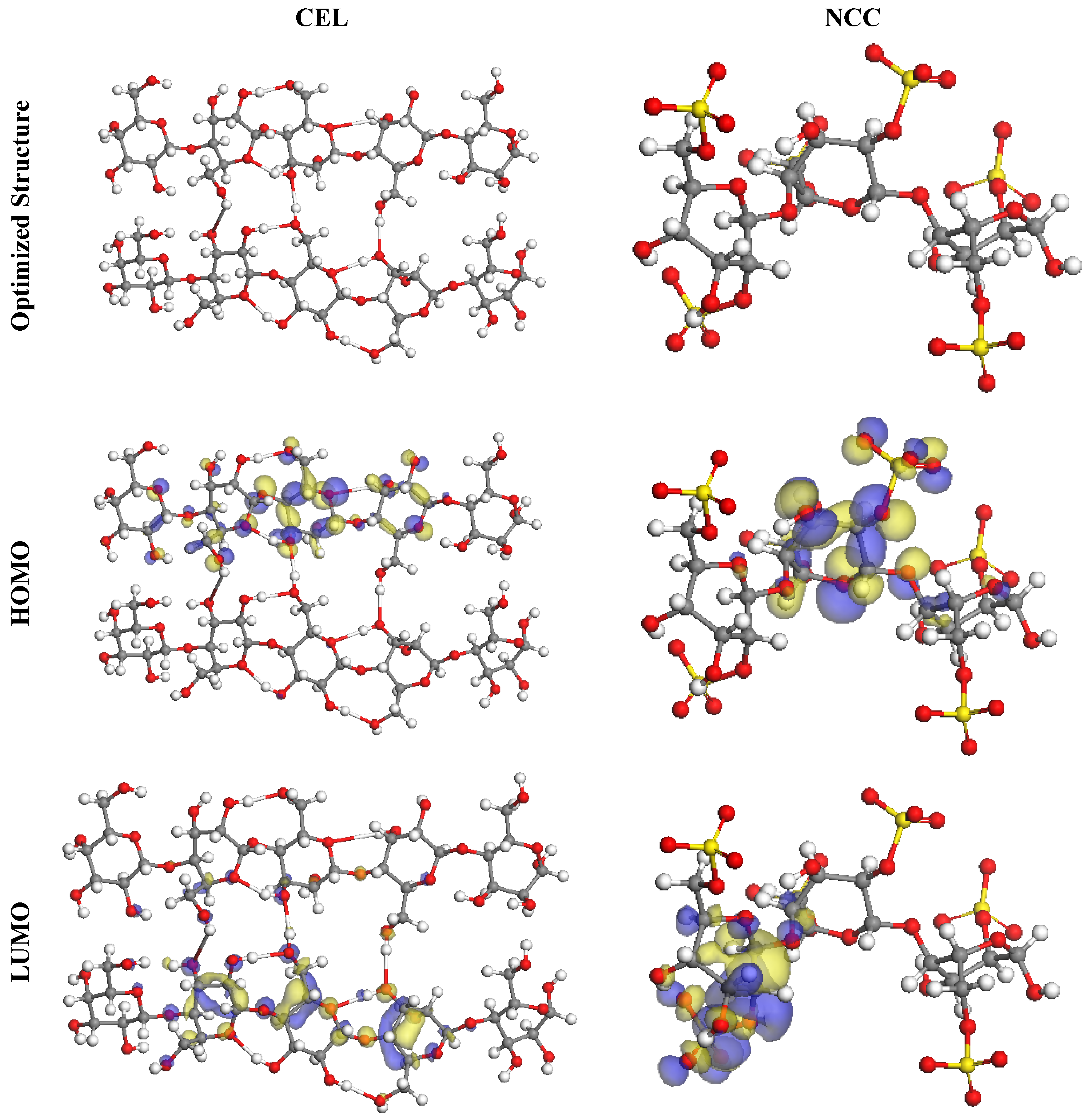
3.3.1. DFT Studies
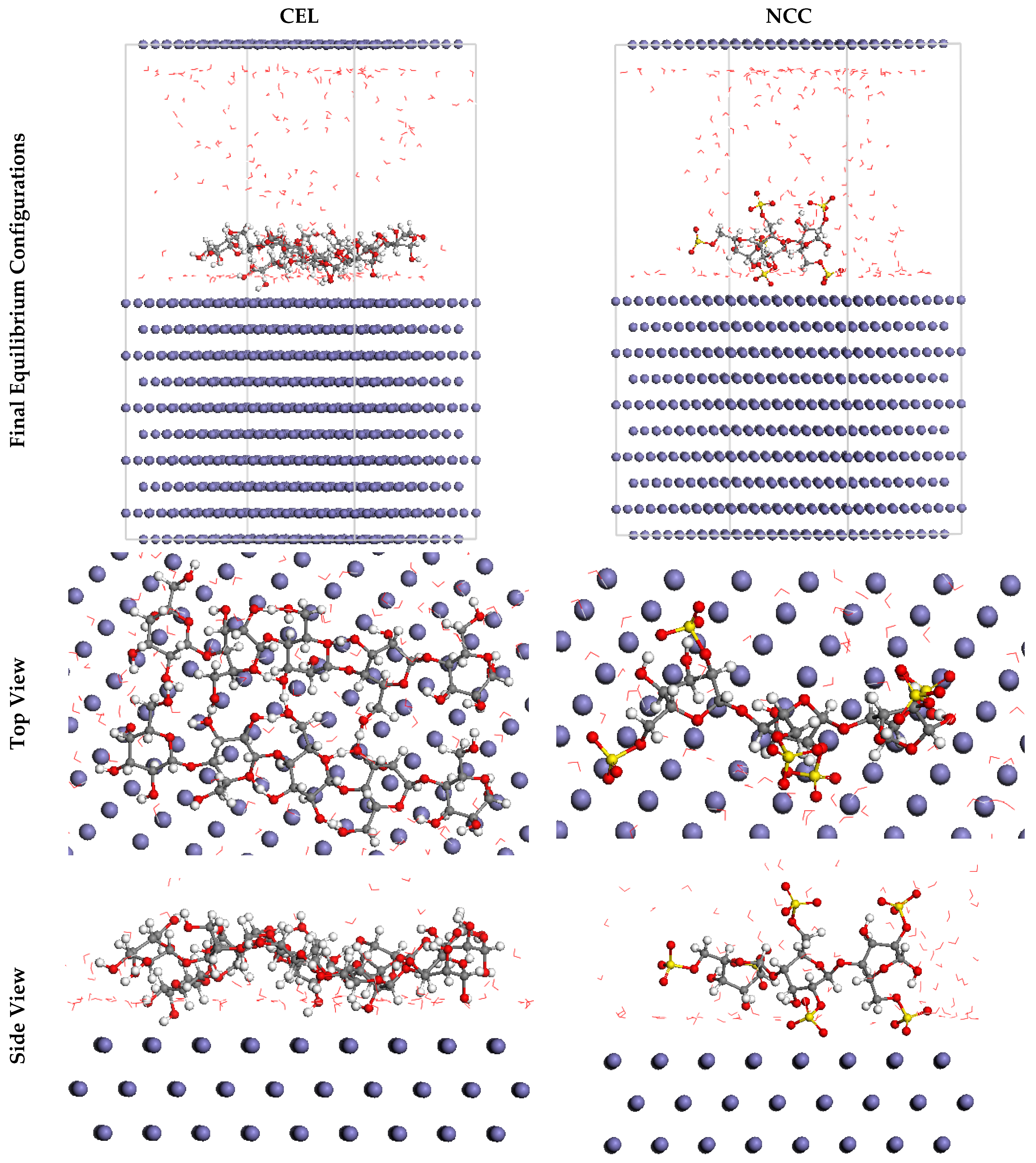
3.3.2. MC Simulations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oguzie, E.E.; Li, Y.; Wang, F.H. Corrosion inhibition and adsorption behavior of methionine on mild steel in sulfuric acid and synergistic effect of iodide ion. J. Colloid. Interface Sci. 2007, 310, 90–98. [Google Scholar] [CrossRef]
- Usher, K.M.; Kaksonen, A.H.; Cole, I.; Marney, D. Critical review: Microbially influenced corrosion of buried carbon steel pipes. Int. Biodeterior. Biodegrad. 2014, 93, 84–106. [Google Scholar] [CrossRef]
- Abdallah, M. Maltodextrin and Chitosan Polymers as Inhibitors for the Corrosion of Carbon Steel in 1.0 M Hydrochloric Acid. Int. J. Electrochem. Sci. 2020, 5650–5663. [Google Scholar] [CrossRef]
- Toghan, A.; Abou-krisha, M.M.; Assaf, F.H.; El-Sheref, F. Effect of Deposition Potential on the Mechanism and Corrosion Behavior of Zn-Fe-Co Thin Coatings Electrochemically Deposited on a Steel Substrate. Int. J. Electrochem. Sci. 2021, 16, 151044. [Google Scholar] [CrossRef]
- Toghan, A.; Abo-Bakr, A.M.; Rageh, H.M.; Abdel-Sabour, M. Green Electrochemical Strategy for One-Step Synthesis of New Catechol Derivatives. RSC Adv. 2019, 9, 13145–13152. [Google Scholar] [CrossRef] [Green Version]
- Heikal, M.; Ali, A.; Ibrahim, B.; Toghan, A. Electrochemical and Physico-Mechanical Characterizations of Fly Ash-Composite Cement. Constr. Build. Mater. 2020, 243, 118309. [Google Scholar] [CrossRef]
- Olajire, A.A. Corrosion Inhibition of Offshore Oil and Gas Production Facilities Using Organic Compound Inhibitors—A Review. J. Mol. Liq. 2017, 248, 775–808. [Google Scholar] [CrossRef]
- Roy, P.; Karfa, P.; Adhikari, U.; Sukul, D. Corrosion Inhibition of Mild Steel in Acidic Medium by Polyacrylamide Grafted Guar Gum with Various Grafting Percentage: Effect of Intramolecular Synergism. Corros. Sci. 2014, 88, 246–253. [Google Scholar] [CrossRef]
- Umoren, S.; Li, Y.; Wang, F. Synergistic Effect of Iodide Ion and Polyacrylic Acid on Corrosion Inhibition of Iron in H2SO4 Investigated by Electrochemical Techniques. Corros. Sci. 2010, 52, 2422–2429. [Google Scholar] [CrossRef]
- Gao, B.; Zhang, X.; Sheng, Y. Studies on Preparing and Corrosion Inhibition Behaviour of Quaternized Polyethyleneimine for Low Carbon Steel in Sulfuric Acid. Mater. Chem. Phys. 2008, 108, 375–381. [Google Scholar] [CrossRef]
- Fekry, A.M.; Gasser, A.A.A.; Ameer, M.A. Corrosion Protection of Mild Steel by Polyvinylsilsesquioxanes Coatings in 3% NaCl Solution. J. Appl. Electrochem. 2010, 40, 739–747. [Google Scholar] [CrossRef]
- Selvaraj, S.K.; Kennedy, A.J.; Amalraj, A.J.; Rajendran, S.; Palaniswamy, N. Corrosion Behaviour of Carbon Steel In The Presence Of Polyvinylpyrrolidone. Corros. Rev. 2004, 22, 219–232. [Google Scholar] [CrossRef]
- Zeni, D.F.M. Preparation of Microcellulose (Mcc) and Nanocellulose (Ncc) from Eucalyptus Kraft Ssp Pulp. Polym. Sci. 2016, 1, 1–7. [Google Scholar] [CrossRef]
- Trache, D.; Hussin, M.H.; Chuin, C.T.H.; Sabar, S.; Fazita, M.N.; Taiwo, O.F.; Hassan, T.; Haafiz, M.M. Microcrystalline Cellulose: Isolation, Characterization and Bio-Composites application—A Review. Int. J. Biol. Macromol. 2016, 93, 789–804. [Google Scholar] [CrossRef]
- Nsor-Atindana, J.; Chen, M.; Goff, H.D.; Zhong, F.; Sharif, H.R.; Li, Y. Functionality and Nutritional Aspects of Microcrystalline Cellulose in Food. Carbohydr. Polym. 2017, 172, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Kian, L.K.; Jawaid, M.; Ariffin, H.; Alothman, O.Y. Isolation and Characterization of Microcrystalline Cellulose from Roselle Fibers. Int. J. Biol. Macromol. 2017, 103, 931–940. [Google Scholar] [CrossRef]
- Chin, K.-M.; Ting, S.S.; Lin, O.H.; Owi, W.T. Extraction of Microcrystalline Cellulose from Rice Straw and Its Effect on Polyvinyl Alcohol Biocomposites Film. In Proceedings of the 3rd International Conference of Global Network for Innovative Technology 2016 (3rd IGNITE-2016): Advanced Materials for Innovative Technologies, Penang, Malaysia, 27–29 January 2017. [Google Scholar] [CrossRef]
- Naduparambath, S.; Jinitha, T.V.; Shaniba, V.; Sreejith, M.P.; Balan, A.K.; Purushothaman, E. Isolation and characterisation of cellulose nanocrystals from sago seed shells. Carbohydr. Polym. 2018, 180, 13–20. [Google Scholar] [CrossRef]
- Bano, S.; Negi, Y.S. Studies on Cellulose Nanocrystals Isolated from Groundnut Shells. Carbohydr. Polym. 2017, 157, 1041–1049. [Google Scholar] [CrossRef]
- Hsieh, Y.-L. Cellulose Nanocrystals and Self-Assembled Nanostructures from Cotton, Rice Straw and Grape Skin: A Source Perspective. J. Mater. Sci. 2013, 48, 7837–7846. [Google Scholar] [CrossRef] [Green Version]
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a Tiny Fiber with Huge Applications. Curr. Opin. Biotechnol. 2016, 39, 76–88. [Google Scholar] [CrossRef]
- Khalil, H.A.; Davoudpour, Y.; Saurabh, C.K.; Hossain, S.; Adnan, A.S.; Dungani, R.; Paridah, M.; Sarker, Z.I.; Fazita, M.N.; Syakir, M.; et al. A Review on Nanocellulosic Fibres as New Material for Sustainable Packaging: Process and Applications. Renew. Sustain. Energy Rev. 2016, 64, 823–836. [Google Scholar] [CrossRef]
- Azeredo, H.M.; Rosa, M.F.; Mattoso, L.H.C. Nanocellulose in Bio-Based Food Packaging Applications. Ind. Crop. Prod. 2017, 97, 664–671. [Google Scholar] [CrossRef]
- Chen, W.; Abe, K.; Uetani, K.; Yu, H.; Liu, Y.; Yano, H. Individual cotton cellulose nanofibers: Pretreatment and fibrillation technique. Cellulose 2014, 21, 1517–1528. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, X.; Li, J.; Wang, F.; Wang, Q.; Zhang, Y.; Kong, L. Homogeneous Isolation of Nanocellulose from Eucalyptus Pulp by High Pressure Homogenization. Ind. Crop. Prod. 2017, 104, 237–241. [Google Scholar] [CrossRef]
- Hideno, A.; Abe, K.; Uchimura, H.; Yano, H. Preparation by Combined Enzymatic and Mechanical Treatment and Characterization of Nanofibrillated Cotton Fibers. Cellulose 2016, 23, 3639–3651. [Google Scholar] [CrossRef]
- ASTM G3—89A. Standard Practice for Conventions Applicable to Electrochemical Measurements in Corrosion Testing; ASTM International: West Conshohocken, PA, USA, 2010; pp. 1–9. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Sayed, A.R.; Shalabi, K. Synthesis and Theoretical Studies of Novel Conjugated Polyazomethines and Their Application as Efficient Inhibitors for C1018 Steel Pickling Corrosion Behavior. Surf. Interfaces 2021, 23, 101037. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Shalabi, K.; Abdelhamid, A.A. One-Pot Synthesis of Novel Triphenyl Hexyl Imidazole Derivatives Catalyzed by Ionic Liquid for Acid Corrosion Inhibition of C1018 Steel: Experimental and Computational Perspectives. J. Mol. Liq. 2021, 334, 116081. [Google Scholar] [CrossRef]
- Verma, D.K.; Aslam, R.; Aslam, J.; Quraishi, M.; Ebenso, E.E.; Verma, C. Computational Modeling: Theoretical Predictive Tools for Designing of Potential Organic Corrosion Inhibitors. J. Mol. Struct. 2021, 1236, 130294. [Google Scholar] [CrossRef]
- Moriana, R.; Vilaplana, F.; Ek, M. Cellulose Nanocrystals from Forest Residues as Reinforcing Agents for Composites: A Study from Macro- to Nano-Dimensions. Carbohydr. Polym. 2016, 139, 139–149. [Google Scholar] [CrossRef]
- Neto, W.P.F.; Silvério, H.A.; Dantas, N.; Pasquini, D. Extraction and Characterization of Cellulose Nanocrystals from Agro-Industrial Residue—Soy Hulls. Ind. Crop. Prod. 2013, 42, 480–488. [Google Scholar] [CrossRef]
- Lu, P.; Hsieh, Y.-L. Preparation and Properties of Cellulose Nanocrystals: Rods, Spheres, and Network. Carbohydr. Polym. 2010, 82, 329–336. [Google Scholar] [CrossRef]
- Wiley, J.H.; Atalla, R.H. Band Assignments in the Raman Spectra of Celluloses. Carbohydr. Res. 1987, 160, 113–129. [Google Scholar] [CrossRef]
- Medel, F.; Gomez-Barrena, E.; García-Alvarez, F.; Ríos, R.; Gracia-Villa, L.; Puértolas, J. Fractography Evolution in Accelerated Aging of UHMWPE After Gamma Irradiation in Air. Biomaterials 2004, 25, 9–21. [Google Scholar] [CrossRef]
- Schenzel, K.; Fischer, S. NIR FT Raman Spectroscopy–a Rapid Analytical Tool for Detecting the Transformation of Cellulose Polymorphs. Cellulose 2001, 8, 49–57. [Google Scholar] [CrossRef]
- Deepa, B.; Abraham, E.; Cordeiro, N.; Mozetic, M.; Mathew, A.P.; Oksman, K.; Faria, M.; Thomas, S.; Pothan, L.A. Utilization of Various Lignocellulosic Biomass for the Production of Nanocellulose: A Comparative Study. Cellulose 2015, 22, 1075–1090. [Google Scholar] [CrossRef]
- Singh, D.K.; Kumar, S.; Udayabhanu, G.; John, R.P. 4(N,N-Dimethylamino) Benzaldehyde Nicotinic Hydrazone As Corrosion Inhibitor for Mild Steel in 1 M HCl Solution: An Experimental and Theoretical Study. J. Mol. Liq. 2016, 216, 738–746. [Google Scholar] [CrossRef]
- Benabdellah, M.; Touzani, R.; Aouniti, A.; Dafali, A.; El Kadiri, S.; Hammouti, B.; Benkaddour, M. Inhibitive Action of Some Bipyrazolic Compounds on the Corrosion of Steel in 1M HCl. Mater. Chem. Phys. 2007, 105, 373–379. [Google Scholar] [CrossRef]
- Saha, S.K.; Ghosh, P.; Hens, A.; Murmu, N.C.; Banerjee, P. Density Functional Theory and Molecular Dynamics Simulation Study on Corrosion Inhibition Performance of Mild Steel by Mercapto-Quinoline Schiff Base Corrosion Inhibitor. Phys. E Low Dimens. Syst. Nanostruct. 2015, 66, 332–341. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.; Haque, J.; Dohare, P.; Lgaz, H.; Salghi, R.; Quraishi, M. Effect of Electron Donating Functional Groups on Corrosion Inhibition of Mild Steel in Hydrochloric Acid: Experimental and Quantum Chemical Study. J. Taiwan Inst. Chem. Eng. 2018, 82, 233–251. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.; Quraishi, M.; Lgaz, H.; Lin, Y. Synthesis and Investigation of Pyran Derivatives as Acidizing Corrosion Inhibitors for N80 Steel in Hydrochloric Acid: Theoretical and Experimental Approaches. J. Alloys Compd. 2018, 762, 347–362. [Google Scholar] [CrossRef]
- Dagdag, O.; Safi, Z.; Hsissou, R.; Erramli, H.; El Bouchti, M.; Wazzan, N.; Guo, L.; Verma, C.; Ebenso, E.E.; El Harfi, A. Epoxy pre-polymers as new and effective materials for corrosion inhibition of carbon steel in acidic medium: Computational and experimental studies. Sci. Rep. 2019, 9, 11715. [Google Scholar] [CrossRef]
- Musa, A.Y.; Kadhum, A.A.H.; Mohamad, A.B.; Takkriff, M.S. Molecular Dynamics and Quantum Chemical Calculation Studies on 4,4-Dimethyl-3-Thiosemicarbazide as Corrosion Inhibitor in 2.5M H2SO4. Mater. Chem. Phys. 2011, 129, 660–665. [Google Scholar] [CrossRef]
- Haque, J.; Ansari, K.; Srivastava, V.; Quraishi, M.; Obot, I. Pyrimidine Derivatives as Novel Acidizing Corrosion Inhibitors for N80 Steel Useful for Petroleum Industry: A Combined Experimental and Theoretical Approach. J. Ind. Eng. Chem. 2017, 49, 176–188. [Google Scholar] [CrossRef]
- Umoren, S.A.; AlAhmary, A.A.; Gasem, Z.M.; Solomon, M.M. Evaluation of Chitosan and Carboxymethyl Cellulose as Ecofriendly Corrosion Inhibitors for Steel. Int. J. Biol. Macromol. 2018, 117, 1017–1028. [Google Scholar] [CrossRef] [PubMed]
- Solomon, M.M.; Gerengi, H.; Umoren, S.A. Carboxymethyl Cellulose/Silver Nanoparticles Composite: Synthesis, Characterization and Application as a Benign Corrosion Inhibitor for St37 Steel in 15% H2SO4 Medium. ACS Appl. Mater. Interfaces 2017, 9, 6376–6389. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, Y.; Meenakshi, S.; Sundaram, C.S. Corrosion Inhibition of Aminated Hydroxyl Ethyl Cellulose on Mild Steel in Acidic Condition. Carbohydr. Polym. 2016, 150, 13–20. [Google Scholar] [CrossRef]
- Toghan, A.; Gadow, H.S.; Dardeer, H.M.; Elabbasy, H.M. New promising halogenated cyclic imides derivatives as Potential Corrosion Inhibitors for Carbon Steel in Acidic Environment. J. Mol. Liq. 2021, 325, 115136. [Google Scholar] [CrossRef]
- Fawzy, A.; Toghan, A. Inhibition Evaluation of Chromotrope Dyes for the Corrosion of Mild Steel in an Acidic Environment: Thermodynamic and Kinetic Aspects. ACS Omega 2021, 6, 4051–4061. [Google Scholar] [CrossRef]
- Tan, J.; Guo, L.; Yang, H.; Zhang, F.; El Bakri, Y. Synergistic Effect of Potassium Iodide and Sodium Dodecyl Sulfonate on the Corrosion Inhibition of Carbon Steel in HCl Medium: A Combined Experimental and Theoretical Investigation. RSC Adv. 2020, 10, 15163–15170. [Google Scholar] [CrossRef] [Green Version]
- Boulhaoua, M.; El Hafi, M.; Zehra, S.; Eddaif, L.; Alrashdi, A.A.; Lahmidi, S.; Guo, L.; Mague, J.T.; Lgaz, H. Synthesis, Structural Analysis and Corrosion Inhibition Application of a New Indazole Derivative on Mild Steel Surface in Acidic Media Complemented with DFT and MD Studies. Colloids Surf. A Physicochem. Eng. Asp. 2021, 617, 126373. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Shalabi, K.; Tantawy, A.H. Corrosion Inhibition and Adsorption Features of Novel Bioactive Cationic Surfactants Bearing Benzenesulphonamide on C1018-Steel under Sweet Conditions: Combined Modeling and Experimental Approaches. J. Mol. Liq. 2020, 320, 114564. [Google Scholar] [CrossRef]
- Palaniappan, N.; Cole, I.S.; Kuznetsov, A.E. Experimental and Computational Studies of Graphene Oxide Covalently Functionalized by Octylamine: Electrochemical Stability, Hydrogen Evolution, and Corrosion Inhibition of the AZ13 Mg Alloy in 3.5% NaCl. RSC Adv. 2020, 10, 11426–11434. [Google Scholar] [CrossRef] [Green Version]
- Obot, I.; Macdonald, D.; Gasem, Z. Density Functional Theory (DFT) As a Powerful Tool for Designing New Organic Corrosion Inhibitors. Part 1: An Overview. Corros. Sci. 2015, 99, 1–30. [Google Scholar] [CrossRef]
- Lukovits, I.; Kálmán, E.; Zucchi, F. Corrosion Inhibitors—Correlation Between Electronic Structure and Efficiency. Corrosion 2001, 57, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Upadhyay, A.; Purohit, A.K.; Mahakur, G.; Dash, S.; Kar, P.K. Verification of Corrosion Inhibition of Mild Steel by Some 4-Aminoantipyrine-Based Schiff Bases—Impact of Adsorbate Substituent and Cross-Conjugation. J. Mol. Liq. 2021, 333, 115960. [Google Scholar] [CrossRef]
- El-Lateef, H.M.A.; Shalabi, K.; Tantawy, A.H. Corrosion Inhibition of Carbon Steel in Hydrochloric Acid Solution Using Newly Synthesized Urea-Based Cationic Fluorosurfactants: Experimental and Computational Investigations. New J. Chem. 2020, 44, 17791–17814. [Google Scholar] [CrossRef]
- Oyebamiji, A.K.; Adeleke, B.B. Quantum chemical studies on inhibition activities of 2,3-dihydroxypropyl-sulfanyl derivative on carbon steel in acidic media. Int. J. Corros. Scale Inhib. 2018, 7, 498–508. [Google Scholar] [CrossRef]
- Madkour, L.H.; Kaya, S.; Obot, I.B. Computational, Monte Carlo Simulation and Experimental Studies of Some Arylazotriazoles (AATR) and Their Copper Complexes in Corrosion Inhibition Process. J. Mol. Liq. 2018, 260, 351–374. [Google Scholar] [CrossRef]
- Gece, G.; Bilgiç, S. Quantum Chemical Study of Some Cyclic Nitrogen Compounds as Corrosion Inhibitors of Steel in NaCl Media. Corros. Sci. 2009, 51, 1876–1878. [Google Scholar] [CrossRef]
- Shalabi, K.; Helmy, A.; El-Askalany, A.; Shahba, M. New Pyridinium Bromide Mono-Cationic Surfactant as Corrosion Inhibitor for Carbon Steel During Chemical Cleaning: Experimental and Theoretical Studies. J. Mol. Liq. 2019, 293, 111480. [Google Scholar] [CrossRef]
- El Aadad, H.; Galai, M.; Ouakki, M.; Elgendy, A.; Touhami, M.E.; Chahine, A. Improvement of the Corrosion Resistance of Mild Steel in Sulfuric Acid by New Organic-Inorganic Hybrids of Benzimidazole-Pyrophosphate: Facile Synthesis, Characterization, Experimental and Theoretical Calculations (DFT and MC). Surf. Interfaces 2021, 24, 101084. [Google Scholar] [CrossRef]
- Dehghani, A.; Mostafatabar, A.H.; Bahlakeh, G.; Ramezanzadeh, B. A Detailed Study on the Synergistic Corrosion Inhibition Impact of the Quercetin Molecules and Trivalent Europium Salt on Mild Steel; electrochemical/Surface Studies, DFT Modeling, and MC/MD Computer Simulation. J. Mol. Liq. 2020, 316, 113914. [Google Scholar] [CrossRef]








| Inhibitors Code | Cinh/ppm | Ecor/V vs. (Ag/AgCl) | jcor/µA cm−2 ± SD | βa/mV dec−1 ± SD | −βc/mV dec−1 ± SD | θ | ζPDP/% |
|---|---|---|---|---|---|---|---|
| Blank | 0.0 | −0.350 | 1054.5 ± 65 | 92.4 ± 6.6 | 172.3 ± 13.1 | - | - |
| CEL | 25 | −0.346 | 728.6 ± 24 | 93.2 ± 4.1 | 169.5 ± 15.3 | 0.309 | 30.9 |
| 50 | −0.345 | 534.6 ± 21 | 89.3 ± 5.2 | 171.3 ± 14.3 | 0.493 | 49.3 | |
| 100 | −0.343 | 314.2 ± 15 | 90.5 ± 6.3 | 166.4 ± 12.8 | 0.702 | 70.2 | |
| 150 | −0.354 | 183.4 ± 9 | 92.8 ± 3.9 | 162.5 ± 11.4 | 0.826 | 82.6 | |
| 200 | −0.334 | 72.76 ± 4 | 95.9 ± 4.8 | 177.6 ± 10.8 | 0.931 | 93.1 | |
| NCC | 25 | −0.353 | 648.5 ± 23 | 96.7 ± 6.5 | 176.6 ± 14.7 | 0.385 | 38.5 |
| 50 | −0.332 | 422.8 ± 13 | 98.4 ± 6.4 | 177.9 ± 12.7 | 0.599 | 59.9 | |
| 100 | −0.344 | 208.7 ± 11 | 90.5 ± 5.4 | 172.8 ± 15.3 | 0.802 | 80.2 | |
| 150 | −0.343 | 126.4 ± 7 | 99.7 ± 7.1 | 173.7 ± 11.8 | 0.887 | 88.7 | |
| 200 | −0.346 | 39. 1 ± 2 | 91.8 ± 7.2 | 177.6 ± 16.2 | 0.963 | 96.3 |
| Inhibitor Codes | Cinh/ppm | Re/Ω cm2 | RP/Ω cm2 | Cdl/µ F cm−2 | QCPE | χ2 × 10−4 | θ | ζE/% | |
|---|---|---|---|---|---|---|---|---|---|
| Y0/μΩ−1 sn cm−2 | n | ||||||||
| Blank | 0.0 | 0.96 | 19.47 | 760.25 | 62.38 | 0.776 | 4.86 | - | - |
| CEL | 25 | 1.14 | 32.25 | 232.49 | 18.29 | 0.851 | 4.97 | 0.396 | 39.6 |
| 50 | 1.15 | 39.38 | 130.15 | 10.21 | 0.881 | 5.03 | 0.505 | 50.5 | |
| 100 | 1.25 | 66.61 | 85.87 | 6.59 | 0.825 | 5.09 | 0.707 | 70.7 | |
| 150 | 1.26 | 103.81 | 75.55 | 5.53 | 0.871 | 5.29 | 0.812 | 81.2 | |
| 200 | 1.28 | 248.28 | 52.24 | 4.94 | 0.831 | 5.15 | 0.921 | 92.1 | |
| NCC | 25 | 1.18 | 34.95 | 227.14 | 17.69 | 0.819 | 4.89 | 0.443 | 344.0 |
| 50 | 1.15 | 48.64 | 118.71 | 9.89 | 0.866 | 5.19 | 0.599 | 59.9 | |
| 100 | 1.28 | 98.66 | 63.18 | 4.932 | 0.873 | 5.33 | 0.802 | 80.2 | |
| 150 | 1.28 | 152.35 | 49.93 | 3.24 | 0.898 | 5.38 | 0.872 | 87.2 | |
| 200 | 1.29 | 398.72 | 37.97 | 2.89 | 0.888 | 5.97 | 0.951 | 95.1 | |
| Inhibitor Name | Corrosive Medium | Optimum Concentration | Max. Protection Capacity/% | Refs. |
|---|---|---|---|---|
| Chitosan | CO2-saturated 3.5% NaCl solution | 100 ppm | 45 | Ref. [46] |
| Commercial inhibitor | CO2-saturated 3.5% NaCl solution | 100 ppm | 88 | Ref. [46] |
| CMC/AgNPs Composite | 15% H2SO4 | 1000 ppm | 89.9 | Ref. [47] |
| Aminated hydroxyl ethyl cellulose | 1.0 M HCl | 900 ppm | 91 | Ref. [48] |
| CEL | 2.0 M HCl | 200 ppm | 93.1 | Current work |
| NCC | 2.0 M HCl | 200 ppm | 96.3 | Current work |
| Inhibitor | CEL | NCC |
|---|---|---|
| EHOMO, eV | −5.73 | −5.45 |
| ELUMO, eV | 0.10 | 0.33 |
| ∆E, eV | 5.83 | 5.78 |
| I | 5.73 | 5.45 |
| A | −0.10 | −0.33 |
| χ | 2.82 | 2.56 |
| η | 2.92 | 2.89 |
| σ | 0.34 | 0.35 |
| ΔN | 0.72 | 0.77 |
| ∆Eback-donation, eV | −0.73 | −0.72 |
| Dipole moment value, debye | 10.24 | 16.64 |
| Molecular surface area, Å2 | 1222.34 | 343.96 |
| Structures | Adsorption Energy/ Kcal mol−1 | Rigid Adsorption Energy/ kcal mol−1 | Deformation Energy/ kcal mol−1 | dEads/dNi: Inhibitor kcal mol−1 | dEads/dNi: Water kcal mol−1 |
|---|---|---|---|---|---|
| Fe (110) | −2133.54 | −2194.93 | 61.39 | −303.04 | −15.82 |
| CEL | |||||
| water | |||||
| Fe (110) | −2831.49 | −2962.22 | 130.73 | −1297.24 | −14.56 |
| NCC | |||||
| water |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toghan, A.; Gouda, M.; Shalabi, K.; El-Lateef, H.M.A. Preparation, Characterization, and Evaluation of Macrocrystalline and Nanocrystalline Cellulose as Potential Corrosion Inhibitors for SS316 Alloy during Acid Pickling Process: Experimental and Computational Methods. Polymers 2021, 13, 2275. https://doi.org/10.3390/polym13142275
Toghan A, Gouda M, Shalabi K, El-Lateef HMA. Preparation, Characterization, and Evaluation of Macrocrystalline and Nanocrystalline Cellulose as Potential Corrosion Inhibitors for SS316 Alloy during Acid Pickling Process: Experimental and Computational Methods. Polymers. 2021; 13(14):2275. https://doi.org/10.3390/polym13142275
Chicago/Turabian StyleToghan, Arafat, Mohamed Gouda, Kamal Shalabi, and Hany M. Abd El-Lateef. 2021. "Preparation, Characterization, and Evaluation of Macrocrystalline and Nanocrystalline Cellulose as Potential Corrosion Inhibitors for SS316 Alloy during Acid Pickling Process: Experimental and Computational Methods" Polymers 13, no. 14: 2275. https://doi.org/10.3390/polym13142275
APA StyleToghan, A., Gouda, M., Shalabi, K., & El-Lateef, H. M. A. (2021). Preparation, Characterization, and Evaluation of Macrocrystalline and Nanocrystalline Cellulose as Potential Corrosion Inhibitors for SS316 Alloy during Acid Pickling Process: Experimental and Computational Methods. Polymers, 13(14), 2275. https://doi.org/10.3390/polym13142275