Review on Interfacial Bonding Mechanism of Functional Polymer Coating on Glass in Atomistic Modeling Perspective
Abstract
:1. Introduction
2. Computational Methodology
2.1. Force Field
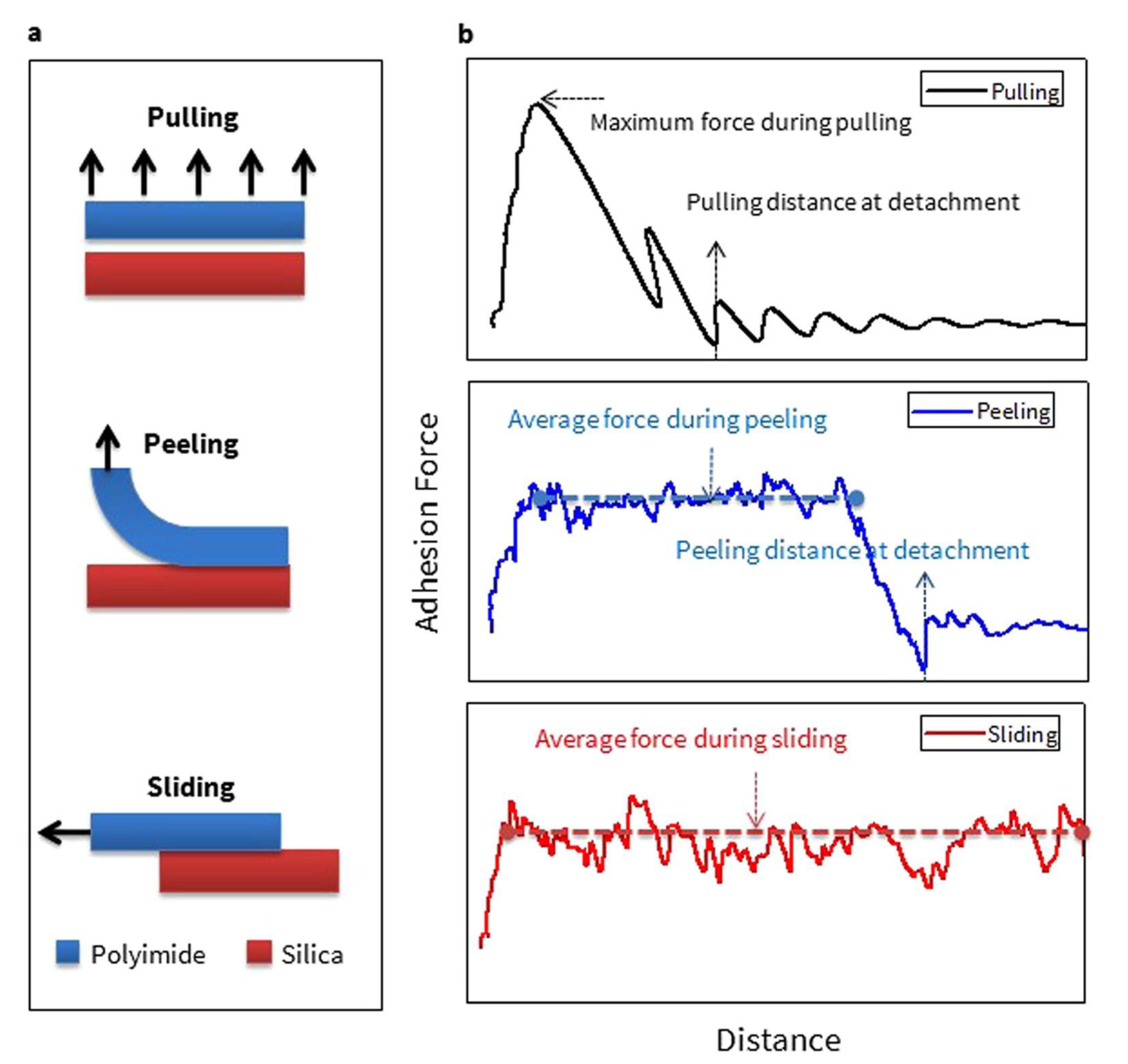
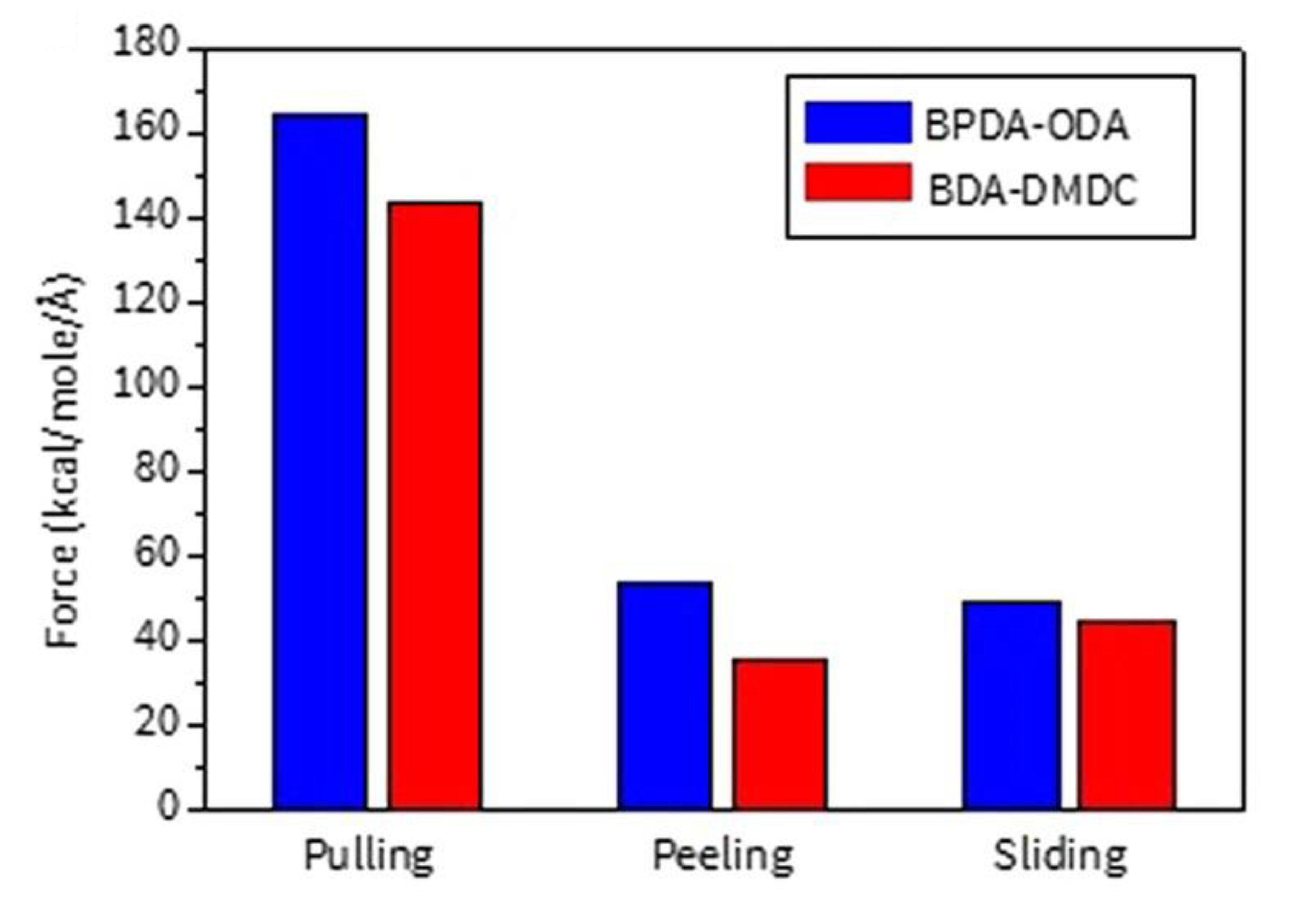
2.2. Pulling, Sliding, and Peeling with Steered Molecular Dynamics
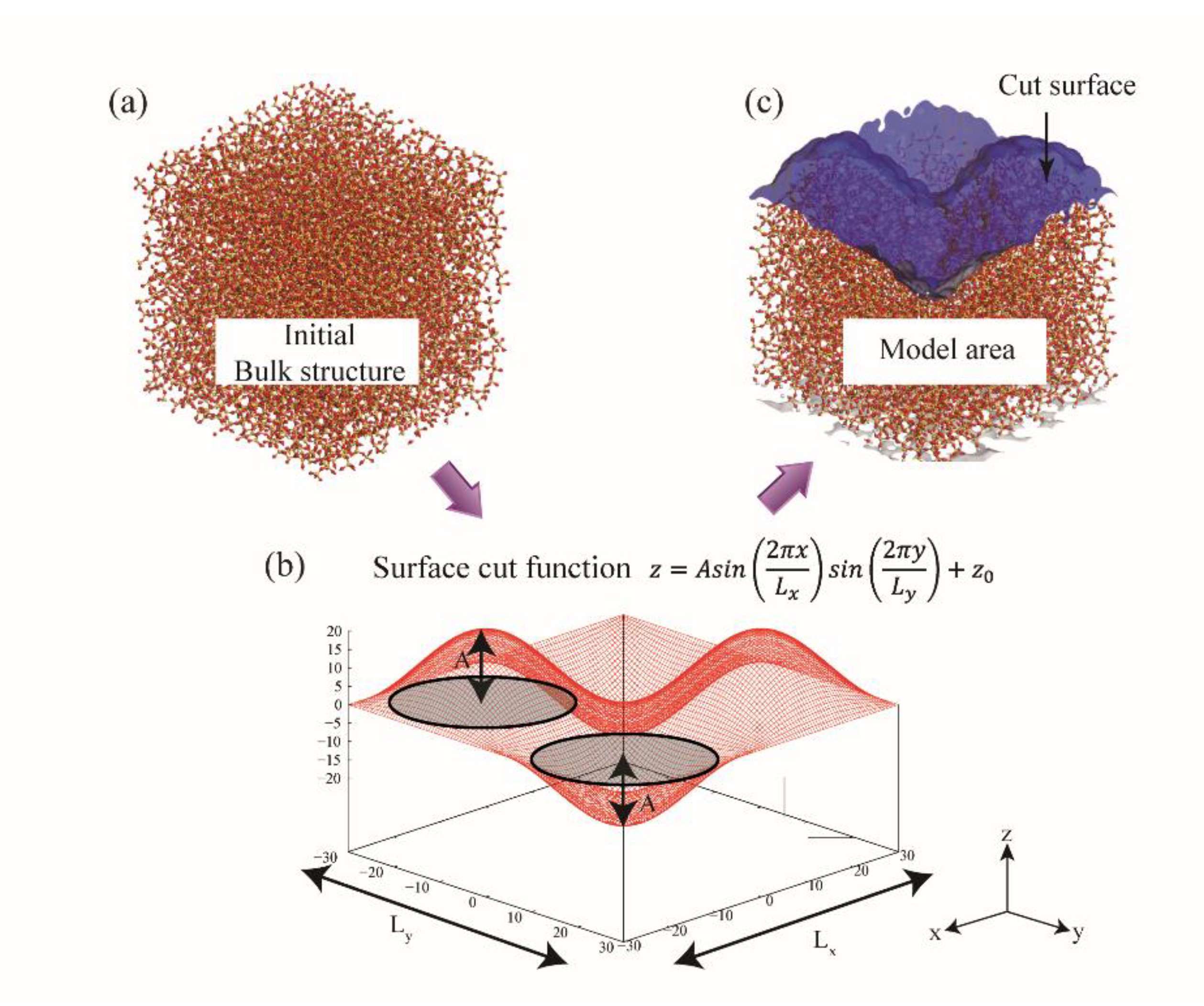
2.3. Generation of Surface Roughness
2.4. Bond Creating/Breaking
3. Mechanism Analysis on Interfacial Interactions
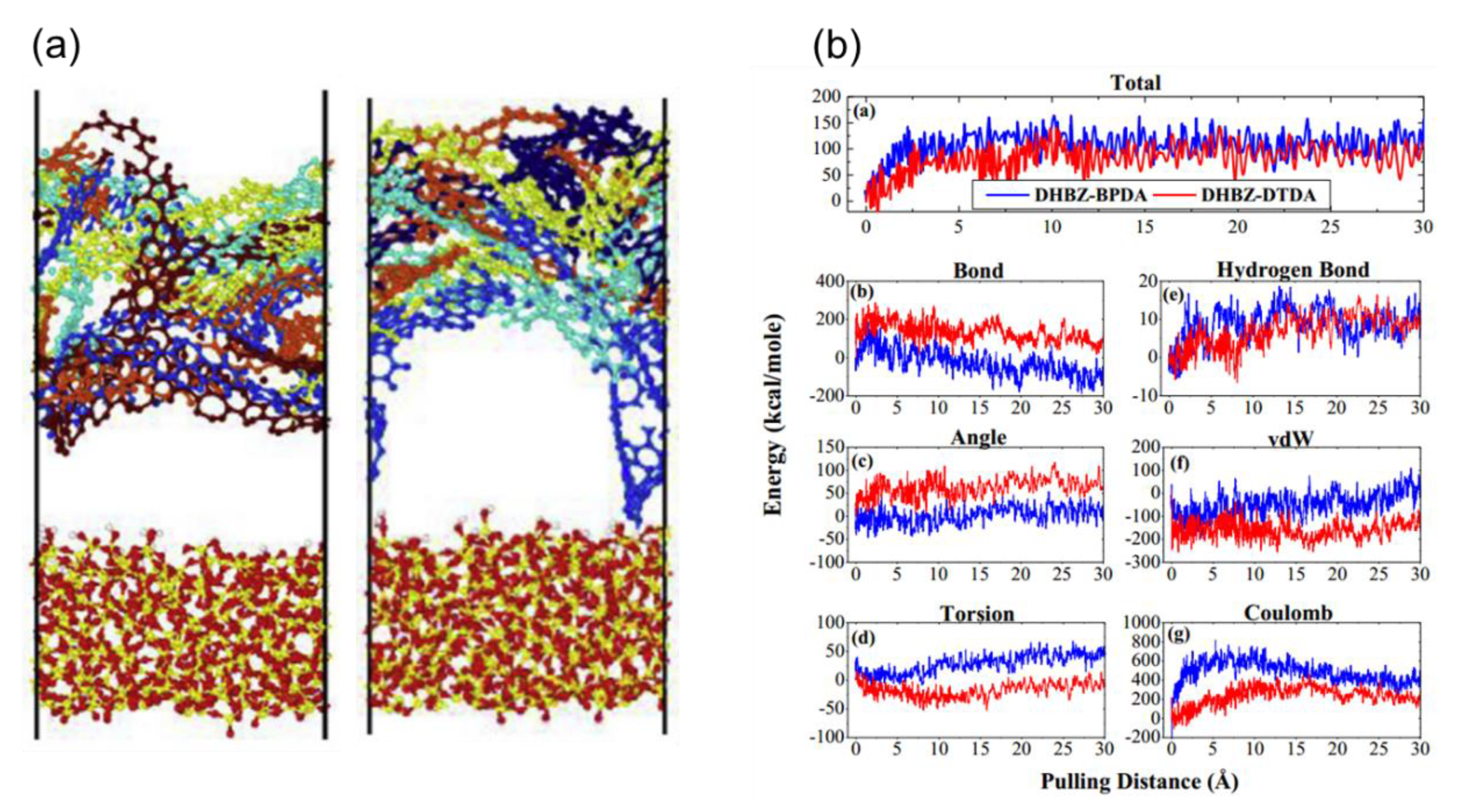
3.1. Non-Bonded Interaction
3.2. Bonded Interaction
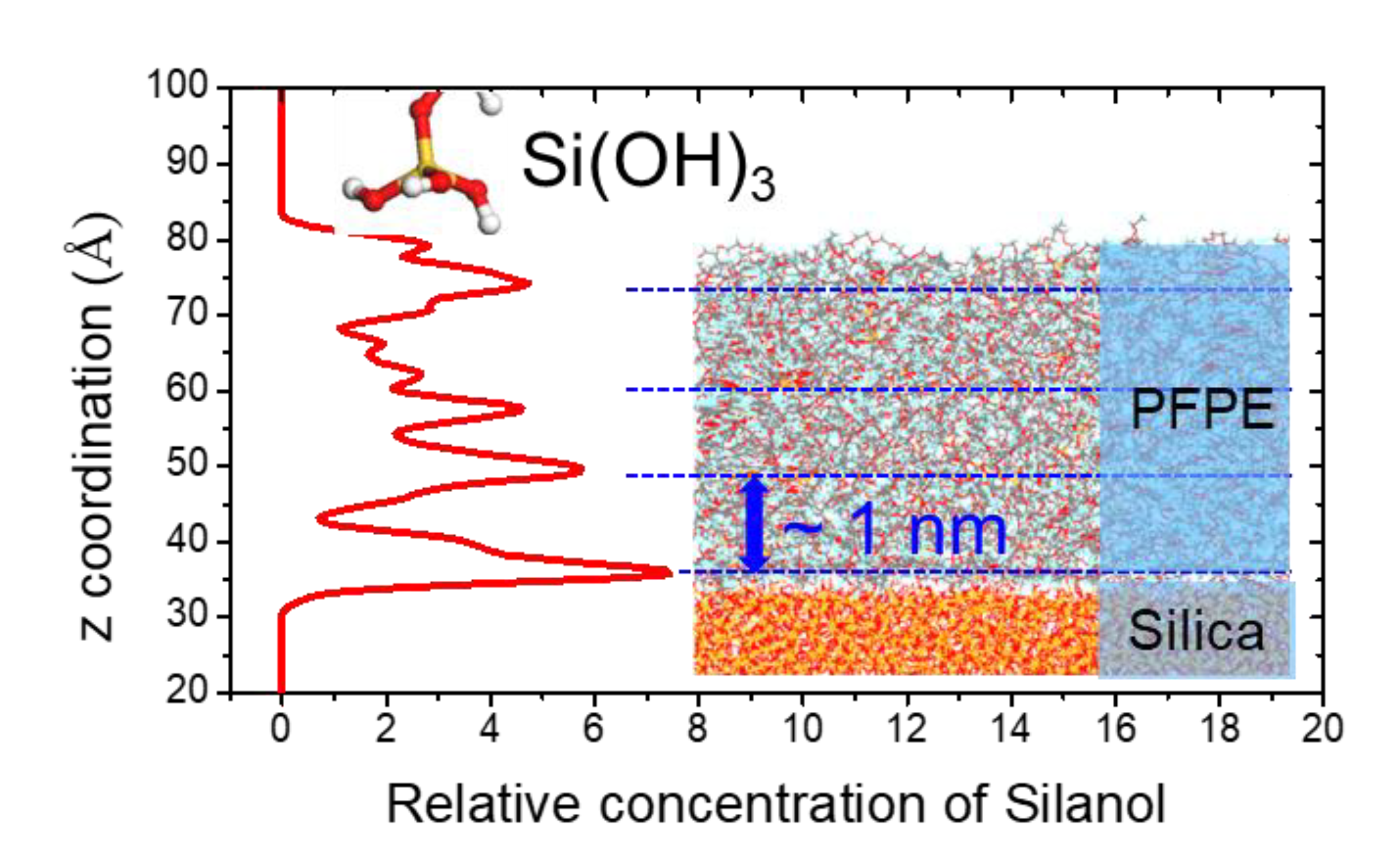
3.2.1. Silane Functionalized Perfluoropolyether (SPFPE) and Siloxane Bond Formation
3.2.2. Film Property and Adhesion of SPFPE
4. Extrinsic Conditions Affecting Interactions
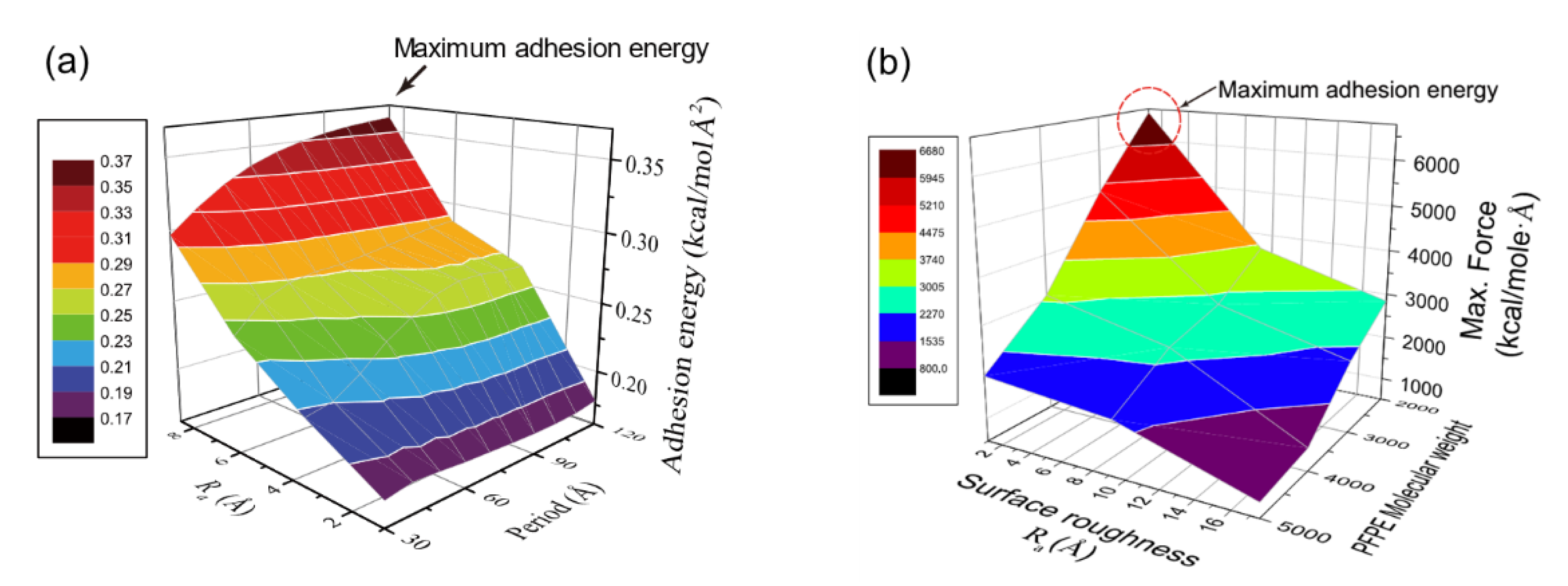
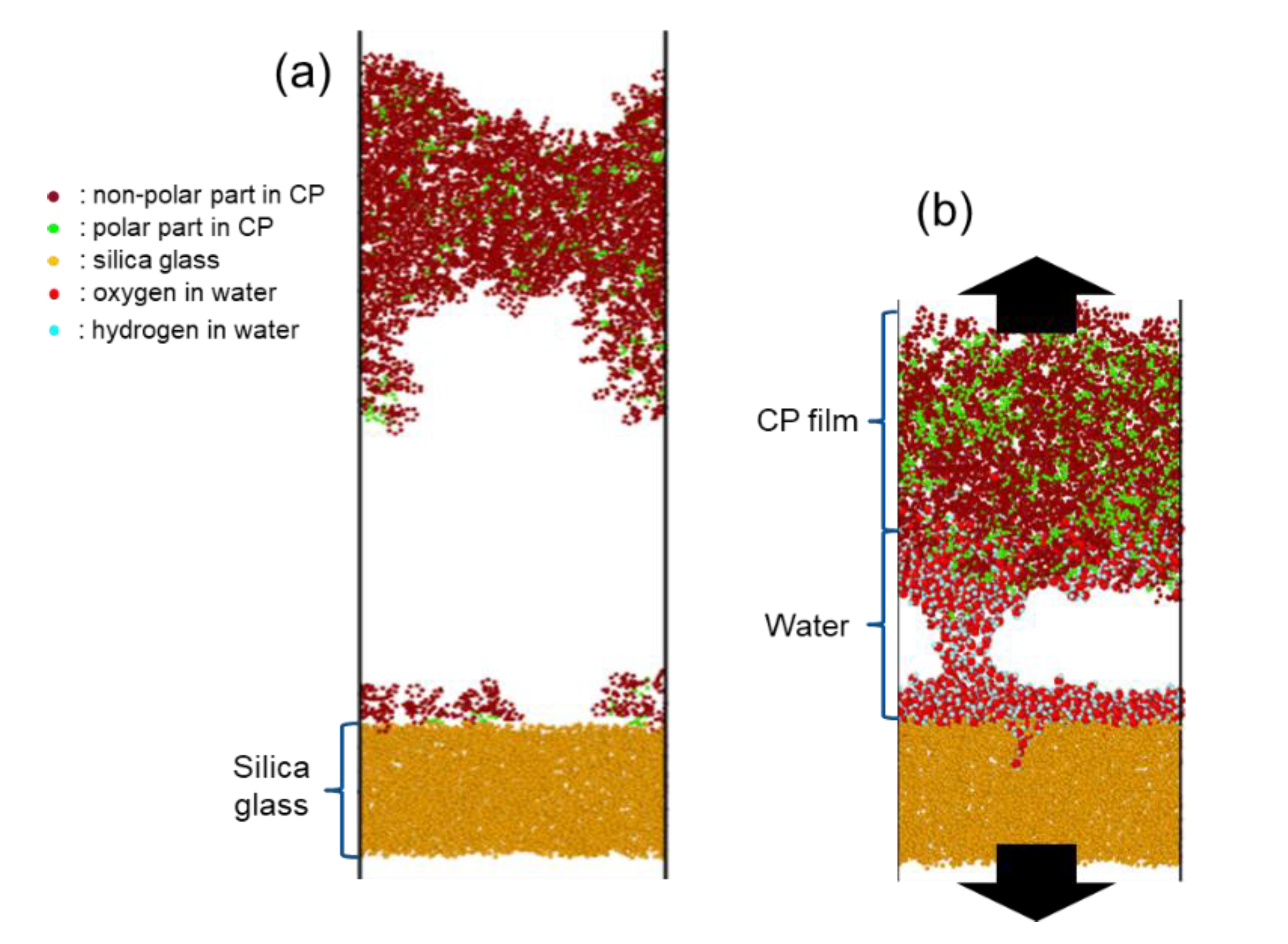
4.1. Surface Morphology: Effect of Nanoscale Roughness on the Adhesion
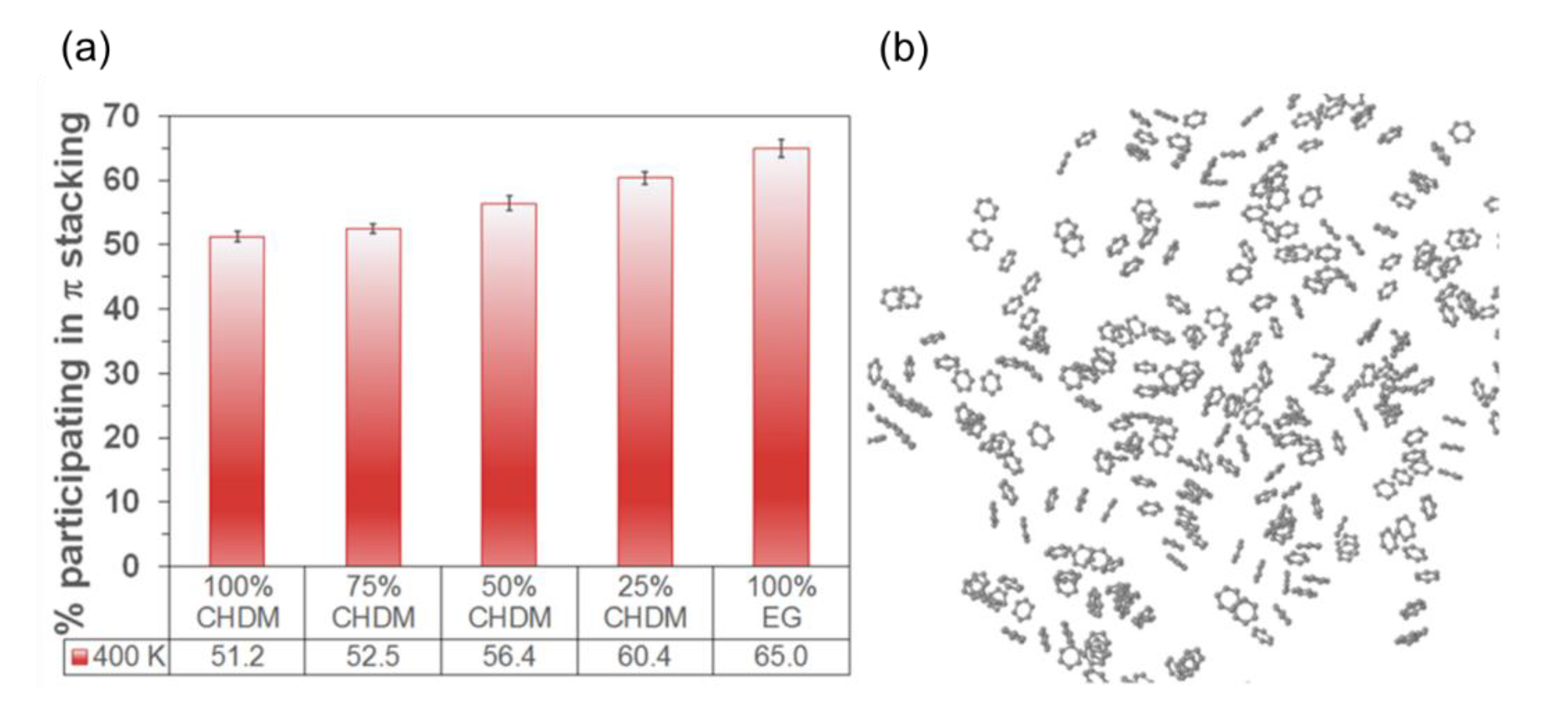
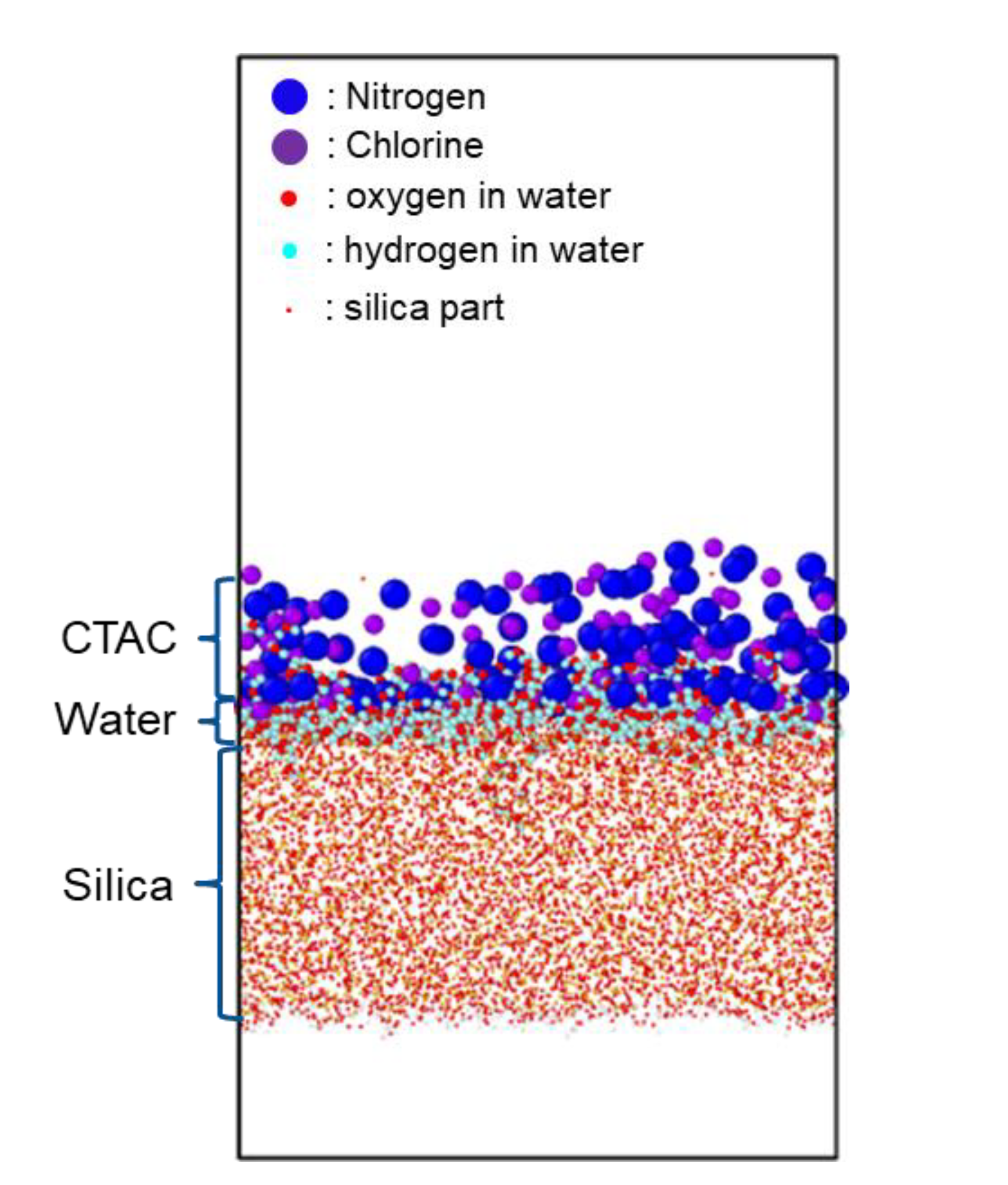
4.2. Humidity
4.2.1. Comparison of Dry and Wet Condition
4.2.2. Control of Relative Humidity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whittingham, M.S. Ultimate Limits to Interaction Reactions for Lithium Batteries. Chem. Rev. 2014, 114, 11414–11443. [Google Scholar] [CrossRef]
- Tallon, M.A.; Liu, X. Industrially Significant Copolymers Containing Maleic Anhydride. In Handbook of Maleic Anhydride Based Materials; Musa, O.M., Ed.; Springer: Cham, Switzerland, 2016; pp. 251–310. [Google Scholar]
- Dilger, K.; Frauenhofer, M. Adhesives in the Automotive Industry. In Handbook of Adhesive Technology; Pizzi, A., Mittal, K.L., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 160–182. [Google Scholar]
- Hussey, R.J.; Wilson, J. Structural Adhesives: Directory and Databook; Springer: London, UK, 2012; pp. 1–13. [Google Scholar]
- Hoshino, T.; Morizawa, Y. Fluorinated Specialty Chemicals—Fluorinated Copolymers for Paints and Perfluoropolyethers for Coatings. In Fluorinated Polymers Volume 2: Applications; Ameduri, B., Sawada, H., Eds.; The Royal Society of Chemistry: Croydon, UK, 2016; Volume 2, p. 110. [Google Scholar]
- Kalyani, N.T.; Swart, H.; Dhoble, S.J. Principles and Applications of Organic Light Emitting Diodes (OLEDs); Elsevier: Duxford, UK, 2017; pp. 205–226. [Google Scholar]
- Moore, E.; Delalat, B.; Vasani, R.; McPhee, G.; Thissen, H.; Voelcker, N.H. Surface-Initiated Hyperbranched Polyglycerol as an Ultralow-Fouling Coating on Glass, Silicon, and Porous Silicon Substrates. Appl. Mater. Interfaces 2014, 6, 15243–15252. [Google Scholar] [CrossRef]
- Chaudhury, M.K.; Gentle, T.M.; Plueddemann, E.P. Adhesion Mechanism of Polyvinyl Chloride to Silane Primed Metal Surfaces. J. Adhesion Sci. Tech. 1987, 1, 29–38. [Google Scholar] [CrossRef]
- Juhl, K.M.; Bovet, N.; Hassenkam, T.; Dideriksen, K.; Pedersen, C.S.; Jensen, C.M.; Okhrimenko, D.V.; Stipp, S.L. Change in Organic Molecule Adhesion on α Alumina (Sapphire) with Change in NaCl and CaCl2 Solution Salinity. Langmuir 2014, 30, 8741–8750. [Google Scholar] [CrossRef]
- Awaja, F.; Gilbert, M.; Kelly, G.; Fox, B.; Pigram, P.J. Adhesion of Polymers. Prog. Polym. Sci. 2009, 34, 948–968. [Google Scholar] [CrossRef]
- Bhushan, B. Adhesion and Stiction: Mechanisms, Measurement Techniques, and Methods for Reduction. J. Vac. Sci. Technol. B 2003, 21, 2262–2296. [Google Scholar] [CrossRef] [Green Version]
- Lacomb, R. Adhesion Measurement Methods Theory and Practice; CRC/Taylor & Francis: Boca Raton, FL, USA, 2006; Volume 1, pp. 7–74. [Google Scholar]
- Hudzinskyy, D.; Lyulin, A.V.; Baljon, A.R.C.; Balabaev, N.K.; Mechels, M.A.J. Effects of Strong Confinement on the Glass-transition Temperature in Simulated Atactic Polystyrene Filmes. Macromolecules 2011, 44, 2299–2310. [Google Scholar] [CrossRef]
- Varshneya, A.K. Fundamentals of Inorganic Glasses, 2nd ed.; Society of Glass Technology: Sheffield, UK, 2013; Volume 1, pp. 477–524. [Google Scholar]
- Miwa, T.; Tawata, R.; Numata, S. Relationship between Structure and Adhesion Properties of Aromatic Polyimides. Polymer 1993, 34, 621–624. [Google Scholar] [CrossRef]
- Israelachvili, J.N. Intermolecular and Surface Forces; Elsevier: Waltham, MA, USA, 2011; Volume 1, pp. 23–52. [Google Scholar]
- Marotzke, A.; Hampe, C. The Energy Release Rate of the Fiber/Polymer Matrix Interface: Measurement. J. Reinf. Plast. Compos. 1997, 16, 341–352. [Google Scholar]
- Pizzi, A.; Mittal, K.L. Handbook of Adhesive Technology, Revised and Expanded; CRC Press: New York, NY, USA, 2003; pp. 159–180. [Google Scholar]
- Pham, T.A.; Lee, D.; Schwegler, E.; Galli, G. Interfacial Effects on the Band Edges of Functionalized Si Surfaces in Liquid Water. J. Am. Chem. Soc. 2014, 136, 17071–17077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wippermann, S.; Voros, M.; Gali, A.; Gygi, F.; Zimanyi, G.T.; Galli, G. Solar Nanocomposites with Complementary Charge Extraction Pathways. Phys. Rev. Rett. 2014, 112, 106801. [Google Scholar]
- Muhich, C.L.; Qui, J.; Holder, A.M.; Wu, Y.C.; Weimer, A.W.; Wei, W.D.; McElwee-White, L.; Musgrave, C.B. Solvent Control of Surface Plasmon-Mediated Chemical Deposition of Au Nanoparticles from Alkylgold Phosphine Complexes. ACS Appl. Mater. Interfaces 2015, 7, 13384–13394. [Google Scholar] [CrossRef] [PubMed]
- Gleizer, A.; Peralta, G.; Kermode, J.R.; De Vita, A.; Shermann, D. Dissociative Chemisorption of O2 Inducing Stress Corrosion Cracking in Silicon Crystals. Phys. Rev. Rett. 2014, 112, 115501. [Google Scholar]
- Ren, J.; Zhou, G.F.; Guo, Z.C.; Zhang, W. Density Functional Theory Study on the Surface Reaction Mechanism of Atomic Layer Deposited Ta2O5 on Si(100) Surfaces. Chem. J. Chin. Univ. 2009, 30, 2279–2283. [Google Scholar]
- Heinz, H.; Lin, T.-J.; Mishra, R.K.; Emami, F.S. Thermodynamically Consistent Force Fields for the Assembly of Inorganic, Organic, and Biological Nanostructures: The INTERFACE Force Field. Langmuir 2013, 29, 1754–1765. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.; Park, H.-H.; Lee, S.H.; Savoy, E.; McKenzie, M.E.; Rammohan, A.R.; Mauro, J.C.; Kim, H.; Min, K.; Cho, E. Characterizing the Fundamental Adhesion of Polyimide Monomers on Crystalline and Glassy Silica Surfaces: A Molecular Dynamics Study. J. Phys. Chem. C 2016, 120, 23631–23639. [Google Scholar] [CrossRef]
- Min, K.; Kim, Y.; Goyal, S.; Lee, S.H.; McKenzie, M.E.; Park, H.; Savoy, E.; Rammohan, A.R.; Mauro, J.C.; Kim, H. Interfacial Adhesion Behavior of Polyimides on Silica Glass: A Molecular Dynamics Study. Polymer 2016, 98, 1–10. [Google Scholar] [CrossRef]
- Min, K.; Rammohan, A.R.; Lee, H.S.; Shin, J.; Lee, S.H.; Goyal, S.; Park, H.; Mauro, J.C.; Stewart, R.; Botu, V.; et al. Computational Approaches for Investigating Interfacial Adhesion Phenomena of Polyimide on Silica Glass. Sci. Rep. 2017, 7, 10475. [Google Scholar] [CrossRef] [Green Version]
- Min, K.; Rammohan, A.R.; Lee, S.H.; Goyal, S.; Park, H.; Stewart, R.; He, X.; Cho, E. Grafting Functional Groups in Polymeric Binder toward Enhancing Structural Integrity of LixSiO2 Anode during Electrochemical Cycling. J. Phys. Chem. C 2018, 122, 17190–17198. [Google Scholar] [CrossRef]
- Ahn, Y.N.; Lee, S.H.; Oh, S.Y. Adsorption characteristics of silane-functionalized perfluoropolyether on hydroxylated glassy silica surfaces: A multiscale approach. Appl. Surf. Sci. 2019, 496, 143699. [Google Scholar] [CrossRef]
- Lee, S.H.; Ahn, Y.N.; Botu, V.; Stewart, R.J.; Oh, S.Y. Enhancement of Adhesion Strength of Perfluoroalkylpolyethers on Rough Glassy Silica for Antismudge Coatings. ACS Appl. Polym. Mater. 2019, 1, 2613–2621. [Google Scholar] [CrossRef]
- Lee, S.H.; Stewart, R.J.; Park, H.; Goyal, S.; Botu, V.; Kim, H.; Min, K.; Cho, E.; Rammohan, A.R.; Mauro, J.C. Effect of Nanoscale Roughness on Adhesion between Glassy Silica and Polyimides: A Molecular Dynamics Study. J. Phys. Chem. C 2017, 121, 24648–24656. [Google Scholar] [CrossRef]
- Park, H.; Lee, S.H. Roles of Paper Composition and Humidity on the Adhesion between Paper Sheet and Glass: A Molecular Dynamics Study. Cellulose, Under review.
- Park, H.; Lee, S.H.; Acquard, D.F.; Agnello, G.; Banerjee, J. Computational Analysis on the Adhesion Mechanism between Organic Coatings and Glass by Molecular Dynamics Simulations. In Proceedings of the International Congress on Glass, Boston, MA, USA, 9–14 June 2019. [Google Scholar]
- Goyal, S.; Park, H.; Lee, S.H.; McKenzie, M.; Rammohan, A.R.; Kim, H.; Mauro, J.; Min, K.; Cho, E.; Botu, V.; et al. Fundamentals of Organic-Glass Adhesion. In Handbook of Materials Modeling; Andreoni, W.Y.S., Ed.; Springer Nature: Cham, Switzerland, 2019; pp. 1–41. [Google Scholar]
- Emami, F.S.; Puddu, V.; Berry, R.J.; Varshney, V.; Patwardhan, S.V.; Perry, C.C.; Heinz, H. Force Field and a Surface Model Database for Silica to Simulate Interfacial Properties in Atomic Resolution. Chem. Mater. 2014, 26, 2647–2658. [Google Scholar] [CrossRef]
- Park, S.; Schulten, K. Calculating potentials of mean force from steered molecular dynamics simulations. J. Chem. Phys. 2004, 120, 5946–5961. [Google Scholar] [CrossRef]
- Jarzynski, C. Nonequilibrium Equality for Free Energy Differences. Phys. Rev. Rett. 1997, 78, 2690. [Google Scholar] [CrossRef] [Green Version]
- Bhushan, B. Modern Tribology Handbook; CRC Press: Boca Raton, FL, USA, 2001; Volume 1, pp. 40–52. [Google Scholar]
- Hanson, B.; Hofmann, J.; Pasquinelli, M.A. Influence of Copolyester Composition on Adhesion to Soda-Lime Glass via Molecular Dynamics Simulations. Appl. Mater. Interfaces 2016, 8, 13583–13589. [Google Scholar] [CrossRef]
- Nishiyama, Y. Structure and Properties of the Cellulose Microfibril. J. Wood. Sci. 2009, 55, 241–249. [Google Scholar] [CrossRef]
- Zugenmaier, P. Crystalline Cellulose and Cellulos Derivatives: Characterization and Structures; Springer: Berlin, Germany, 2008; pp. 1–6. [Google Scholar]
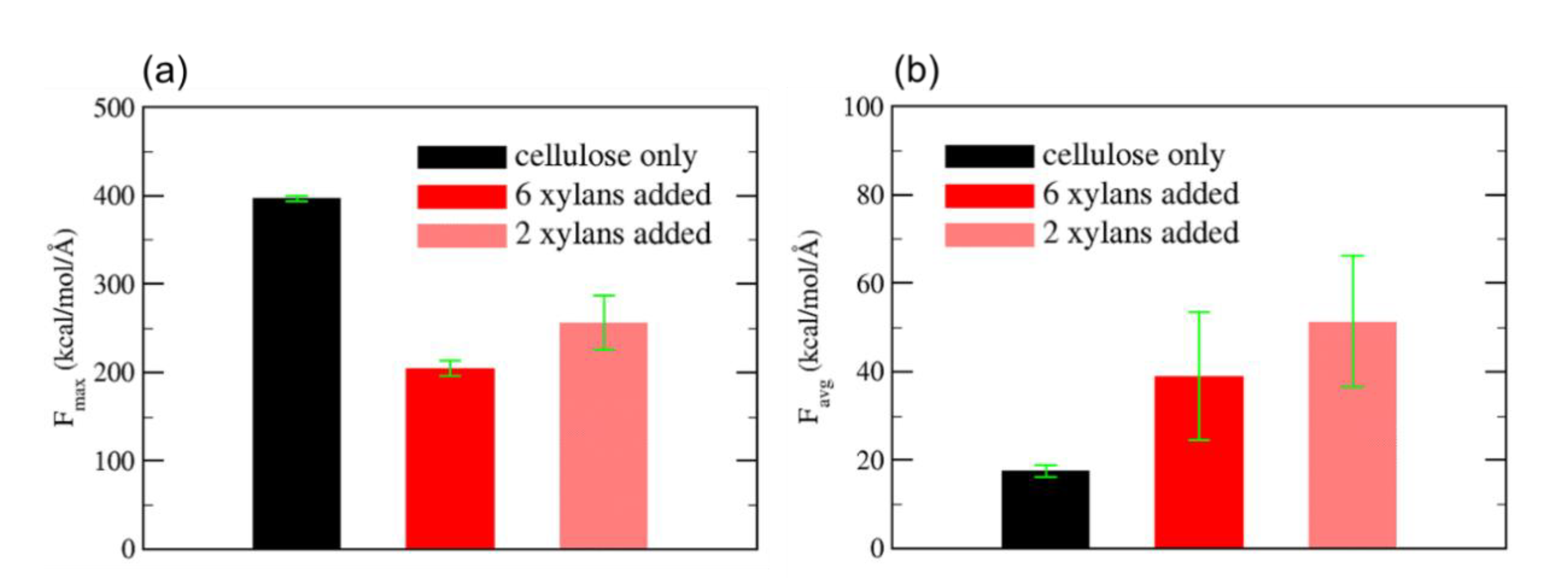
- Zhang, N.; Li, S.; Xiong, L.; Hong, Y.; Chen, Y. Cellulose-Hemicellulose Interaction in Wood Secondary Cell-Wall. Modeling Simul. Mater. Sci. Eng. 2015, 23, 1–15. [Google Scholar] [CrossRef]
- Mazeau, K.; Charlier, L. The Molecular Basis of the Adsorption of Xylans on Cellulose Surface. Cellulose 2012, 19, 337–349. [Google Scholar] [CrossRef]
- Wang, M.; Liechti, K.M.; Wang, Q.; White, J.M. Self-Assembled Silane Monolayers: Fabrication with Nanoscale Uniformity. Langmuir 2005, 21, 1848–1857. [Google Scholar] [CrossRef]
- Kyaw, H.H.; Al-Harthi, S.H.; Sellai, A.; Dutta, J. Self-organization of gold nanoparticles on silanated surfaces. Beilstein J. Nanotechnol. 2015, 6, 2345–2353. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Cho, J.; Seidler, P.M.; Kurland, N.E.; Yadavalli, V.K. Investigations of Chemical Modifications of Amino-Terminated Organic Films on Silicon Substrates and Controlled Protein Immobilization. Langmuir 2010, 26, 2599–2608. [Google Scholar] [CrossRef] [PubMed]
- Pasternack, R.M.; Rivillon Amy, S.; Chabal, Y.J. Attachment of 3-(Aminopropyl)triethoxysilane on Silicon Oxide Surfaces: Dependence on Solution Temperature. Langmuir 2008, 24, 12963–12971. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Hou, S.; Wang, L.; Feng, X.; Wang, R.; Yang, Y.; Wang, C.; Yu, L.; Shao, B.; Qiao, M. Two methods for glass surface modification and their application in protein immobilization. Colloids Surfaces B 2007, 60, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Roscioni, O.M.; Muccioli, L.; Mityashin, A.; Cornil, J.; Zannoni, C. Structural Characterization of Alkylsilane and Fluoroalkylsilane Self-Assembled Monolayers on SiO2 by Molecular Dynamics Simulations. J. Phys. Chem. C 2016, 120, 14652–14662. [Google Scholar] [CrossRef]
- Kim, H.; Saha, J.K.; Zhang, Z.; Jang, J.; Matin, M.A.; Jang, J. Molecular Dynamics Study on the Self-Assembled Monolayer Grown from a Droplet of Alkanethiol. J. Phys. Chem. C 2014, 118, 11149–11157. [Google Scholar] [CrossRef]
- Ewers, B.W.; Batteas, J.D. Molecular Dynamics Simulations of Alkylsilane Monolayers on Silica Nanoasperities: Impact of Surface Curvature on Monolayer Structure and Pathways for Energy Dissipation in Tribological Contacts. J. Phys. Chem. C 2012, 116, 25165–25177. [Google Scholar] [CrossRef]
- Lewis, J.B.; Vilt, S.G.; Rivera, J.L.; Jennings, G.K.; McCabe, C. Frictional Properties of Mixed Fluorocarbon/Hydrocarbon Silane Monolayers: A Simulation Study. Langmuir 2012, 28, 14218–14226. [Google Scholar] [CrossRef]
- Virkar, A.; Mannsfeld, S.; Oh, J.H.; Toney, M.F.; Tan, Y.H.; Liu, G.-y.; Scott, J.C.; Miller, R.; Bao, Z. The Role of OTS Density on Pentacene and C60 Nucleation, Thin Film Growth, and Transistor Performance. Adv. Funct. Mater. 2009, 19, 1962–1970. [Google Scholar] [CrossRef]
- Schollmeyer, H.; Struth, B.; Riegler, H. Long Chain n-Alkanes at SiO2/Air Interfaces: Molecular Ordering, Annealing, and Surface Freezing of Triacontane in the Case of Excess and Submonolayer Coverage. Langmuir 2003, 19, 5042–5051. [Google Scholar] [CrossRef]
- Barriga, J.; Coto, B.; Fernandez, B. Molecular dynamics study of optimal packing structure of OTS self-assembled monolayers on SiO2 surfaces. Tribol. Int. 2007, 40, 960–966. [Google Scholar] [CrossRef]
- Tonelli, C.; Valsecchi, R.; Mashlyakovsky, L.N.; Khomko, E.V. Reactive perfluoropolyoxyalkylene oligomers. I. Isothermal equilibrium on silica gel: Fundamental parameters. J. Fluorine Chem. 2014, 161, 76–82. [Google Scholar] [CrossRef]
- Verdaguer, A.; Weis, C.; Oncins, G.; Ketteler, G.; Bluhm, H.; Salmeron, M. Growth and Structure of Water on SiO2 Films on Si Investigated by Kelvin Probe Microscopy and in Situ X-Ray Spectroscopies. Langmuir 2007, 23, 9699–9703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, K.; Chiu, P.; Jain, M.; Fortes, J.; Moudgil, B.M.; Sinnott, S. Morphology and Mechanical Properties of Surfactant Aggregates at Water-Silica Interfaces: Molecular Dynamics Simulations. Langmuir 2005, 21, 5337. [Google Scholar] [CrossRef] [PubMed]
- Gutig, C.; Grady, B.P.; Striolo, A. Experimental Studies on the Adsorption of Two Surfactants on Solid-Aqueous Interfaces: Adsorption Isotherms and Kinetics. Langmuir 2008, 24, 4806–4816. [Google Scholar] [CrossRef] [PubMed]
- Tyrode, E.; Rutland, M.W.; Bain, C.D. Adsorption of CTAB on Hydrophilic Silica Studied by Linear and Nonlinear Optical Spectroscopy. J. Am. Chem. Soc. 2008, 130, 17434–17445. [Google Scholar] [CrossRef]
- Angeles, F.J.; Khoshnood, A.; Firrozabadi, A. Molecular Dynamics Simulation of the Adsorption and Aggregation of Ionic Surfactants at Liquid-Solid Interfaces. J. Phys. Chem. C 2017, 121, 25908–25920. [Google Scholar] [CrossRef]
- Liu, J.-F.; Min, G.; Ducker, W.A. AFM Study of Adsorption of Cationic Surfactants and Cationic Polyelectrolytes at the Silica-Water Interface. Langmuir 2001, 17, 4895–4903. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.; Lee, S.H. Review on Interfacial Bonding Mechanism of Functional Polymer Coating on Glass in Atomistic Modeling Perspective. Polymers 2021, 13, 2244. https://doi.org/10.3390/polym13142244
Park H, Lee SH. Review on Interfacial Bonding Mechanism of Functional Polymer Coating on Glass in Atomistic Modeling Perspective. Polymers. 2021; 13(14):2244. https://doi.org/10.3390/polym13142244
Chicago/Turabian StylePark, Hyunhang, and Sung Hoon Lee. 2021. "Review on Interfacial Bonding Mechanism of Functional Polymer Coating on Glass in Atomistic Modeling Perspective" Polymers 13, no. 14: 2244. https://doi.org/10.3390/polym13142244
APA StylePark, H., & Lee, S. H. (2021). Review on Interfacial Bonding Mechanism of Functional Polymer Coating on Glass in Atomistic Modeling Perspective. Polymers, 13(14), 2244. https://doi.org/10.3390/polym13142244




