Using Plasma Etching to Access the Polymer Density Distribution and Diffusivity of Gel Particles
Abstract
:1. Introduction
2. Theory
2.1. Gels at Equilibrium
2.2. Evolution to the Equilibrium
3. Materials and Methods
3.1. Synthesis of Minigels
3.2. Polymer Oxidation
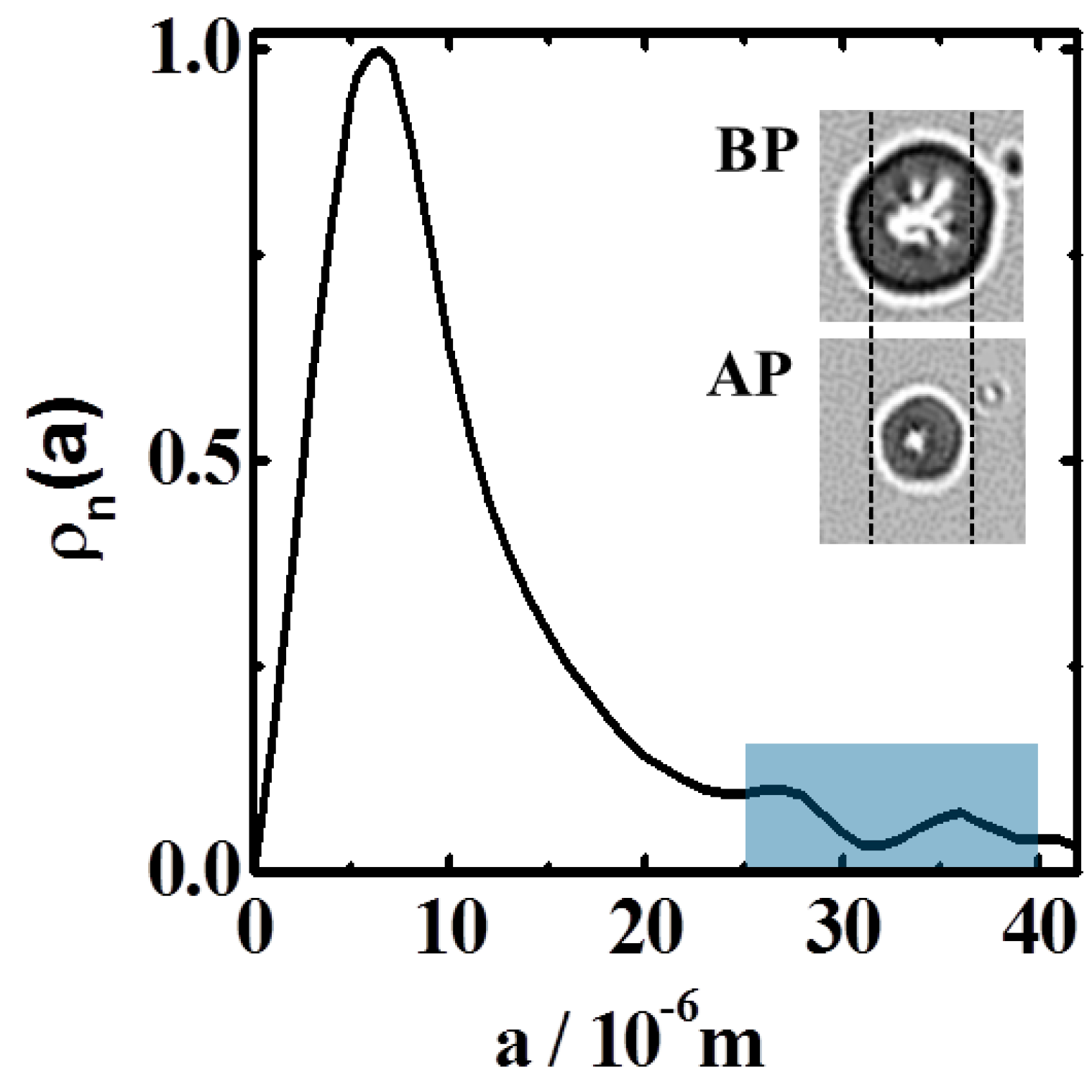
3.3. Particle Size-Distribution
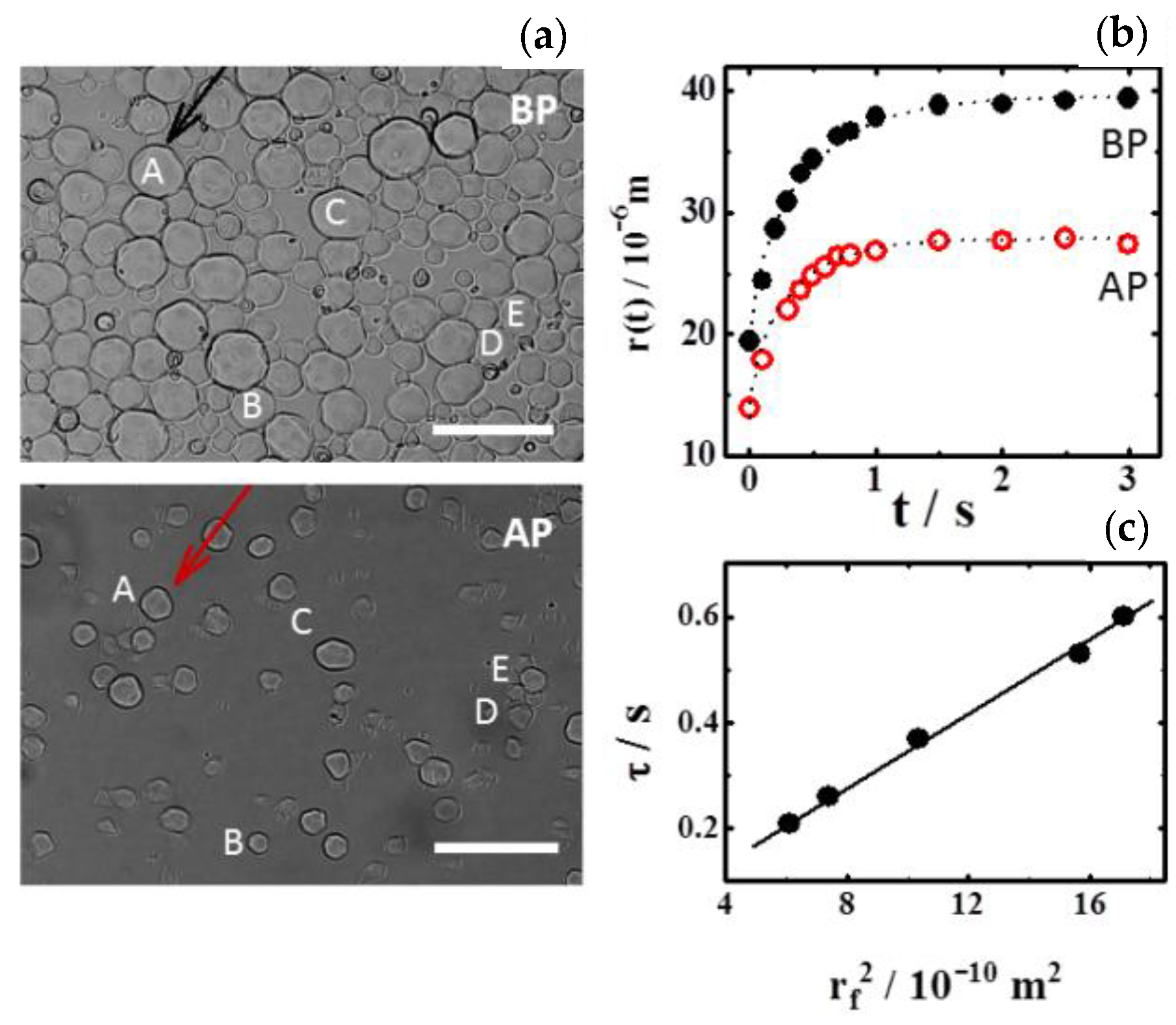
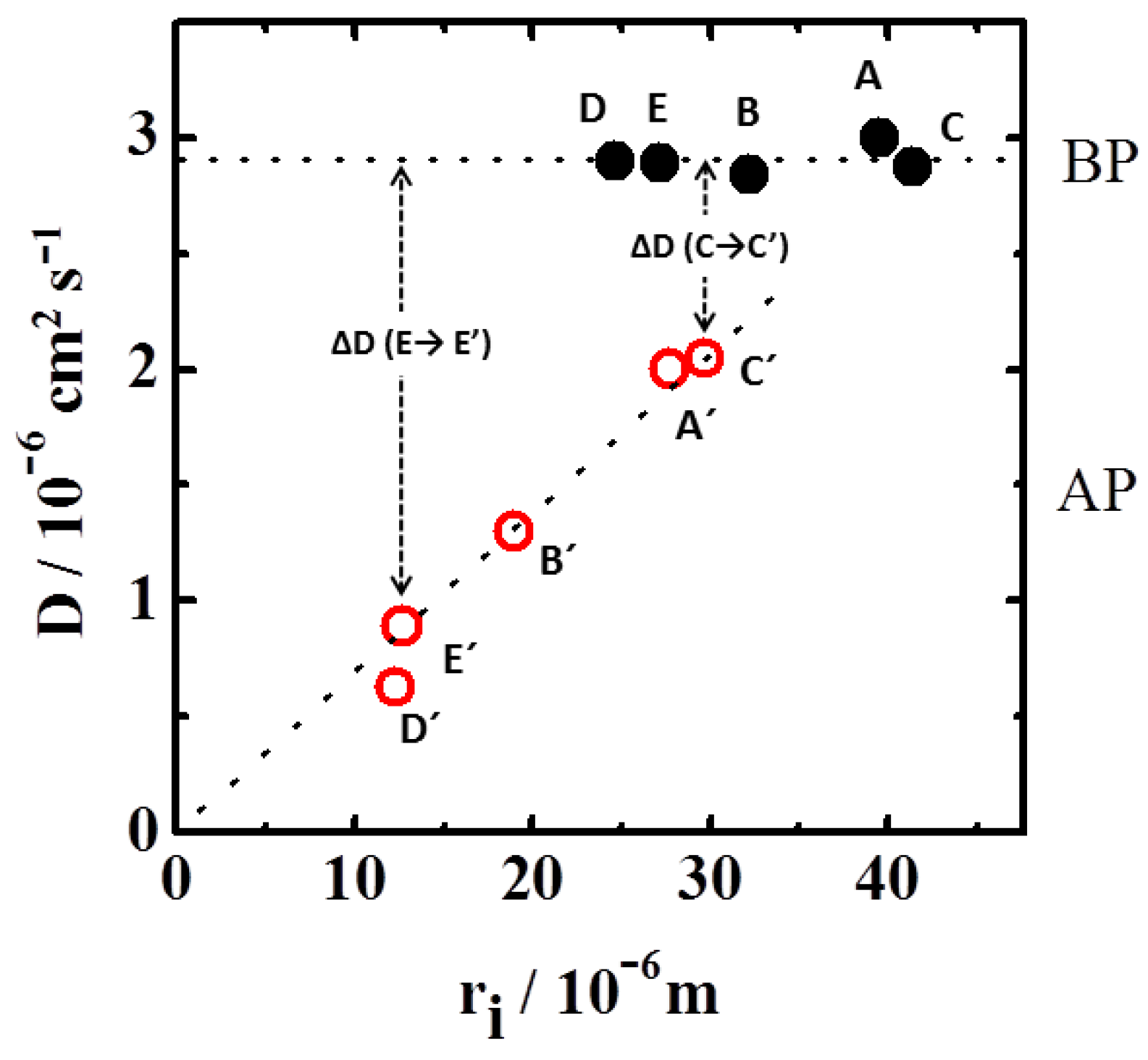
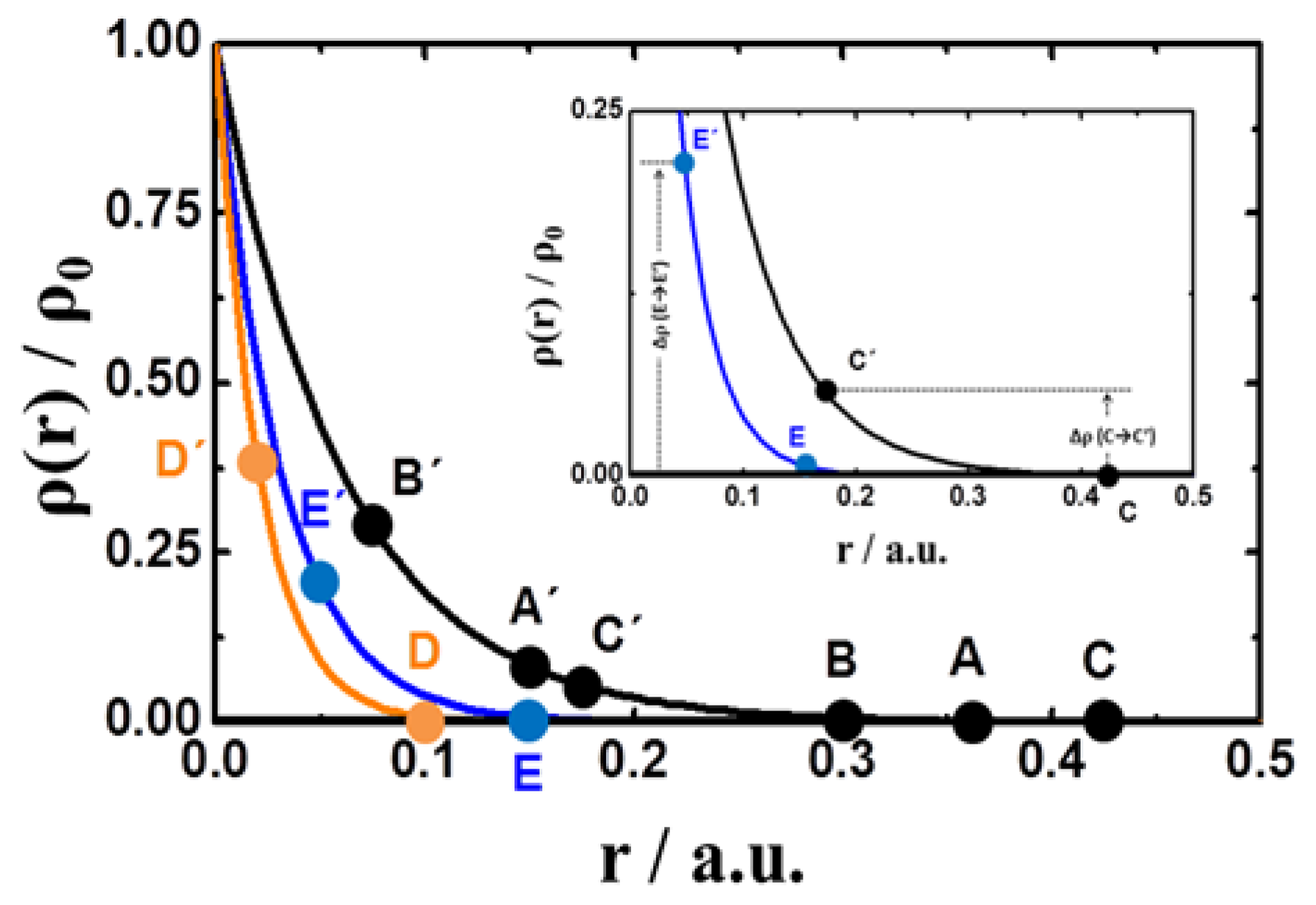
4. Results and Discussion
4.1. Radial Density-Distribution
4.2. Dynamics of Swelling
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoare, T.; Pelton, R. Functionalized Microgel Swelling: Comparing Theory and Experiment. J. Phys. Chem. B 2007, 111, 11895–11906. [Google Scholar] [CrossRef] [PubMed]
- Hirotsu, S. Static and Time-Dependent Properties of Polymer Gels around the Volume Phase Transition. Phase Transit. 1994, 47, 183–240. [Google Scholar] [CrossRef]
- Suzuki, A.; Tanaka, T. Phase Transition in Polymer Gels Induced by Visible Light. Nature 1990, 346, 345–347. [Google Scholar] [CrossRef]
- Lehmann, M.; Tabaka, W.; Möller, T.; Oppermann, A.; Wöll, D.; Volodkin, D.; Wellert, S.; Klitzing, R. von DLS Setup for in Situ Measurements of Photoinduced Size Changes of Microgel-Based Hybrid Particles. Langmuir 2018, 34, 3597–3603. [Google Scholar] [CrossRef]
- Introduction to Physical Polymer Science, 4th Edition | Wiley. Available online: https://www.wiley.com/en-us/Introduction+to+Physical+Polymer+Science%2C+4th+Edition-p-9780471706069 (accessed on 15 March 2021).
- Nickel, A.C.; Scotti, A.; Houston, J.E.; Ito, T.; Crassous, J.; Pedersen, J.S.; Richtering, W. Anisotropic Hollow Microgels That Can Adapt Their Size, Shape, and Softness. Nano Lett. 2019, 19, 8161–8170. [Google Scholar] [CrossRef]
- Witte, J.; Kyrey, T.; Lutzki, J.; Dahl, A.M.; Kühnhammer, M.; von Klitzing, R.; Holderer, O.; Wellert, S. Looking inside Poly(N-Isopropylacrylamide) Microgels: Nanomechanics and Dynamics at Solid–Liquid Interfaces. ACS Appl. Polym. Mater. 2021, 3, 976–985. [Google Scholar] [CrossRef]
- Wu, X.; Pelton, R.H.; Hamielec, A.E.; Woods, D.R.; McPhee, W. The Kinetics of Poly(N-Isopropylacrylamide) Microgel Latex Formation. Colloid. Polym. Sci. 1994, 272, 467–477. [Google Scholar] [CrossRef]
- Acciaro, R.; Gilányi, T.; Varga, I. Preparation of Monodisperse Poly(N-Isopropylacrylamide) Microgel Particles with Homogenous Cross-Link Density Distribution. Langmuir 2011, 27, 7917–7925. [Google Scholar] [CrossRef] [PubMed]
- Varga, I.; Gilányi, T.; Mészáros, R.; Filipcsei, G.; Zrínyi, M. Effect of Cross-Link Density on the Internal Structure of Poly(N-Isopropylacrylamide) Microgels. J. Phys. Chem. B 2001, 105, 9071–9076. [Google Scholar] [CrossRef]
- Crowther, H.M.; Saunders, B.R.; Mears, S.J.; Cosgrove, T.; Vincent, B.; King, S.M.; Yu, G.-E. Poly(NIPAM) Microgel Particle de-Swelling: A Light Scattering and Small-Angle Neutron Scattering Study. Colloids Surf. A Physicochem. Eng. Asp. 1999, 152, 327–333. [Google Scholar] [CrossRef]
- Kratz, K.; Hellweg, T.; Eimer, W. Structural Changes in PNIPAM Microgel Particles as Seen by SANS, DLS, and EM Techniques. Polymer 2001, 42, 6631–6639. [Google Scholar] [CrossRef]
- Fernández-Barbero, A.; Fernández-Nieves, A.; Grillo, I.; López-Cabarcos, E. Structural Modifications in the Swelling of Inhomogeneous Microgels by Light and Neutron Scattering. Phys. Rev. E 2002, 66, 051803. [Google Scholar] [CrossRef] [PubMed]
- Kratz, K.; Lapp, A.; Eimer, W.; Hellweg, T. Volume Transition and Structure of Triethyleneglycol Dimethacrylate, Ethylenglykol Dimethacrylate, and N,N′-Methylene Bis-Acrylamide Cross-Linked Poly(N-Isopropyl Acrylamide) Microgels: A Small Angle Neutron and Dynamic Light Scattering Study. Colloids Surf. A Physicochem. Eng. Asp. 2002, 197, 55–67. [Google Scholar] [CrossRef]
- Saunders, B.R. On the Structure of Poly(N-Isopropylacrylamide) Microgel Particles. Langmuir 2004, 20, 3925–3932. [Google Scholar] [CrossRef]
- Wellert, S.; Radulescu, A.; Carl, A.; von Klitzing, R.; Gawlitza, K. Evolution of Size and Structure during the Polymerization Process: A SANS Study on EG-Based Microgels. Macromolecules 2015, 48, 4901–4909. [Google Scholar] [CrossRef]
- Di Lorenzo, F.; Hellwig, J.; von Klitzing, R.; Seiffert, S. Macroscopic and Microscopic Elasticity of Heterogeneous Polymer Gels. ACS Macro Lett. 2015, 4, 698–703. [Google Scholar] [CrossRef]
- Fernández-Nieves, A.; de las Nieves, F.J.; Fernández-Barbero, A. Static Light Scattering from Microgel Particles: Model of Variable Dielectric Permittivity. J. Chem. Phys. 2003, 120, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Conley, G.M.; Nöjd, S.; Braibanti, M.; Schurtenberger, P.; Scheffold, F. Superresolution Microscopy of the Volume Phase Transition of PNIPAM Microgels. Colloids Surf. A Physicochem. Eng. Asp. 2016, 499, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Saunders, B.R.; Vincent, B. Thermal and Osmotic Deswelling of Poly(NIPAM) Microgel Particles. J. Chem. Soc. Faraday Trans. 1996, 92, 3385–3389. [Google Scholar] [CrossRef]
- Fernández-Nieves, A.; Fernández-Barbero, A.; Vincent, B.; de las Nieves, F.J. Charge Controlled Swelling of Microgel Particles. Macromolecules 2000, 33, 2114–2118. [Google Scholar] [CrossRef]
- Fernández-Nieves, A.; Fernández-Barbero, A.; de las Nieves, F.J. Salt Effects over the Swelling of Ionized Mesoscopic Gels. J. Chem. Phys. 2001, 115, 7644–7649. [Google Scholar] [CrossRef]
- Tanaka, T.; Fillmore, D.J. Kinetics of Swelling of Gels. J. Chem. Phys. 1979, 70, 1214–1218. [Google Scholar] [CrossRef]
- Shibayama, M.; Takata, S.; Norisuye, T. Static Inhomogeneities and Dynamic Fluctuations of Temperature Sensitive Polymer Gels. Phys. A Stat. Mech. Appl. 1998, 249, 245–252. [Google Scholar] [CrossRef]
- Suárez, I.J.; Fernández-Nieves, A.; Márquez, M. Swelling Kinetics of Poly(N-Isopropylacrylamide) Minigels. J. Phys. Chem. B 2006, 110, 25729–25733. [Google Scholar] [CrossRef] [PubMed]
- Martínez, H.; Rodríguez-Lazcano, Y. N-Isopropylacrylamide Decomposition Process in Helium Plasma. J. Appl. Polym. Sci. 2007, 103, 2591–2596. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suarez, I.J.; Sierra-Martin, B.; Fernandez-Barbero, A. Using Plasma Etching to Access the Polymer Density Distribution and Diffusivity of Gel Particles. Polymers 2021, 13, 2537. https://doi.org/10.3390/polym13152537
Suarez IJ, Sierra-Martin B, Fernandez-Barbero A. Using Plasma Etching to Access the Polymer Density Distribution and Diffusivity of Gel Particles. Polymers. 2021; 13(15):2537. https://doi.org/10.3390/polym13152537
Chicago/Turabian StyleSuarez, Ivan J., Benjamin Sierra-Martin, and Antonio Fernandez-Barbero. 2021. "Using Plasma Etching to Access the Polymer Density Distribution and Diffusivity of Gel Particles" Polymers 13, no. 15: 2537. https://doi.org/10.3390/polym13152537
APA StyleSuarez, I. J., Sierra-Martin, B., & Fernandez-Barbero, A. (2021). Using Plasma Etching to Access the Polymer Density Distribution and Diffusivity of Gel Particles. Polymers, 13(15), 2537. https://doi.org/10.3390/polym13152537






