Fiber Selection for Reinforced Additive Manufacturing
Abstract
:1. Introduction
2. Characteristics of Fibers
2.1. Natural Fibers
2.1.1. Cellulose Fibers
Vegetable Plant Fibers
Seed Fibers
Stem (Bast) Fibers
Flax Fiber
Hemp Fiber
Jute Fiber
Ramie Fiber
Leaf Fibers
Abaca Fiber
Sisal Fiber
Husk/Fruit Fibers
Wood Fibers
Regenerated Cellulose Fibers
Rayon Fibers
Acetate Fibers
2.1.2. Keratin Fibers
Wool Fibers
Silk Fibers
2.1.3. Comparison of Natural Fibers
2.2. Synthetic Polymer Fibers
2.2.1. Amide Fibers
2.2.2. Polyester Fibers
2.2.3. Liquid Crystalline Polymer Fibers
Aramid Fibers
Para-Aramid Fibers
Meta-Aramid Fibers
Aromatic Heterocycle Liquid Crystalline Fibers
PBO Fibers
PBI Fibers
PIPD Fibers
Copolyester Liquid Crystalline Fibers
2.2.4. Olefin Fibers
Polyethylene Fibers
Polypropylene Fibers
2.2.5. Acrylic Fibers
2.3. Carbon Fibers
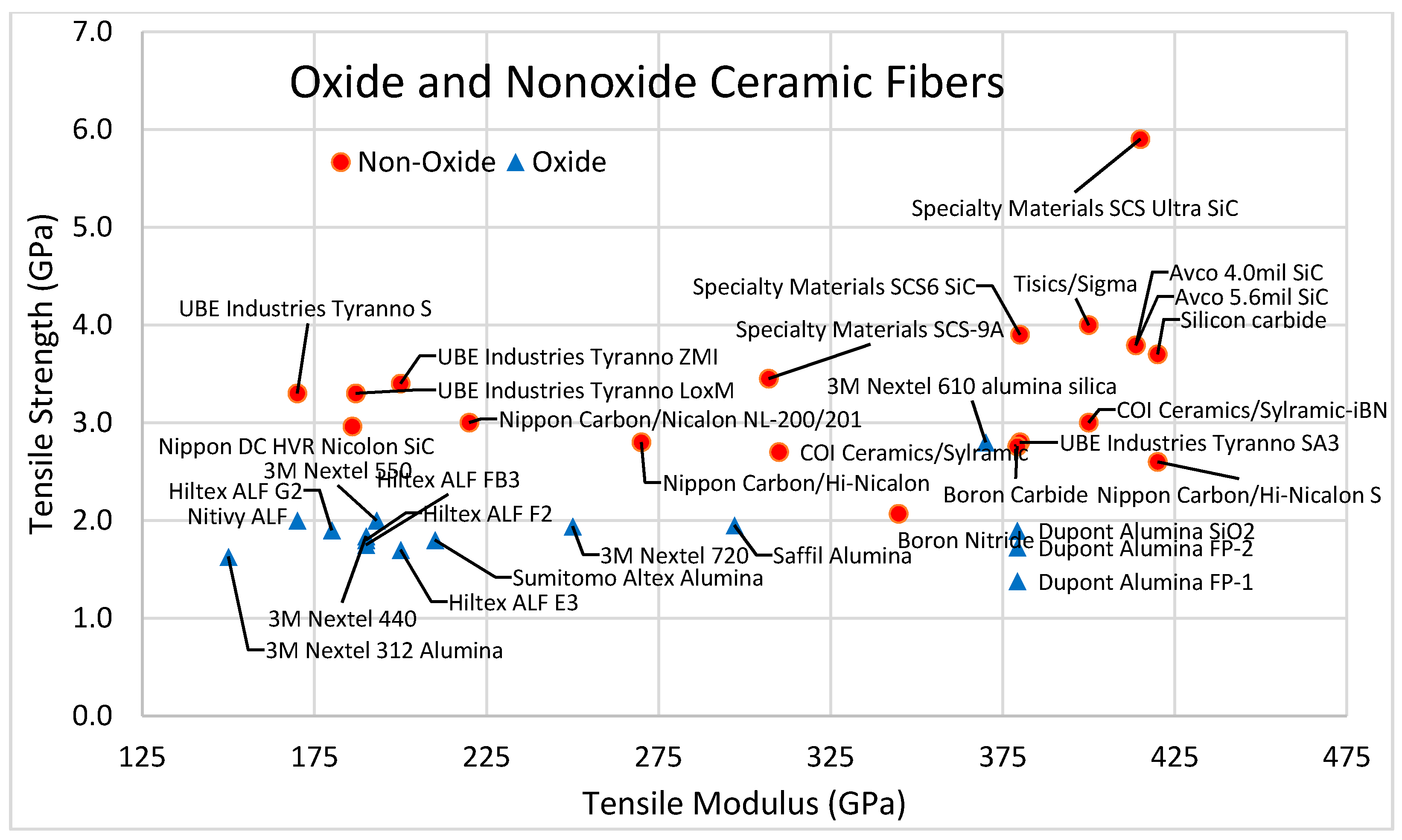
2.4. Ceramic Fibers
2.4.1. Nonoxide Ceramic Fibers
2.4.2. Oxide Ceramic Fibers
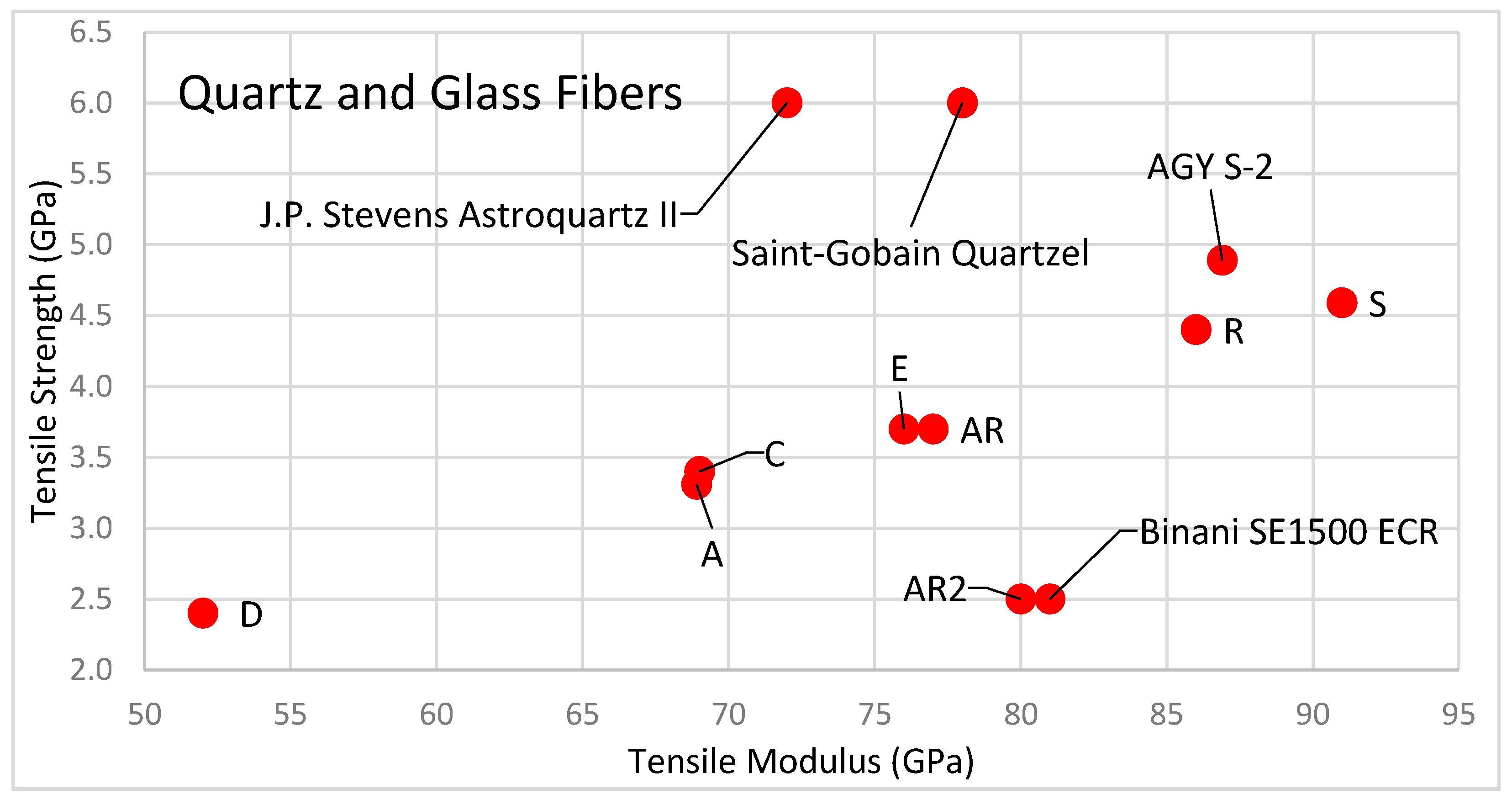
2.5. Oxide Fibers: Quartz and Glass
2.5.1. Quartz Fiber
2.5.2. Glass Fiber
2.6. Mineral Fibers
2.6.1. Asbestos Fibers
2.6.2. Boron Fibers
2.7. Volcanic Rock: Basalt Fibers
2.8. Metal Fibers
3. Mechanical, Thermal, and Chemical Considerations for Fiber Selection
3.1. Anisotropic and Isotropic Characteristics
3.2. Mechanical Considerations
3.2.1. Density
3.2.2. Strength
Tensile Strength
Compressive Strength
Interfacial Strength
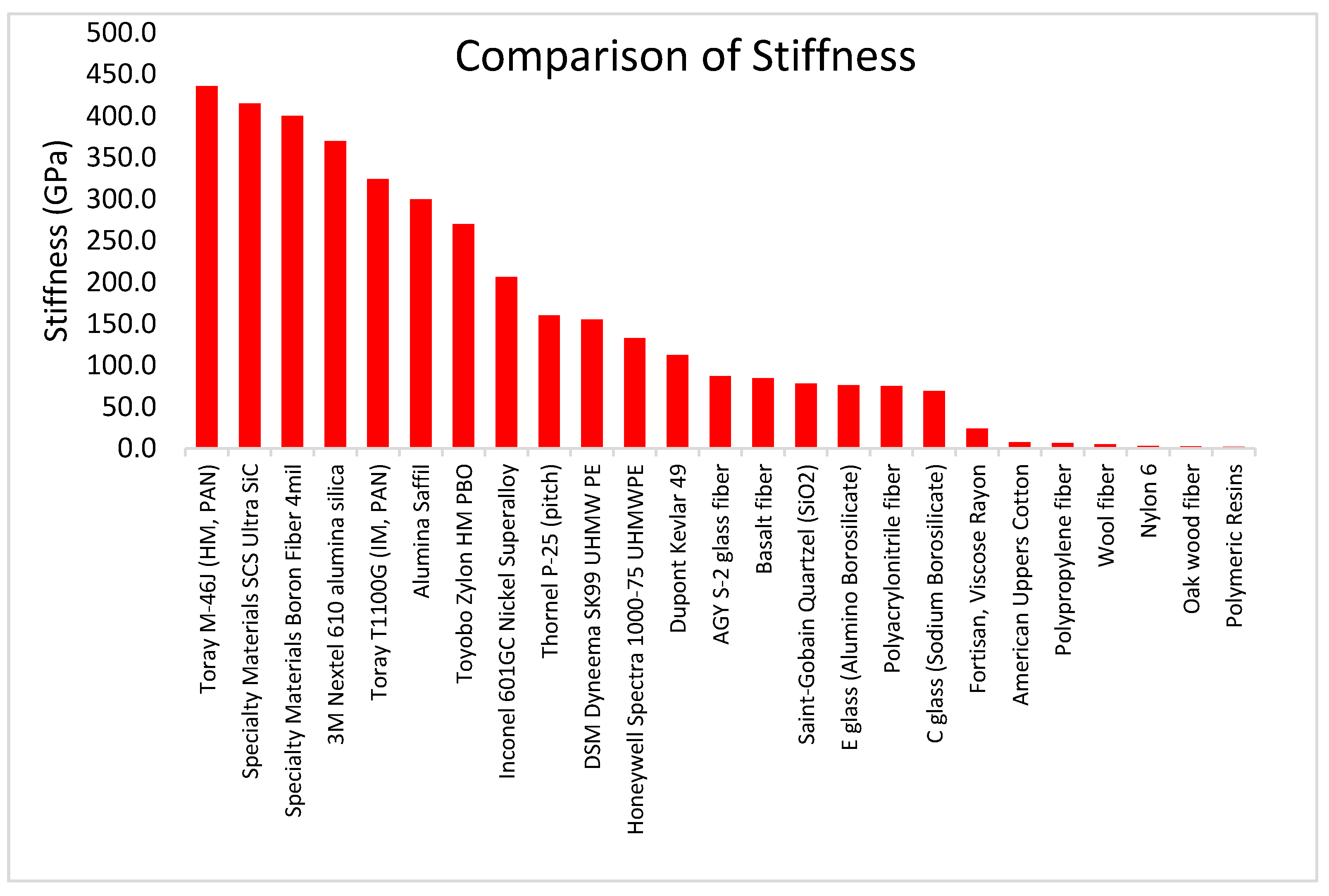
3.2.3. Stiffness
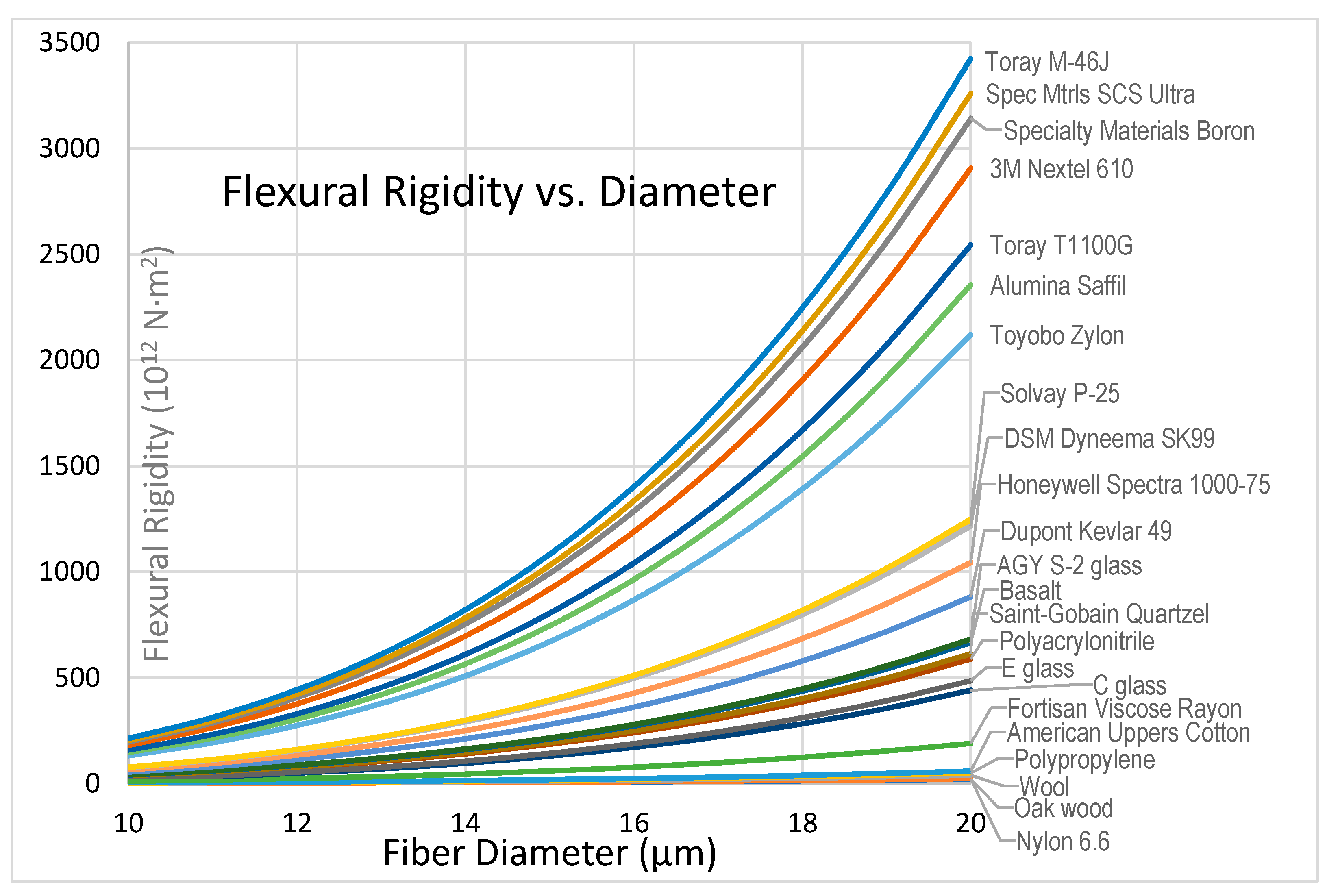
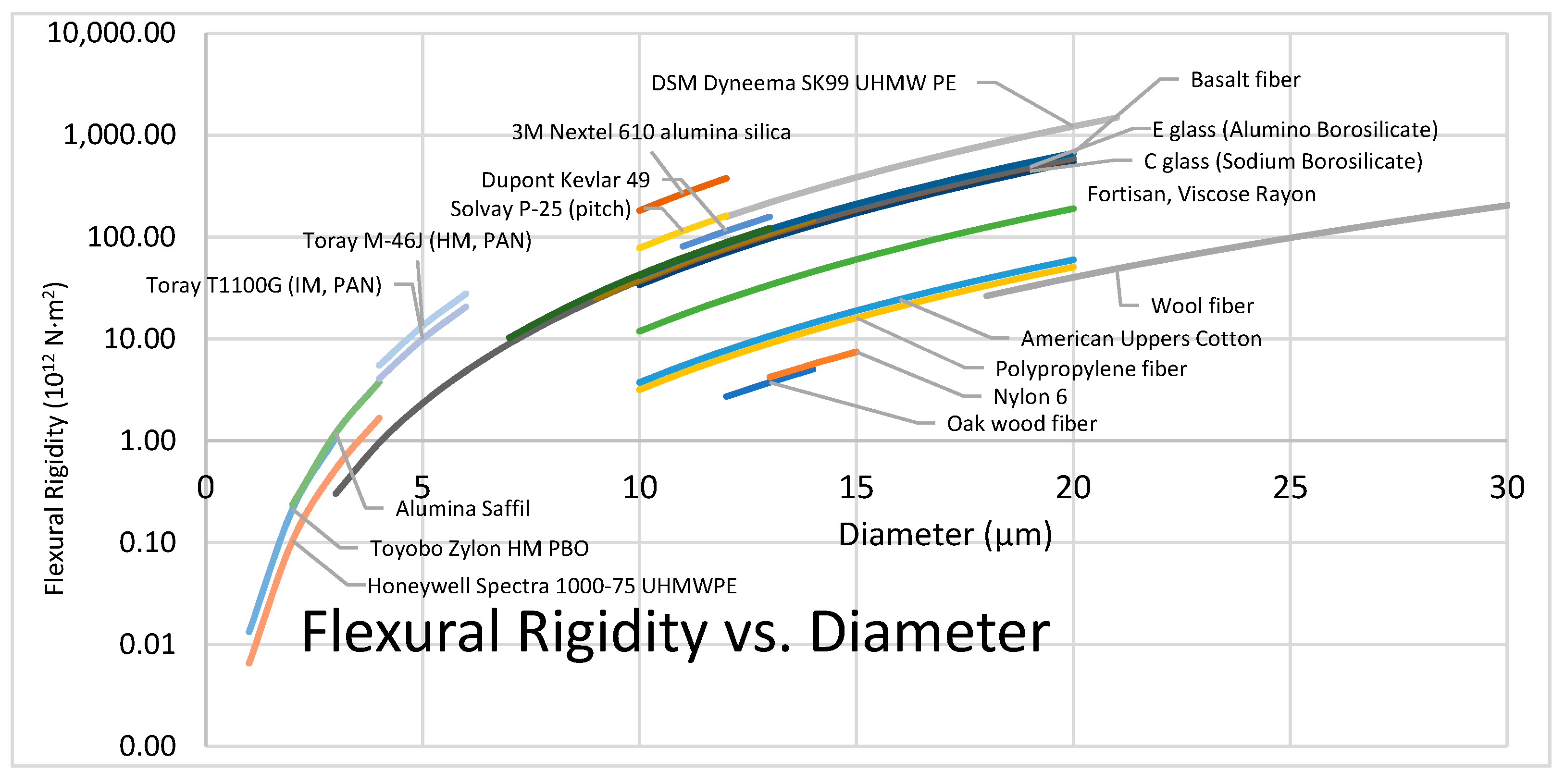
3.2.4. Flexibility and Flexural Rigidity
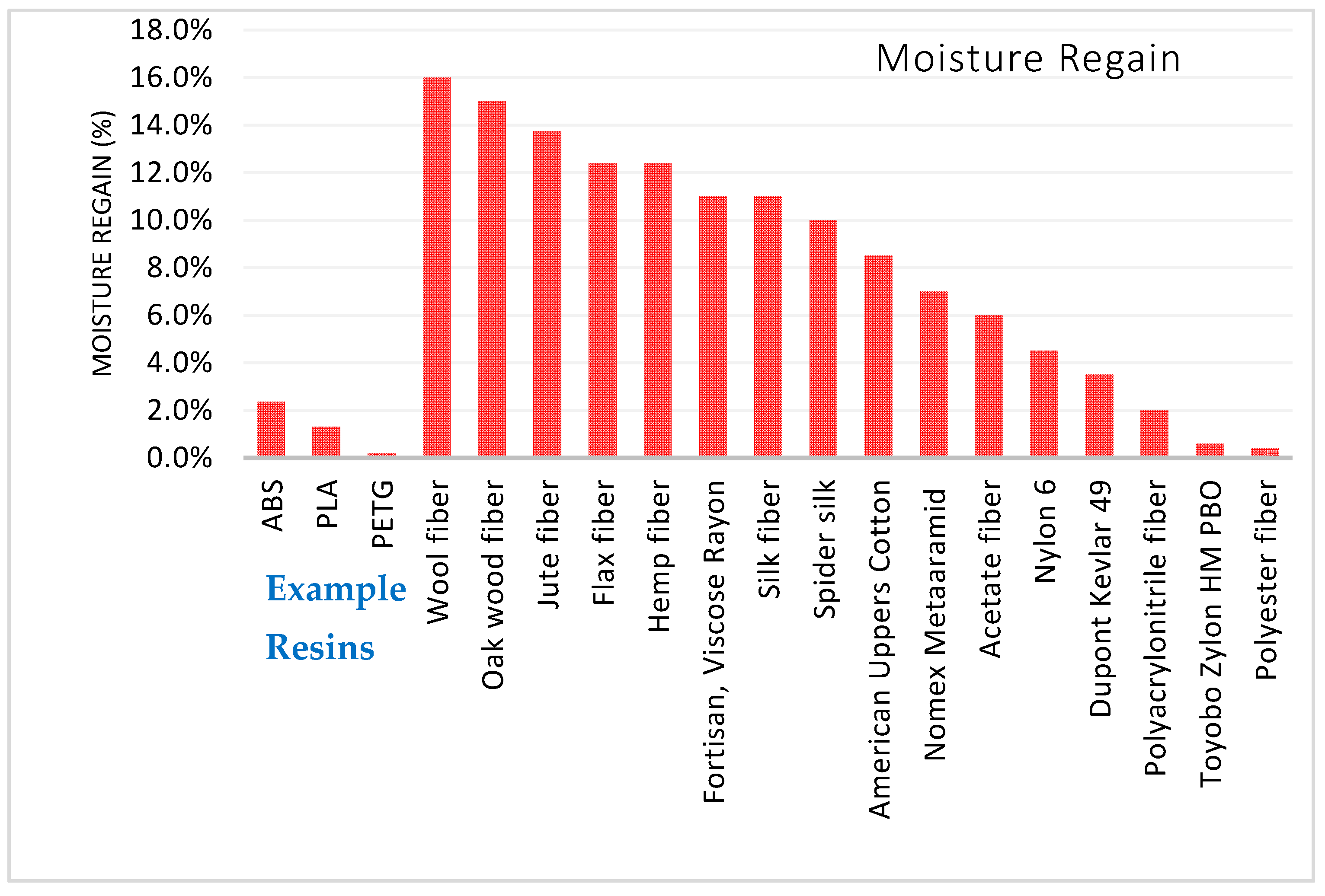
3.2.5. Moisture Regain
3.3. Thermal Considerations
3.3.1. Maximum Temperature
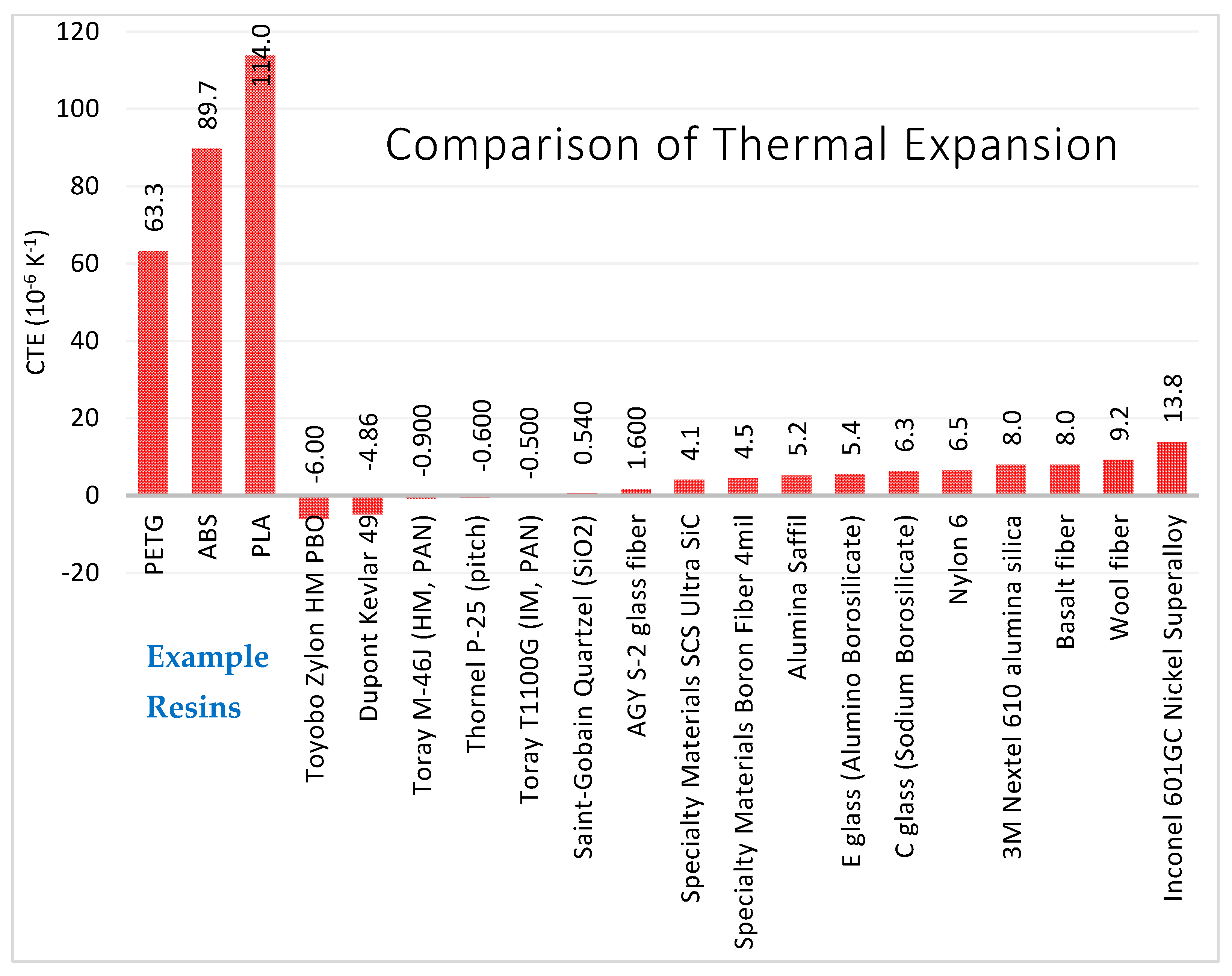
3.3.2. Thermal Expansion
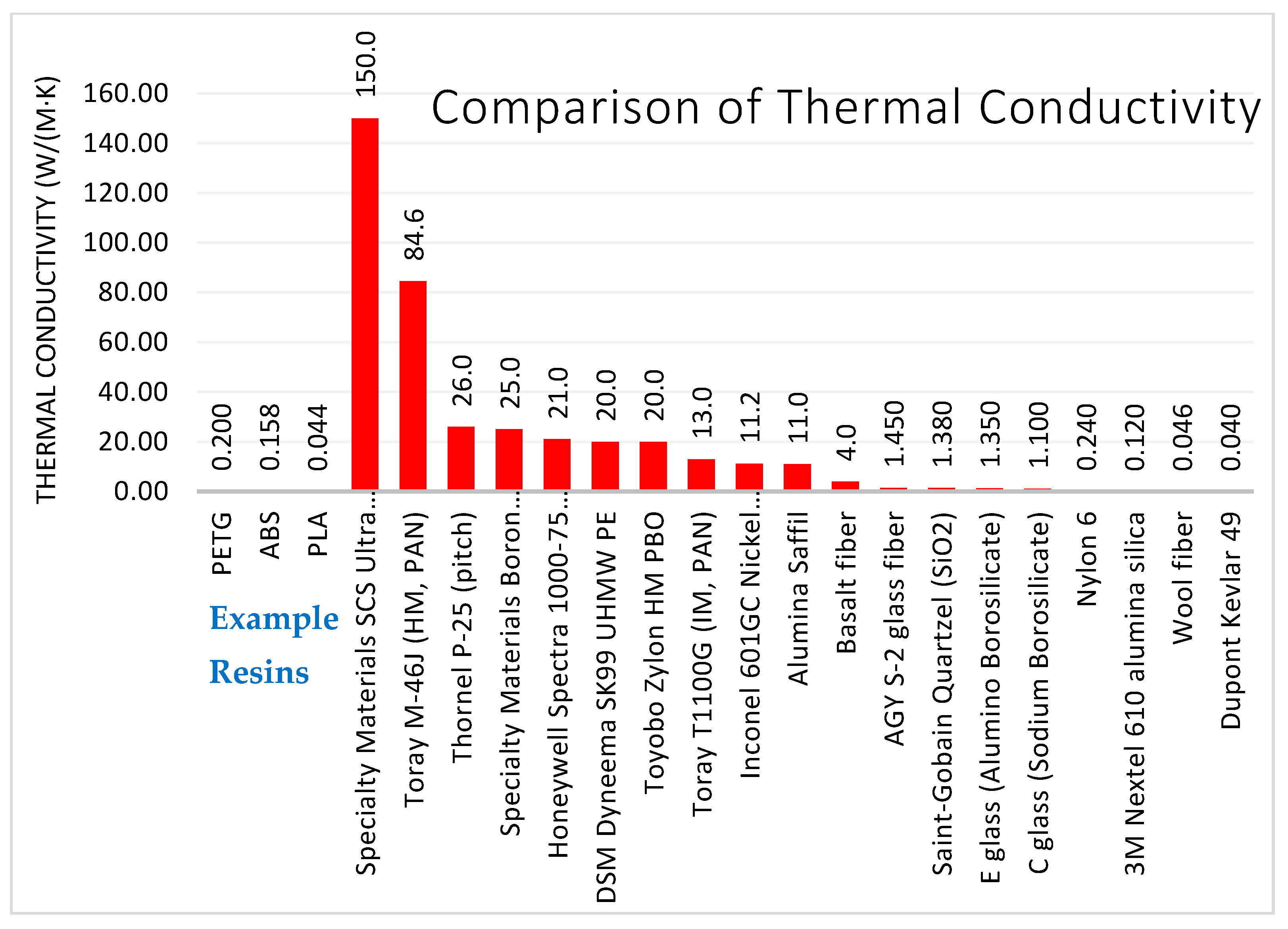
3.3.3. Thermal Conductivity
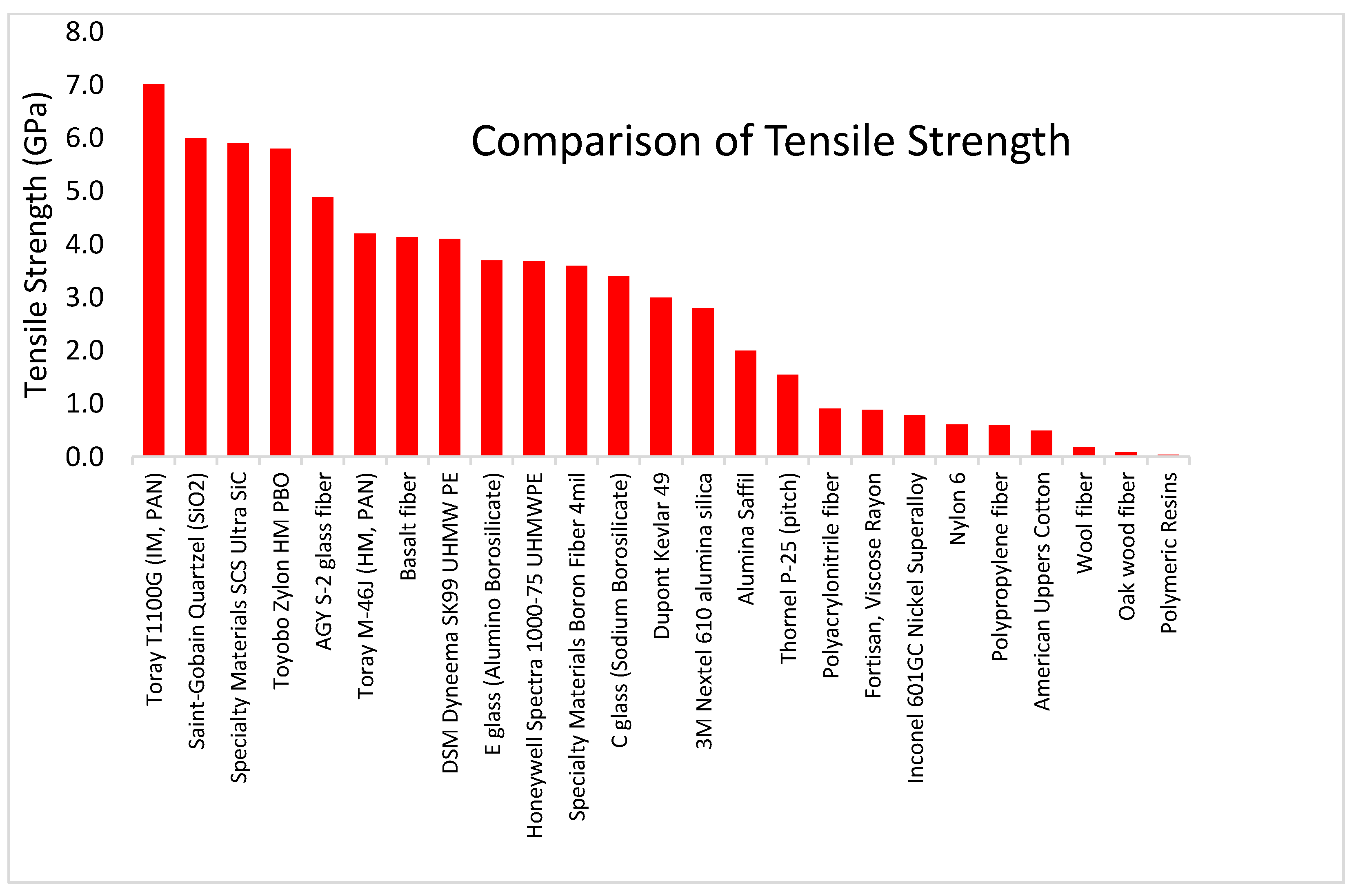
4. Fiber Comparison Summary
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
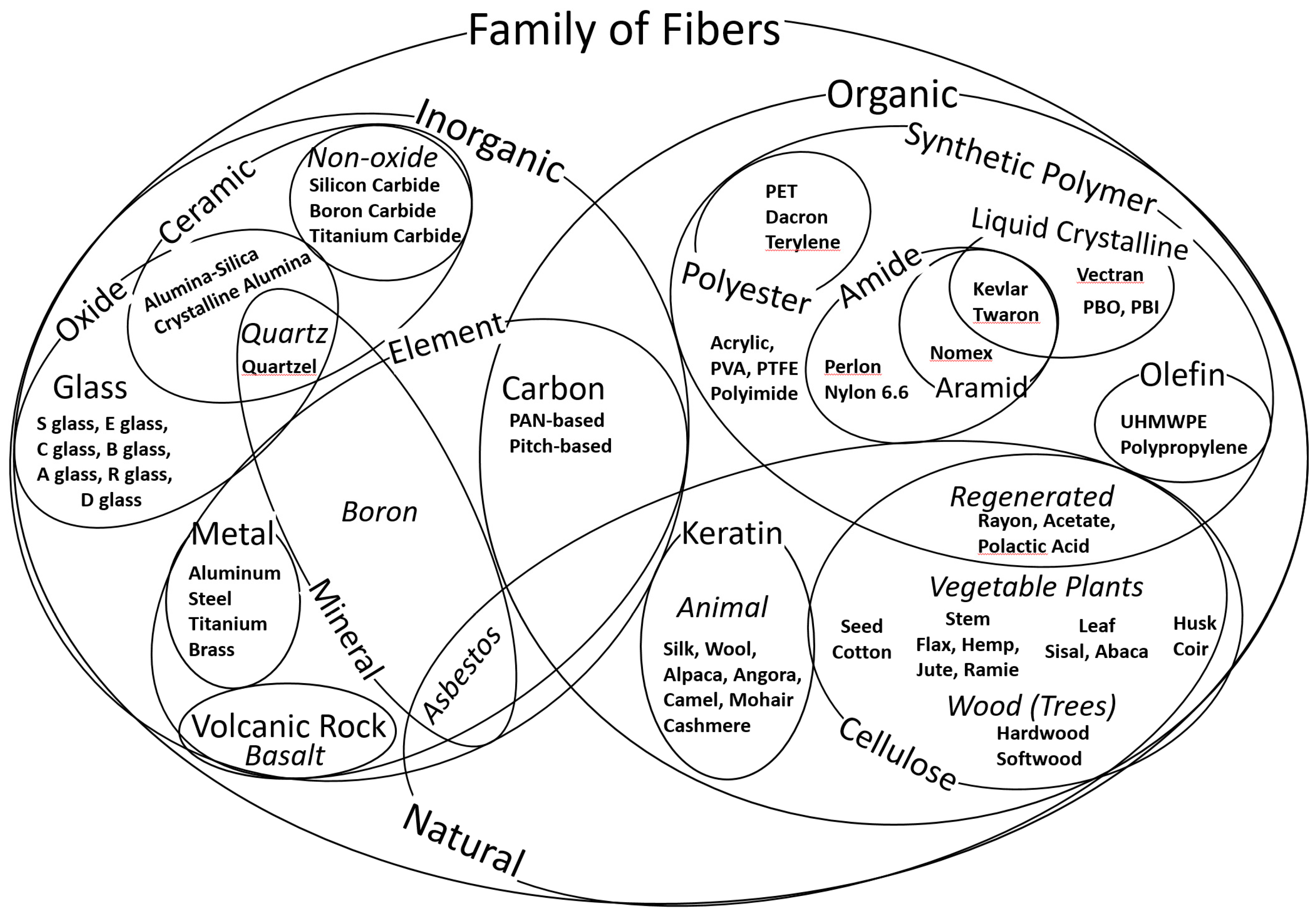
Appendix A. The Family of Fibers
| Family of Fibers | Organic | Natural | Cellulose | Plants (seed, stem, leaf, husk) (cotton, ramie, jute, hemp, flax, abaca) |
| Trees (pulp of hardwood or softwood) | ||||
| Regenerated Cellulose | Viscose Rayon (Fortisan) | |||
| Acetate, Triacetate | ||||
| Polylactic Acid | ||||
| Animal | Keratin (silk, wool, alpaca, angora, camel, cashmere, mohair) | |||
| Synthetic | Amide | Nylon, Perlon | ||
| Liquid Crystalline | Para-aramid (Kevlar, Twaron) | |||
| Meta-aramid (Nomex) | ||||
| PBO, PBI | ||||
| HBA/HNA copolyester, Vectran | ||||
| Olefin | Ultra High MW Polyethylene (UHMWPE) (Spectra, Dyneema, IZANAS, Tsunooga) | |||
| High Density Polyethylene (HDPE) | ||||
| Low Density Polyethylene (LDPE) | ||||
| Polypropylene (Typar, Tekton) | ||||
| Common | Acrylic, Polyacrylonitrile (PAN) | |||
| Polyimide (P84) | ||||
| Polystyrene | ||||
| Polyvinylalcohol (PVA) (Nycon) | ||||
| Polyurethane (Spandex) | ||||
| Polytetrafluorethylene (PTFE) (Teflon) | ||||
| Inorganic | Element | Carbon | PAN based high modulus | |
| PAN based intermediate modulus | ||||
| Pitch based | ||||
| Graphite, activated carbon | ||||
| Volcanic Rock | Basalt | |||
| Mineral | Boron | |||
| Asbestos | ||||
| Metallic | Aluminum | |||
| Steel | ||||
| Nickel and nickel alloys | ||||
| Titanium, Brass | ||||
| Ceramic | Nonoxide | Silicon Carbide, Boron Carbide | ||
| Oxide | Alumina-silica | |||
| Quartz | Quartzel, Astroquartz, pure silica | |||
| Glass | S-Glass | S, S2, S3-glass | ||
| E-Glass | E glass (Alumino Borosilicate), ECR | |||
| C-Glass | C-glass (Sodium Borosilicate) | |||
| B-Glass | B-glass | |||
| A-Glass | A-glass, AR1, AR2 |
| Fiber | Tensile Strength | Tensile Modulus | Bending | Moisture Regain | Nozzle Temp | Thermal Expansion | Thermal Conductivity |
|---|---|---|---|---|---|---|---|
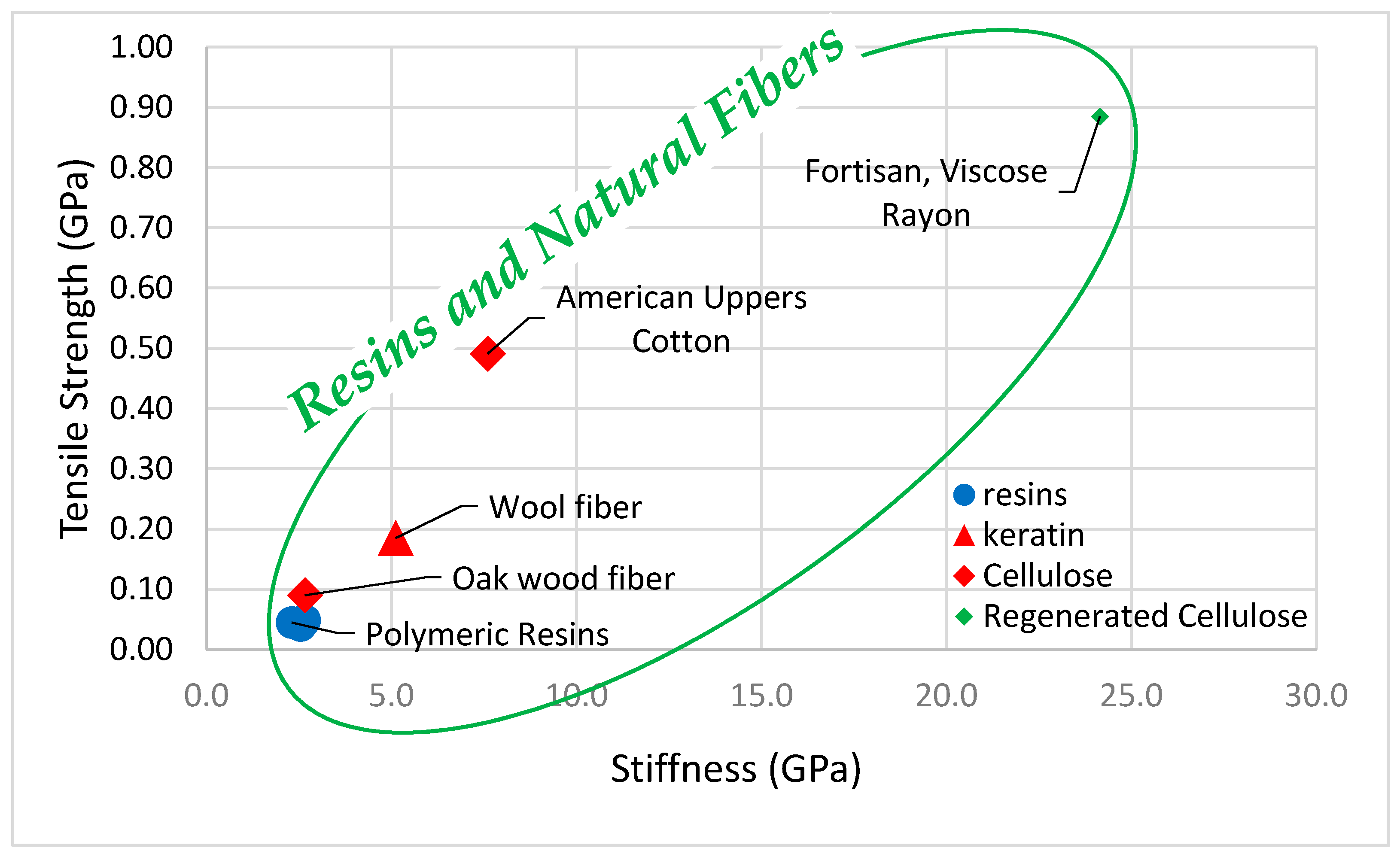
| American Uppers Cotton [30] (Organic, Natural, Cellulose, Vegetable, Plant, Seed) | Fair | Elastic | Flexible | Very High | Safe | Low | Very Low |
| Oak wood fibers [103] (Organic, Natural, Cellulose, Hard Wood) | Fair | Elastic | Flexible | Very High | Safe | Low | Very Low |
| Fortisan (regenerated rayon) [29] (Organic, Natural, Regenerated Cellulose) | Fair | Elastic | Flexible | Very High | Safe | Low | Low |
| Wool [31] (Organic, Natural, Animal, Keratin) | Fair | Elastic | Flexible | Very High | Safe | Low | Very Low |
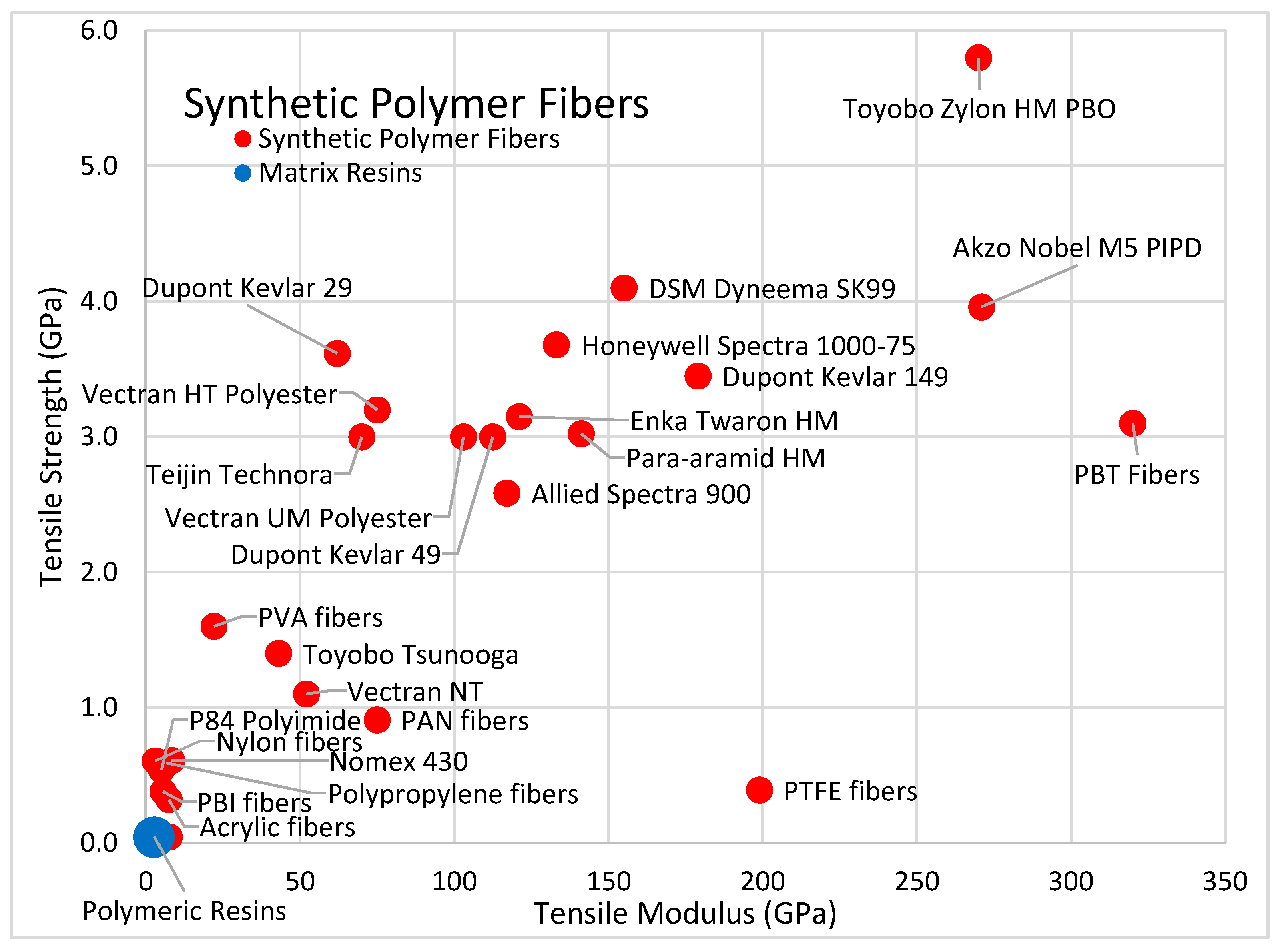
| DSM Dyneema SK99 [53] (Organic, Synthetic Polymer, UHMWPE) | High | Stiff | Moderate | None | Melt | Negative | High |
| Honeywell Spectra 1000-75 [57] (Organic, Synthetic Polymer, UHMWPE) | High | Stiff | Moderate | None | Melt | Negative | High |
| Nylon 6/6.6 [104] (Organic, Synthetic Polymer, Polyamide) | Fair | Elastic | Flexible | High | Melt | Low | Very Low |
| Dupont Kevlar 49 [47] (Organic, Syntetic Polymer, Polyamide Para aramid) | Moderate | Stiff | Moderate | Moderate | Near Max | Negative | Lowest |
| Toyobo Zylon HM [46] (Organic, Syntetic Polymer, Polybenzoxazole) | Very High | Stiff | Rigid | Moderate | Near Max | Negative | High |
| Polyacrylonitrile [105] (Organic, Synthetic Polymer, Acrylic) | Fair | Stiff | Moderate | Moderate | Melt | Low | Low |
| Polypropylene [29] (Organic, Synthetic Polymer, Polypropylene) | Fair | Elastic | Flexible | None | Melt | Low | Low |
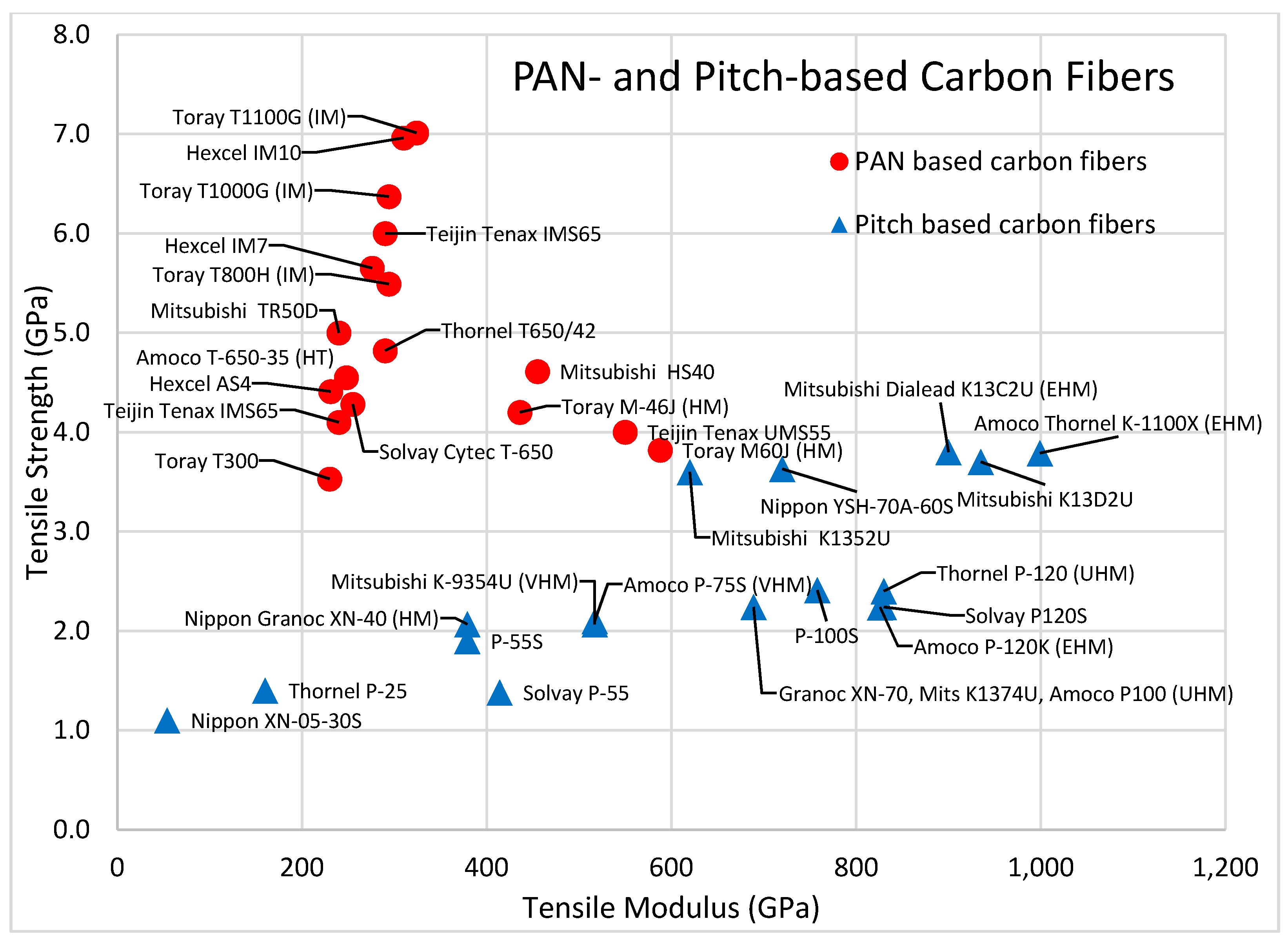
| Toray M-46J (HM, PAN) [64] (Inorganic, Carbon, PAN-based) | High | Most Stiff | Most Rigid | None | Safe | Negative | Very High |
| Toray T1100G (IM, PAN) [64] (Inorganic, Carbon, PAN-based) | Very High | Stiff | Rigid | None | Safe | Very Low | High |
| Solvay Thornel P-25 [106] (Inorganic, Carbon, Pitch-based) | Fair | Stiff | Moderate | None | Safe | Very Low | High |
| Spec Mtrls Boron 4mil [109] (Inorganic, Mineral) | Moderate | Very Stiff | Highly Rigid | None | Safe | Low | High |
| Basalt [110] (Inorganic, Volcanic Rock) | High | Stiff | Moderate | None | Safe | Low | Moderate |
| Inconel 601GC Nickel Superalloy [111] (Inorganic, Metallic, Steel Alloy) | Fair | Stiff | Rigid | None | Safe | Moderate | High |
| Saffil [108] (Inorganic, Ceramic, Oxide) | Moderate | Stiff | Rigid | None | Safe | Low | High |
| 3M Nextel 610 Al Si [77] (Inorganic, Ceramic, Nonoxide) | Moderate | Very Stiff | Rigid | None | Safe | Low | Very Low |
| Spec Mtrls SCS Ultra SiC [72] (Inorganic, Ceramic, Nonoxide) | Very High | Very Stiff | Highly Rigid | None | Safe | Low | Highest |
| Saint-Gobain Quartzel (SiO2) [82] (Inorganic, Ceramic, Quartz) | Very High | Stiff | Moderate | None | Safe | Very Low | Low |
| AGY S2-glass fiber [107] (Inorganic, Ceramic, Glass) | High | Stiff | Moderate | None | Safe | Very Low | Low |
| E-glass [79] (Inorganic, Ceramic, Glass) | High | Stiff | Moderate | None | Safe | Low | Low |
| C-glass [79] (Inorganic, Ceramic, Glass) | Moderate | Stiff | Moderate | None | Safe | Low | Low |
Appendix B. Explanation of Terminology and Units
| Symbol | Name | Dimension | Derivation |
|---|---|---|---|
| m | meter | length | base unit |
| cm | centimeter | length | 0.01 m |
| mm | milimeter | length | 0.001 m |
| μm | micrometer | length | 10−6 m |
| nm | nanometer | length | 10−9 m |
| g/cm3 | grams per cubic centimeter | density | 0.001 kg⋅m−3 |
| GPa | gigapascal | stress | 109 kg⋅m−1⋅s−2 |
| MPa | megapascal | stress | 106 kg⋅m−1⋅s−2 |
| MY | megaYuri | specific strength | 106 m2⋅s−2 |
| m/min | meters per minute | linear rate | 0.01667 m⋅s−1 |
| W/(m⋅K) | watts per meter Kelvin | thermal conductivity | kg⋅m⋅K−1⋅s−3 |
| CTE | coefficient of thermal expansion | strain per rate of temperature change | K−1 |
Appendix C. Linear Density, Specific Strength, and Tenacity
| Equivalent Value | Units of Specific Strength (SI Base Units of m2/s2) |
|---|---|
| 1,000,000 | Yuri |
| 1 | megaYuri (MY) |
| 1 | Newtons per tex (N/tex) |
| 1 | GPa/(g/cm3) |
| 1000 | MPa/(g/cm3) |
| 11.33 | grams per denier (g/den) |
Appendix D. Composition of Fibers
| Quartz | Glass | Basalt | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| D | A | C | S2 | AR | ECR | R | E | |||
| Density (g/cm3) | 2.15 | 2.1 | 2.44 | 2.49 | 2.46 | 2.68 | 2.62 | 2.52 | 2.53 | 2.64 |
| Specific Strength (MY) | 1.58 | 1.14 | 1.36 | 1.37 | 1.99 | 1.38 | 1.18 | 1.75 | 1.46 | 1.57 |
| Specific Modulus (MY) | 32.1 | 24.76 | 28.2 | 27.7 | 35.33 | 28.73 | 30.53 | 34.1 | 30.04 | 32.12 |
| Component (wt %) | - | - | - | - | - | - | - | - | - | - |
| SiO2 Silica | 100 | 75.5 | 71.8 | 65 | 65 | 61 | 60 | 60 | 55.2 | 51 |
| Al2O3 Alumina | - | 0.5 | 1 | 4 | 25 | 1 | 13.2 | 25 | 14.8 | 18 |
| B2O3 Boron Trioxide | - | 20 | - | 5 | - | - | - | - | 6.9 | - |
| ZrO2 Zirconia | - | - | - | - | - | 17 | - | - | - | - |
| MgO Magnesia | - | 0.5 | 3.8 | 3 | 10 | - | 3.1 | 6 | 3.3 | 7 |
| CaO Calcia | - | 0.5 | 8.8 | 14 | - | 2 | 22.1 | 9 | 18.7 | 5 |
| Na2O Sodium Oxide | - | - | - | - | - | - | 0.5 | - | - | 1 |
| K2O Potash | - | 3 | 13.6 | 8.7 | - | 16 | 0.6 | - | 0.3 | 6.4 |
| LiO2 Lithium Oxide | - | - | 0.6 | - | - | 3 | 0.2 | - | 0.2 | 4.5 |
| F2 Fluorine | - | - | - | - | - | - | 0.1 | - | 0.3 | - |
| Fe2O3 Iron Oxide | - | - | 0.4 | 0.3 | - | - | 0.2 | - | 0.3 | 6.1 |
| MnO Manganese Oxide | - | - | - | - | - | - | - | - | - | 0.3 |
| H2O Water | - | - | - | - | - | - | - | - | - | 0.4 |
| P2O5 Phosphorous Oxide | - | - | - | - | - | - | - | - | - | 0.3 |
| Oxide Ceramic Fibers | Type | Density (g/cm3) | Tensile Strength (GPa) | Specific Strength (MY) | E (GPa) | Es (MY) | Composition | ||
|---|---|---|---|---|---|---|---|---|---|
| Al2O3 | SiO2 | B2O3 | |||||||
| 3M Nextel 610 alumina silica | Crystalline | 3.90 | 2.800 | 0.718 | 370.00 | 94.87 | 100% | - | - |
| 3M Nextel 720 | Crystalline | 3.40 | 1.940 | 0.571 | 250.00 | 73.53 | 85% | 15% | - |
| Nitivy ALF | Alumina-Silica | 2.90 | 2.000 | 0.690 | 170.00 | 58.62 | 72% | 28% | - |
| 3M Nextel 550 | Alumina-Silica | 3.03 | 2.000 | 0.660 | 193.00 | 63.70 | 73% | 27% | - |
| 3M Nextel 440 | Alumina-Silica | 3.00 | 1.840 | 0.613 | 190.00 | 63.33 | 70% | 28% | 2% |
| 3M Nextel 312 | Alumina-Silica | 2.80 | 1.630 | 0.582 | 150.00 | 53.57 | 63% | 25% | 13% |
| Sumitomo Altex | Alumina-Silica | 3.30 | 1.800 | 0.545 | 210.00 | 63.64 | 85% | 15% | - |
| Unifrax Saffil | Alumina-Silica | 3.28 | 1.950 | 0.595 | 297.00 | 90.55 | - | - | - |
| Nonoxide Ceramic Fibers | Production Technique | Density (g/cm3) | Tensile Strength (Gpa) | Specific Strength (MY) | E (GPa) | Es (MY) | Composition | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Si | C | O | Al | Zr | Ti | |||||||
| Nippon Hi-Nicalon S | Polycarbosilane | 3.10 | 2.600 | 0.839 | 420.00 | 135.48 | 68.9% | 30.9% | 0.2% | - | - | - |
| Nippon Hi-Nicalon | Polycarbosilane | 2.74 | 2.800 | 1.022 | 270.00 | 98.54 | 63.7% | 35.8% | 0.5% | - | - | - |
| Nippon Nicalon NL-200/201 | Polycarbosilane | 2.55 | 3.000 | 1.176 | 220.00 | 86.27 | 56.5% | 31.2% | 12.3% | - | - | - |
| UBE Industries Tyranno SA3 | Polycarbosilane | 3.10 | 2.800 | 0.903 | 380.00 | 122.58 | 67.8% | 31.3% | 0.3% | 0.6% | - | - |
| UBE Industries Tyranno ZMI | Polycarbosilane | 2.48 | 3.400 | 1.371 | 200.00 | 80.65 | 56.1% | 34.2% | 8.7% | - | 1.0% | - |
| UBE Industries Tyranno LoxM | Polycarbosilane | 2.48 | 3.300 | 1.331 | 187.00 | 75.40 | 55.4% | 32.4% | 10.2% | - | - | 2.0% |
| UBE Industries Tyranno S | Polycarbosilane | 2.35 | 3.300 | 1.404 | 170.00 | 72.34 | 50.4% | 29.7% | 17.9% | - | - | 2.0% |
| Specialty Materials SCS Ultra SiC | CVD | 3.08 | 5.900 | 1.916 | 415.00 | 134.74 | SiC on carbon | |||||
| Specialty Materials SCS6 SiC | CVD | 3.08 | 3.900 | 1.266 | 380.00 | 123.38 | SiC on carbon | |||||
| Specialty Materials SCS-9A | CVD | 2.80 | 3.450 | 1.232 | 307.00 | 109.64 | SiC on carbon | |||||
| Tisics/Sigma | CVD | 3.40 | 4.000 | 1.176 | 400.00 | 117.65 | SiC on tungsten | |||||
| COI Ceramics/Sylramic-iBN | Precursor | 3.00 | 3.000 | 1.000 | 400.00 | 133.33 | ||||||
| COI Ceramics/Sylramic | Precursor | 2.95 | 2.700 | 0.915 | 310.00 | 105.08 | ||||||
| Avco 5.6mil SiC | 3.07 | 3.792 | 1.235 | 413.69 | 134.75 | |||||||
| Avco 4.0mil SiC | 3.30 | 3.792 | 1.149 | 413.69 | 125.36 | |||||||
| Nippon DC HVR Nicolon SiC | 2.36 | 2.962 | 1.255 | 186.00 | 78.81 | |||||||
| Nicalon SiC | 2.55 | 2.760 | 1.082 | 193.00 | 75.69 | |||||||
| Boron Carbide | 2.00 | 2.758 | 1.379 | 379.21 | 189.61 | |||||||
| Boron Nitride | 1.80 | 2.068 | 1.149 | 344.74 | 191.52 | |||||||
| Metal Fibers | Density (g/cm3) | Tensile Strength (GPa) | Specific Strength (MY) | E (GPa) | Es (MY) | Composition | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fe | Ni | Cr | Mo | Co | ||||||
| AISI 316 Stainless | 8.00 | 0.580 | 0.073 | 193 | 24.13 | 66 | 12 | 17 | 2 | - |
| Inconel 601GC Nickel Superalloy | 8.11 | 0.790 | 0.097 | 206.50 | 25.46 | 15 | 57 | 25 | - | - |
| Haynes HR160 Nickel alloy | 8.08 | 0.767 | 0.095 | 211 | 26.11 | 2 | 35 | 27 | 1 | 30 |
| Hastelloy X alloy | 8.22 | 0.755 | 0.092 | 205.00 | 24.94 | 18 | 47 | 22 | 9 | 1.5 |
Appendix E. Production of Carbon Fibers from PAN
- Oxidation. In the oxidation reaction, oxygen atoms attach to the carbon chain, ejecting two hydrogen atoms in the form of N2 gas. The fibers incorporate approximately 8% oxygen during this exothermic process. Oxidation is the gain of oxygen atoms and loss of hydrogen atoms.
- Hydrogenation. In the dehydrogenation reaction, double bonds are formed between carbon atoms to stabilize the carbon chain and eject oxygen and hydrogen atoms in the form of water vapor and N2 gas.
- Cyclization. In the cyano group cyclization reaction, the C≡N triple bonds are broken, and formation of single and double bonds in a continuous ladder structure between alternating carbon and nitrogen atoms.
- Aromatization. The aromatization reaction is the formation of a heterocyclic system created by cyclization of the nitrogen atoms.
- Crosslinking. The crosslinking reaction is the bonding of one polymer chain to another. The structure results in aromatic pyridine groups as the carbon atoms lose hydrogen atoms and give off hydrogen gas. The crosslinking reaction sets the carbon structure.
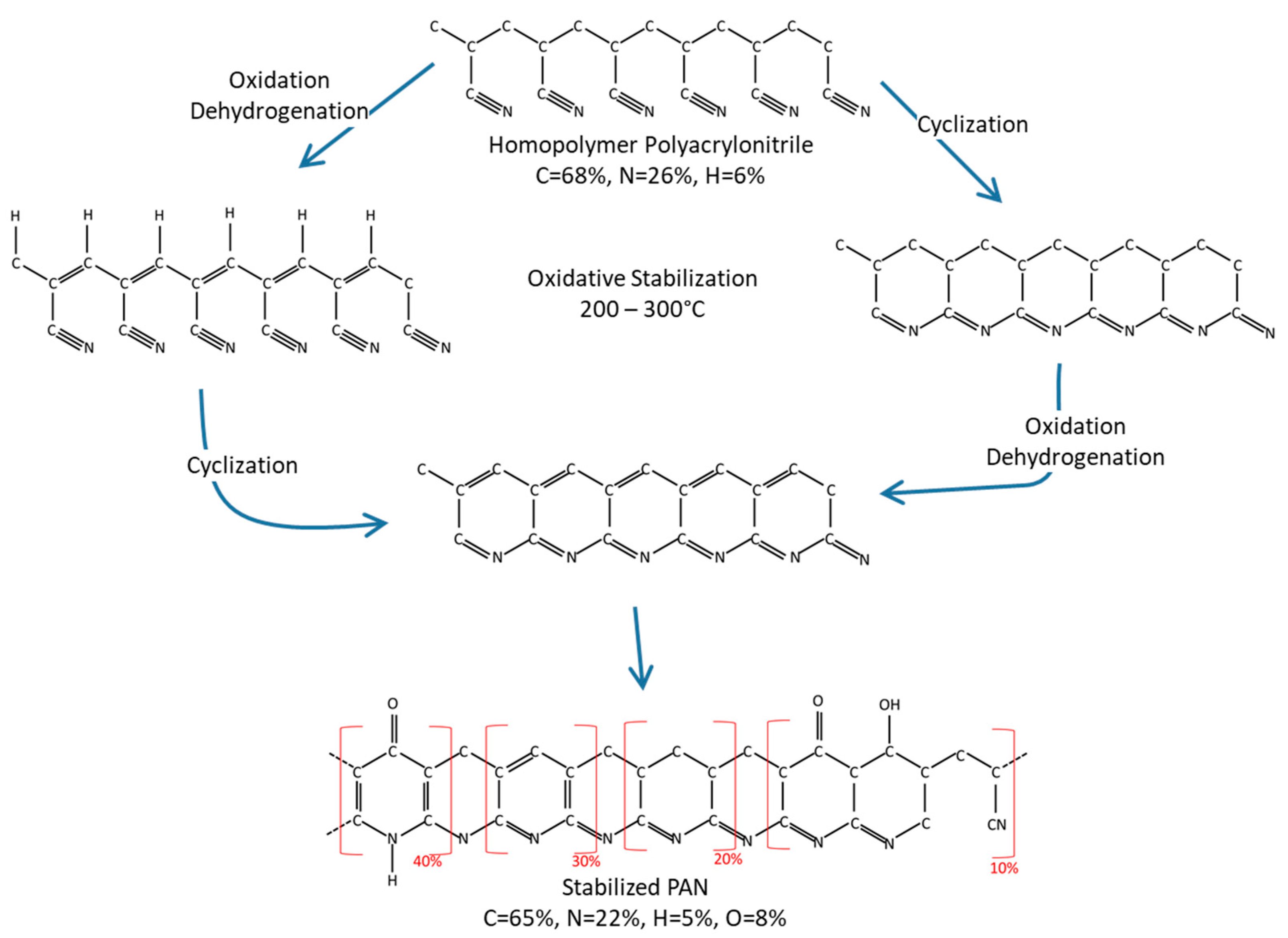
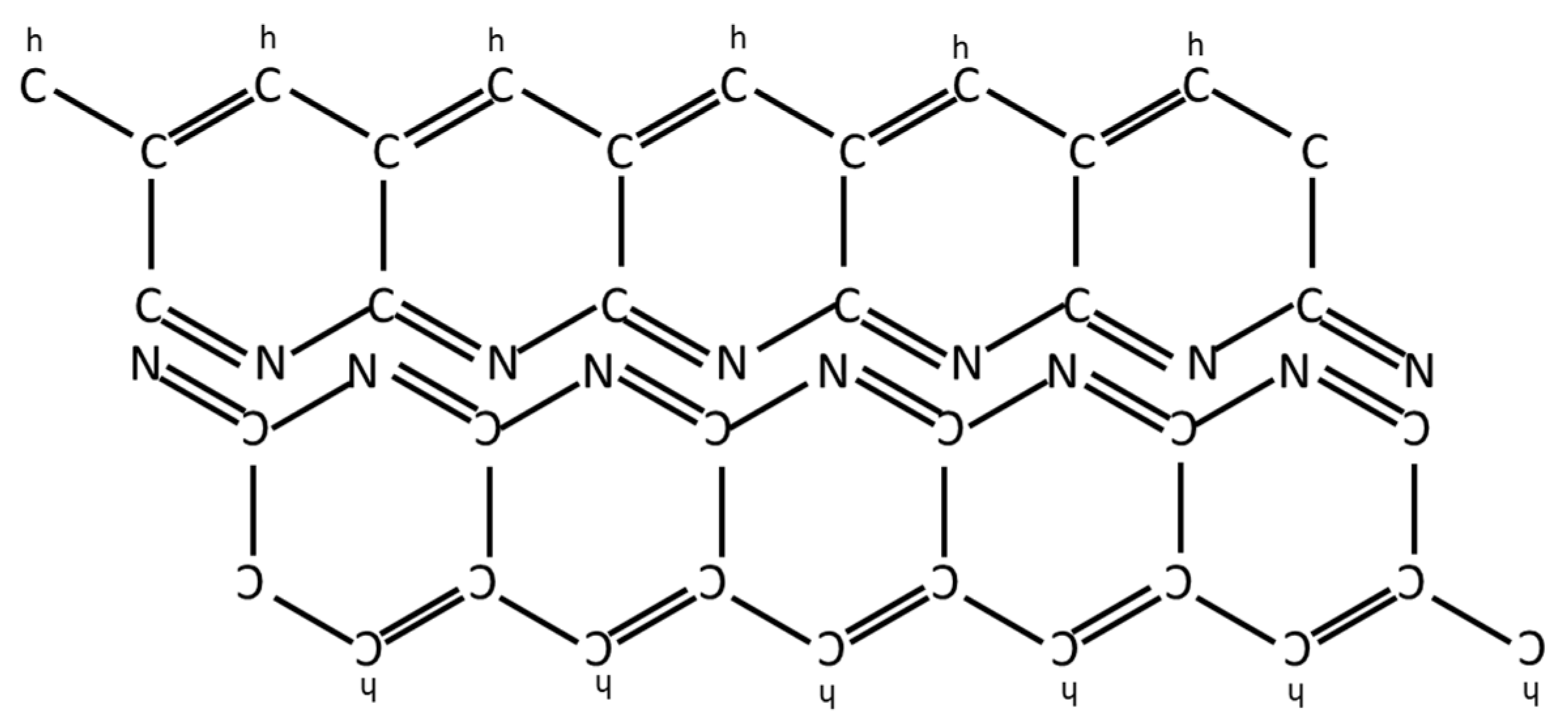
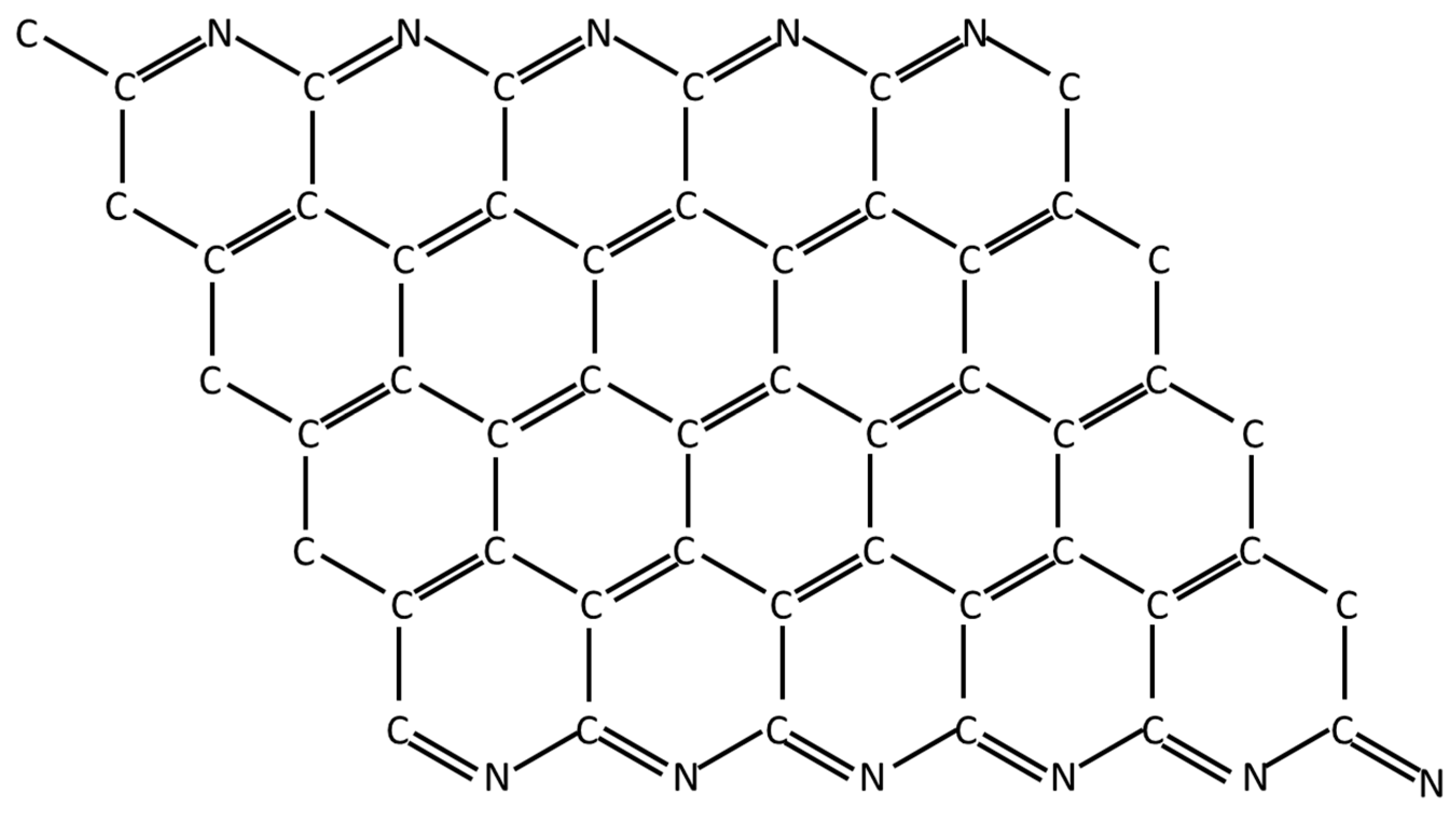
Appendix F. Comparison of Specific Strength of Fiber Families
| Fiber Family | Density (g/cm3) | Yield Strength (GPa) | Specific Strength (MY) |
|---|---|---|---|
| Carbon Fibers | 1.74 to 2.20 | 2.2 to 7.0 | 1.2 to 4.0 |
| Intermediate Modulus Carbon Fiber (from PAN) | 1.80 to 1.95 | 6.2 to 7.0 | 3.5 to 4.0 |
| High Strength Carbon Fiber | 1.74 to 1.80 | 3.5 to 4.9 | 2.0 to 2.7 |
| High Modulus Carbon Fiber (from Pitch) | 1.83 to 2.20 | 2.2 to 3.0 | 1.2 to 1.5 |
| Polymer Fibers | - | - | - |
| Synthetic Polymer UHMW Polyethylene (Dyneema, Spectra, Tsunooga) | 0.97 to 0.98 | 3.3 to 3.6 | 2.6 to 3.7 |
| Synthetic Polymer PBO Polybenzoxazole (Zylon) | 1.54 | 5.8 | 3.8 |
| Synthetic Polymer, Polyester (Vectran) | 1.4 | 2.9 | 2.21 |
| Synthetic Polymer (Polyester, Polyimide, PVA) | 1.39 to 1.43 | 0.52 to 1.85 | 0.37 to 1.32 |
| Synthetic Polymer (Polypropylene, Nylon, Acrylic) | 0.90 to 1.18 | 0.33 to 1.20 | 0.27 to 0.88 |
| Aramid Polymer Fibers | - | - | - |
| Synthetic Polymer, Para Aramid (Kevlar) | 1.44 to 1.47 | 2.8 to 3.8 | 1.9 to 2.6 |
| Synthetic Polymer, Meta Aramid (Nomex) | 1.38 to 1.38 | 0.3 to 0.6 | 0.3 to 0.5 |
| Glass Fibers | - | - | - |
| Glass (A, C, E, and S type) | 2.45 to 2.60 | 2.00 to 4.83 | 0.78 to 1.94 |
| Ceramic Fibers | - | - | - |
| Nonoxides (Silicon Carbide, Silicon Nitride) | 1.8 to 3.4 | 2.1 to 5.9 | 0.8 to 1.9 |
| Oxides (Alumina) | 2.8 to 3.9 | 1.4 to 2.8 | 0.4 to 0.7 |
| Mineral Fibers | - | - | - |
| Basalt Fiber | 2.64 to 2.70 | 2.5 to 4.8 | 1.0 to 1.8 |
| Keratin Fibers | - | - | - |
| Spider Silk | 1.31 | 1.40 | 1.07 |
| Natural Wool | 1.31 | 0.19 | 0.14 |
| Regenerated Cellulose Fibers | - | - | - |
| Fortisan, Rayon, Tenasco, Acetate | 1.25 to 1.52 | 0.17 to 0.89 | 0.13 to 0.59 |
| Cellulose Fibers | - | - | - |
| Natural (Cotton, Silk, Flax, Jute, Hemp, Ramie) | 1.27 to 1.54 | 0.29 to 0.89 | 0.19 to 0.59 |
| Natural Wood fibers | 0.14 to 0.74 | 0.07 to 0.9 | 0.12 to 0.52 |
| Metallic Fibers | - | - | - |
| Steel, Aluminum | 1.74 to 8.92 | 0.22 to 2.21 | 0.03 to 0.28 |
References
- Dickson, A.; Abourayana, H.; Dowling, D. 3D Printing of Fibre-Reinforced Thermoplastic Composites Using Fused Filament Fabrication—A Review. Polymers 2020, 12, 2188. [Google Scholar] [CrossRef]
- Fidan, I.; Imeri, A.; Gupta, A.; Hasanov, S.; Nasirov, A.; Elliott, A.; Alifui-Segbaya, F.; Nanami, N. The trends and challenges of fiber reinforced additive manufacturing. Int. J. Adv. Manuf. Technol. 2019, 102, 1801–1818. [Google Scholar] [CrossRef]
- 3DXTECH. Pellets & Colorants. Available online: https://www.3dxtech.com/products/pellets-colorants/ (accessed on 4 July 2021).
- Markforged. 3D Printing Materials. Available online: https://markforged.com/materials (accessed on 4 July 2021).
- U.S. Customs and Border Protection. Fiber Trade Names & Generic Terms. Available online: https://www.cbp.gov/document/guidance/fiber-trade-names-generic-terms (accessed on 4 July 2021).
- MatWeb. Online Materials Information Resource. Available online: http://www.matweb.com/ (accessed on 4 July 2021).
- Chawla, K.K. Fibrous Reinforcements for Composites: Overview. In Encyclopedia of Materials: Science and Technology; Buschow, K.H.J., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., Veyssière, P., Eds.; Elsevier: Oxford, UK, 2001; pp. 3160–3167. [Google Scholar] [CrossRef]
- Denton, M.J.; Daniels, P.N. Textile Institute., and Textile Terms and Definitions Committee. In Textile Terms and Definitions; Textile Institute: Manchester, UK, 2002. [Google Scholar]
- Labib, W.A. Fibre Reinforced Cement Composites. In Cement Based Materials; Saleh, H.E.-D.M., Rahman, R.O.A., Eds.; InTech: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Dahy, H. Natural Fibre-Reinforced Polymer Composites (NFRP) Fabricated from Lignocellulosic Fibres for Future Sustainable Architectural Applications, Case Studies: Segmented-Shell Construction, Acoustic Panels, and Furniture. Sensors 2019, 19, 738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Maharma, A.Y.; Al-Huniti, N. Critical Review of the Parameters Affecting the Effectiveness of Moisture Absorption Treatments Used for Natural Composites. J. Compos. Sci. 2019, 3, 27. [Google Scholar] [CrossRef] [Green Version]
- Elmogahzy, Y.; Farag, R. Tensile properties of cotton fibers. In Handbook of Properties of Textile and Technical Fibres; Elsevier: Amsterdam, The Netherlands, 2018; pp. 223–273. [Google Scholar] [CrossRef]
- Hutten, I.M. Handbook of Nonwoven Filter Media, 2nd ed.; Butterworth-Heinemann: Oxford, UK, 2016; pp. 1–52. [Google Scholar] [CrossRef]
- Solaiman Hoque, M.B.; Alam, A.B.M.H.; Mahmud, H.; Nobi, A. Mechanical, Degradation and Water Uptake Properties of Fabric Reinforced Polypropylene Based Composites: Effect of Alkali on Composites. Fibers 2018, 6, 94. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.; Chouw, N.; Jayaraman, K. Flax fibre and its composites—A review. Compos. Part B Eng. 2014, 56, 296–317. [Google Scholar] [CrossRef]
- Chapman, M.; Dhakal, H.N. Effects of Hybridisation on the Low Velocity Falling Weight Impact and Flexural Properties of Flax-Carbon/Epoxy Hybrid Composites. Fibers 2019, 7, 95. [Google Scholar] [CrossRef] [Green Version]
- Small, E.; Marcus, D. Hemp: A New Crop with New Uses for North America; ASHS Press: Alexandria, VA, USA, 2002; p. 43. [Google Scholar]
- Manaia, J.P.; Manaia, A.T.; Rodriges, L. Industrial Hemp Fibers: An Overview. Fibers 2019, 7, 106. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, M.A.; Zwawi, M.; Mehran, M.T.; Kanthasamy, R.; Bahadar, A. Jute Based Bio and Hybrid Composites and Their Applications. Fibers 2019, 7, 77. [Google Scholar] [CrossRef] [Green Version]
- Jose, S.; Rajna, S.; Ghosh, P. Ramie Fibre Processing and Value Addition. Asian J. Text. 2016, 7. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Mai, Y.-W.; Ye, L. Sisal fibre and its composites: A review of recent developments. Compos. Sci. Technol. 2000, 60, 2037–2055. [Google Scholar] [CrossRef]
- Liang, Z.; Wu, H.; Liu, R.; Wu, C. Preparation of Long Sisal Fiber-Reinforced Polylactic Acid Biocomposites with Highly Improved Mechanical Performance. Polymers 2021, 13, 1124. [Google Scholar] [CrossRef]
- Tran, L.Q.N.; Fuentes, C.; Verpoest, I.; van Vuure, A.W.; Vuure, V. Tensile Behavior of Unidirectional Bamboo/Coir Fiber Hybrid Composites. Fibers 2019, 7, 62. [Google Scholar] [CrossRef] [Green Version]
- §303.7 Generic Names and Definitions for Manufactured Fibers, Vol. 303.7. Available online: https://www.ecfr.gov/cgi-bin/text-idx?SID=c61f170ac1704d3e06bbcd41988a6f1e&mc=true&node=se16.1.303_17&rgn=div8 (accessed on 4 July 2021).
- Hearle, J.W.S. Textile Fibers: A Comparative Overview. In Encyclopedia of Materials: Science and Technology; Buschow, K.H.J., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., Veyssière, P., Eds.; Elsevier: Oxford, UK, 2001; pp. 9100–9116. [Google Scholar] [CrossRef]
- Ko, F.K.; Wan, L.Y. Engineering properties of spider silk. In Handbook of Properties of Textile and Technical Fibres; Elsevier: Amsterdam, The Netherlands, 2018; pp. 185–220. [Google Scholar] [CrossRef]
- Colomban, P.; Jauzein, V. Silk. In Handbook of Properties of Textile and Technical Fibres; Elsevier: Amsterdam, The Netherlands, 2018; pp. 137–183. [Google Scholar] [CrossRef]
- Griffiths, J.R.; Salanitri, V.R. The strength of spider silk. J. Mater. Sci. 1980, 15, 491–496. [Google Scholar] [CrossRef]
- de Araújo, M. 1-Natural and man-made fibres: Physical and mechanical properties. In Fibrous and Composite Materials for Civil Engineering Applications; Fangueiro, R., Ed.; Woodhead Publishing: Sawston, UK, 2011; pp. 3–28. [Google Scholar] [CrossRef]
- Yan, Y. 2-Developments in fibers for technical nonwovens. In Advances in Technical Nonwovens; Kellie, G., Ed.; Woodhead Publishing: Sawston, UK, 2016; pp. 19–96. [Google Scholar] [CrossRef]
- Ingeo. Technical Bulletin 180904. Available online: https://www.natureworksllc.com/~/media/Technical_Resources/Fact_Sheets/Fibers/FactSheet_Fabrics_Fiber_FabricProperties_pdf.pdf (accessed on 4 July 2021).
- Environmental Data. 2007. Available online: https://web.archive.org/web/20071009144917/http://www.io.tudelft.nl/research/dfs/idemat/Onl_db/Id192p.htm (accessed on 4 July 2021).
- Grishanov, S. Structure and properties of textile materials. In Handbook of Textile and Industrial Dyeing; Elsevier: Amsterdam, The Netherlands, 2011; pp. 28–63. [Google Scholar] [CrossRef]
- Militký, J.; Venkataraman, M.; Mishra, R. The chemistry, manufacture, and tensile behavior of polyamide fibers. In Handbook of Properties of Textile and Technical Fibres; Elsevier: Amsterdam, The Netherlands, 2018; pp. 367–419. [Google Scholar] [CrossRef]
- Fiberline Polyester Fiber. Available online: https://www.fiber-line.com/uploads/pdf%20US/fl.us.datasheet-petpolyester.pdf (accessed on 4 July 2021).
- Militky, J. The chemistry, manufacture and tensile behaviour of polyester fibers. In Handbook of Tensile Properties of Textile and Technical Fibres; Elsevier: Amsterdam, The Netherlands, 2009; pp. 223–314. [Google Scholar] [CrossRef]
- Bellis, M. The History of Polyester. Available online: https://www.thoughtco.com/history-of-polyester-4072579 (accessed on 4 July 2021).
- Pegoretti, A.; Traina, M. 17-Liquid crystalline organic fibers and their mechanical behavior. In Handbook of Properties of Textile and Technical Fibres, 2nd ed.; Bunsell, A.R., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 621–697. [Google Scholar] [CrossRef]
- DeMeuse, M.T.; Kiss, G. Liquid crystal polymers (LCPs) as a reinforcement in high temperature polymer blends. In High Temperature Polymer Blends; Elsevier: Amsterdam, The Netherlands, 2014; pp. 141–164. [Google Scholar] [CrossRef]
- Uppal, R.; Ramaswamy, G.N.; Loughin, T. A novel method to assess degree of crystallinity of aramid filament yarns. J. Ind. Text. 2012, 43, 3–19. [Google Scholar] [CrossRef]
- Trigo-López, M.; García, J.M.; Ruiz, J.A.R.; García, F.C.; Ferrer, R. Aromatic Polyamides: AROMATIC POLYAMIDES. In Encyclopedia of Polymer Science and Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2018; pp. 1–51. [Google Scholar] [CrossRef]
- Jain, A.; Vijayan, K. Thermally induced structural changes in Nomex fibres. Bull. Mater. Sci. 2002, 25, 341–346. [Google Scholar] [CrossRef]
- Zhang, T.; Jin, J.; Yang, S.; Li, G.; Jiang, J. Preparation and properties of novel PIPD fibers. Chin. Sci. Bull. 2010, 55, 4203–4207. [Google Scholar] [CrossRef]
- Cunniff, P.M.A.; Auerbach, M.A.; Vetter, E.; Sikkema, D.J. High Performance ‘M5’ Fiber for Ballistics/Structural Composites. In Proceedings of the 23rd Army Science Conference, Orlando, FL, USA, 2–5 December 2002. [Google Scholar]
- Lammers, M.; Klop, E.; Northolt, M.; Sikkema, D. Mechanical properties and structural transitions in the new rigid-rod polymer fibre PIPD (‘M5’) during the manufacturing process. Polymer 1998, 39, 5999–6005. [Google Scholar] [CrossRef]
- Toyobo. Available online: https://www.toyobo-global.com/ (accessed on 4 July 2021).
- Dupont. Kevlar® Aramid Fiber Technical Guide. p. 24. Available online: https://www.dupont.com/content/dam/dupont/amer/us/en/safety/public/documents/en/Kevlar_Technical_Guide_0319.pdf (accessed on 4 July 2021).
- Teijin. Twaron—A Versatile High-Performance Fiber. Available online: https://www.teijinaramid.com/wp-content/uploads/2016/07/Product-Brochure-Twaron.pdf (accessed on 4 July 2021).
- Kuraray. Vectran Liquid Crystal Polymer Fiber Technology. Available online: https://www.vectranfiber.com/wp-content/uploads/2015/12/Kuraray-Vectran-Brochure.pdf (accessed on 4 July 2021).
- Dupont. Nomex Fiber Technical Guide. p. 38. Available online: https://www.dupont.com/content/dam/dupont/amer/us/en/personal-protection/public/documents/en/Nomex(R)%20Fiber%20Technical%20Guide.pdf (accessed on 4 July 2021).
- Sandor, R. PBI (Polybenzimidazole): Synthesis, Properties and Applications. High Perform. Polym. 1990, 2, 25–37. [Google Scholar] [CrossRef]
- Pennings, A.J.; Roukema, M. Polyethylene Fibers by High Speed Spinning of Ultra-High-Molecular-Weight Polyethylene. U.S. Patent 5,068,073, 26 November 1991. [Google Scholar]
- DSM. Ultra High Molecular Weight Polyethylene Fiber from DSM Dyneema. Available online: https://extreemasoftslings.com/wp-content/uploads/2019/02/Dyneema-UHMWPF.pdf (accessed on 4 July 2021).
- Vlasblom, M. The manufacture, properties, and applications of high-strength, high-modulus polyethylene fibers. In Handbook of Properties of Textile and Technical Fibres; Elsevier: Amsterdam, The Netherlands, 2018; pp. 699–755. [Google Scholar] [CrossRef]
- Oransi. ERIK650A Air Filtration Unit. Available online: https://oransi.com/products/650a-portable-commercial-air-purifier?variant=37157373477045 (accessed on 4 July 2021).
- Richaud, E.; Fayolle, B.; Davies, P. Tensile properties of polypropylene fibers. In Handbook of Properties of Textile and Technical Fibres; Elsevier: Amsterdam, The Netherlands, 2018; pp. 515–543. [Google Scholar] [CrossRef]
- Honeywell. Honeywell Spectra® Fiber. Available online: https://www.packagingcomposites-honeywell.com/spectra/product-info/spectra-fiber/ (accessed on 4 July 2021).
- Toyobo. TsunoogaTM. Available online: https://www.toyobo-global.com/seihin/dn/tsunooga/ (accessed on 4 July 2021).
- Gupta, B.S.; Afshari, M. Polyacrylonitrile fibers. In Handbook of Properties of Textile and Technical Fibres; Elsevier: Amsterdam, The Netherlands, 2018; pp. 545–593. [Google Scholar] [CrossRef]
- Newcomb, B.A.; Chae, H.G. The properties of carbon fibers. In Handbook of Properties of Textile and Technical Fibres; Elsevier: Amsterdam, The Netherlands, 2018; pp. 841–871. [Google Scholar] [CrossRef]
- Bengtsson, A.; Bengtsson, J.; Sedin, M.; Sjöholm, E. Carbon Fibers from Lignin-Cellulose Precursors: Effect of Stabilization Conditions. ACS Sustain. Chem. Eng. 2019, 7, 8440–8448. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Liu, Y.; Chen, S.; Ni, Y. Current overview of carbon fiber: Toward green sustainable raw materials. BioResources 2020, 15, 7234–7259. [Google Scholar] [CrossRef]
- American Chemical Society. High Performance Carbon Fibers—National Historic Chemical Landmark. Available online: https://www.acs.org/content/acs/en/education/whatischemistry/landmarks/carbonfibers.html (accessed on 4 July 2021).
- Toray Composite Materials America, Inc. TORAYCA® Carbon Fiber. Available online: https://www.toraycma.com/page.php?id=661 (accessed on 4 July 2021).
- Hexcel. Carbon Fiber Data Sheets. Available online: https://www.hexcel.com/Resources/DataSheets/Carbon-Fiber (accessed on 4 July 2021).
- Teijin Carbon. TenaxTM Filament Yarn. Available online: https://www.teijincarbon.com/products/tenaxr-carbon-fiber/tenaxr-filament-yarn (accessed on 4 July 2021).
- Mitsubishi Chemical Carbon Fiber Composites. Carbon Fiber. Available online: http://mccfc.com/carbon-fiber/ (accessed on 4 July 2021).
- Solvay. Carbon Fiber Product Catalog. Available online: https://www.solvay.com/en/chemical-categories/our-composite-materials-solutions/carbon-fiber/product-catalog (accessed on 4 July 2021).
- Nippon Graphite Fiber Corporation. Unique Properties of GRANOC Contribute to the Development of Advanced Technology. Available online: http://www.ngfworld.com/en/en_skill.html#skill (accessed on 4 July 2021).
- Clauß, B.; Stacey, M.H. Fibers, 13. Ceramic Fibers. In Ullmann’s Encyclopedia of Industrial Chemistry; American Cancer Society: Atlanta, GA, USA, 2012. [Google Scholar] [CrossRef]
- Bunsell, A.R. Small-diameter silicon carbide fibers. In Handbook of Properties of Textile and Technical Fibres; Elsevier: Amsterdam, The Netherlands, 2018; pp. 873–902. [Google Scholar] [CrossRef]
- Specialty Materials. Specialty Materials Silicon Carbide Fibers. Available online: https://www.specmaterials.com/silicon-carbide-fiber-1 (accessed on 4 July 2021).
- Military Standard MIL-STD-1944. Composite Fiber Properties, Table I. Available online: http://www.waveequation.com/composite_fiber_properties.htm (accessed on 4 July 2021).
- Clauß, B. Fibers for Ceramic Matrix Composites. In Ceramic Matrix Composites; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; pp. 1–20. [Google Scholar] [CrossRef] [Green Version]
- Wilson, D. 23-Continuous oxide fibers. In Handbook of Properties of Textile and Technical Fibres, 2nd ed.; Bunsell, A.R., Ed.; Woodhead Publishing: Sawston, UK, 2018; pp. 903–927. [Google Scholar] [CrossRef]
- Kamiya, K. Sol–Gel-Prepared Glass and Ceramic Fibers. In Handbook of Sol-Gel Science and Technology; Klein, L., Aparicio, M., Jitianu, A., Eds.; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- 3M™ Nextel™ Ceramic Fibers and Textiles Technical Reference Guide. Available online: https://multimedia.3m.com/mws/media/1327055O/3m-nextel-technical-reference-guide.pdf (accessed on 4 July 2021).
- Hiltex. Alf the Ultimate Solution. Available online: https://www.hiltex.com/mod/Upload01/_Files/Hiltex%20Semi%20Products%20ALF%20The%20Ultimate%20Solution.pdf (accessed on 4 July 2021).
- Jones, F.R.; Huff, N.T. The structure and properties of glass fibers. In Handbook of Properties of Textile and Technical Fibres; Elsevier: Amsterdam, The Netherlands, 2018; pp. 757–803. [Google Scholar] [CrossRef]
- Pico, D.; Wilms, C.; Seide, G.; Gries, T.; Kleinholz, R.; Tiesler, H. Fibers, 12. Glass Fibers. In Ullmann’s Encyclopedia of Industrial Chemistry; American Cancer Society: Atlanta, GA, USA, 2012. [Google Scholar] [CrossRef]
- U.S. Department of Defense. Composite Materials Handbook MIL 17: Materials Usage, Design, and Analysis; CRC Press: Boca Raton, FL, USA, 2002; Volume 3. [Google Scholar]
- Saint-Gobain. Quartzel®. Available online: https://www.quartz.saint-gobain.com/products/quartzel (accessed on 4 July 2021).
- JPS Composite Materials, a Handy and Harman Company. Technical Reference Handbook. Available online: https://jpscm.com/wp-content/uploads/2017/10/2017-Data-Book-Small-1.pdf (accessed on 4 July 2021).
- AGY. S-2 Glass® Fibers. Available online: https://www.agy.com/wp-content/uploads/2014/03/S2_Glass_Fibers-Technical.pdf (accessed on 4 July 2021).
- 3B the Fibreglass Company. Products. Available online: https://www.3b-fibreglass.com/ (accessed on 4 July 2021).
- Bloise, A.; Punturo, R.; Kusiorowski, R.; Gómez, D.P. Editorial for Special Issue “Mineral Fibres”. Fibers 2019, 7, 54. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Jin, N. Fibers made by chemical vapor deposition. In Handbook of Properties of Textile and Technical Fibres; Elsevier: Amsterdam, The Netherlands, 2018; pp. 929–991. [Google Scholar] [CrossRef]
- Militký, J.; Mishra, R.; Jamshaid, H. Basalt fibers. In Handbook of Properties of Textile and Technical Fibres; Elsevier: Amsterdam, The Netherlands, 2018; pp. 805–840. [Google Scholar] [CrossRef]
- Bekaert. Bekipor® HEPA/ULPA Filter Media. Available online: https://www.bekaert.com/en/products/basic-materials/filtration/bekipor-hepa-ulpa-filter-media (accessed on 4 July 2021).
- Bekaert. Bekipor® Metal Fiber Media. Available online: https://www.bekaert.com/-/media/Files/Download-Files/BFT/Bekipor-metal-fiber-media.pdf (accessed on 4 July 2021).
- Bruyne, R.D.; Lefever, I. Fibers, 14. Metal Fibers. In Ullmann’s Encyclopedia of Industrial Chemistry; American Cancer Society: Atlanta, GA, USA, 2012. [Google Scholar] [CrossRef]
- Ning, F.; Cong, W.; Qiu, J.; Wei, J.; Wang, S. Additive manufacturing of carbon fiber reinforced thermoplastic composites using fused deposition modeling. Compos. Part B Eng. 2015, 80, 369–378. [Google Scholar] [CrossRef]
- Yu, S.; Hwang, Y.H.; Hwang, J.Y.; Hong, S.H. Analytical study on the 3D-printed structure and mechanical properties of basalt fiber-reinforced PLA composites using X-ray microscopy. Compos. Sci. Technol. 2019, 175, 18–27. [Google Scholar] [CrossRef]
- Hull, D.; Clyne, T.W. An Introduction to Composite Materials, 2nd ed.; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar] [CrossRef]
- Adams, D. Transverse Flexure Testing. Available online: https://www.compositesworld.com/articles/transverse-flexure-testing (accessed on 4 July 2021).
- Adams, D. Fiber-Matrix Interfacial Bond Test Methods. Available online: https://www.compositesworld.com/articles/fiber-matrix-interfacial-bond-test-methods (accessed on 4 July 2021).
- 3DXTech Advanced Materials. Technical Data Sheet: 3DXSTAT ESD-PETG 3D Printing Filament. Available online: https://www.3dxtech.com/product/3dxstat-esd-petg/ (accessed on 4 July 2021).
- Textile Study Center. Flexural Properties of Textile Materials. Available online: https://textilestudycenter.com/flexural-properties-of-textile-materials/ (accessed on 4 July 2020).
- Carlene, P.W. 9—The Relation between Fibre and Yarn Flexural Rigidity in Continuous Filament Viscose Yarns. J. Text. Inst. Trans. 1950, 41, T159–T172. [Google Scholar] [CrossRef]
- Wiedemann, H.G.; McKarns, T.; Bayer, G. Thermal characteristics of polymer fibers. Thermochim. Acta 1990, 169. [Google Scholar] [CrossRef]
- Trznadel, M.; Kryszewski, M. Thermal Shrinkage of Oriented Polymers. J. Macromol. Sci. Part C 1992, 32, 259–300. [Google Scholar] [CrossRef]
- Saint-Gobain. Quartzel® Fused Quartz Textiles. Available online: https://docplayer.net/58637421-Quartzel-fused-quartz-textiles.html#download_tab_content (accessed on 4 July 2021).
- Wikipedia. Specific Strength. Available online: https://en.wikipedia.org/w/index.php?title=Specific_strength&oldid=931470309 (accessed on 4 July 2021).
- MatWeb. Overview of Materials for Nylon 6 Fiber. Available online: http://www.matweb.com/search/DataSheet.aspx?MatGUID=675b77f996b142f59e4cb60d69d64872 (accessed on 4 July 2021).
- MatWeb. ZoltekTM OX Continuous Tow Oxidized PAN Fiber. Available online: http://www.matweb.com/search/DataSheet.aspx?MatGUID=7965dc42c7474350917b4b6086f572d1 (accessed on 4 July 2021).
- MatWeb. Solvay THORNEL® P-25 Pitch-Based Fiber. Available online: http://www.matweb.com/search/DataSheet.aspx?MatGUID=36772f241b654d6a8f6a1b0c322485ce (accessed on 4 July 2021).
- AGY. S-2 Glass® Fibers. Available online: https://www.agy.com/products/s-glass/ (accessed on 4 July 2021).
- MatWeb. Thermal Ceramics Saffil® Bulk Fiber. Available online: http://www.matweb.com/search/DataSheet.aspx?MatGUID=92453918bf9c4c039b6eb7350f5faec5 (accessed on 4 July 2021).
- Specialty Materials. Boron Fiber Properties. Available online: https://www.specmaterials.com/boron-fiber-test-page (accessed on 4 July 2021).
- Texonic. Fiber Properties Guide. Available online: https://texonic.net/en/tableau/technical/ (accessed on 4 July 2021).
- MatWeb. Special Metals INCONEL® Alloy 601. Available online: http://www.matweb.com/search/DataSheet.aspx?MatGUID=84d2284cc7de4ed788f3e1e04a4df017 (accessed on 4 July 2021).
- UMaine Composites Center Receives Three Guinness World Records Related to Largest 3D Printer—UMaine News—University of Maine. UMaine News, 10 October 2019. Available online: https://umaine.edu/news/blog/2019/10/10/umaine-composites-center-receives-three-guinness-world-records-related-to-largest-3d-printer/ (accessed on 4 July 2021).
- The International Space Elevator Consortium. Strong Tether Challenge. 2013. Available online: https://web.archive.org/web/20160114223616/http://www.isec.org/images/StrongTetherChallenge/2013/Handbook-ts2013.rev0.pdf (accessed on 4 July 2021).

| Polymeric Resins | Fiber | Glass TransitionTemp (°C) | Extrusion Temp (°C) | |
|---|---|---|---|---|
| PLA | Poly(L-lactic acid), polyactide [3] | Carbon, Glass | 60 | 215 |
| ABS | Acrylonitrile butadiene styrene [3] | Carbon, Glass | 105 | 230 |
| PETG | Poly(ethylene terephthalate) [3] | Carbon | 80 | 245 |
| PP | Polypropylene [3] | Carbon, Glass | −20 | 265 |
| PA6 | Nylon 6 [3] | Carbon, Glass | 70 | 275 |
| PA12 | Nylon 12 [3] | Carbon | 158 | 285 |
| PC | Polycarbonate [3] | Carbon | 143 | 300 |
| PPS | Polyphenylene Sulfide [6] | Glass | 90 | 309 |
| PEI | Polyetherimide [3] | Carbon, Glass | 217 | 385 |
| PEKK | Polyketone [3] | Carbon | 165 | 390 |
| PEEK | Polyether ether ketone [3] | Carbon, Glass | 143 | 400 |
| Seed | Stem (Bast) | Leaf | Husk/Fruit |
|---|---|---|---|
| Cotton, Kapok | Flax, Hemp, Jute, Ramie | Sisal, Abaca | Coconut (Coir) |
| Akon, Milk Wheat | Kenaf, Kudzu, Linden | Palm, Manila | Banana |
| Rice Husk | Milkweed, Nettle, Okra | Caraua | Agave |
| Wool | Hair | Silk |
|---|---|---|
| Sheep, Alpaca, Angora Rabbit | Horse, | Spider silk |
| American Bison, Cashmere Goat | Camel | Silkworm silk |
| Mohair (Angora Sheep), Muskox |
| Natural Fiber | Protein Type | Density (g/cm3) | Tensile Strength (GPa) | Specific Strength (MY) |
|---|---|---|---|---|
| Spider silk [26,28] | Keratin | 1.31 | 1.400 | 1.069 |
| Ramie fiber [29] | Cellulose | 1.50 | 0.885 | 0.590 |
| Flax fiber [29] | Cellulose | 1.54 | 0.831 | 0.540 |
| Hemp fiber [29] | Cellulose | 1.50 | 0.705 | 0.470 |
| Cotton [30] | Cellulose | 1.52 | 0.684 | 0.450 |
| Silkworm silk [25] | Keratin | 1.34 | 0.509 | 0.380 |
| Jute fiber [29] | Cellulose | 1.50 | 0.465 | 0.310 |
| Wool fiber [31] | Keratin | 1.31 | 0.185 | 0.141 |
| Oak wood fiber [32] | Cellulose | 0.74 | 0.090 | 0.122 |
| Pine wood fiber [6] | Cellulose | 0.35 | 0.078 | 0.223 |
| Balsa fiber [6] | Cellulose | 0.16 | 0.073 | 0.456 |
| Amide | Polyester | Liquid Crystalline | Olefin | Common Polymers |
|---|---|---|---|---|
| Perlon | PET | Para-aramid | Polypropylene | PVA, PTFE |
| Nylon 6.6 | Dacron | Meta-aramid | Polyethylene | Polyurethane |
| Terylene | Aromatic Heterocycle, Copolyester | (HDPE, LDPE, UHMWPE), | Acrylic |
| Amide Synthetic Fibers | Type | Density (g/cm3) | Tensile Strength (GPa) | Specific Strength (MY) |
|---|---|---|---|---|
| Perlon (nylon 6) [6] | Polyamide | 1.14 | 0.578 | 0.507 |
| Nylon (nylon 6.6) [6] | Polyamide | 1.14 | 0.508 | 0.446 |
| PET [35] | Polyester | 1.38 | 1.133 | 0.821 |
| Liquid Crystalline Synthetic Fibers | Type | Density (g/cm3) | Tensile Strength (GPa) | Specific Strength (MY) |
|---|---|---|---|---|
| Toyobo Zylon HM (PBO) [46] | Aromatic Heterocycle | 1.56 | 5.800 | 3.718 |
| Akzo Nobel M5 (PIPD) [44] | Aromatic Heterocycle | 1.70 | 3.960 | 2.329 |
| Dupont Kevlar 29 [47] | Para Aramid | 1.44 | 3.617 | 2.512 |
| Teijin Twaron HT [48] | Para Aramid | 1.45 | 3.600 | 2.500 |
| Teijin Technora [48] | Para Aramid | 1.39 | 3.500 | 2.518 |
| Dupont Kevlar 149 [47] | Para Aramid | 1.47 | 3.450 | 2.347 |
| Teijin Twaron HM [48] | Para Aramid | 1.44 | 3.312 | 2.300 |
| Teijin Twaron SM [48] | Para Aramid | 1.44 | 3.240 | 2.250 |
| Vectran HT (HBA/HNA) [49] | Copolyester | 1.41 | 3.200 | 2.270 |
| Vectran UM (HBA/HNA) [49] | Copolyester | 1.40 | 3.000 | 2.143 |
| Dupont Kevlar 49 [47] | Para Aramid | 1.44 | 3.000 | 2.083 |
| Vectran NT (HBA/HNA) [49] | Copolyester | 1.40 | 1.100 | 0.786 |
| Teijinconex [48] | Meta Aramid | 1.38 | 0.860 | 0.623 |
| Dupont Nomex 430 [50] | Meta Aramid | 1.38 | 0.609 | 0.441 |
| Polybenzimidazole fiber (PBI) [51] | Aromatic Heterocycle | 1.43 | 0.341 | 0.238 |
| Olefin Synthetic Fibers | Type | Density (g/cm3) | Tensile Strength (GPa) | Specific Strength (MY) |
|---|---|---|---|---|
| DSM Dyneema SK99 UHMWPE [53] | Olefin | 0.97 | 4.100 | 4.227 |
| Honeywell Spectra 1000-75 UHMWPE [57] | Olefin | 0.97 | 3.680 | 3.794 |
| Honeywell Spectra 900 UHMWPE [57] | Olefin | 0.97 | 2.585 | 2.670 |
| Toyobo Tsunooga UHMWPE [58] | Olefin | 0.97 | 1.400 | 1.433 |
| Polypropylene fiber [29] | Olefin | 0.91 | 0.591 | 0.649 |
| Polyethylene fiber [13] | Olefin | 0.90 | 0.477 | 0.530 |
| Carbon Fiber | Type | Density (g/cm3) | Tensile Strength (GPa) | Specific Strength (MY) |
|---|---|---|---|---|
| Toray T1100G (IM) [64] | PAN-based | 1.79 | 7.012 | 3.917 |
| Hexcel IM10 [65] | PAN-based | 1.83 | 6.826 | 3.813 |
| Toray T1000G (IM) [64] | PAN-based | 1.80 | 6.371 | 3.539 |
| Teijin Tenax IMS65 [66] | PAN-based | 1.78 | 6.000 | 3.371 |
| Hexcel IM7 [65] | PAN-based | 1.78 | 5.688 | 3.196 |
| Toray T800H (IM) [64] | PAN-based | 1.81 | 5.490 | 3.033 |
| Mitsubishi TRH50 [67] | PAN-based | 1.82 | 5.300 | 2.912 |
| Mitsubishi HS40 [67] | PAN-based | 1.85 | 4.610 | 2.492 |
| Solvay Thornel T650/35 [68] | PAN-based | 1.78 | 4.275 | 2.415 |
| Hexcel AS4 [65] | PAN-based | 1.79 | 4.447 | 2.483 |
| Toray M-46J (HM) [64] | PAN-based | 1.84 | 4.200 | 2.283 |
| Teijin Tenax UMS40 [66] | PAN-based | 1.79 | 4.700 | 2.626 |
| Toray M60J (HM) [64] | PAN-based | 1.93 | 3.820 | 1.979 |
| Mitsubishi K13C2U (EHM) [67] | Pitch-based | 2.20 | 3.800 | 1.727 |
| Mitsubishi K13D2U [67] | Pitch-based | 2.20 | 3.700 | 1.682 |
| Nippon YSH-70A-60S [69] | Pitch-based | 2.14 | 3.630 | 1.696 |
| Mitsubishi K1352U [67] | Pitch-based | 2.12 | 3.600 | 1.698 |
| Toray T300 [64] | PAN-based | 1.76 | 3.530 | 2.006 |
| Thornel P-120 (UHM) [6] | Pitch-based | 2.13 | 2.400 | 1.127 |
| Solvay P-25 [68] | Pitch-based | 1.95 | 1.380 | 0.707 |
| Solvay P-55 [68] | Pitch-based | 2.10 | 1.380 | 0.657 |
| Nippon XN-05-30S [69] | Pitch-based | 1.65 | 1.100 | 0.667 |
| Nonoxide Ceramic Fiber | Type | Density (g/cm3) | Tensile Strength (GPa) | Specific Strength (MY) |
|---|---|---|---|---|
| Specialty Materials SCS Ultra [72] | Silicon Carbide | 2.89 | 5.900 | 1.916 |
| Avco 5.6mil [73] | Silicon Carbide | 3.07 | 3.792 | 1.235 |
| Avco 4.0mil [73] | Silicon Carbide | 3.30 | 3.792 | 1.149 |
| Specialty Materials SCS6 [72] | Silicon Carbide | 3.08 | 3.450 | 1.150 |
| UBE Industries Tyranno ZMI [74] | Silicon Carbide | 2.48 | 3.400 | 1.371 |
| UBE Industries Tyranno LoxM [74] | Silicon Carbide | 2.48 | 3.300 | 1.331 |
| UBE Industries Tyranno S [74] | Silicon Carbide | 2.35 | 3.300 | 1.404 |
| Nippon Carbon/Nicalon NL-200/201 [74] | Silicon Carbide | 2.55 | 3.000 | 1.176 |
| COI Ceramics/Sylramic-iBN | Silicon Carbide | 3.00 | 3.000 | 1.000 |
| Nippon DC HVR Nicalon [74] | Silicon Carbide | 2.36 | 2.962 | 1.255 |
| Nippon Carbon/Hi-Nicalon [74] | Silicon Carbide | 2.74 | 2.800 | 1.022 |
| UBE Industries Tyranno SA3 [74] | Silicon Carbide | 3.10 | 2.800 | 0.903 |
| Nicalon [6] | Silicon Carbide | 2.55 | 2.760 | 1.082 |
| Boron Carbide [73] | Boron Carbide | 2.00 | 2.758 | 1.379 |
| COI Ceramics/Sylramic [74] | Silicon Carbide | 2.95 | 2.700 | 0.915 |
| Nippon Carbon/Hi-Nicalon S | Silicon Carbide | 3.10 | 2.600 | 0.839 |
| Boron Nitride [73] | Boron Nitride | 1.80 | 2.068 | 1.149 |
| Ceramic Oxide Fiber | Density (g/cm3) | Tensile Strength (GPa) | Specific Strength (MY) |
|---|---|---|---|
| 3M Nextel 610 alumina silica [77] | 3.90 | 2.800 | 0.718 |
| Alumina Saffil [6] | 3.30 | 2.000 | 0.606 |
| Hiltex ALF G2 [78] | 2.80 | 1.900 | 0.679 |
| Hiltex ALF F2 [78] | 2.90 | 1.800 | 0.621 |
| Hiltex ALF E3 [78] | 3.00 | 1.700 | 0.567 |
| Hiltex ALF FB3 [78] | 3.00 | 1.750 | 0.583 |
| Nitivy ALF [74] | 2.90 | 2.000 | 0.690 |
| 3M Nextel 550 [6] | 3.03 | 2.000 | 0.660 |
| 3M Nextel 440 [6] | 3.00 | 1.840 | 0.613 |
| 3M Nextel 720 [6] | 3.40 | 1.940 | 0.571 |
| Dupont Alumina SiO2 [73] | 3.70 | 1.896 | 0.512 |
| Sumitomo Altex Alumina [74] | 3.30 | 1.800 | 0.545 |
| Dupont Alumina FP-2 [74] | 3.70 | 1.724 | 0.466 |
| 3M Nextel 312 Alumina [6] | 2.80 | 1.630 | 0.582 |
| Dupont Alumina FP-1 [74] | 3.70 | 1.379 | 0.373 |
| Glass Fiber | Type | Density (g/cm3) | Tensile Strength (GPa) | Specific Strength (MY) |
|---|---|---|---|---|
| Saint-Gobain Quartzel [82] | Pure Silica (Quartz) | 2.20 | 6.000 | 2.727 |
| J.P. Stevens Astroquartz II [83] | Pure Silica (Quartz) | 2.20 | 6.000 | 2.727 |
| AGY S2-glass fiber [84] | Magnesium Borosilicate | 2.46 | 4.890 | 1.988 |
| S-glass fiber [6] | Alumino Silicate | 2.49 | 4.585 | 1.841 |
| R-glass fiber [6] | Alumino Silicate | 2.54 | 4.135 | 1.628 |
| AGY Advantex (ECR) [6] | Calcia Silicate | 2.62 | 3.750 | 1.431 |
| E-glass fiber [6] | Alumino Borosilicate | 2.57 | 3.620 | 1.409 |
| AGY S3-glass fiber [84] | Alumino Silicate | 2.83 | 3.338 | 1.180 |
| C-glass fiber [6] | Sodium Borosilicate | 2.54 | 3.310 | 1.303 |
| A-glass fiber [6] | High alkali | 2.44 | 3.310 | 1.357 |
| Binani SE1500 (ECR) [85] | Calcia Silicate | 2.62 | 2.500 | 0.954 |
| AR2-glass fiber [6] | Zirconia and Potash | 2.74 | 2.500 | 0.912 |
| D-glass fiber [6] | Boron Trioxide | 2.11 | 2.415 | 1.145 |
| Serpentine Asbestos | Amphiboles Asbestos |
|---|---|
| Chrysotile | Amosite (asbestos grunerite), Crocidolite, Tremolite, Anthophyllite, Actinolite |
| Isotropic Fibers | Anisotropic Fibers |
|---|---|
| Glass, Quartz, Ceramic, Metallic | Cellulose, Keratin, Carbon, Mineral, Synthetic Polymer |
| Fiber Family | Density (g/cm3) |
|---|---|
| Cellulose Fibers | 0.14 to 1.54 |
| Polymer Fibers | 0.90 to 1.54 |
| Polymeric Resins | 1.07 to 1.29 |
| Keratin Fibers | 1.30 to 1.34 |
| Regenerated Cellulose | 1.25 to 1.52 |
| Carbon Fibers | 1.74 to 2.20 |
| Glass and Quartz Fibers | 2.20 to 2.60 |
| Ceramic Fibers | 1.80 to 3.90 |
| Mineral Fibers | 2.64 to 2.70 |
| Metallic Fibers | 1.74 to 8.92 |
| Fiber Family | Yield Strength (GPa) | Specific Strength (MY) |
|---|---|---|
| Carbon Fibers | 2.20 to 7.00 | 1.20 to 4.00 |
| Glass and Quartz Fibers | 2.00 to 6.00 | 0.78 to 2.72 |
| Polymer Fibers | 0.33 to 5.80 | 0.27 to 3.80 |
| Ceramic Fibers | 1.40 to 5.90 | 0.40 to 1.90 |
| Mineral Fibers | 2.50 to 4.80 | 1.00 to 1.80 |
| Metallic Fibers | 0.22 to 2.21 | 0.03 to 0.28 |
| Keratin Fibers | 0.19 to 1.40 | 0.14 to 1.07 |
| Regenerated Cellulose | 0.17 to 0.89 | 0.13 to 0.59 |
| Cellulose Fibers | 0.07 to 0.89 | 0.12 to 0.59 |
| Resin Material (PLA, PETG, ABS) | 0.04 to 0.05 | 0.03 to 0.04 |
| Type | Fiber | Density (g/cm3) | Compressive Strength (GPa) [7] | Tensile Strength (GPa) |
|---|---|---|---|---|
| Alumina (Oxide Ceramic) | 3M Nextel 610 | 3.90 | 6.90 | 2.80 |
| Boron (Mineral, CVD) | Spec. Materials | 2.6 | 5.90 | 3.60 |
| Silicon Carbide (Nonoxide Ceramic) | Nicalon HI COI | 2.55 | 3.10 | 2.80 |
| PIPD (Aromatic Heterocycle) | Akzo Nobel M5 | 1.70 | 1.70 | 3.96 |
| Carbon (PAN, High Modulus) | Toray M60J | 1.93 | 1.67 | 3.82 |
| S-Glass (Alumino Silicate) | AGY S2-Glass | 2.46 | 1.10 | 4.89 |
| Carbon (Pitch) | Solvay Thornel P100 | 2.13 | 0.48 | 2.40 |
| Para Aramid | Dupont Kevlar 149 | 1.47 | 0.46 | 3.45 |
| Polybenzazole (LC Synthetic) | Toyobo Zylon PBO | 1.56 | 0.41 | 5.80 |
| UHMWPE (Olefin) | Spectra 1000 | 0.97 | 0.17 | 3.68 |
| Polyamide | Nylon 6 | 1.14 | 0.10 | 0.61 |
| Polyester | PET | 1.39 | 0.09 | 1.20 |
| Hygroscopic Filaments (Moisture Absorption) | Nonhygroscopic Filaments |
|---|---|
| PLA (1.3%), ABS (2.3%), PC (0.7%), PETG (0.2%), PET (0.8%) | PVC, Polypropylene, Polyethylene, Polystyrene |
| Fiber | Tg (°C) | Tm (°C) | Td (°C) |
|---|---|---|---|
| Honeywell Spectra (UHMWPE) | - | 147 | 65 |
| DSM Dyneema (UHMWPE) | - | 144 | 65 |
| Polypropylene (polyethylene) | 0 | 162 | - |
| Nylon 6 (polyamide) | 47 | 214 | - |
| Nylon 6.6 (polyamide) | 70 | 236 | - |
| Polyacrylonitrile | 140 | 300 | 300 |
| Kevlar (para aramid) | 321 | - | 427 |
| Zylon (PBO) | - | - | 650 |
| Cotton (Natural Cellulose) | - | - | 246 |
| Oak wood (Natural Cellulose) | - | - | 250 |
| Silk (Keratin) | - | - | 170 |
| Wool (Keratin) | - | - | 132 |
| Viscose Rayon (Regenerated) | - | - | 240 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beckman, I.P.; Lozano, C.; Freeman, E.; Riveros, G. Fiber Selection for Reinforced Additive Manufacturing. Polymers 2021, 13, 2231. https://doi.org/10.3390/polym13142231
Beckman IP, Lozano C, Freeman E, Riveros G. Fiber Selection for Reinforced Additive Manufacturing. Polymers. 2021; 13(14):2231. https://doi.org/10.3390/polym13142231
Chicago/Turabian StyleBeckman, Ivan Philip, Christine Lozano, Elton Freeman, and Guillermo Riveros. 2021. "Fiber Selection for Reinforced Additive Manufacturing" Polymers 13, no. 14: 2231. https://doi.org/10.3390/polym13142231
APA StyleBeckman, I. P., Lozano, C., Freeman, E., & Riveros, G. (2021). Fiber Selection for Reinforced Additive Manufacturing. Polymers, 13(14), 2231. https://doi.org/10.3390/polym13142231