New Aspects of Degradation in Silicone Rubber under UVA and UVB Irradiation: A Gas Chromatography–Mass Spectrometry Study
Abstract
:1. Introduction
2. Experiments
2.1. Materials Preparation
2.2. Ultraviolet Irradiation
2.3. Extraction of the Uncrosslinked Siloxanes
2.4. GC–MS and GC Analysis
2.5. Positron Annihilation Lifetime Spectroscopy
3. Results and Discussion
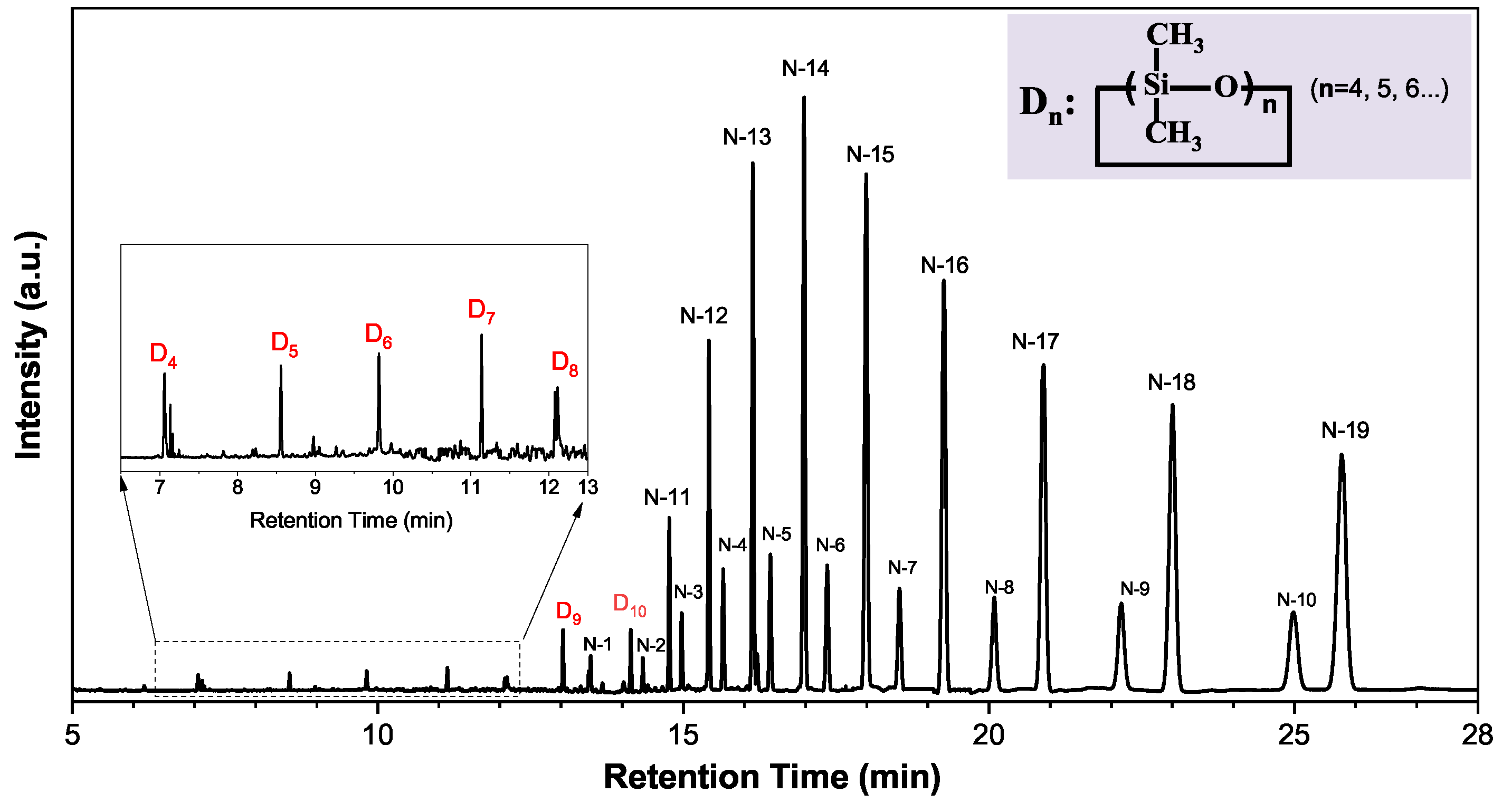
3.1. Extracts in Silicone Rubber Identified by GC–MS
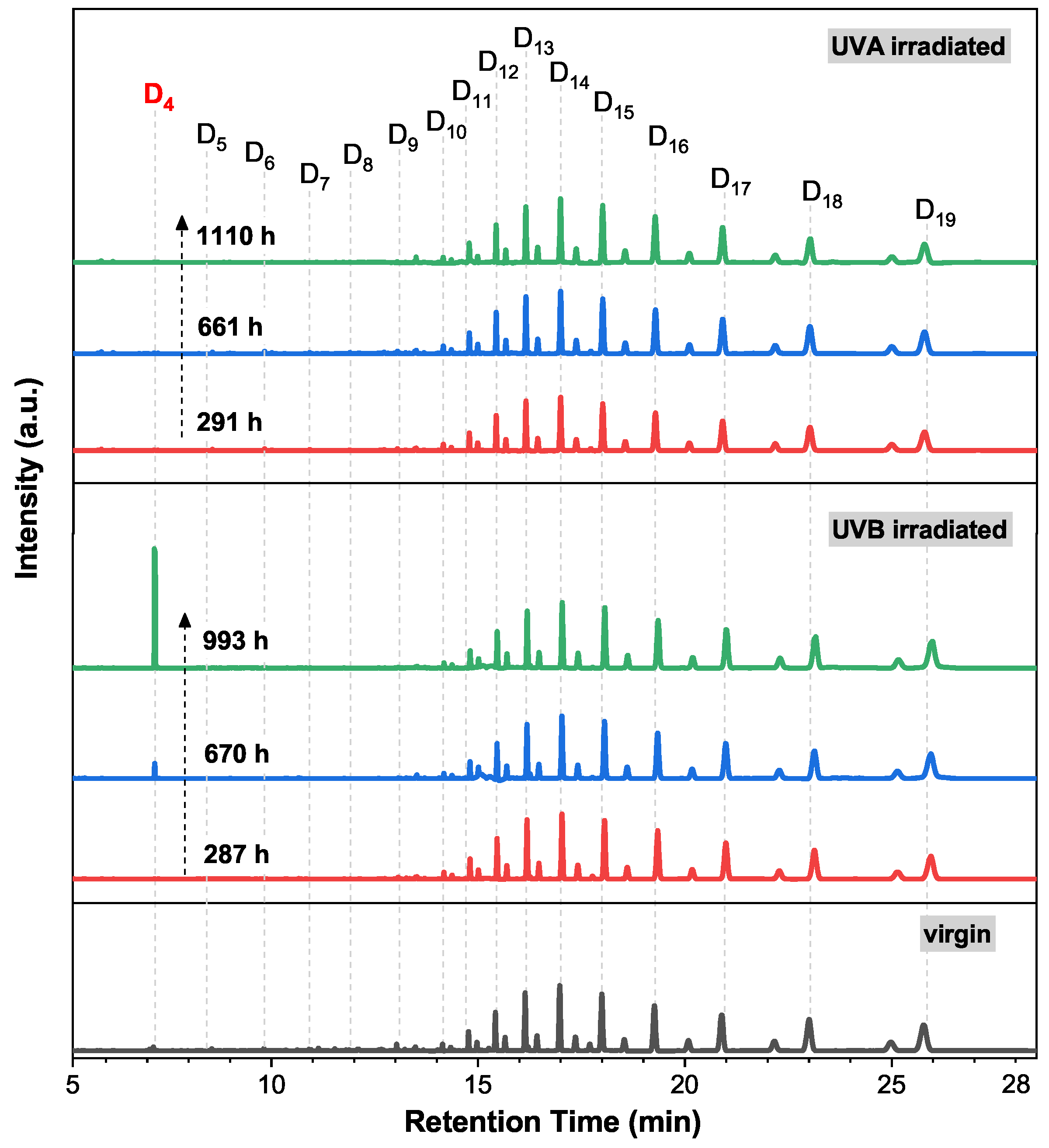
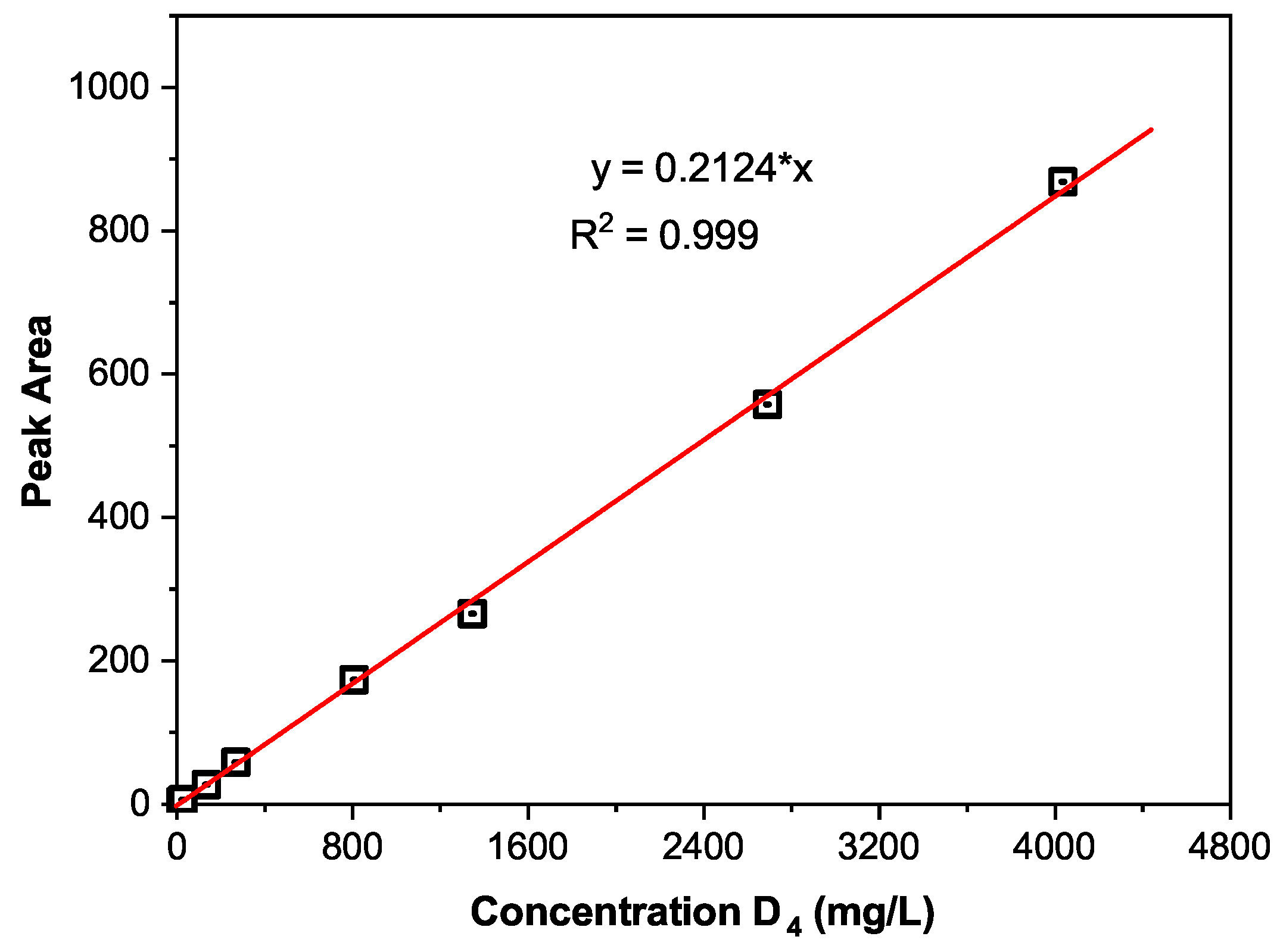
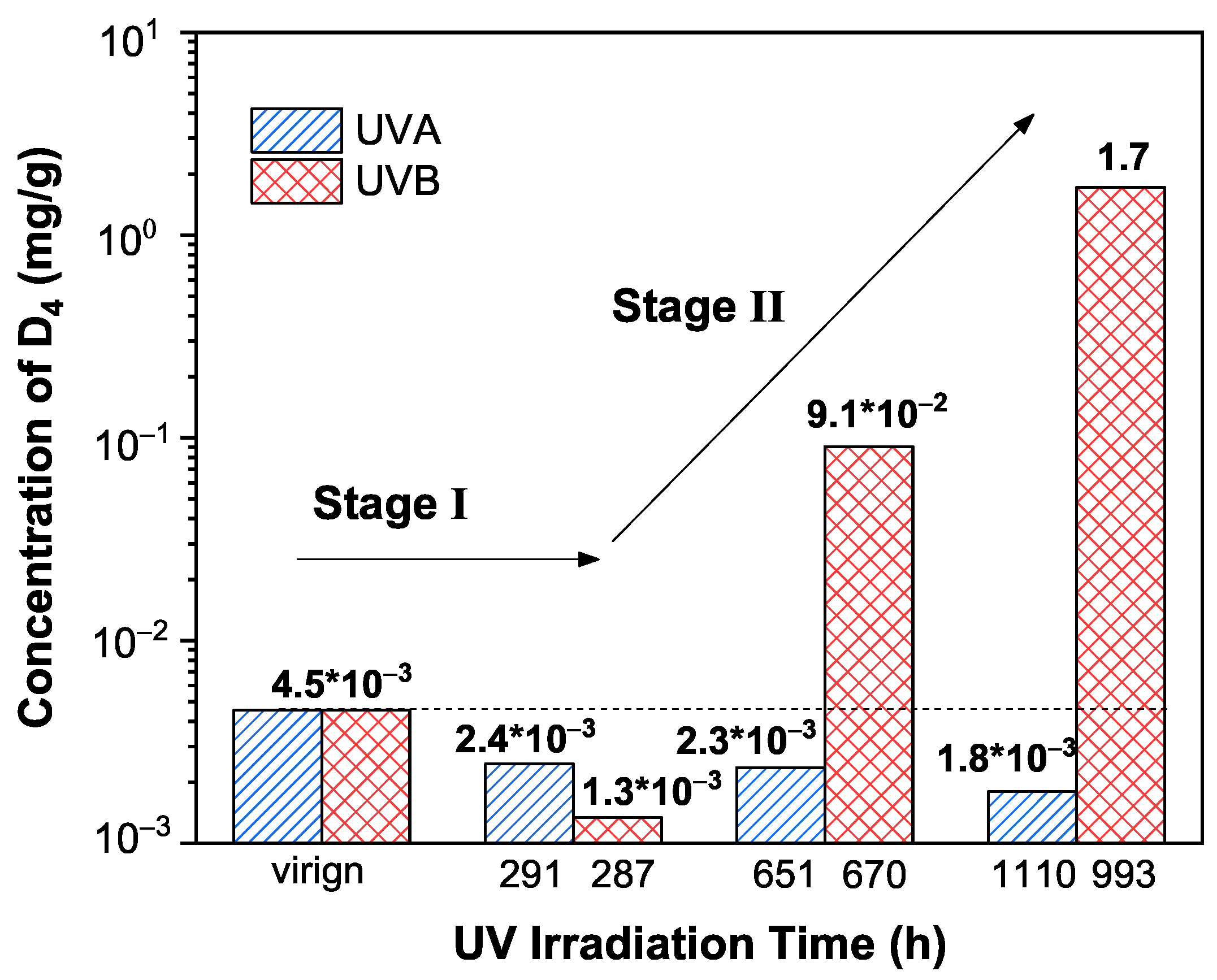
3.2. Quantitative Analysis of Extracts in Silicone Rubber Irradiated by Sunlight UV
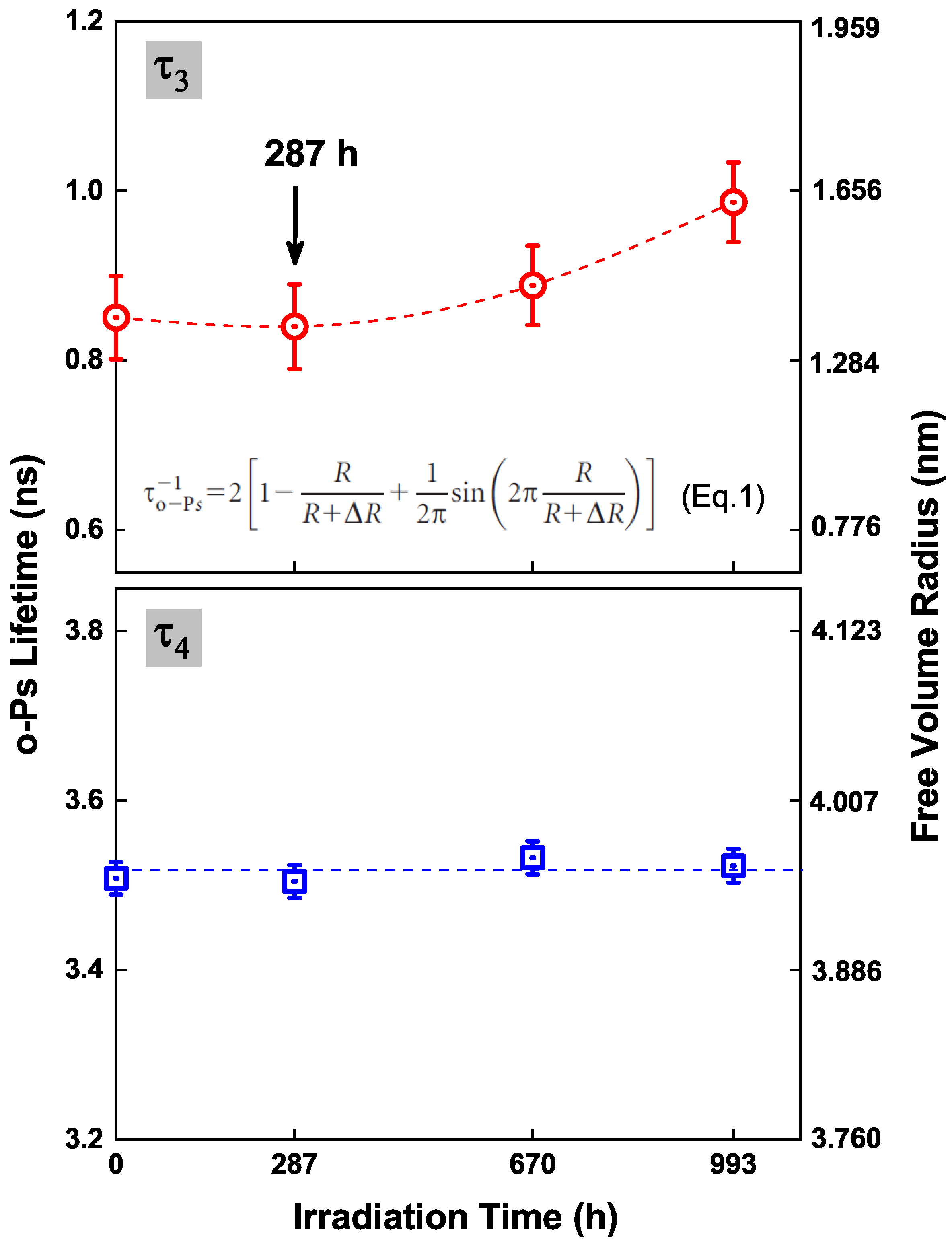
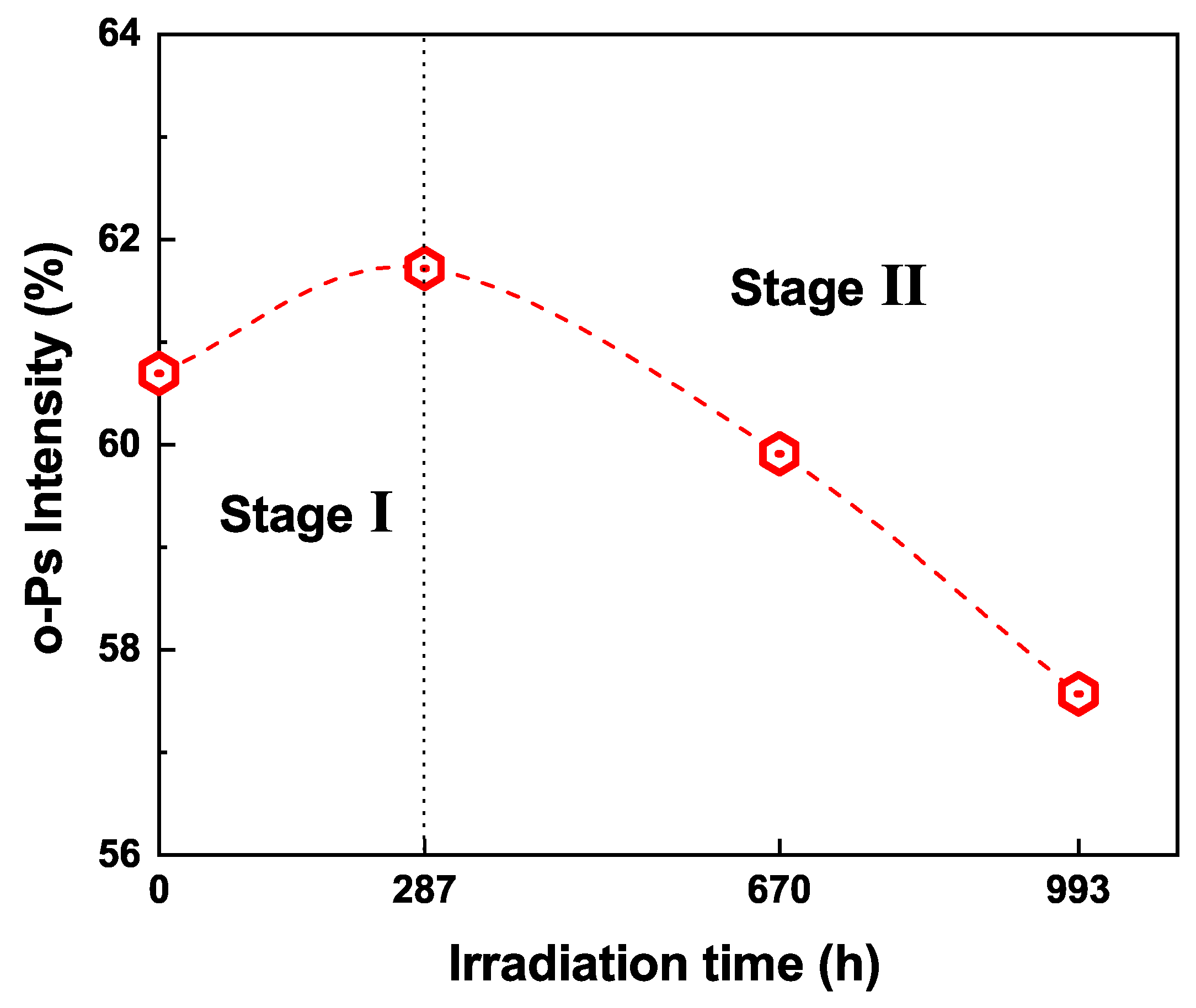
3.3. Free Volumes Characterized by PALS
3.4. Aging Mechanism of Silicone Rubber under UVA/UVB Irradiation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, X.; Bachman, M.; Sims, C.; Li, G.P.; Allbritton, N. Electroosmotic properties of microfluidic channels composed of poly(dimethylsiloxane). J. Chromatogr. B 2001, 762, 117. [Google Scholar] [CrossRef]
- Vozzi, G.; Morelli, I.; Vozzi, F.; Andreoni, C.; Salsedo, E.; Morachioli, A.; Giusti, P.; Ciardelli, G. SOFT-MI: A novel microfabrication technique integrating soft-lithography and molecular imprinting for tissue engineering applications. Biotechnol. Bioeng. 2010, 106, 804–817. [Google Scholar] [CrossRef]
- Ali, M.; Hackam, R. Effects of saline water and temperature on surface properties of HTV silicone rubber. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 1368–1378. [Google Scholar] [CrossRef]
- Yilgör, E.; Yilgör, I. Silicone containing copolymers: Synthesis, properties and applications. Prog. Polym. Sci. 2014, 39, 1165–1195. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Gao, Y.; Liang, X.; Gubanski, S.M.; Wang, Q.; Bao, W.; Li, S. Manifestation of Interactions of Nano-Silica in Silicone Rubber Investigated by Low-Frequency Dielectric Spectroscopy and Mechanical Tests. Polymers 2019, 11, 717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, M.; Hackam, R. Recovery of Hydrophobicity of HTV Silicone Rubber after Accelerated Aging in Saline Solutions. IEEE Trans. Dielectr. Electr. Insul. 2009, 16, 842–852. [Google Scholar] [CrossRef]
- Hillborg, H.; Gedde, U.W. Hydrophobicity recovery of polydimethylsiloxane after exposure to corona discharges. Polymer 1998, 39, 1991–1998. [Google Scholar] [CrossRef]
- Peng, X.; Wang, Z.; Xu, Z.; Li, Z.; Huang, Z.; Fang, P. Influence of acid and alkali aging on hydrophobicity of silicone rubber composite insulator. Guangdong Electr. Power 2017, 30, 110–114. [Google Scholar]
- Kim, J.; Chaudhury, M.K.; Owen, M.J. Hydrophobicity loss and recovery of silicone HV insulation. IEEE Trans. Dielectr. Electr. Insul. 1999, 6, 695–702. [Google Scholar] [CrossRef]
- Kim, J.; Chaudhury, M.K.; Owen, M.J.; Orbeck, T. The mechanisms of hydrophobic recovery of polydimethylsiloxane elastomers exposed to partial electrical discharges. J. Colloid Interface Sci. 2001, 244, 200–207. [Google Scholar] [CrossRef] [Green Version]
- Hillborg, H.; Karlsson, S.; Gedde, U.W. Characterisation of low molar mass siloxanes extracted from crosslinked polydimethylsiloxanes exposed to corona discharges. Polym. Commun. 2001, 42, 8883–8889. [Google Scholar] [CrossRef]
- Kaali, P.; Momcilovic, D.; Markström, A.; Aune, R.; Czel, G.; Karlsson, S. Degradation of biomedical polydimethylsiloxanes during exposure to in vivo biofilm environment monitored by FE-SEM, ATR-FTIR, and MALDI-TOF MS. J. Appl. Polym. Sci. 2010, 115, 802–810. [Google Scholar] [CrossRef]
- Hillborg, H.; Gedde, U.W. Hydrophobicity changes in silicone rubbers. IEEE Trans. Dielectr. Electr. Insul. 1999, 6, 703–717. [Google Scholar] [CrossRef]
- Vasilets, V.N.; Nakamura, K.; Uyama, Y.; Ogata, S.; Ikada, Y. Improvement of the micro-wear resistance of silicone by vacuum ultraviolet irradiation. Polymer 1998, 39, 2875–2881. [Google Scholar] [CrossRef]
- Hillborg, H.C. Loss and Recovery of Hydrophobicity of Polydimethlsiloxane after Exposure to Electrical Discharges. Ph.D. Thesis, Royal Institute of Technology, Stockholm, Sweden, 2001. [Google Scholar]
- Zheng, F.; He, C.Q.; Fang, P.F.; Wang, J.G.; Xiong, B.Y.; Wang, K.; Liu, F.W.; Peng, X.Y.; Xu, X.G.; Xu, Z.H.; et al. The surface structure of UV exposed poly-dimethylsiloxane (PDMS) insulator studied by slow positron beam. Appl. Surf. Sci. 2013, 283, 327–331. [Google Scholar] [CrossRef]
- Kirkegaard, P.; Eldrup, M.; Mogensen, O.E.; Pedersen, N.J. Program system for analysing positron lifetime spectra and angular correlation curves. Comput. Phys. Commun. 1981, 23, 307–335. [Google Scholar] [CrossRef]
- Wang, Z.; Yin, C.; Luo, Y.; Chen, L.; Zhou, Y.; He, C.; Fang, P.; Peng, X.; Huang, Z. Effect of aluminum hydroxide on low-molecular-weight siloxane distribution and microstructure of high-temperature vulcanized silicone rubber. J. Appl. Polym. Sci. 2018, 135, 45803. [Google Scholar] [CrossRef]
- Homma, H.; Kuroyagi, T.; Izumi, K.; Mirley, C.; Ronzello, J.; Boggs, S.A. Evaluation of surface degradation of silicone rubber using gas chromatography/mass spectroscopy. IEEE Trans. Power Deliv. 2000, 15, 796–803. [Google Scholar] [CrossRef]
- Hunt, S.M.; George, G.A. Characterization of siloxane residues from polydimethylsiloxane elastomers by MALDI-TOF-MS. Polym. Int. 2000, 49, 633–635. [Google Scholar] [CrossRef]
- Liu, F.; Yin, M.; Xiong, B.; Zheng, F.; Mao, W.; Chen, Z.; He, C.; Zhao, X.; Fang, P. Evolution of microstructure of epoxy coating during UV degradation progress studied by slow positron annihilation spectroscopy and electrochemical impedance spectroscopy. Electrochim. Acta 2014, 133, 283–293. [Google Scholar] [CrossRef]
- Wang, B.; Gong, W.; Liu, W.; Wang, Z.; Qi, N.; Li, X.; Liu, M.; Li, S. Influence of physical aging and side group on the free volume of epoxy resins probed by positron. Polymer 2003, 44, 4047–4052. [Google Scholar] [CrossRef]
- Cao, H.; Yuan, J.P.; Zhang, R.; Huang, C.M.; He, Y.; Sandreczki, T.C.; Jean, Y.C.; Nielsen, B.; Suzuki, R.; Ohdaira, T. Degradation of polymer coating systems studied by positron annihilation spectroscopy. 3. Wavelength dependence of UV irradiation effect. Macromolecules 1999, 32, 5925–5933. [Google Scholar] [CrossRef]
- Chen, H.; Hung, W.S.; Lo, C.H.; Huang, S.H.; Cheng, M.L.; Liu, G.; Lee, K.R.; Lai, J.Y.; Sun, Y.M.; Hu, C.C.; et al. Free-Volume Depth Profile of Polymeric Membranes Studied by Positron Annihilation Spectroscopy: Layer Structure from Interfacial Polymerization. Macromolecules 2007, 40, 7542–7557. [Google Scholar] [CrossRef]
- He, C.; Shantarovich, V.; Suzuki, T.; Stepanov, S.; Suzuki, R.; Matsuo, M. Mechanism of enhanced positronium formation in low-temperature polymers. J. Chem. Phys. 2005, 122, 214907. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, O.E. Positron Annihilation in Chemistry; Springer: Berlin/Heidelberg, Germany, 1995. [Google Scholar]
- Jean, Y.C. Positron annihilation spectroscopy for chemical analysis: A novel probe for microstructural analysis of polymers. Microchem. J. 1990, 42, 72–102. [Google Scholar] [CrossRef]
- Yin, C.; Li, J.; Zhou, Y.; Zhang, H.; Fang, P.; He, C. Enhancement in Proton Conductivity and Thermal Stability in Nafion Membranes Induced by Incorporation of Sulfonated Carbon Nanotubes. ACS Appl. Mater. Interfaces 2018, 10, 14026–14035. [Google Scholar] [CrossRef]
- Zheng, W.; Yang, Y.; Peng, X.; Huang, Z.; Qian, L.; He, C.; Fang, P. Water diffusivity transition in fumed silica-filled polydimethylsiloxane composite: Correlation with the interfacial free volumes characterized by positron annihilation lifetime spectroscopy. J. Mater. Sci. 2021, 56, 3095–3110. [Google Scholar]
- Tao, S.J. Positronium annihilation in molecular substances. J. Chem. Phys. 1972, 56, 5499–5510. [Google Scholar] [CrossRef]
- Dreiss, C.A.; Cosgrove, T.; Benton, N.J.; Kilburn, D.; Alam, M.A.; Schmidt, R.G.; Gordon, G.V. Effect of crosslinking on the mobility of PDMS filled with polysilicate nanoparticles: Positron lifetime, rheology and NMR relaxation studies. Polymer 2007, 48, 4419–4428. [Google Scholar] [CrossRef]
- Yoshimura, N.; Kumagai, S. Electrical and environmental aging of silicone rubber used in outdoor insulation. IEEE Trans. Dielectr. Electr. Insul. 1999, 6, 632–649. [Google Scholar] [CrossRef]
- Thomas, T.H.; Kendrick, T.C. Thermal analysis of polydimethylsiloxanes. I. Thermal degradation in controlled atmospheres. J. Polym. Sci. 1969, 7, 537–549. [Google Scholar] [CrossRef]


| Raw Material Type | Raw Material Name | Weight | Percentage |
|---|---|---|---|
| (phr) | (wt.%) | ||
| organic component | methyl-terminated PDMS | 100 | 46.51 |
| inorganic fillers | aluminum hydroxide (ATH) | 70 | 32.56 |
| amorphous silica (SiO) | 25 | 11.63 | |
| zinc oxide (ZnO) | 2 | 0.93 | |
| assistant additives | hydride-functional silicone oil | 10 | 2.79 |
| silane coupling agent | 6 | 4.65 | |
| 2,5-dimethyl-2,5-di(tert-butylperoxy) | 2 | 0.93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Qian, L.; Peng, X.; Huang, Z.; Yang, Y.; He, C.; Fang, P. New Aspects of Degradation in Silicone Rubber under UVA and UVB Irradiation: A Gas Chromatography–Mass Spectrometry Study. Polymers 2021, 13, 2215. https://doi.org/10.3390/polym13132215
Wang Z, Qian L, Peng X, Huang Z, Yang Y, He C, Fang P. New Aspects of Degradation in Silicone Rubber under UVA and UVB Irradiation: A Gas Chromatography–Mass Spectrometry Study. Polymers. 2021; 13(13):2215. https://doi.org/10.3390/polym13132215
Chicago/Turabian StyleWang, Zheng, Libing Qian, Xiangyang Peng, Zhen Huang, Yue Yang, Chunqing He, and Pengfei Fang. 2021. "New Aspects of Degradation in Silicone Rubber under UVA and UVB Irradiation: A Gas Chromatography–Mass Spectrometry Study" Polymers 13, no. 13: 2215. https://doi.org/10.3390/polym13132215







