Macro and Micro Routes to High Performance Bioplastics: Bioplastic Biodegradability and Mechanical and Barrier Properties
Abstract
:1. Introduction
2. Bioplastic Production
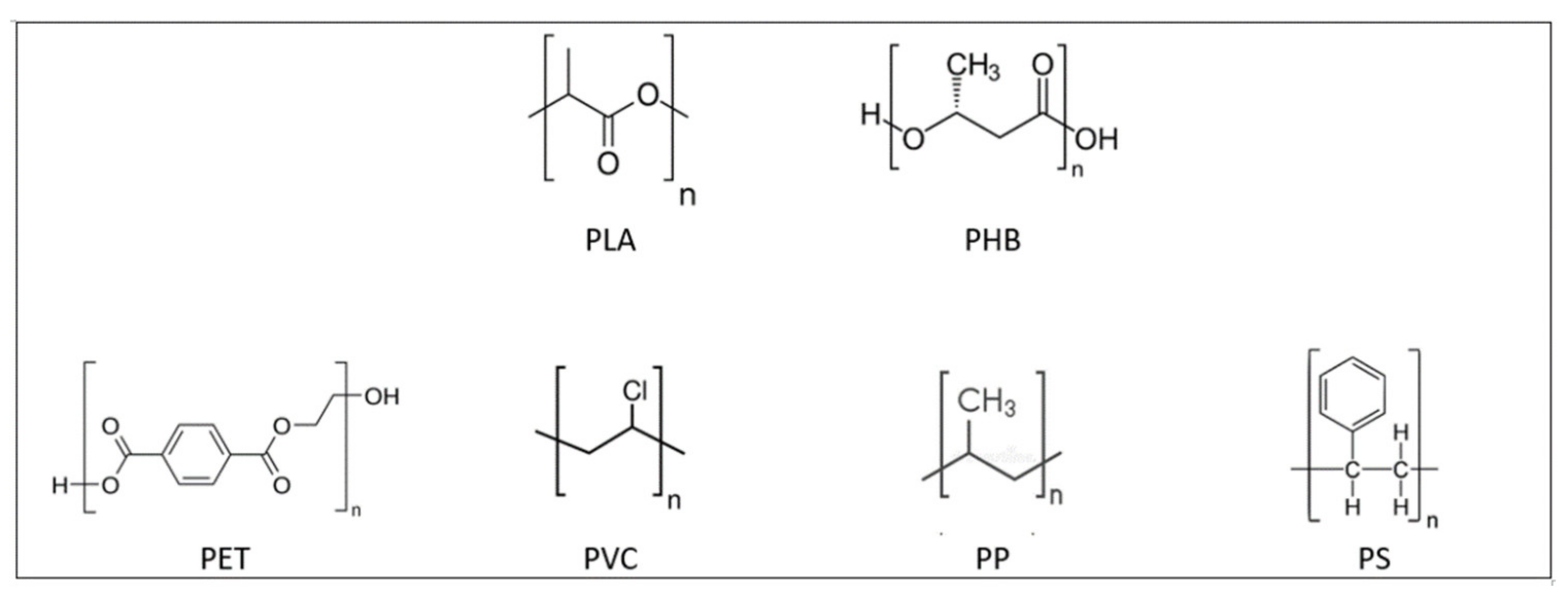
3. Biosynthesised Plastics
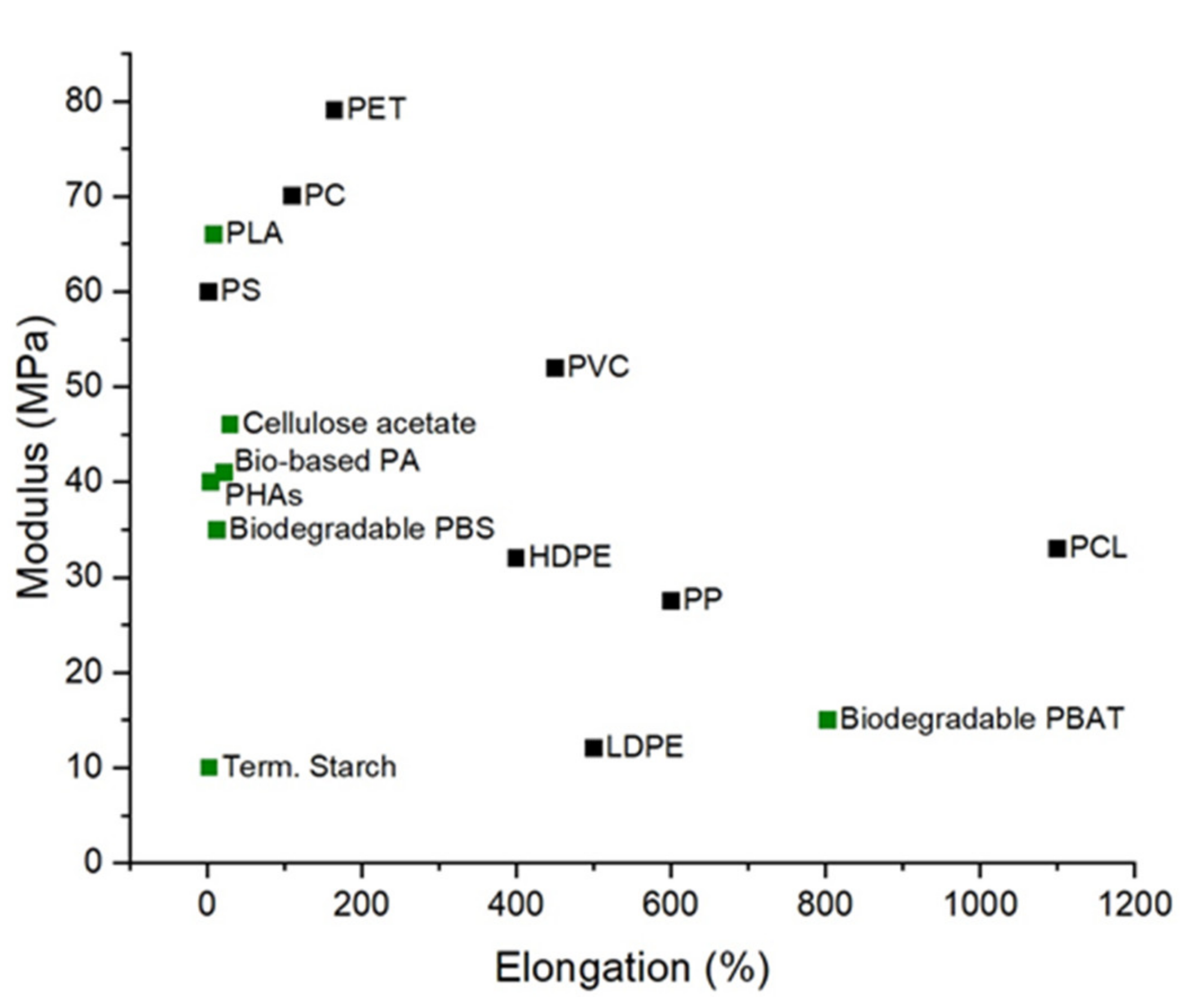
3.1. Bioplastic Mechanical Performance
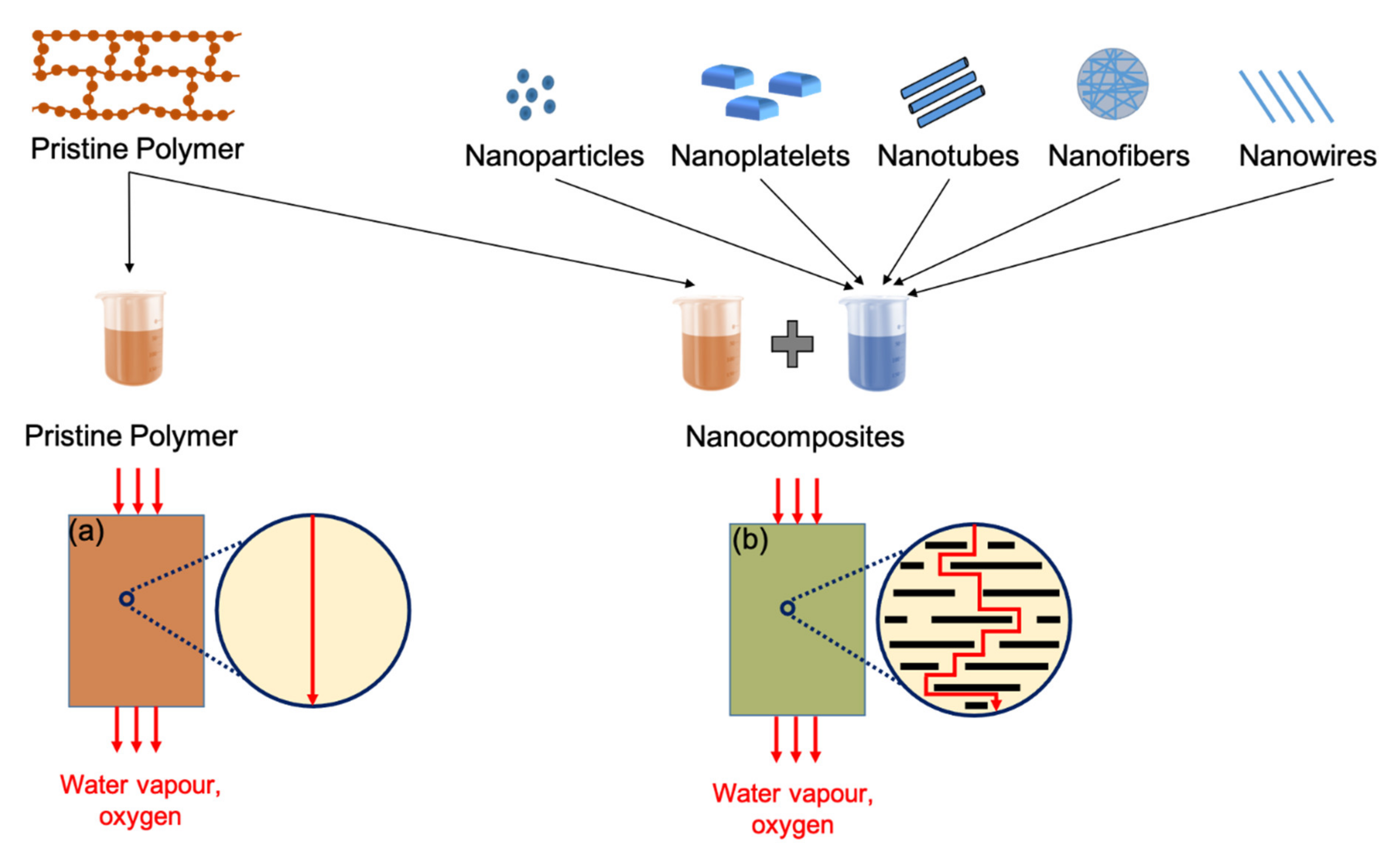
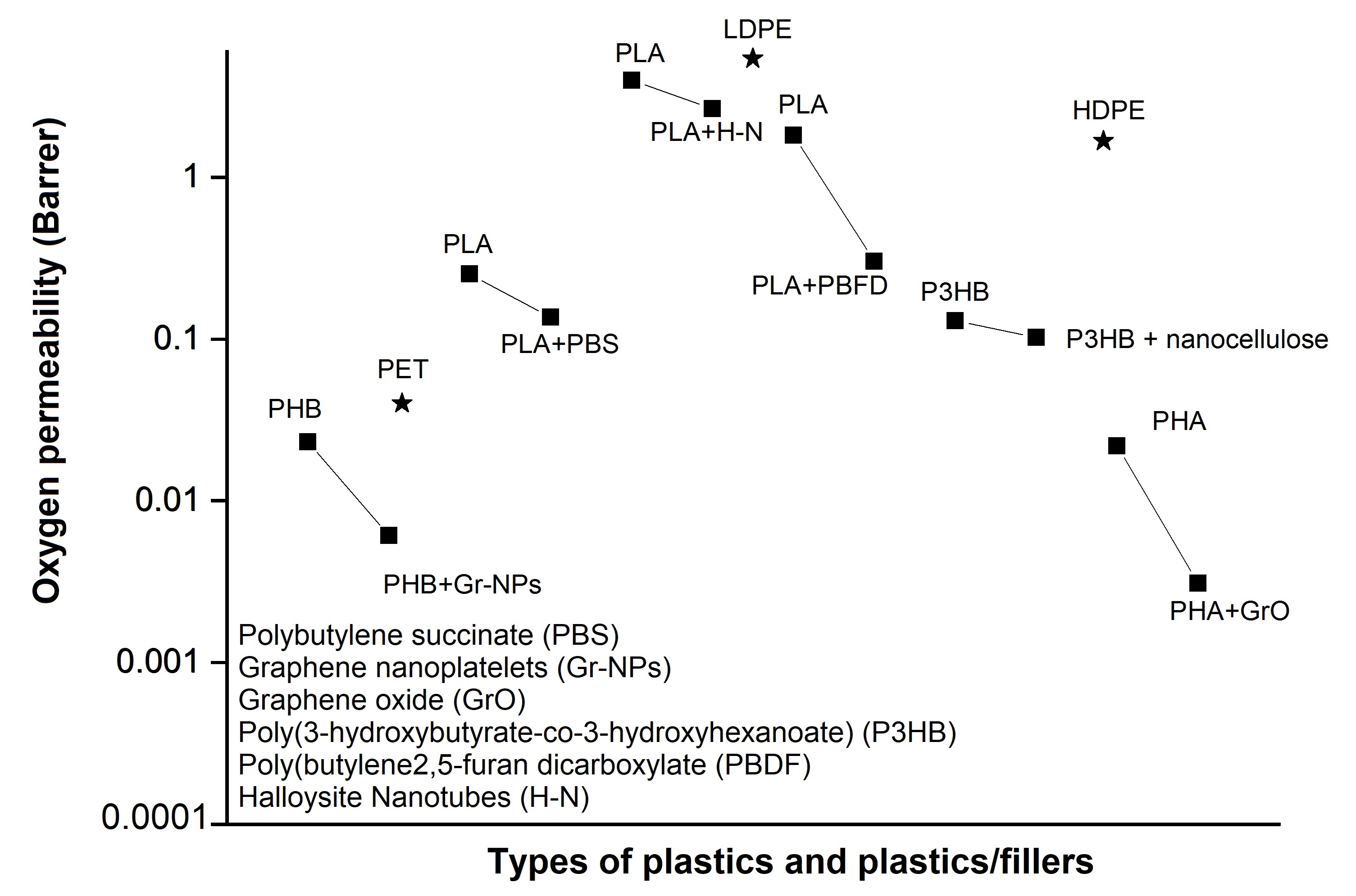
3.2. Bioplastic Barrier Performance
3.3. Bioplastic Processing and Formulation
3.3.1. Blends and Composites
3.3.2. Compatibilisers and Plasticisers
3.3.3. Natural Fillers
3.3.4. Bio-Coatings
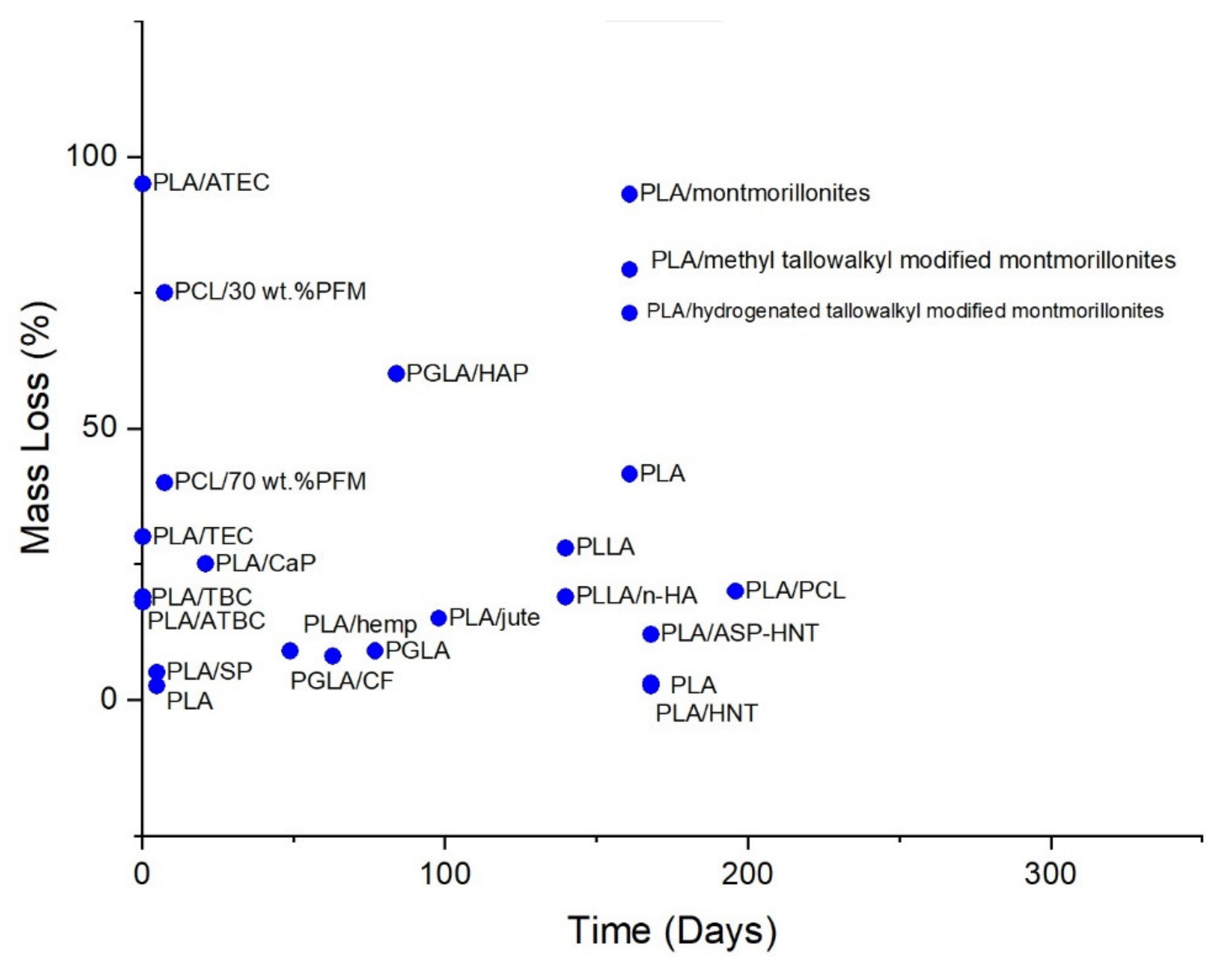
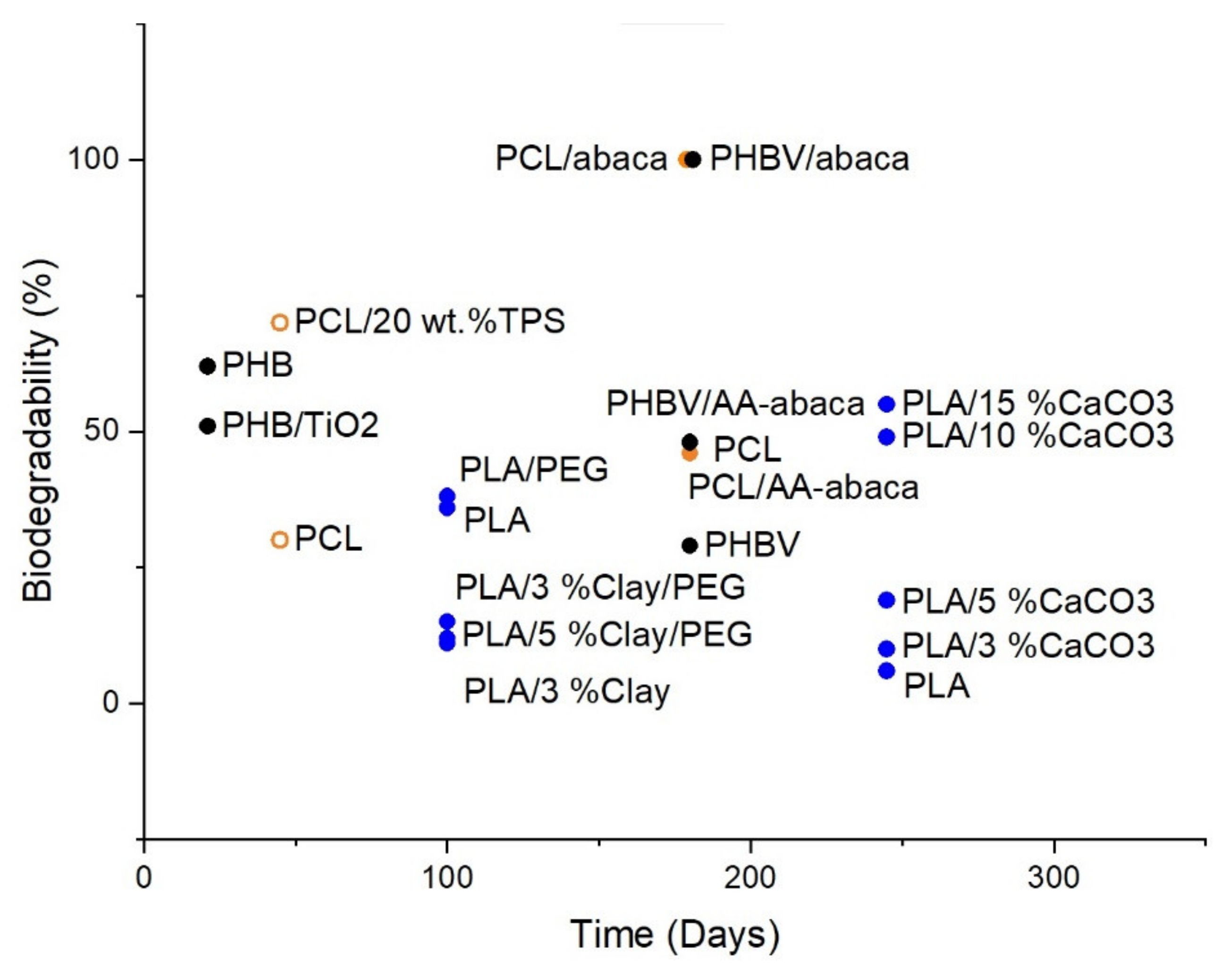
4. Bioplastics Biodegradability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Madison, L.L.; Huisman, G.W. Metabolic Engineering of Poly(3-Hydroxyalkanoates): From DNA to Plastic. Microbiol. Mol. Biol. Rev. 1999, 63, 21–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koller, M.; Maršálek, L.; Dias, M.M.D.S.; Braunegg, G. Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner. New Biotechnol. 2017, 37, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Koller, M. A Review on Established and Emerging Fermentation Schemes for Microbial Production of Polyhydroxy-alkanoate (PHA) Biopolyesters. Fermentation 2018, 4, 30. [Google Scholar] [CrossRef] [Green Version]
- Rajwade, J.M.; Paknikar, K.; Kumbhar, J.V. Applications of bacterial cellulose and its composites in biomedicine. Appl. Microbiol. Biotechnol. 2015, 99, 2491–2511. [Google Scholar] [CrossRef]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Muneer, F.; Rasul, I.; Azeem, F.; Siddique, M.H.; Zubair, M.; Nadeem, H. Microbial Polyhydroxyalkanoates (PHAs): Efficient Replacement of Synthetic Polymers. J. Polym. Environ. 2020, 28, 2301–2323. [Google Scholar] [CrossRef]
- Ciesielski, S.; Mozejko-Ciesielska, J.; Pisutpaisal, N. Plant oils as promising substrates for polyhydroxyalkanoates pro-duction. J. Clean. Prod. 2015, 106, 408–421. [Google Scholar] [CrossRef]
- Shuai, X.-T.; Jedlinski, Z.; Luo, Q.; Farhod, N. Synthesis of novel block copolymers of poly(3-hydroxybutyric acid) with poly(ethylene glycol) through anionic polymerisation. Chin. J. Polym. Sci. 2000, 18, 19–23. [Google Scholar]
- Fu, J.; Sharma, P.; Spicer, V.; Krokhin, O.V.; Zhang, X.; Fristensky, B.; Wilkins, J.A.; Cicek, N.; Sparling, R.; Levin, D.B. Effects of impurities in biodiesel-derived glycerol on growth and expression of heavy metal ion homeostasis genes and gene products in Pseudomonas putida LS46. Appl. Microbiol. Biotechnol. 2015, 99, 5583–5592. [Google Scholar] [CrossRef]
- Noda, I.; Lindsey, S.B.; Caraway, D. Nodax™ Class PHA Copolymers: Their Properties and Applications. In Beneficial Microorganisms in Food and Nutraceuticals; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2010; pp. 237–255. [Google Scholar]
- Kunioka, M.; Doi, Y. Thermal degradation of microbial copolyesters: Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and poly(3-hydroxybutyrate-co-4-hydroxybutyrate). Macromolecules 1990, 23, 1933–1936. [Google Scholar] [CrossRef]
- Avella, M.; Martuscelli, E.; Raimo, M. Review Properties of blends and composites based on poly(3-hydroxy)butyrate (PHB) and poly(3-hydroxybutyrate-hydroxyvalerate) (PHBV) copolymers. J. Mater. Sci. 2000, 35, 523–545. [Google Scholar] [CrossRef]
- Qiu, Y.-Z.; Han, J.; Guo, J.-J.; Chen, G.-Q. Production of Poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from Gluconate and Glucose by Recombinant Aeromonas hydrophila and Pseudomonas putida. Biotechnol. Lett. 2005, 27, 1381–1386. [Google Scholar] [CrossRef]
- McAdam, B.; Fournet, M.B.; McDonald, P.; Mojicevic, M. Production of Polyhydroxybutyrate (PHB) and Factors Impacting Its Chemical and Mechanical Characteristics. Polymers 2020, 12, 2908. [Google Scholar] [CrossRef]
- Ravenelle, F.; Marchessault, R.H. One-Step Synthesis of Amphiphilic Diblock Copolymers from Bacterial Poly([R]-3-hydroxybutyric acid). Biomacromolecules 2002, 3, 1057–1064. [Google Scholar] [CrossRef]
- Amaro, T.M.M.M.; Rosa, D.; Comi, G.; Iacumin, L. Prospects for the Use of Whey for Polyhydroxyalkanoate (PHA) Pro-duction. Front. Microbiol. 2019, 10, 992. [Google Scholar] [CrossRef]
- Colombo, B.; Favini, F.; Scaglia, B.; Sciarria, T.P.; D’Imporzano, G.; Pognani, M.; Alekseeva, A.; Eisele, G.; Cosentino, C.; Adani, F. Enhanced polyhydroxyalkanoate (PHA) production from the organic fraction of municipal solid waste by using mixed microbial culture. Biotechnol. Biofuels 2017, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, N.; Sharanya, P.; Sneha Raj, M.; Ruth Angeeleena, B.; Rajam, C. Seaweeds can be a new source for bio-plastics. J. Pharm. Res. 2012, 5, 1476–1479. [Google Scholar]
- Chee, J.Y.; Tan, Y.; Samian, R.; Sudesh, K. Isolation and Characterization of a Burkholderia sp. USM (JCM15050) Capable of Producing Polyhydroxyalkanoate (PHA) from Triglycerides, Fatty Acids and Glycerols. J. Polym. Environ. 2010, 18, 584–592. [Google Scholar] [CrossRef]
- Koller, M.; Salerno, A.; Dias, M.; Reiterer, A.; Braunegg, G. Modern biotechnological polymer synthesis: A review. Food Technol. Biotechnol. 2010, 48, 255–269. [Google Scholar]
- Pandey, A. Solid-state fermentation. Biochem. Eng. J. 2003, 13, 81–84. [Google Scholar] [CrossRef]
- Sindhu, R.; Pandey, A.; Binod, P. Solid-state fermentation for the production of Poly(hydroxyalkanoates). Chem. Biochem. Eng. Q. 2015, 29, 173–181. [Google Scholar] [CrossRef]
- Reiniati, I.; Hrymak, A.N.; Margaritis, A. Recent developments in the production and applications of bacterial cellulose fibers and nanocrystals. Crit. Rev. Biotechnol. 2017, 37, 510–524. [Google Scholar] [CrossRef]
- Florea, M.; Hagemann, H.; Santosa, G.; Abbott, J.; Micklem, C.; Spencer-Milnes, X.; Garcia, L.D.A.; Paschou, D.; Lazenbatt, C.; Kong, D.; et al. Engineering control of bacterial cellulose production using a genetic toolkit and a new cellulose-producing strain. Proc. Natl. Acad. Sci. USA 2016, 113, E3431–E3440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Li, S.; Xie, Y.; Jia, S.; Hou, Y.; Zou, Y.; Zhong, C. Enhanced bacterial cellulose production by Gluconacetobacter xylinus via expression of Vitreoscilla hemoglobin and oxygen tension regulation. Appl. Microbiol. Biotechnol. 2018, 102, 1155–1165. [Google Scholar] [CrossRef]
- Hungund, B.; Gupta, S. Strain improvement of Gluconacetobacter xylinus NCIM 2526 for bacterial cellulose production. Afr. J. Biotechnol. 2010, 9, 32. [Google Scholar]
- Neves, N.M.; Reis, R.L. (Eds.) Biomaterials from Nature for Advanced Devices and Therapies; Wiley: Hoboken, NJ, USA, 2016; pp. 384–399. [Google Scholar]
- Volova, T.G.; Prudnikova, S.; Sukovatyi, A.G.; Shishatskaya, E. Production and properties of bacterial cellulose by the strain Komagataeibacter xylinus B-12068. Appl. Microbiol. Biotechnol. 2018, 102, 7417–7428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.-P.; Calvar, I.L.; Catchmark, J.M.; Liu, J.-R.; Demirci, A.; Cheng, K.-C. Biosynthesis, production and applications of bacterial cellulose. Cellulose 2013, 20, 2191–2219. [Google Scholar] [CrossRef]
- Shah, N.; Ul-Islam, M.; Khattak, W.A.; Park, J.K. Overview of bacterial cellulose composites: A multipurpose advanced material. Carbohydr. Polym. 2013, 98, 1585–1598. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J.-T.; Wang, M.-J.; Lai, J.-T.; Liu, H.-S. A novel static cultivation of bacterial cellulose production by intermittent feeding strategy. J. Taiwan Inst. Chem. Eng. 2016, 63, 46–51. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Khan, S.; Ullah, M.W.; Park, J.K. Bacterial cellulose composites: Synthetic strategies and multiple applications in bio-medical and electro-conductive fields. Biotechnol. J. 2015, 10, 1847–1861. [Google Scholar] [CrossRef] [PubMed]
- Singhsa, P.; Narain, R.; Manuspiya, H. Physical structure variations of bacterial cellulose produced by different Komaga-taeibacter xylinus strains and carbon sources in static and agitated conditions. Cellulose 2018, 25, 1571–1581. [Google Scholar] [CrossRef]
- Parte, F.G.B.; Santoso, S.P.; Chou, C.-C.; Verma, V.; Wang, H.-T.; Ismadji, S.; Cheng, K.-C. Current progress on the production, modification, and applications of bacterial cellulose. Crit. Rev. Biotechnol. 2020, 40, 397–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auras, R.; Harte, B.; Selke, S. An Overview of Polylactides as Packaging Materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef]
- Zhao, X.; Cornish, K.; Vodovotz, Y. Narrowing the Gap for Bioplastic Use in Food Packaging: An Update. Environ. Sci. Technol. 2020, 54, 4712–4732. [Google Scholar] [CrossRef]
- Chen, S.-Q.; Lopez-Sanchez, P.; Wang, D.; Mikkelsen, D.; Gidley, M.J. Mechanical properties of bacterial cellulose synthesised by diverse strains of the genus Komagataeibacter. Food Hydrocoll. 2018, 81, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Jem, K.J.; Tan, B. The development and challenges of poly (lactic acid) and poly (glycolic acid). Adv. Ind. Eng. Polym. Res. 2020, 3, 60–70. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread appli-cations—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visakh, P.M. CHAPTER 1. Polyhydroxyalkanoates (PHAs), their Blends, Composites and Nanocomposites: State of the Art, New Challenges and Opportunities. In Polyhydroxyalkanoate (PHA) Based Blends, Composites and Nanocomposites; Green Chemistry Series; Royal Society of Chemistry: Cambridge, UK, 2014; pp. 1–17. [Google Scholar] [CrossRef]
- Goh, L.-K.; Purama, R.K.; Sudesh, K. Enhancement of Stress Tolerance in the Polyhydroxyalkanoate Producers without Mobilization of the Accumulated Granules. Appl. Biochem. Biotechnol. 2014, 172, 1585–1598. [Google Scholar] [CrossRef]
- Dos Santos, A.J.; Valentina, L.V.O.D.; Schulz, A.A.H.; Duarte, M.A.T. From Obtaining to Degradation of PHB:Material Properties. Part I. Ing. Cienc. 2017, 13, 269–298. [Google Scholar] [CrossRef] [Green Version]
- El-Hadi, A.M.; Schnabel, R.; Straube, E.; Müller, G.; Henning, S. Correlation between degree of crystallinity, morphology, glass temperature, mechanical properties and biodegradation of poly (3-hydroxyalkanoate) PHAs and their blends. Polym. Test. 2002, 21, 665–674. [Google Scholar] [CrossRef]
- Smith, M.K.M.; Paleri, D.M.; Abdelwahab, M.; Mielewski, D.F.; Misra, M.; Mohanty, A.K. Sustainable composites from poly(3-hydroxybutyrate) (PHB) bioplastic and agave natural fibre. Green Chem. 2020, 22, 3906–3916. [Google Scholar] [CrossRef]
- Barham, P.J.; Keller, A.; Otun, E.L.; Wills, H.H.; Holmes, P.A. Crystallization and morphology of a bacterial thermoplastic: Poly-3-hyd roxybutyrate. J. Mater. Sci. 1984, 19. [Google Scholar] [CrossRef]
- Barham, P.J.; Keller, A. The relationship between microstructure and mode of fracture in polyhydroxybutyrate. J. Polym. Sci. Part B Polym. Phys. 1986, 24, 69–77. [Google Scholar] [CrossRef]
- Janigová, I.; Lacıík, I.; Chodák, I. Thermal degradation of plasticized poly(3-hydroxybutyrate) investigated by DSC. Polym. Degrad. Stab. 2002, 77, 35–41. [Google Scholar] [CrossRef]
- Shim, S.H.; Kim, K.T.; Lee, J.U.; Jo, W.H. Facile Method to Functionalize Graphene Oxide and Its Application to Poly(ethylene terephthalate)/Graphene Composite. ACS Appl. Mater. Interfaces 2012, 4, 4184–4191. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, E.L.; Basnett, P.; Paul, U.C.; Marras, S.; Ceseracciu, L.; Roy, I.; Athanassiou, A. Green Composites of Poly(3-hydroxybutyrate) Containing Graphene Nanoplatelets with Desirable Electrical Conductivity and Oxygen Barrier Properties. ACS Omega 2019, 4, 19746–19755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhar, P.; Gaur, S.S.; Soundararajan, N.; Gupta, A.; Bhasney, S.M.; Milli, M.; Kumar, A.; Katiyar, V. Reactive Extrusion of Polylactic Acid/Cellulose Nanocrystal Films for Food Packaging Applications: Influence of Filler Type on Thermomechanical, Rheological, and Barrier Properties. Ind. Eng. Chem. Res. 2017, 56, 4718–4735. [Google Scholar] [CrossRef]
- Azeem, M.; Jan, R.; Farrukh, S.; Hussain, A. Improving gas barrier properties with boron nitride nanosheets in poly-mer-composites. Results Phys. 2019, 12, 1535–1541. [Google Scholar] [CrossRef]
- Bharadwaj, R.K. Modeling the Barrier Properties of Polymer-Layered Silicate Nanocomposites. Macromolecules 2001, 34, 9189–9192. [Google Scholar] [CrossRef]
- Moggridge, G.; Lape, N.K.; Yang, C.; Cussler, E. Barrier films using flakes and reactive additives. Prog. Org. Coat. 2003, 46, 231–240. [Google Scholar] [CrossRef]
- Cui, Z.; Martinez, A.P.; Adamson, D.H. PMMA functionalized boron nitride sheets as nanofillers. Nanoscale 2015, 7, 10193–10197. [Google Scholar] [CrossRef]
- Gitari, B.; Chang, B.P.; Misra, M.; Navabi, A.; Mohanty, A.K. A comparative study on the mechanical, thermal, and water barrier properties of PLA nanocomposite films prepared with bacterial nanocellulose and cellulose nanofibrils. BioResources 2019, 14, 1867–1889. [Google Scholar]
- Xu, P.; Yang, W.; Niu, D.; Yu, M.; Du, M.; Dong, W.; Chen, M.; Lemstra, P.J.; Ma, P. Multifunctional and robust polyhydroxyalkanoate nanocomposites with superior gas barrier, heat resistant and inherent antibacterial performances. Chem. Eng. J. 2020, 382, 122864. [Google Scholar] [CrossRef]
- Jandas, P.J.; Mohanty, S.; Nayak, S.K. Morphology and Thermal Properties of Renewable Resource-Based Polymer Blend Nanocomposites Influenced by a Reactive Compatibilizer. ACS Sustain. Chem. Eng. 2013, 2, 377–386. [Google Scholar] [CrossRef]
- Kick, T.; Grethe, T.; Mahltig, B. A Natural Based Method for Hydrophobic Treatment of Natural Fiber Material. Acta Chim. Slov. 2017, 64, 373–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, N.; Oza, S.; Tajabadi, M.G. Surface Modification of Natural Fibers for Reinforcement in Polymeric Composites. In Surface Modification of Biopolymers; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 224–237. [Google Scholar]
- Zhijiang, C.; Yi, X.; Haizheng, Y.; Jia, J.; Liu, Y. Poly(hydroxybutyrate)/cellulose acetate blend nanofiber scaffolds: Prepa-ration, characterization and cytocompatibility. Mater. Sci. Eng. C 2016, 58, 757–767. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Nicolae, C.A.; Gabor, A.R.; Trusca, R. Thermal and mechanical properties of poly(3-hydroxybutyrate) reinforced with cellulose fibers from wood waste. Ind. Crop. Prod. 2020, 145, 112071. [Google Scholar] [CrossRef]
- Mokhothu, T.H.; John, M.J. Review on hygroscopic aging of cellulose fibres and their biocomposites. Carbohydr. Polym. 2015, 131, 337–354. [Google Scholar] [CrossRef]
- Joffe, R.; Pupure, L.; Berthold, F.; Varna, J. Micro-structure and Mechanical Properties in PLA Reinforced with Cellulosic Fiber Sheets Made by Wet Forming Method. In Proceedings of the 8th International Conference on Composites Testing and Model Identification (CompTest 2017), Leuven, Belgium, 5–7 April 2017. [Google Scholar]
- Masmoudi, F.; Bessadok, A.; Dammak, M.; Jaziri, M.; Ammar, E. Biodegradable packaging materials conception based on starch and polylactic acid (PLA) reinforced with cellulose. Environ. Sci. Pollut. Res. 2016, 23, 20904–20914. [Google Scholar] [CrossRef]
- Kyutoku, H.; Maeda, N.; Sakamoto, H.; Nishimura, H.; Yamada, K. Effect of surface treatment of cellulose fiber (CF) on durability of PLA/CF bio-composites. Carbohydr. Polym. 2019, 203, 95–102. [Google Scholar] [CrossRef]
- Piekarska, K.; Sowinski, P.; Piorkowska, E.; Haque, M.M.U.; Pracella, M. Structure and properties of hybrid PLA nano-composites with inorganic nanofillers and cellulose fibers. Compos. Part A Appl. Sci. Manuf. 2016, 82, 34–41. [Google Scholar] [CrossRef]
- Hu, R.; Lim, J.-K. Fabrication and Mechanical Properties of Completely Biodegradable Hemp Fiber Reinforced Polylactic Acid Composites. J. Compos. Mater. 2007, 41, 1655–1669. [Google Scholar] [CrossRef]
- Pommet, M.; Juntaro, J.; Heng, J.; Mantalaris, A.; Lee, A.; Wilson, K.; Kalinka, G.; Shaffer, M.S.P.; Bismarck, A. Surface Modification of Natural Fibers Using Bacteria: Depositing Bacterial Cellulose onto Natural Fibers To Create Hierarchical Fiber Reinforced Nanocomposites. Biomacromolecules 2008, 9, 1643–1651. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Yang, L.; Ni, Q.; Ruan, F.; Wang, H. Fabrication of high-performance green hemp/polylactic acid fibre composites. J. Eng. Fibers Fabr. 2019, 14. [Google Scholar] [CrossRef] [Green Version]
- Michel, A.; Billington, S. Characterization of poly-hydroxybutyrate films and hemp fiber reinforced composites exposed to accelerated weathering. Polym. Degrad. Stab. 2012, 97, 870–878. [Google Scholar] [CrossRef]
- Keller, A. Compounding and mechanical properties of biodegradable hemp fibre composites. Compos. Sci. Technol. 2003, 63, 1307–1316. [Google Scholar] [CrossRef]
- Sawpan, M.A.; Pickering, K.L.; Fernyhough, A. Characterisation of hemp fibre reinforced Poly(Lactic Acid) composites. Int. J. Mater. Prod. Technol. 2009, 36, 229. [Google Scholar] [CrossRef]
- Smoca, A.; Kukle, S.; Zelca, Z. Properties of Hemp Fibres Reinforced PLA Composites. Key Eng. Mater. 2019, 800, 205–209. [Google Scholar] [CrossRef]
- Avella, M.; Buzõarovska, A.; Errico, M.E.; Gentile, G.; Grozdanov, A.; Bogoeva-Gaceva, G. Poly(3-hydroxybutyrate-co-3-hydroxyvalerate)-based biocomposites reinforced with kenaf fibers. J. Appl. Polym. Sci. 2007, 104, 3192–3200. [Google Scholar] [CrossRef]
- Hassan, A.; Isa, M.R.M.; Ishak, Z.A.M. Improving thermal and mechanical properties of injection moulded Kenaf Fibre-reinforced Polyhydroxy-butyrate composites through fibre surface treatment. BioResources 2019, 14, 3101–3116. [Google Scholar]
- Buzarovska, A.; Bogoeva-Gaceva, G.; Grozdanov, A.; Avella, M. Crystallization behavior of polyhydroxybutyrate in model composites with kenaf fibers. J. Appl. Polym. Sci. 2006, 102, 804–809. [Google Scholar] [CrossRef]
- Graupner, N.; Müssig, J. A comparison of the mechanical characteristics of kenaf and lyocell fibre reinforced poly(lactic acid) (PLA) and poly(3-hydroxybutyrate) (PHB) composites. Compos. Part A Appl. Sci. Manuf. 2011, 42, 2010–2019. [Google Scholar] [CrossRef]
- Kuciel, S.; Liber-Knec, A. Biocomposites based on PHB filled with wood or kenaf fibers. Polimery 2011, 56, 218–223. [Google Scholar] [CrossRef]
- Bayart, M.; Gauvin, F.; Foruzanmehr, M.R.; Elkoun, S.; Robert, M. Mechanical and moisture absorption characterization of PLA composites reinforced with nano-coated flax fibers. Fibers Polym. 2017, 18, 1288–1295. [Google Scholar] [CrossRef]
- Georgiopoulos, P.; Kontou, E.; Georgousis, G. Effect of silane treatment loading on the flexural properties of PLA/flax unidirectional composites. Compos. Commun. 2018, 10, 6–10. [Google Scholar] [CrossRef]
- Karsli, N.G.; Aytac, A. Properties of alkali treated short flax fiber reinforced poly(lactic acid)/polycarbonate composites. Fibers Polym. 2014, 15, 2607–2612. [Google Scholar] [CrossRef]
- Aydın, M.; Tozlu, H.; Kemaloglu, S.; Aytac, A.; Ozkoc, G. Effects of Alkali Treatment on the Properties of Short Flax Fi-ber-Poly(Lactic Acid) Eco-Composites. J. Polym. Environ. 2011, 19, 11–17. [Google Scholar] [CrossRef]
- Shanks, R.A.; Hodzic, A.; Ridderhof, D. Composites of poly(lactic acid) with flax fibers modified by interstitial polymeri-zation. J. Appl. Polym. Sci. 2006, 99, 2305–2313. [Google Scholar] [CrossRef]
- Foruzanmehr, M.; Vuillaume, P.; Elkoun, S.; Robert, M. Physical and mechanical properties of PLA composites reinforced by TiO2 grafted flax fibers. Mater. Des. 2016, 106, 295–304. [Google Scholar] [CrossRef]
- Ventura, H.; Claramunt, J.; Rodríguez-Pérez, M.; Ardanuy, M. Effects of hydrothermal aging on the water uptake and tensile properties of PHB/flax fabric biocomposites. Polym. Degrad. Stab. 2017, 142, 129–138. [Google Scholar] [CrossRef]
- Chilali, A.; Assarar, M.; Zouari, W.; Kebir, H.; Ayad, R. Mechanical characterization and damage events of flax fabric-reinforced biopolymer composites. Polym. Polym. Compos. 2019, 28, 631–644. [Google Scholar] [CrossRef]
- Rytlewski, P.; Stepczyńska, M.; Moraczewski, K.; Malinowski, R.; Karasiewicz, T.; Sikorska, W.; Żenkiewicz, M. Flax fibers reinforced polycaprolactone modified by triallyl isocyanurate and electron radiation. Polym. Compos. 2019, 40, 481–488. [Google Scholar] [CrossRef]
- Lee, S.G.; Choi, S.-S.; Park, W.H.; Cho, D. Characterization of surface modified flax fibers and their biocomposites with PHB. Macromol. Symp. 2003, 197, 089–100. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Samper, M.D.; Aldas, M.; López, J. On the Use of PLA-PHB Blends for Sustainable Food Packaging Applications. Materials 2017, 10, 1008. [Google Scholar] [CrossRef]
- Zhang, M.; Thomas, N.L. Blending polylactic acid with polyhydroxybutyrate: The effect on thermal, mechanical, and biodegradation properties. Adv. Polym. Technol. 2011, 30, 67–79. [Google Scholar] [CrossRef]
- Armentano, I.; Fortunati, E.; Burgos, N.; Dominici, F.; Luzi, F.; Fiori, S.; Jimenez, A.; Yoon, K.; Ahn, J.; Kang, S.; et al. Processing and characterization of plasticized PLA/PHB blends for biodegradable multiphase systems. Express Polym. Lett. 2015, 9, 583–596. [Google Scholar] [CrossRef]
- Armentano, I.; Fortunati, E.; Burgos, N.; Dominici, F.; Luzi, F.; Fiori, S.; Jiménez, A.; Yoon, K.; Ahn, J.; Kang, S.; et al. Bio-based PLA_PHB plasticized blend films: Processing and structural characterization. LWT 2015, 64, 980–988. [Google Scholar] [CrossRef] [Green Version]
- Abdelwahab, M.; Flynn, A.; Chiou, B.-S.; Imam, S.; Orts, W.; Chiellini, E. Thermal, mechanical and morphological characterization of plasticized PLA–PHB blends. Polym. Degrad. Stab. 2012, 97, 1822–1828. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Samper, M.D.; Lopez, J.; Jiménez, A. Combined Effect of Poly(hydroxybutyrate) and Plasticizers on Polylactic acid Properties for Film Intended for Food Packaging. J. Polym. Environ. 2014, 22, 460–470. [Google Scholar] [CrossRef]
- Rodrigues, J.A.F.R.; Parra, D.F.; Lugão, A.B. Crystallization on films of PHB/PEG blends. J. Therm. Anal. Calorim. 2005, 79, 379–381. [Google Scholar] [CrossRef]
- Ali, A.; Shaker, K.; Nawab, Y.; Jabbar, M.; Hussain, T.; Militky, J.; Baheti, V. Hydrophobic treatment of natural fibers and their composites—A review. J. Ind. Text. 2018, 47, 2153–2183. [Google Scholar] [CrossRef]
- Lalit, R.; Mayank, P.; Ankur, K. Natural Fibers and Biopolymers Characterization: A Future Potential Composite Material. Stroj. Cas. 2018, 68, 33–50. [Google Scholar] [CrossRef] [Green Version]
- Awasthi, S.; Agarwal, D. Influence of cycloaliphatic compounds on the properties of polyurethane coatings. J. Coat. Technol. Res. 2007, 4, 67–73. [Google Scholar] [CrossRef]
- Alagi, P.; Choi, Y.J.; Hong, S.C. Preparation of vegetable oil-based polyols with controlled hydroxyl functionalities for thermoplastic polyurethane. Eur. Polym. J. 2016, 78, 46–60. [Google Scholar] [CrossRef]
- Miao, S.; Wang, P.; Su, Z.; Zhang, S. Vegetable-oil-based polymers as future polymeric biomaterials. Acta Biomater. 2014, 10, 1692–1704. [Google Scholar] [CrossRef] [PubMed]
- Deka, H.; Karak, N. Bio-based hyperbranched polyurethanes for surface coating applications. Prog. Org. Coat. 2009, 66, 192–198. [Google Scholar] [CrossRef]
- Kong, X.; Liu, G.; Curtis, J.M. Novel polyurethane produced from canola oil based poly(ether ester) polyols: Synthesis, characterization and properties. Eur. Polym. J. 2012, 48, 2097–2106. [Google Scholar] [CrossRef]
- Tan, S.; Abraham, T.; Ference, D.; Macosko, C.W. Rigid polyurethane foams from a soybean oil-based Polyol. Polymer 2011, 52, 2840–2846. [Google Scholar] [CrossRef]
- Ismail, E.A.; Motawie, A.; Sadek, E.; Ismail, E.A.; Motawie, A.; Sadek, E. Synthesis and characterization of polyurethane coatings based on soybean oil–polyester polyols. Egypt. J. Pet. 2011, 20, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Datta, J.; Głowińska, E. Effect of hydroxylated soybean oil and bio-based propanediol on the structure and thermal properties of synthesized bio-polyurethanes. Ind. Crop. Prod. 2014, 61, 84–91. [Google Scholar] [CrossRef]
- Fu, C.; Zheng, Z.; Yang, Z.; Chen, Y.; Shen, L. A fully bio-based waterborne polyurethane dispersion from vegetable oils: From synthesis of precursors by thiol-ene reaction to study of final material. Prog. Org. Coat. 2014, 77, 53–60. [Google Scholar] [CrossRef]
- Thakur, S.; Karak, N. Castor oil-based hyperbranched polyurethanes as advanced surface coating materials. Prog. Org. Coat. 2013, 76, 157–164. [Google Scholar] [CrossRef]
- Gurunathan, T.; Mohanty, S.; Nayak, S.K. Isocyanate terminated castor oil-based polyurethane prepolymer: Synthesis and characterization. Prog. Org. Coat. 2015, 80, 39–48. [Google Scholar] [CrossRef]
- Mokhothu, T.H.; John, M.J. Bio-based coatings for reducing water sorption in natural fibre reinforced composites. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Van Krevelen, D.W.; Te Nijenhuis, K. Properties of Polymers: Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction from Additive Group Contributions; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Sanchez, P.; A Perez-Maqueda, L.; Perejon, A.; Criado, J.M. A new model for the kinetic analysis of thermal degradation of polymers driven by random scission. Polym. Degrad. Stab. 2010, 95, 733–739. [Google Scholar] [CrossRef]
- Ghosh, P. Polymer Science and Technology; Tata McGraw-Hill Education: New York, NY, USA, 1990. [Google Scholar]
- Tokiwa, Y.; Calabia, B.P.; Ugwu, C.U.; Aiba, S. Biodegradability of Plastics. Int. J. Mol. Sci. 2009, 10, 3722–3742. [Google Scholar] [CrossRef]
- Bernard, M. Industrial Potential of Polyhydroxyalkanoate Bioplastic: A Brief Review. USURJ: Univ. Sask. Undergrad. Res. J. 2014, 1. [Google Scholar] [CrossRef]
- Muhammadi, S.; Afzal, M.; Hameed, S. Bacterial polyhydroxyalkanoates-eco-friendly next generation plastic: Production, biocompatibility, biodegradation, physical properties and applications. Green Chem. Lett. Rev. 2015, 8, 56–77. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, T.; Shahid, M.; Azeem, F.; Rasul, I.; Shah, A.A.; Noman, M.; Hameed, A.; Manzoor, N.; Manzoor, I.; Muhammad, S. Biodegradation of plastics: Current scenario and future prospects for environmental safety. Environ. Sci. Pollut. Res. 2018, 25, 7287–7298. [Google Scholar] [CrossRef] [PubMed]
- Knoll, M.; Hamm, T.M.; Wagner, F.; Martinez, V.; Pleiss, J. The PHA Depolymerase Engineering Database: A systematic analysis tool for the diverse family of polyhydroxyalkanoate (PHA) depolymerases. BMC Bioinform. 2009, 10, 89. [Google Scholar] [CrossRef] [Green Version]
- Mergaert, J.; Webb, A.; Anderson, C.; Wouters, A.; Swings, J. Microbial degradation of poly(3-hydroxybutyrate) and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) in soils. Appl. Environ. Microbiol. 1993, 59, 3233–3238. [Google Scholar] [CrossRef] [Green Version]
- Jendrossek, D.; Handrick, R. Microbial Degradation of Polyhydroxyalkanoates. Annu. Rev. Microbiol. 2002, 56, 403–432. [Google Scholar] [CrossRef]
- Manna, A.; Paul, A. Degradation of microbial polyester poly(3-hydroxybutyrate) in environmental samples and in culture. Biodegradation 2000, 11, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Kusaka, S.; Iwata, T.; Doi, Y. Properties and biodegradability of ultra-high-molecular-weight poly[(R)-3-hydroxybutyrate] produced by a recombinant Escherichia coli. Int. J. Biol. Macromol. 1999, 25, 87–94. [Google Scholar] [CrossRef]
- Boyandin, A.N.; Prudnikova, S.; Filipenko, M.L.; Khrapov, E.A.; Vasil’Ev, A.D.; Volova, T. Biodegradation of polyhydroxyalkanoates by soil microbial communities of different structures and detection of PHA degrading microorganisms. Appl. Biochem. Microbiol. 2011, 48, 28–36. [Google Scholar] [CrossRef]
- Murphy, J. Chapter 4—Modifying Specific Properties: Mechanical Properties—Fillers. In Additives for Plastics Handbook, 2nd ed.; Murphy, J., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2001; Volume 1, pp. 19–35. [Google Scholar]
- Aframehr, W.M.; Molki, B.; Heidarian, P.; Behzad, T.; Sadeghi, M.; Bagheri, R. Effect of calcium carbonate nanoparticles on barrier properties and biodegradability of polylactic acid. Fibers Polym. 2017, 18, 2041–2048. [Google Scholar] [CrossRef]
- Teramoto, N.; Urata, K.; Ozawa, K.; Shibata, M. Biodegradation of aliphatic polyester composites reinforced by abaca fiber. Polym. Degrad. Stab. 2004, 86, 401–409. [Google Scholar] [CrossRef]
- Altaee, N.; El-Hiti, G.A.; Fahdil, A.; Sudesh, K.; Yousif, E. Biodegradation of different formulations of polyhydroxybutyrate films in soil. SpringerPlus 2016, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Paul, M.-A.; Delcourt, C.; Alexandre, M.; Degée, P.; Monteverde, F.; Dubois, P. Polylactide/montmorillonite nanocomposites: Study of the hydrolytic degradation. Polym. Degrad. Stab. 2005, 87, 535–542. [Google Scholar] [CrossRef]
- Chen, Y.; Murphy, A.; Scholz, D.; Geever, L.M.; Lyons, J.G.; Devine, D.M. Surface-modified halloysite nanotubes reinforced poly(lactic acid) for use in biodegradable coronary stents. J. Appl. Polym. Sci. 2018, 135, 46521. [Google Scholar] [CrossRef]
- Navarro, M.; Ginebra, M.; Planell, J.; Barrias, C.; Barbosa, M. In vitro degradation behavior of a novel bioresorbable composite material based on PLA and a soluble CaP glass. Acta Biomater. 2005, 1, 411–419. [Google Scholar] [CrossRef]
- Huang, J.; Xiong, J.; Liu, J.; Zhu, W.; Wang, D. Investigation of the in vitro degradation of a novel polylactide/nanohydroxyapatite composite for artificial bone. J. Nanomater. 2013, 2013, 3. [Google Scholar] [CrossRef] [Green Version]
- Valapa, R.B.; Pugazhenthi, G.; Katiyar, V. Hydrolytic degradation behaviour of sucrose palmitate reinforced poly(lactic acid) nanocomposites. Int. J. Biol. Macromol. 2016, 89, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Jamarani, R.; Erythropel, H.C.; Nicell, J.A.; Leask, R.L.; Marić, M. How Green is Your Plasticizer? Polymers 2018, 10, 834. [Google Scholar] [CrossRef] [Green Version]
- Boll, M.; Geiger, R.; Junghare, M.; Schink, B. Microbial degradation of phthalates: Biochemistry and environmental impli-cations. Environ. Microbiol. Rep. 2020, 12, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Labrecque, L.V.; Kumar, R.A.; Gross, R.A.; McCarthy, S.P. Citrate esters as plasticizers for poly(lactic acid). J. Appl. Polym. Sci. 1997, 66, 1507–1513. [Google Scholar] [CrossRef]
- Ozkoc, G.; Kemaloglu, S. Morphology, biodegradability, mechanical, and thermal properties of nanocomposite films based on PLA and plasticized PLA. J. Appl. Polym. Sci. 2009, 114, 2481–2487. [Google Scholar] [CrossRef]



| 3-Hidroxyacids | Structure |
|---|---|
| butyric (3HB) | 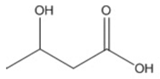 |
| hexanoic (3HHx) |  |
| octanoic (3HO) | 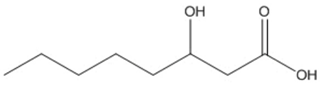 |
| decanoic (3HD) | 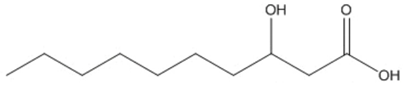 |
| dodecanoic (3HDD) |  |
| Polymer | Structure |
|---|---|
| poly(6-hydroxyhexanoate) |  |
| poly(3-hydroxyoctanoate) | 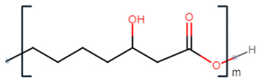 |
| monomethoxy-terminated poly(ethylene glycol) (mPEG) | 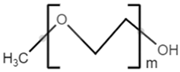 |
| poly(ethylene glycol) |  |
| Process | Natural Fibre Used | Biopolymer Matrix | Outcomes | Ref. | ||
|---|---|---|---|---|---|---|
| Interfacial Adhesion | Mechanical Properties | Barrier Properties | ||||
| Treatment with compatibilisers; Polyglycerol polyglycidyl ether (SR-4GL), Trimethylol propane polyglycidyl ether (SR-TMP), and (Polyglycerol polypropyleneoxide (SC-P1000) | Cellulose fibres | PLA | Improved interfacial adhesion between fibres and PLA and | Inhibited degradation of the PLA matrix | [65] | |
| STEFAC TM 8170, surfactant modification | Cellulose fibres | PLA/PHB | Enhanced mechanical performance | Improved water resistance, reduced oxygen and UV-light transmission, as well as appropriate disintegration in compost | [94] | |
| Alkali treatment | Kenaf fibre | PHB | Reduction in the crystallinity of PHB (up to 6% reduction), making it more ductile, and improvement of the flexural modulus by up to 11%. | [75] | ||
| Silane treatment | Flax fibre | PLA | Improvement to fibre/matrix adhesion with 2% w/w silane content, yet further improvement of the fibre-matrix interface can be partially resolved by silane/alkali treatment combination. | Improved mechanical properties | [80] | |
| Alkali treatment | Flax fibres | PLA | Tg values of fabricated bio-composites were lowered by 10 °C for 10% NaOH treatment and 15 °C for 30% NaOH treatment | [82] | ||
| Treatment with ethylene plasma | Flax fibres | PHB | Improved interfacial adhesion strength in the bio-composite | Improved thermal resistance | [88] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attallah, O.A.; Mojicevic, M.; Garcia, E.L.; Azeem, M.; Chen, Y.; Asmawi, S.; Brenan Fournet, M. Macro and Micro Routes to High Performance Bioplastics: Bioplastic Biodegradability and Mechanical and Barrier Properties. Polymers 2021, 13, 2155. https://doi.org/10.3390/polym13132155
Attallah OA, Mojicevic M, Garcia EL, Azeem M, Chen Y, Asmawi S, Brenan Fournet M. Macro and Micro Routes to High Performance Bioplastics: Bioplastic Biodegradability and Mechanical and Barrier Properties. Polymers. 2021; 13(13):2155. https://doi.org/10.3390/polym13132155
Chicago/Turabian StyleAttallah, Olivia A., Marija Mojicevic, Eduardo Lanzagorta Garcia, Muhammad Azeem, Yuanyuan Chen, Shumayl Asmawi, and Margaret Brenan Fournet. 2021. "Macro and Micro Routes to High Performance Bioplastics: Bioplastic Biodegradability and Mechanical and Barrier Properties" Polymers 13, no. 13: 2155. https://doi.org/10.3390/polym13132155
APA StyleAttallah, O. A., Mojicevic, M., Garcia, E. L., Azeem, M., Chen, Y., Asmawi, S., & Brenan Fournet, M. (2021). Macro and Micro Routes to High Performance Bioplastics: Bioplastic Biodegradability and Mechanical and Barrier Properties. Polymers, 13(13), 2155. https://doi.org/10.3390/polym13132155











