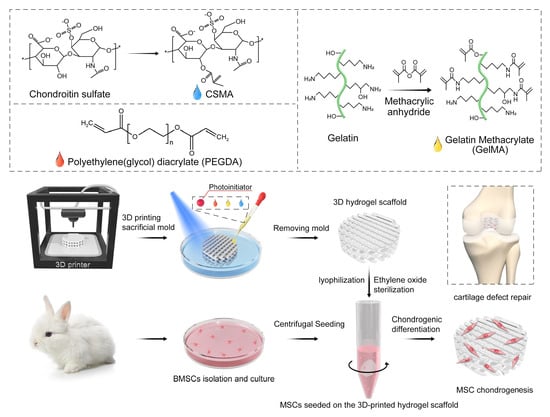
Fabrication of 3D-Printed Interpenetrating Hydrogel Scaffolds for Promoting Chondrogenic Differentiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Designing of Mold
2.3. Synthesis of Photo-Polymerizable Mixture Gel Precursor
2.4. Preparation of Photocurable IPN Hydrogel Porous Scaffolds Using 3D Composite-Printing and Lyophilization
2.5. Characterization of Macromers and 3D-Printed Hydrogel Scaffolds
2.6. In Vitro Degradation
2.7. Isolation and Culturing of BMSCs
2.8. Cell Seeding on 3D Hydrogel Scaffolds
2.9. Cytocompatibility
2.10. Live/Dead Staining
2.11. Cytoskeleton Staining
2.12. RT-PCR Test
2.13. Immunofluorescence Staining
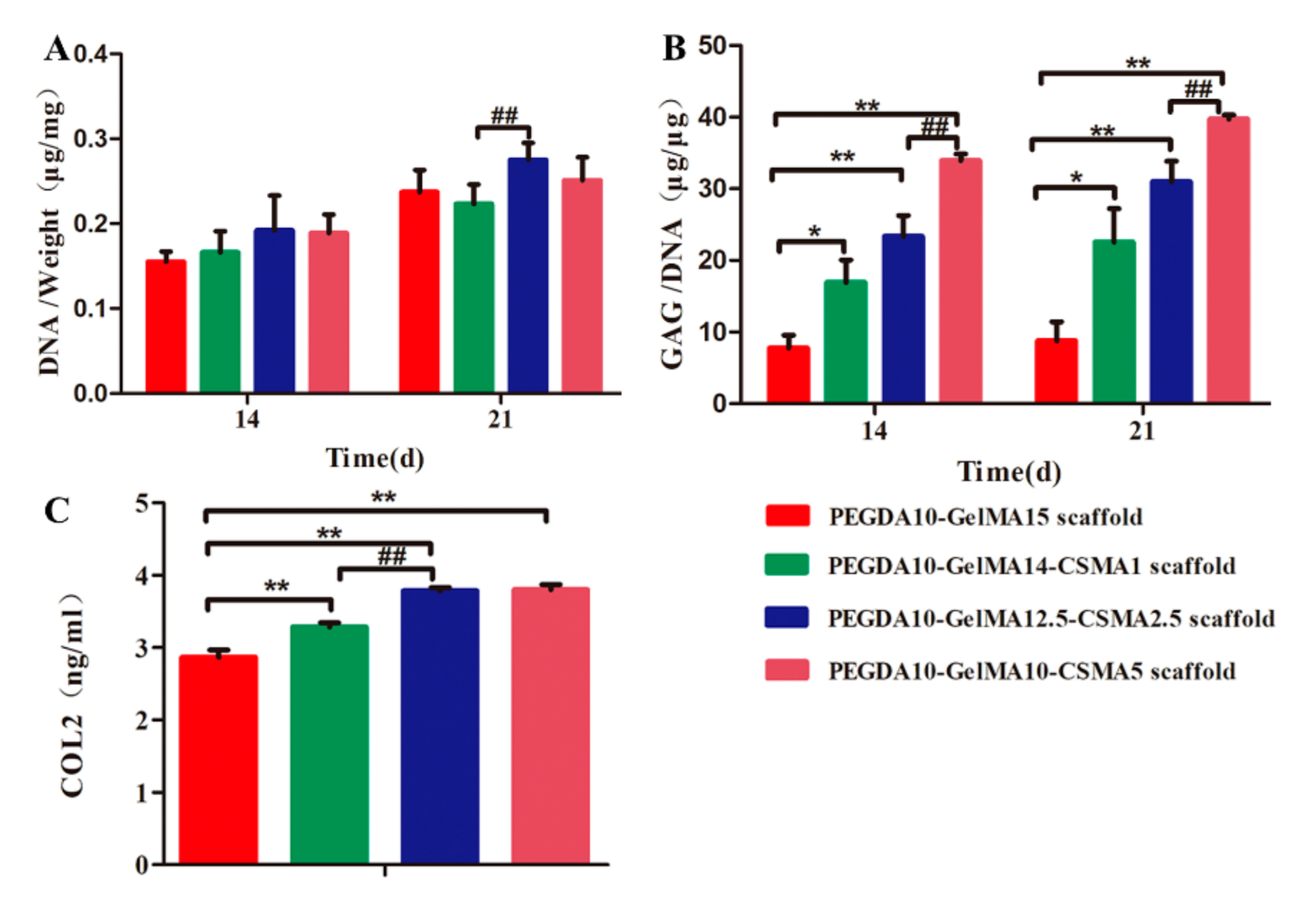
2.14. Quantification of DNA, GAG, and COL2 Content
2.15. Statistical Analysis
3. Results
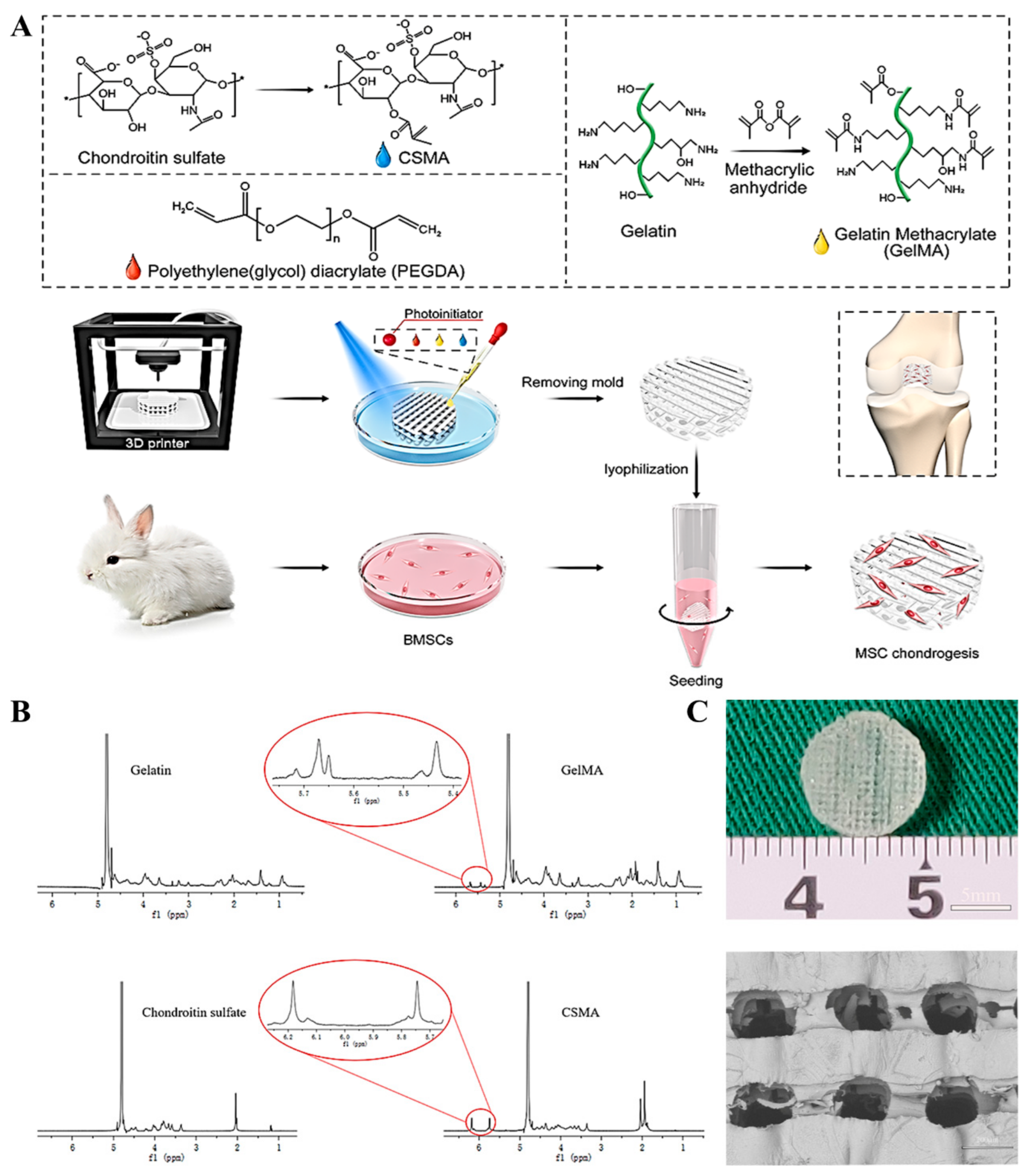
3.1. Preparation and Characterization of GelMA and CSMA
3.2. Mechanical Properties and Biodegradation of 3D-Printed Hydrogel Scaffolds
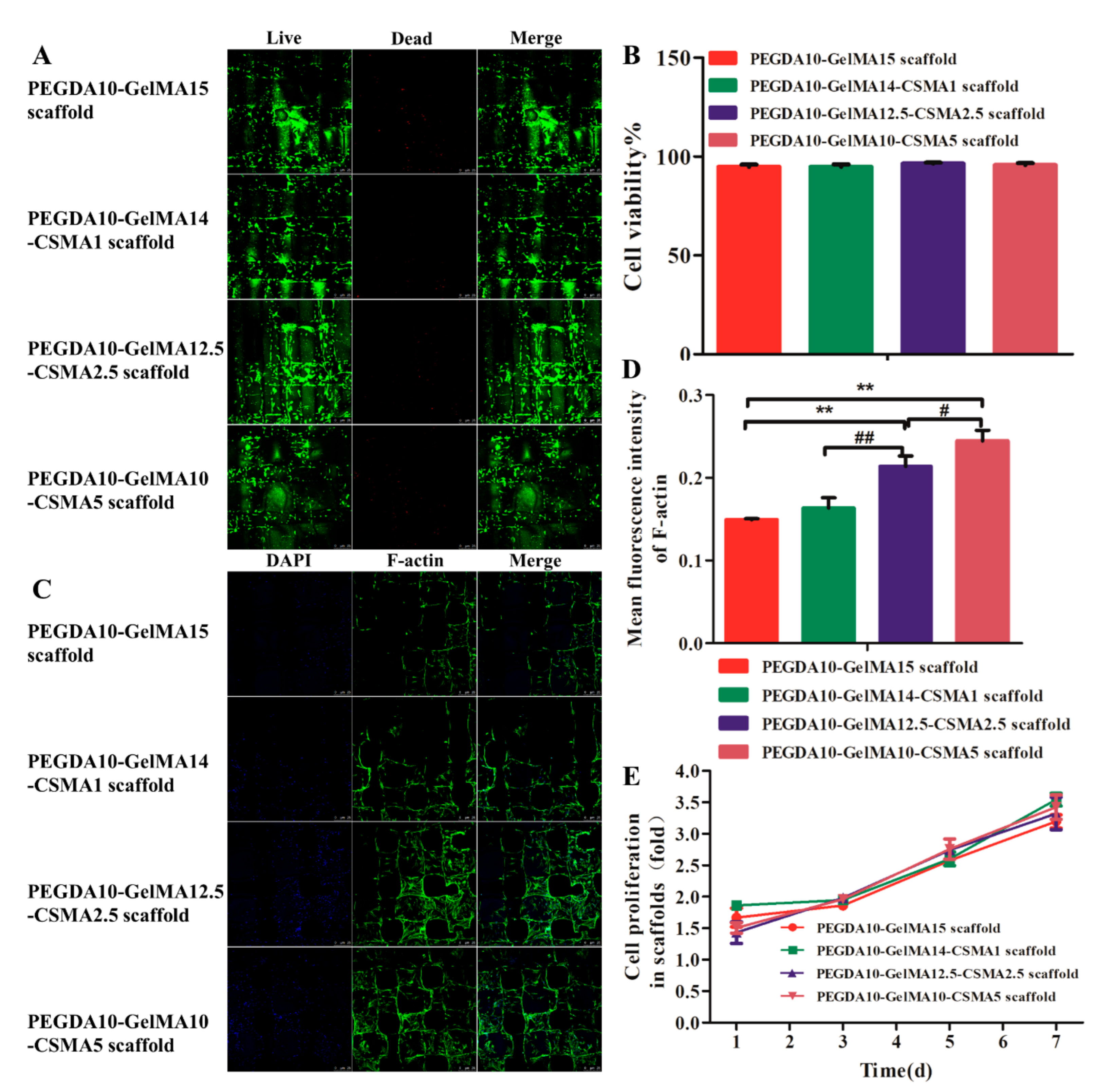
3.3. Cytocompatibility of 3D-Printed Scaffolds
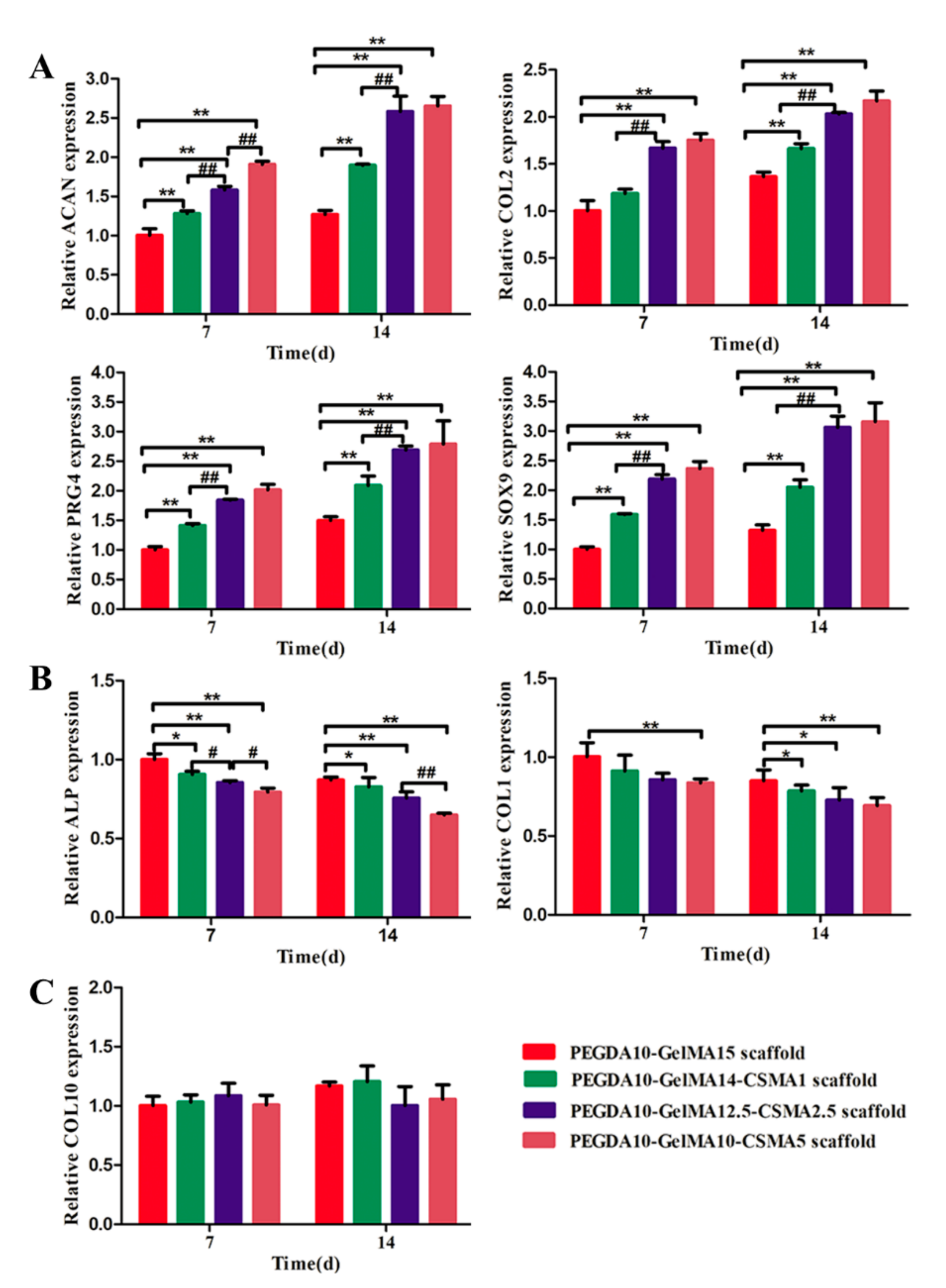
3.4. RT-PCR Analysis
3.5. COL 2, ACAN, and SOX 9 Production Analysis by Immunofluorescence
3.6. ECM Deposition on Scaffolds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huey, D.J.; Hu, J.; Athanasiou, K.A. Unlike Bone, Cartilage Regeneration Remains Elusive. Science 2012, 338, 917–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.-R.; Zhou, Z.-X.; Zhang, J.-Y.; Yuan, F.-Z.; Xu, B.-B.; Guan, J.; Han, C.; Jiang, D.; Yang, Y.; Yu, J.-K. Low-Molecular-Weight Heparin-Functionalized Chitosan-Chondroitin Sulfate Hydrogels for Controlled Release of TGF-β3 and in vitro Neocartilage Formation. Front. Chem. 2019, 7, 745. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.-J.; Zhang, Z.-Z.; Jiang, D.; Qi, Y.-S.; Wang, H.-J.; Zhang, J.-Y.; Ding, J.-X.; Yu, J.-K. Thermogel-coated Poly(epsilon-caprolactone) composite scaffold for enhanced cartilage tissue engineering. Polymers 2016, 8, 200. [Google Scholar] [CrossRef] [Green Version]
- Izadifar, Z.; Chen, X.; Kulyk, W. Strategic Design and Fabrication of Engineered Scaffolds for Articular Cartilage Repair. J. Funct. Biomater. 2012, 3, 799–838. [Google Scholar] [CrossRef] [Green Version]
- Armiento, A.; Stoddart, M.; Alini, M.; Eglin, D. Biomaterials for articular cartilage tissue engineering: Learning from biology. Acta Biomater. 2018, 65, 1–20. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.S.; Yue, K.; Khademhosseini, A. Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater. 2017, 57, 1–25. [Google Scholar] [CrossRef]
- Naahidi, S.; Jafari, M.; Logan, M.; Wang, Y.; Yuan, Y.; Bae, H.; Dixon, B.; Chen, P. Biocompatibility of hydrogel-based scaffolds for tissue engineering applications. Biotechnol. Adv. 2017, 35, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Talaat, W.; Aryal, A.C.S.; Al Kawas, S.; Samsudin, A.B.R.; Kandile, N.G.; Harding, D.R.K.; Ghoneim, M.M.; Zeiada, W.; Jagal, J.; Aboelnaga, A.; et al. Nanoscale Thermosensitive Hydrogel Scaffolds Promote the Chondrogenic Differentiation of Dental Pulp Stem and Progenitor Cells: A Minimally Invasive Approach for Cartilage Regeneration. Int. J. Nanomed. 2020, 15, 7775–7789. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Li, R.; Jiang, W.; Sun, Y.; Li, H. Comparison of three-dimensional printing and vacuum freeze-dried techniques for fabricating composite scaffolds. Biochem. Biophys. Res. Commun. 2016, 477, 1085–1091. [Google Scholar] [CrossRef]
- Shi, W.; Sun, M.; Hu, X.; Ren, B.; Cheng, J.; Li, C.; Duan, X.; Fu, X.; Zhang, J.; Chen, H.; et al. Structurally and Functionally Optimized Silk-Fibroin-Gelatin Scaffold Using 3D Printing to Repair Cartilage Injury In Vitro and In Vivo. Adv. Mater. 2017, 29, 1701089. [Google Scholar] [CrossRef]
- Daly, A.; Freeman, F.; Gonzalez-Fernandez, T.; Critchley, S.E.; Nulty, J.; Kelly, D.J. 3D Bioprinting for Cartilage and Osteochondral Tissue Engineering. Adv. Healthc. Mater. 2017, 6, 1700298. [Google Scholar] [CrossRef]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011, 1–19. [Google Scholar] [CrossRef]
- Gao, J.; Ding, X.; Yu, X.; Chen, X.; Zhang, X.; Cui, S.; Shi, J.; Chen, J.; Yu, L.; Chen, S.; et al. Cell-Free Bilayered Porous Scaffolds for Osteochondral Regeneration Fabricated by Continuous 3D-Printing Using Nascent Physical Hydrogel as Ink. Adv. Healthc. Mater. 2021, 10, e2001404. [Google Scholar] [CrossRef]
- Abbadessa, A.; Blokzijl, M.; Mouser, V.; Marica, P.; Malda, J.; Hennink, W.; Vermonden, T. A thermo-responsive and photo-polymerizable chondroitin sulfate-based hydrogel for 3D printing applications. Carbohydr. Polym. 2016, 149, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Strehin, I.; Nahas, Z.; Arora, K.; Nguyen, T.; Elisseeff, J. A versatile pH sensitive chondroitin sulfate–PEG tissue adhesive and hydrogel. Biomaterials 2010, 31, 2788–2797. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Sycks, D.; Chan, H.F.; Lin, S.; Lopez, G.P.; Guilak, F.; Leong, K.W.; Zhao, X. 3D Printing of Highly Stretchable and Tough Hydrogels into Complex, Cellularized Structures. Adv. Mater. 2015, 27, 4035–4040. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Sun, X.; Wang, Z.; Guo, S.; Yu, G.; Yang, H. Synthesis and Properties of Gelatin Methacryloyl (GelMA) Hydrogels and Their Recent Applications in Load-Bearing Tissue. Polymers 2018, 10, 1290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Li, L.; Suo, H.; Yan, M.; Yin, J.; Fu, J. 3D printing of biomimetic multi-layered GelMA/nHA scaffold for osteochondral defect repair. Mater. Des. 2019, 171, 107708. [Google Scholar] [CrossRef]
- Guo, Y.; Yuan, T.; Xiao, Z.; Tang, P.; Xiao, Y.; Fan, Y.; Zhang, X. Hydrogels of collagen/chondroitin sulfate/hyaluronan interpenetrating polymer network for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 2012, 23, 2267–2279. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, Y.; Ferracci, G.; Zheng, J.; Cho, N.-J.; Lee, B.H. Gelatin methacryloyl and its hydrogels with an exceptional degree of controllability and batch-to-batch consistency. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.F.; Shen, S.-S.; Lu, S.-C. Synthesis and characterization of chondroitin sulfate–methacrylate hydrogels. Carbohydr. Polym. 2003, 52, 389–396. [Google Scholar] [CrossRef]
- Zhang, Z.-Z.; Jiang, D.; Wang, S.-J.; Qi, Y.-S.; Zhang, J.-Y.; Yu, J.-K. Potential of Centrifugal Seeding Method in Improving Cells Distribution and Proliferation on Demineralized Cancellous Bone Scaffolds for Tissue-Engineered Meniscus. ACS Appl. Mater. Interfaces 2015, 7, 15294–15302. [Google Scholar] [CrossRef] [PubMed]
- Makris, E.A.; Gomoll, A.H.; Malizos, K.N.; Hu, J.C.; Athanasiou, K.A. Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol. 2015, 11, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Duan, P.; Gao, J.; Qu, Z.; Li, X.; He, Y.; Yao, H.; Ding, J. Bilayered PLGA/PLGA-HAp composite scaffold for osteochondral tissue engineering and tissue regeneration. ACS Biomater. Sci. Eng. 2018, 4, 3506–3521. [Google Scholar] [CrossRef]
- Li, L.; Li, J.; Guo, J.; Zhang, H.; Zhang, X.; Yin, C.; Wang, L.; Zhu, Y.; Yao, Q. 3D Molecularly Functionalized Cell-Free Biomimetic Scaffolds for Osteochondral Regeneration. Adv. Funct. Mater. 2019, 29, 1807356. [Google Scholar] [CrossRef]
- Zhou, Z.; Wu, W.; Fang, J.; Yin, J. Polymer-based porous microcarriers as cell delivery systems for applications in bone and cartilage tissue engineering. Int. Mater. Rev. 2021, 66, 77–113. [Google Scholar] [CrossRef]
- Markstedt, K.; Mantas, A.; Tournier, I.; Ávila, H.M.; Hägg, D.; Gatenholm, P. 3D Bioprinting Human Chondrocytes with Nanocellulose–Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef]
- Weigelt, B.; Bissell, M.J. Unraveling the microenvironmental influences on the normal mammary gland and breast cancer. Semin. Cancer Biol. 2008, 18, 311–321. [Google Scholar] [CrossRef] [Green Version]
- Cui, H.; Nowicki, M.; Fisher, J.P.; Zhang, L.G. 3D Bioprinting for Organ Regeneration. Adv. Healthc. Mater. 2017, 6, 27995751. [Google Scholar] [CrossRef] [Green Version]
- Stanton, M.; Samitier, J.; Sánchez, S. Bioprinting of 3D hydrogels. Lab A Chip 2015, 15, 3111–3115. [Google Scholar] [CrossRef] [Green Version]
- Spiller, K.L.; Maher, S.A.; Lowman, A.M. Hydrogels for the repair of articular cartilage defects. Tissue Eng. Part. B Rev. 2011, 17, 281–299. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Cui, H.; Boualam, B.; Masood, F.; Flynn, E.; Rao, R.D.; Zhang, Z.-Y.; Zhang, L.G. 3D bioprinting mesenchymal stem cell-laden construct with core–shell nanospheres for cartilage tissue engineering. Nanotechnology 2018, 29, 185101. [Google Scholar] [CrossRef]
- Liang, X.; Gao, J.; Xu, W.; Wang, X.; Shen, Y.; Tang, J.; Cui, S.; Yang, X.; Liu, Q.; Yu, L.; et al. Structural mechanics of 3D-printed poly(lactic acid) scaffolds with tetragonal, hexagonal and wheel-like designs. Biofabrication 2019, 11, 0350090. [Google Scholar] [CrossRef] [PubMed]
- Gupte, M.J.; Swanson, W.B.; Hu, J.; Jin, X.; Ma, H.; Zhang, Z.; Liu, Z.; Feng, K.; Feng, G.; Xiao, G.; et al. Pore size directs bone marrow stromal cell fate and tissue regeneration in nanofibrous macroporous scaffolds by mediating vascularization. Acta Biomater. 2018, 82, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Pan, Z.; Cao, L.; He, Y.; Wang, H.; Qu, Z.; Dong, J.; Ding, J. The effects of pore size in bilayered poly(lactide-co-glycolide) scaffolds on restoring osteochondral defects in rabbits. J. Biomed. Mater. Res. Part. A 2014, 102, 180–192. [Google Scholar] [CrossRef]
- Pan, Z.; Duan, P.; Liu, X.; Wang, H.; Cao, L.; He, Y.; Dong, J.; Ding, J. Effect of porosities of bilayered porous scaffolds on spontaneous osteochondral repair in cartilage tissue engineering. Regen. Biomater. 2015, 2, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Zustiak, S.P.; Leach, J.B. Hydrolytically Degradable Poly(Ethylene Glycol) Hydrogel Scaffolds with Tunable Degradation and Mechanical Properties. Biomacromolecules 2010, 11, 1348–1357. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-C.; Lin, R.-Z.; Qi, H.; Yang, Y.; Bae, H.; Melero-Martin, J.M.; Khademhosseini, A. Functional human vascular network generated in photocrosslinkable gelatin methacrylate hydrogels. Adv. Funct. Mater. 2012, 22, 2027–2039. [Google Scholar] [CrossRef] [Green Version]
- Ovsianikov, A.; Deiwick, A.; Van Vlierberghe, S.; Dubruel, P.; Moöller, L.; Draäger, G.; Chichkov, B. Laser Fabrication of Three-Dimensional CAD Scaffolds from Photosensitive Gelatin for Applications in Tissue Engineering. Biomacromolecules 2011, 12, 851–858. [Google Scholar] [CrossRef]
- Gao, F.; Xu, Z.; Liang, Q.; Li, H.; Peng, L.; Wu, M.; Zhao, X.; Cui, X.; Ruan, C.; Liu, W. Osteochondral regeneration with 3D-printed biodegradable high-strength supramolecular polymer reinforced-gelatin hydrogel scaffolds. Adv. Sci. 2019, 6, 1900867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koshy, S.T.; Ferrante, T.C.; Lewin, S.A.; Mooney, D.J. Injectable, porous, and cell-responsive gelatin cryogels. Biomaterials 2014, 35, 2477–2487. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Huang, S.; Liu, Y.; Yao, B.; Hu, T.; Shi, H.; Xie, J.; Fu, X. Tuning Alginate-Gelatin Bioink Properties by Varying Solvent and Their Impact on Stem Cell Behavior. Sci. Rep. 2018, 8, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, H.; Zhao, D.; Zhu, H.; Hua, Y.; Xiao, K.; Xu, Y.; Liu, Y.; Chen, W.; Liu, Y.; Zhang, W.; et al. Lyophilized Scaffolds Fabricated from 3D-Printed Photocurable Natural Hydrogel for Cartilage Regeneration. ACS Appl. Mater. Interfaces 2018, 10, 31704–31715. [Google Scholar] [CrossRef] [PubMed]
- Camci-Unal, G.; Cuttica, D.; Annabi, N.; Demarchi, D.; Khademhosseini, A. Synthesis and Characterization of Hybrid Hyaluronic Acid-Gelatin Hydrogels. Biomacromolecules 2013, 14, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Levett, P.A.; Melchels, F.P.; Schrobback, K.; Hutmacher, D.W.; Malda, J.; Klein, T.J. A biomimetic extracellular matrix for cartilage tissue engineering centered on photocurable gelatin, hyaluronic acid and chondroitin sulfate. Acta Biomater. 2014, 10, 214–223. [Google Scholar] [CrossRef] [Green Version]
- Lalitha, S.S.; Schneider, M.C.; Chu, S.; Roucy, G.; Bryant, S.J.; Vernerey, F.J. Heterogeneity is key to hydrogel-based cartilage tissue regeneration. Soft Matter 2017, 13, 4841–4855. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, Q.-J.; He, T.; Ye, K.; Yao, X.; Ding, J. Degradation rate affords a dynamic cue to regulate stem cells beyond varied matrix stiffness. Biomaterials 2018, 178, 467–480. [Google Scholar] [CrossRef]
- Bianco, P.; Robey, P.G. Stem cells in tissue engineering. Nature 2001, 414, 118–121. [Google Scholar] [CrossRef]
- Karp, J.M.; Leng, T.G. Mesenchymal stem cell homing: The devil is in the details. Cell Stem Cell 2009, 4, 206–216. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.H.; Cook, J.L.; Mendelson, A.; Moioli, E.K.; Yao, H.; Mao, J. Regeneration of the articular surface of the rabbit synovial joint by cell homing: A proof of concept study. Lancet 2010, 376, 440–448. [Google Scholar] [CrossRef] [Green Version]
- Brittberg, M. Cell carriers as the next generation of cell therapy for cartilage repair: A review of the matrix-induced au-tologous chondrocyte implantation procedure. Am. J. Sports Med. 2010, 38, 1259–1271. [Google Scholar] [CrossRef] [PubMed]
- Korhonen, R.; Laasanen, M.; Töyräs, J.; Rieppo, J.; Hirvonen, J.; Helminen, H.; Jurvelin, J. Comparison of the equilibrium response of articular cartilage in unconfined compression, confined compression and indentation. J. Biomech. 2002, 35, 903–909. [Google Scholar] [CrossRef]
- Watanabe, H.; Yamada, Y.; Kimata, K. Roles of Aggrecan, a Large Chondroitin Sulfate Proteoglycan, in Cartilage Structure and Function. J. Biochem. 1998, 124, 687–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Gene | Forward Primers (5′-3′) | Reverse Primers (5′-3′) |
|---|---|---|
| COL1 | ACCCCAGAAACAGACGACAAACAAC | ATGAATGCAACGGCAAAAACAAATC |
| COL2 | GCAGCTGTGTGCAGGAGGGGAAG | TGGCAGTGGCGAGGTCAGTAGGG |
| ACAN | GACTCATTGTTAGAGGACAGCCA | CACTCCCAAAAAGAACTCCAGAT |
| PRG4 | GGCAGGGAATGTGACTGTGATG | TGGGTGAGCGTTTAGTTGTTGA |
| SOX9 | CGGCGGAGGAAGTCGGTGAAGA | AGTGGTGGGTGGGGTGGTGGTG |
| ALP | CCGCAAGTATATGTATCCCAAA | CCCAAGAGGTAGTCCACAGTGT |
| GAPDH | CATCAAGAAGGTGGTGAAGCAGG | AGCATCGAAGGTAGAGGAGTGGG |
| Composition (wt. %) | Notation |
|---|---|
| 10% PEGDA, 15% GelMA | PEGDA10-GelMA15 |
| 10% PEGDA, 14% GelMA, 1% CSMA | PEGDA10-GelMA14-CSMA1 |
| 10% PEGDA, 12.5% GelMA, 2.5% CSMA | PEGDA10-GelMA12.5-CSMA2.5 |
| 10% PEGDA, 10% GelMA, 5% CSMA | PEGDA10-GelMA10-CSMA5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, J.; Yuan, F.-z.; Mao, Z.-m.; Zhu, H.-l.; Lin, L.; Chen, H.H.; Yu, J.-k. Fabrication of 3D-Printed Interpenetrating Hydrogel Scaffolds for Promoting Chondrogenic Differentiation. Polymers 2021, 13, 2146. https://doi.org/10.3390/polym13132146
Guan J, Yuan F-z, Mao Z-m, Zhu H-l, Lin L, Chen HH, Yu J-k. Fabrication of 3D-Printed Interpenetrating Hydrogel Scaffolds for Promoting Chondrogenic Differentiation. Polymers. 2021; 13(13):2146. https://doi.org/10.3390/polym13132146
Chicago/Turabian StyleGuan, Jian, Fu-zhen Yuan, Zi-mu Mao, Hai-lin Zhu, Lin Lin, Harry Huimin Chen, and Jia-kuo Yu. 2021. "Fabrication of 3D-Printed Interpenetrating Hydrogel Scaffolds for Promoting Chondrogenic Differentiation" Polymers 13, no. 13: 2146. https://doi.org/10.3390/polym13132146
APA StyleGuan, J., Yuan, F.-z., Mao, Z.-m., Zhu, H.-l., Lin, L., Chen, H. H., & Yu, J.-k. (2021). Fabrication of 3D-Printed Interpenetrating Hydrogel Scaffolds for Promoting Chondrogenic Differentiation. Polymers, 13(13), 2146. https://doi.org/10.3390/polym13132146