Gelation and Crystallization Phenomena in Polyethylene Plastomers Modified with Waxes
Abstract
1. Introduction
2. Materials and Methods
- -
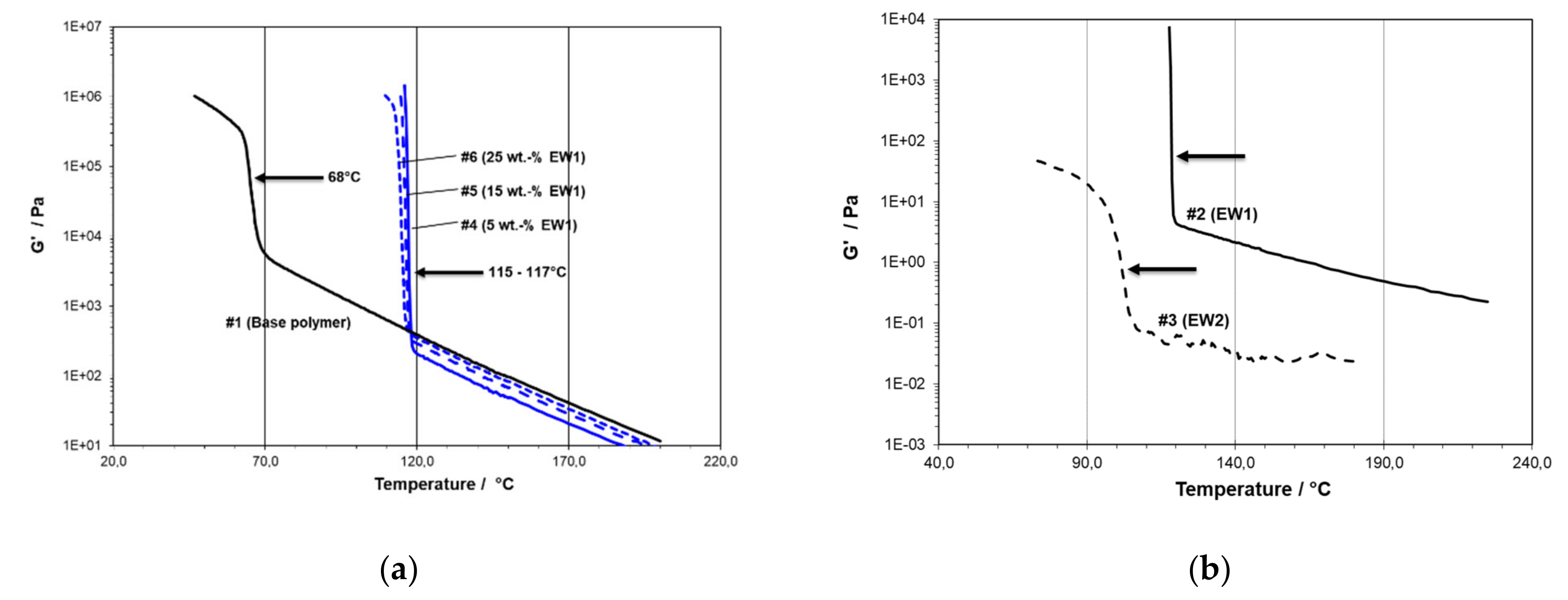
- EW1 is the commercial product Licowax PE190 from Clariant, Germany, an HDPE wax with a melt viscosity of 25 Pa s at 140 °C and a melting point TM of 126 °C.
- -
- EW2 is the commercial product Licolub H4 from Clariant, Germany, a paraffin wax with a melt viscosity of 8.10–3 Pa.s at 120 °C and a softening temperature (TSOFT) of 111 °C.
- -
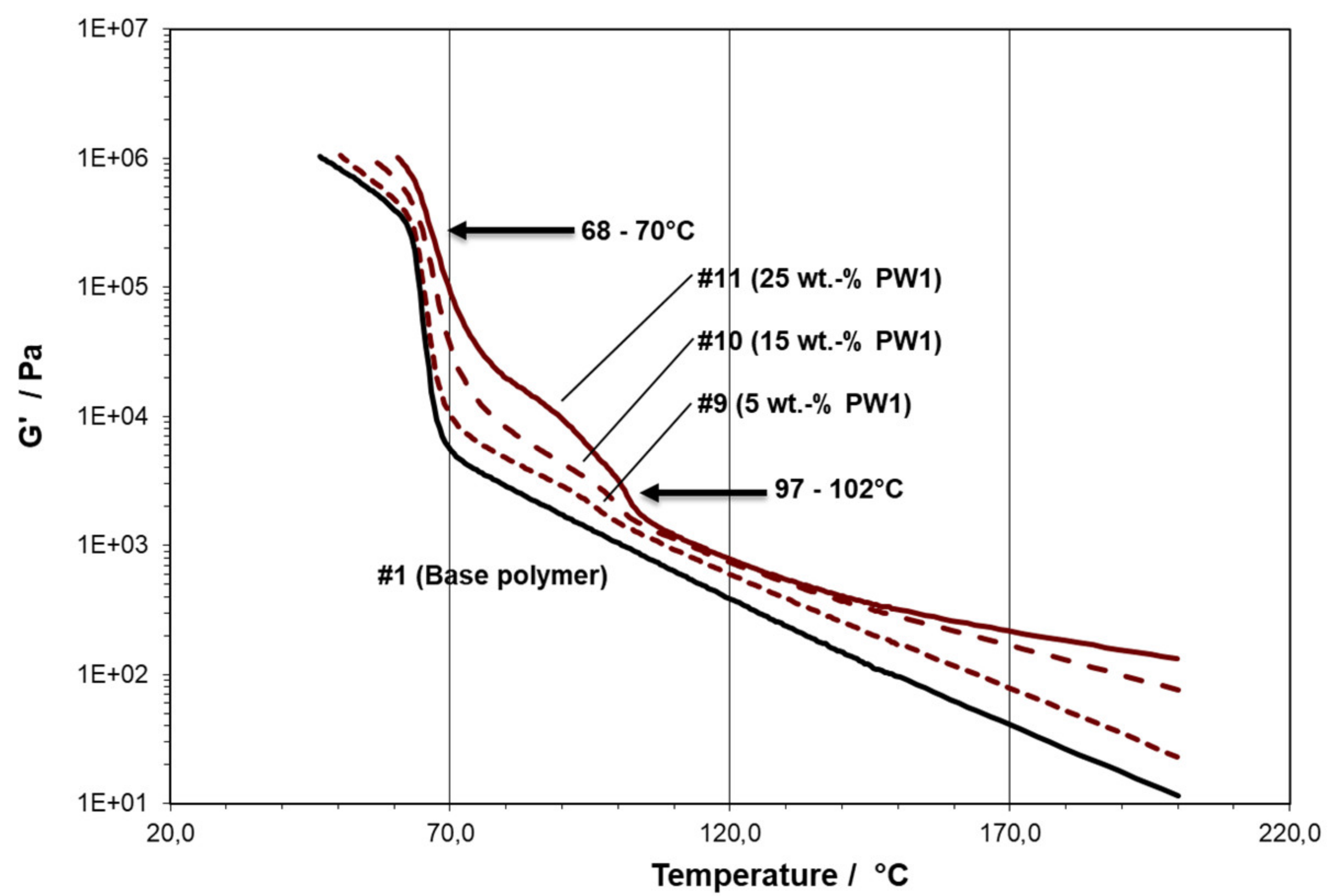
- PW1 is an ethylene-propylene random copolymer (RACO) wax, produced by peroxide-mediated visbreaking [37] from the commercial ethylene-propylene random copolymer grade RJ377MO of Borealis AG, Austria, with an ethylene (C2) content of 3.7 wt.-%, an MFR (230 °C/2.16 kg) of 500 g/10 min, and a TM of 150 °C.
- -
- PW2 is a PP terpolymer wax produced by peroxide-mediated visbreaking from the commercial ethylene-propylene-butene terpolymer grade TD109CF of Borealis AG, Austria, with an MFR (230 °C/2.16 kg) of 500 g/10 min and a TM of 133 °C.
3. Results and Discussion
- -
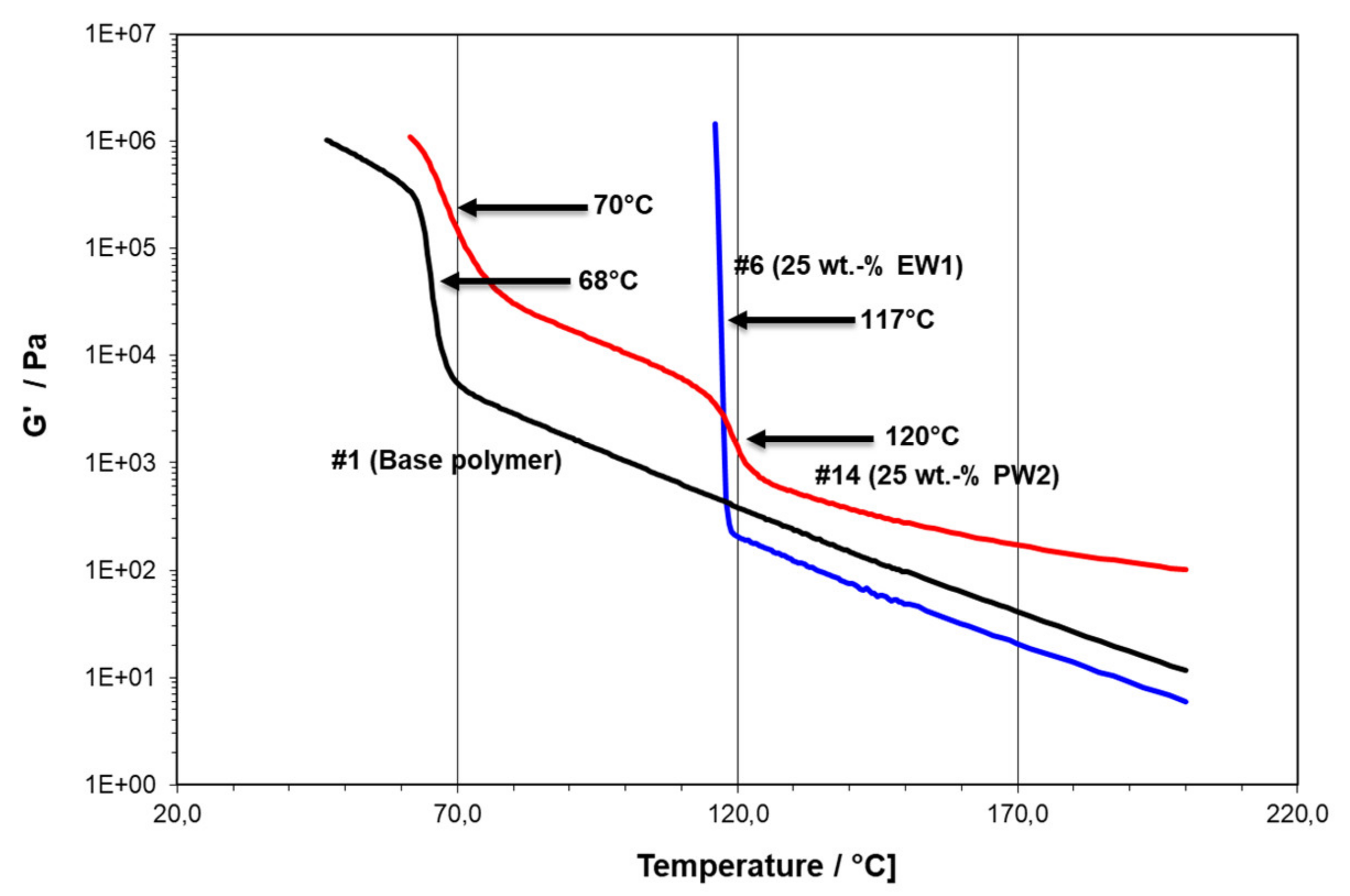
- -#1 is the pure PE plastomer type Queo 8085LA, with a clear solidification at 68 °C (TC,RHEO) and a measurable modulus in solid state;
- -
- -#6 is modified with 25 wt.-% of PE wax EW1, with a gelation to a non-measurable modulus at 117 °C; and
- -
- -#14 is modified with 25 wt.-% PP wax PW2, showing a first “network” transition at 120 °C (TN,RHEO) and a solidification at 70 °C (TC,RHEO).
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wittmann, J.C.; Lotz, B. Epitaxial Crystallization of Polyethylene on Organic Substrates: A Reappraisal of the Mode of Action of Selected Nucleating Agents. J. Polym. Sci. Polym. Phys. Ed. 1981, 19, 1837–1851. [Google Scholar] [CrossRef]
- Blomenhofer, M.; Ganzleben, S.; Hanft, D.; Schmidt, H.-W.; Kristiansen, M.; Smith, P.; Stoll, K.; Mäder, D.; Hoffmann, K. “Designer” Nucleating Agents for Polypropylene. Macromolecules 2005, 38, 3688–3695. [Google Scholar] [CrossRef]
- Gahleitner, M.; Grein, C.; Kheirandish, S.; Wolfschwenger, J. Nucleation of Polypropylene Homo- and Copolymers. Intern. Polym. Proc. 2011, 26, 2–20. [Google Scholar] [CrossRef]
- Horváth, Z.; Menyhárd, A.; Doshev, P.; Gahleitner, M.; Friel, D.; Varga, J.; Pukánszky, B. Improvement of the impact strength of ethylene-propylene random copolymers by nucleation. J. Appl. Polym. Sci. 2016, 133, 43823. [Google Scholar] [CrossRef]
- Dotson, D. Nucleating Agents for Polyethylene. In Handbook of Industrial Polyethylene and Technology: Definitive Guide to Manufacturing, Properties, Processing, Applications and Markets, 1st ed.; Spalding, M.A., Chatterjee, A., Eds.; John Wiley & Sons: Hoboken, NY, USA, 2017; pp. 935–965. [Google Scholar]
- Nguon, O.J.; Charlton, Z.; Kumar, M.; Lefas, J.; Vabsco, G.J. Interactions between sorbitol-type nucleator and additives for polypropylene. Polym. Eng. Sci. 2020, 60, 3046–3055. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Z.; Tao, Y.; Mai, K. Effects of polyamide 6 on the crystallization and melting behavior of β-nucleated polypropylene. Eur. Polym. J. 2008, 44, 3754–3763. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, X.; Yang, Q.; Ai, T.; Jia, S.; Zhou, S. Crystallization and Microstructure Evolution of Microinjection Molded Isotactic Polypropylene with the Assistance of Poly(Ethylene Terephthalate). Polymers 2020, 12, 219. [Google Scholar] [CrossRef]
- Bensason, S.; Minick, J.; Moet, A.; Chum, S.; Hiltner, A.; Baer, E. Classification of homogeneous ethylene-octene copolymers based on comonomer content. J. Polym. Sci. B Polym. Phys. 1996, 34, 1301–1315. [Google Scholar] [CrossRef]
- Chum, P.S.; Swogger, K.W. Olefin polymer technologies—History and recent progress at The Dow Chemical Company. Prog. Polym. Sci. 2008, 33, 797–819. [Google Scholar] [CrossRef]
- Strobl, G. Crystallization and melting of bulk polymers: New observations, conclusions and a thermodynamic scheme. Prog. Polym. Sci. 2006, 31, 398–442. [Google Scholar] [CrossRef]
- Mirabella, F.M. Crystallization and melting of a polyethylene copolymer: In situ observation by atomic force microscopy. J. Appl. Polym. Sci. 2008, 108, 987–994. [Google Scholar] [CrossRef]
- Zhuravlev, E.; Madhavi, V.; Lustiger, A.; Androsch, R.; Schick, C. Crystallization of Polyethylene at Large Undercooling. ACS Macro Lett. 2016, 5, 365–370. [Google Scholar] [CrossRef]
- Gahleitner, M.; Bachner, C.; Ratajski, E.; Rohaczek, G.; Neißl, W. Effects of the Catalyst System on the Crystallization of Polypropylene. J. Appl. Polym. Sci. 1999, 73, 2507–2515. [Google Scholar] [CrossRef]
- Janeschitz-Kriegl, H. How to understand nucleation in crystallizing polymer melts under real processing conditions. Coll. Polym. Sci. 2003, 281, 1157–1171. [Google Scholar] [CrossRef]
- Rana, D.; Lee, C.H.; Cho, K.; Lee, B.H.; Choe, S. Thermal and Mechanical Properties of Binary Blends of Metallocene Polyethylene with Conventional Polyolefins. J. Appl. Polym. Sci. 1998, 69, 2441–2450. [Google Scholar] [CrossRef]
- Galante, M.J.; Mandelkern, L.; Alamo, R.G. The crystallization of blends of different types of polyethylene: The role of crystallization conditions. Polymer 1998, 39, 5105–5119. [Google Scholar] [CrossRef]
- Pogodina, N.V.; Winter, H.H. Polypropylene Crystallization as Physical gelation Process. Macromolecules 1998, 31, 8164–8172. [Google Scholar] [CrossRef]
- Pogodina, N.V.; Siddiquee, S.K.; van Egmond, J.W.; Winter, H.H. Correlation of Rheology and Light Scattering in Isotactic Polypropylene during Early Stages of Crystallization. Macromolecules 1999, 32, 1167–1174. [Google Scholar] [CrossRef]
- Pogodina, N.V.; Lavrenko, V.P.; Srinivas, S.; Winter, H.H. Rheology and structure of polypropylene near the gel point: Quiescent and shear-induced crystallization. Polymer 2001, 42, 9031–9043. [Google Scholar] [CrossRef]
- Gelfer, M.; Horst, R.H.; Winter, H.H.; Heintz, A.M.; Hsu, S.L. Physical gelation of crystallizing metallocene and Ziegler-Natta ethylene-hexene copolymers. Polymer 2003, 44, 2363–2371. [Google Scholar] [CrossRef][Green Version]
- Kobayashi, T.; Hashimoto, T. Development of Self-Assembling Nucleators for Highly Transparent Semi-Crystalline Polypropylene. Bull. Chem. Soc. Jap. 2005, 78, 218–235. [Google Scholar] [CrossRef]
- Kheirandish, S.; Gahleitner, M.; Obadal, M.; Doshev, P.; Pukánszky, B.; Menyhárd, A. The Use or Rheological and Thermal Fractionation Methods for the Assessment of Nucleating Agent Efficiency in Polypropylene. 2010, p. 547. Available online: https://www.researchgate.net/publication/288310698_The_use_of_rheological_and_thermal_fractionation_methods_for_the_assessment_of_nucleating_agent_efficiency_in_polypropylene (accessed on 28 June 2021).
- Wilsens, C.H.R.M.; Hawke, L.G.D.; de Kort, G.W.; Saidi, S.; Roy, M.; Leoné, N.; Hermida-Merino, D.; Peters, G.W.M.; Rastogi, S. Effect of Thermal History and Shear on the Viscoelastic Response of iPP Containing an Oxalamide-Based Organic Compound. Macromolecules 2019, 52, 2789–2802. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, S.; Inoue, M.; Takei, Y.; Nishikawa, R.; Yamaguchi, M. Modulus enhancement of polypropylene by sorbitol nucleating agent in flow field. Polym. Cryst. 2021, 4, e10170. [Google Scholar] [CrossRef]
- Menyhárd, A.; Gahleitner, M.; Varga, J.; Bernreitner, K.; Jääskeläinen, P.; Øysæd, H.; Pukánszky, B. The influence of nucleus density on optical properties in nucleated isotactic polypropylene. Eur. Polym. J. 2009, 45, 3138–3148. [Google Scholar] [CrossRef]
- Ma, Z.; Steenbakkers, R.J.A.; Giboz, J.; Peters, G.W.M. Using rheometry to determine nucleation density in a colored system containing a nucleating agent. Rheol. Acta 2011, 50, 909–915. [Google Scholar] [CrossRef]
- Chen, L.; Ma, C.-G.; Wang, H.-Y.; Zhang, J.-X.; Xiong, X.-M. Effect of continuous oscillatory shear on isothermal crystallization of non-nucleated and nucleating-agent-treated isotactic polypropylene assessed by dynamic mechanical analysis. J. Appl. Polym. Sci. 2015, 132, 41685. [Google Scholar] [CrossRef]
- Lamberti, G. Flow induced crystallisation of polymers. Chem. Soc. Rev. 2014, 43, 2240–2252. [Google Scholar] [CrossRef] [PubMed]
- Koscher, E.; Fulchiron, R. Influence of shear on polypropylene crystallization: Morphology development and kinetics. Polymer 2002, 43, 6931–6942. [Google Scholar] [CrossRef]
- Pantani, R.; Nappo, V.; De, S.F.; Titomanlio, G. Fibrillar Morphology in Shear-Induced Crystallization of Polypropylene. Macromol. Mat. Eng. 2014, 299, 1465–1473. [Google Scholar] [CrossRef]
- Yang, L.; Somani, R.H.; Sics, I.; Hsiao, B.S.; Kolb, R.; Fruitwala, H.; Ong, C. Shear-Induced Crystallization Precursor Studies in Model Polyethylene Blends by In-Situ Rheo-SAXS and Rheo-WAXD. Macromolecules 2004, 37, 4845–4859. [Google Scholar] [CrossRef]
- Pantani, R.; Balzano, L.; Peters, G.W.M. Flow-Induced Morphology of iPP Solidified in a Shear Device. Macromol. Mat. Eng. 2012, 297, 60–67. [Google Scholar] [CrossRef]
- Hato, M.J.; Luyt, A.S. Thermal fractionation and properties of different polyethylene/wax blends. J. Appl. Polym. Sci. 2007, 104, 2225–2236. [Google Scholar] [CrossRef]
- Molefi, J.A.; Luyt, A.S.; Krupa, I. Comparison of LDPE, LLDPE and HDPE as matrices for phase change materials based on a soft Fischer–Tropsch paraffin wax. Thermochim. Acta 2010, 500, 88–92. [Google Scholar] [CrossRef]
- Estan-Carezo, G.; Martín-Martínez, J.M. Thermal, viscoelastic and adhesion properties of EVA (ethylene-co-vinyl acetate) hot melts containing polypropylene waxes of different nature. J. Adhes. Sci. Technol. 2015, 29, 875–889. [Google Scholar] [CrossRef]
- Tzoganakis, C.; Vlachopoulos, J.; Hamielec, A.E. Production of controlled-rheology polypropylene resins by peroxide promoted degradation during extrusion. Polym. Eng. Sci. 1988, 28, 170–180. [Google Scholar] [CrossRef]
- Kim, J.-H.; Robertson, R.E. Theromreversible gelation of polyethylene/decalin mixtures by self-nucleated crystallization. Polymer 1988, 39, 537–5378. [Google Scholar] [CrossRef]
- Kock, C.; Gahleitner, M.; Schausberger, A.; Ingolic, E. Polypropylene/polyethylene blends as models for high-impact propylene-ethylene copolymers. 1: Interaction between rheology and morphology. J. Appl. Polym. Sci. 2013, 128, 1484–1496. [Google Scholar] [CrossRef]
- Kock, C.; Aust, N.; Grein, C.; Gahleitner, M. Polypropylene/polyethylene blends as models for high-impact propylene-ethylene copolymers. 2: Relation between composition and mechanical performance. J. Appl. Polym. Sci. 2013, 130, 287–296. [Google Scholar] [CrossRef]
- Gahleitner, M.; Wang, J.; Ek, C.-G.; Bernreitner, K.; Prades, F. Polyethylene and Propylene Wax for Hot Melt Adhesive. EP Patent 3498799 B1, 14 December 2017. [Google Scholar]



| Composition | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|
| Queo 8085L | Wt.-% | 100 | 0 | 0 | 95 | 85 | 75 | 95 | 75 |
| EW1 | Wt.-% | 0 | 100 | 0 | 5 | 15 | 25 | 0 | 0 |
| EW2 | Wt.-% | 0 | 0 | 100 | 0 | 0 | 0 | 5 | 25 |
| MFR 190 °C | g/10 min | 85 | n.d. * | n.d. * | 98 | 109 | 133 | 110 | 205 |
| DSC | |||||||||
| TC,1 | °C | - | 114 | 97 | 109 | 110 | 113 | 60 | 62 |
| TC,2 | °C | 56 | - | - | 61 | 61 | 60 | - | 85 |
| TM,1 | °C | 54 | - | - | 72 | 74 | 73 | 62 | 62 |
| TM,2 | °C | 76 | 126 | 109 | 121 | 124 | 79 | 78 | 86 |
| Composition | 1 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|
| Queo 8085L | Wt.-% | 100 | 95 | 85 | 75 | 95 | 85 | 75 |
| PW1 | Wt.-% | 0 | 5 | 15 | 25 | 0 | 0 | 0 |
| PW2 | Wt.-% | 0 | 0 | 0 | 0 | 5 | 15 | 25 |
| MFR 190 °C | g/10 min | 85 | 83 | 79 | 78 | 84 | 82 | 80 |
| DSC | ||||||||
| TC,1 | °C | - | - | - | 109 | - | - | 96 |
| TC,2 | °C | 56 | 56 | 58 | 57 | 56 | 56 | 58 |
| TM,1 | °C | 54 | 75 | 75 | 75 | 75 | 74 | 75 |
| TM,2 | °C | 76 | 147 | 147 | 144 | 128 | 132 | 131 |
| Composition | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|
| Queo 8085L | Wt.-% | 100 | 0 | 0 | 95 | 85 | 75 | 95 | 75 |
| EW1 | Wt.-% | 0 | 100 | 0 | 5 | 15 | 25 | 0 | 0 |
| EW2 | Wt.-% | 0 | 0 | 100 | 0 | 0 | 0 | 5 | 25 |
| Rheology | |||||||||
| G’(200 °C) | Pa | 12.0 | 0.60 | 0.02 | 9.2 | 8.0 | 5.9 | 8.4 | 1.2 |
| TN,RHEO | °C | - | - | - | - | - | - | 96 | 107 |
| TC,RHEO | °C | 67 | 120 | 102 | 115 | 116 | 117 | 69 | 70 |
| DMA | °C | ||||||||
| TG | °C | –40 | n.d. | n.d. | –41 | –42 | –43 | –42 | –42 |
| G’(23 °C) | Pa | 10 | n.d. | n.d. | 50 | 76 | 116 | 19 | 61 |
| TSOFT | °C | 24 | n.d. | n.d. | 71 | 85 | 112 | 38 | 57 |
| Composition | 1 | 9 | 10 | 11 | 12 | 13 | 14 | |
|---|---|---|---|---|---|---|---|---|
| Queo 8085L | Wt.-% | 100 | 95 | 85 | 75 | 95 | 85 | 75 |
| PW1 | Wt.-% | 0 | 5 | 15 | 25 | 0 | 0 | 0 |
| PW2 | Wt.-% | 0 | 0 | 0 | 0 | 5 | 15 | 25 |
| Rheology | ||||||||
| G’(200 °C) | Pa | 120 | 22 | 69 | 100 | 23 | 76 | 132 |
| TN,RHEO | °C | - | 97 | 100 | 102 | 115 | 117 | 120 |
| TC,RHEO | °C | 67 | 68 | 69 | 70 | 68 | 69 | 70 |
| DMA | ||||||||
| TG | °C | –40 | –39 | –39 | –38 | –39 | –38 | –38 |
| G’(23 °C) | Pa | 10 | 13 | 18 | 24 | 13 | 30 | 22 |
| TSOFT | °C | 24 | 132 | 39 | 42 | 33 | 37 | 41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gahleitner, M.; Wang, J.; Prades, F.; Bernreitner, K. Gelation and Crystallization Phenomena in Polyethylene Plastomers Modified with Waxes. Polymers 2021, 13, 2147. https://doi.org/10.3390/polym13132147
Gahleitner M, Wang J, Prades F, Bernreitner K. Gelation and Crystallization Phenomena in Polyethylene Plastomers Modified with Waxes. Polymers. 2021; 13(13):2147. https://doi.org/10.3390/polym13132147
Chicago/Turabian StyleGahleitner, Markus, Jingbo Wang, Floran Prades, and Klaus Bernreitner. 2021. "Gelation and Crystallization Phenomena in Polyethylene Plastomers Modified with Waxes" Polymers 13, no. 13: 2147. https://doi.org/10.3390/polym13132147
APA StyleGahleitner, M., Wang, J., Prades, F., & Bernreitner, K. (2021). Gelation and Crystallization Phenomena in Polyethylene Plastomers Modified with Waxes. Polymers, 13(13), 2147. https://doi.org/10.3390/polym13132147






