3.1. Water Sorption
The results of the water sorption of the four groups of samples with different mass fractions of the silicone modifier (m0, m5, m10 and m15) are shown in
Figure 4.
The water sorption curves shown in
Figure 4 can be divided into two categories: The first category (hereinafter called Case I) includes unmodified m0 and m15. The water absorption process of Case I can be divided into two stages—fast water absorption stage and slow water absorption stage. Both stages showed a linear relationship between water sorption and
, but the slope of the first stage is greater than that of the second one. The second category (hereinafter called Case II) includes m5 and m10, whose water absorption curve can be divided into three stages. The first and the third stage of Case II are similar to the first and second stage of Case I, which can also be called the fast water absorption stage and the slow water absorption stage. The noticeable difference between Case I and Case II is that there is a pseudo-saturation stage between the first stage and the second stage, where the slope is much smaller than that of the fast and slow water absorption stage.
As for the diffusion process of water in the epoxy resin system, the most simple and widely used mathematical model is the Fickian diffusion model [
16]. The Fickian diffusion model assumes that the water in the resin system exists in the form of free water. Water molecules diffuse through the long-chain molecules of resin under the concentration gradients’ force, and the system finally reaches equilibrium. The typical Fickian absorption-time square root curve consists of two stages. The Fickian diffusion model suggests the samples first experience a fast water absorption stage where the water uptake grows linearly with the square root of time, and they finally reach the saturation stage where the water uptake of the samples remains stable.
The Fickian diffusion model can be described by the following expression [
17]:
where
is the water absorption rate of epoxy resin in the saturation state;
D is the coefficient of water diffusion, which is proportional to the slope S of the water sorption curve at the origin. The relation between
D and
S can be expressed by the following expression:
In the experiment, none of the four groups of the samples reached the final saturation stage, but they all followed the Fickian diffusion law during the fast water absorption stage; therefore, the Fickian model can be used to analyze the free diffusion of water in the epoxy resin system. The slope S at the origin of the four groups of resins and the fitting result for water absorption in a saturated state are shown in
Table 2; the fitting results are shown in
Figure 5.
As shown in
Table 2, under 10 wt%, the silicone material slowed down the diffusion process, and the water absorption rate in the saturated state decreased. It is generally accepted that diffusion of water molecules and the water absorption rate in the saturated state in the Fickian model are related to the molecular polarity, free volume, hydrogen bond and other properties of the material [
18]. From the perspective of molecular polarity, the encapsulation of alcohol hydroxyl groups during the synthesis and the symmetrical structure of diphenylsilanediol reduces the polarity of the insulation material. From the perspective of free volume, after the grafting reaction, the side chain of the epoxy resin becomes more flexible; the movement of the side chain may prevent the combination of water and nanopores and hinder the diffusion and storage of water molecules in the resin system. From the perspective of the hydrogen bond, the grafting reaction consumes hydroxyl groups; therefore, the binding ability of epoxy resin molecules to water molecules decreases, and, thus, the saturated water absorption of the epoxy resin system further decreases. The increase in diffusion rate and water absorption rate in the saturated state at 15 wt% may have two possible explanations: (1) The incomplete grafting reaction. Unreacted diphenylsilanediol molecules may become the impurities within the system, thus reducing the performance of the insulating material. (2) The polymerization between diphenylsilanediol molecules. Long side chains may make the structure less compact and increase the free volume within the material.
The water absorption curve in the experiment continued to rise after the fast water absorption period and significantly exceeded the saturated water absorption predicted by the Fickian model. The reasons are the following: (1) In addition to free water, there is bound water in the epoxy resin system, which is not considered by the Fickian model. (2) In addition to the Fickian diffusion, the water molecules can also enter epoxy resin by rapid transmission through macropores and the slow absorption process through micropores. In m0 and m15, some microcracks were generated on the surface, and water entered the samples through the microcracks quickly. Therefore, the moisture content of the four samples reached a higher value than that predicted by the Fickian model at the end of the fast water absorption period. After the rapid water absorption period, the cracks of m0 and m15 continued to grow and become the dominant factors for subsequent water absorption. In m5 and m10 samples, the cracks were still not obvious in the pseudo-saturation stage. At this time, the moisture content in the pores of m5 and m10 approached saturation; thus, the water absorption rate of the samples decreased rapidly, forming a platform called the pseudo-saturation period. When m5 and m10 reached 16 h0.5, the cracks in the sample began to occur. The samples then reached the third stage of the rate-controlling stage, and the diffusion of water into the macropores appeared again; therefore, the water absorption started to increase again but at a slower rate compared to the fast absorption period.
3.2. Partial Discharge Inception Voltage
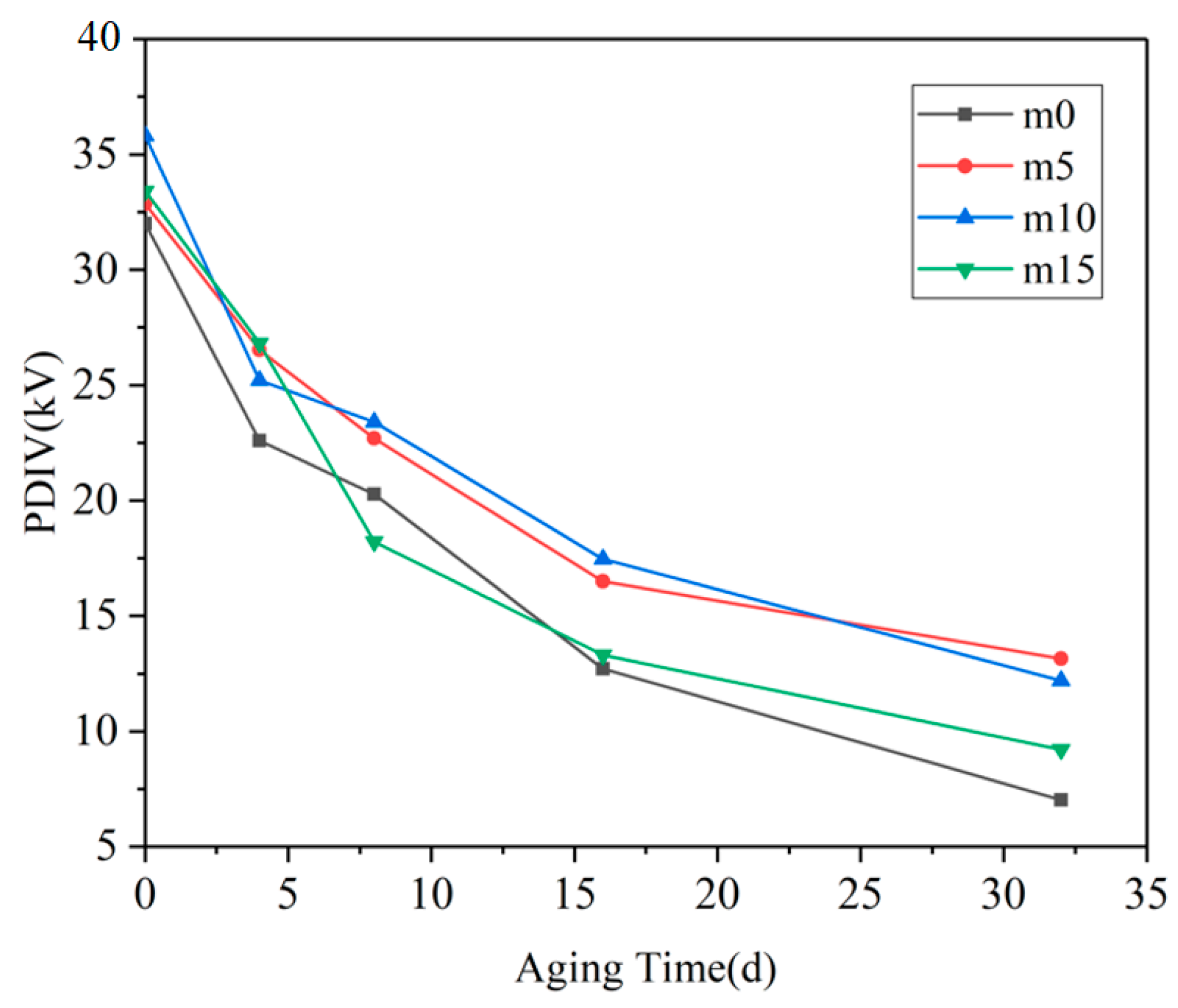
The partial discharge inception voltage results of the four groups of samples are shown in
Figure 6 below.
When the samples were not aged, the PDIV data of the four groups were quite close. With the progress of hygrothermal aging, the PDIV of the four groups of samples decreased significantly, and the fastest decline was in the range of 0 days–4 days, and the slowest decline was after 16 days. The PDIV of m0 and m15 (Case I) from 8 days to the end of the experiment was significantly lower than that of m5 and m10 (Case II) at the same aging time. It can be observed that m5 and m10 showed similarity in PDIV; the possible explanation for this phenomenon may be that the optimal filling content of diphenylsilanediol may be between 5 wt% and 10 wt%. At 10 wt%, this filling content may have passed the optimal filling content; therefore, the PDIV of m10 may decline to a level that is similar to that of m5.
The PDIV of insulating materials is mainly determined by the nature of the material itself and internal defects. At the early stage of hygrothermal aging, the rapid decline of the PDIV of epoxy resin material is mainly due to the change of the dielectric constant of the material. One of the reasons for the partial discharge phenomenon in insulating materials is distortion of the electric field at the inevitable tiny pores generated during production. The dielectric constant of water is very high (
); therefore, the dielectric constant of epoxy resin increases as water diffuses into the resin system [
19]. The gap between the dielectric constant of the material and the tiny pores further expands, resulting in the aggravation of the local electric field distortion at the pores and the decrease in PDIV. At day 4, the PDIV value of m0 was the lowest because its coefficient of water diffusion was higher than that of the other three groups, indicating that m0 absorbed the greatest amount of water.
After 8 days of hydrothermal aging, although water could still be absorbed into the material through hydrolysis and diffusion and the dielectric properties of the material continued to degrade, the number and size of the pores in the material gradually became the main factors. At day 16, the PDIV of the four groups of materials was less than half of that before aging, indicating that there were more pores and defects inside. The above analysis shows that after 16 days of hygrothermal aging, the main changes that happened in the material were the growth and coalescence of cracks and pores. The reason for the slowing down of the PDIV curve after 16 days may be that there are many pores that are large enough to cause PD, and the further expansion of these pore sizes has a smaller effect on the PDIV of the material.
3.3. SEM Analysis
According to the above analysis, in this experiment, the hygrothermal degradation process of Case I and Case II was slightly different. Therefore, in this part, the SEM images of samples of m0 and m10 were selected to represent Case I and Case II, respectively, and show the damage of hygrothermal aging on the two types of epoxy resin systems.
For Case I samples, the SEM images of 0/4/16 days of aging were selected to represent the three key time points of the epoxy resin material: unaged, at the end of the rapid water absorption period and the slow water absorption period.
Figure 7 and
Figure 8 show the micromorphology of the outer surface and the cross-section of group m0, respectively, after 0/4/16 days of hygrothermal aging.
As shown in
Figure 7, before aging, the outer surface of the resin material was smooth, and no obvious defects were found. When the fast water absorption stage ended (4 days), small cracks and clustered white spots could be observed, as shown in
Figure 7b. When the hygrothermal aging developed to 16 days, the damage of water on the resin surface was more obvious, and large-scale cracks and holes could be observed on the material surface.
In
Figure 8, it can be observed that with the progress of aging time, inhomogeneous black blotches began to appear on the resin section. At day 16, large-scale holes could be observed inside the resin system.
For Case II samples, the SEM images of 0/4/16/32 days of aging were selected to represent the four time points (unaged, end of the fast water absorption stage, end of pseudo-saturation stage and during the slow water absorption stage, respectively). The SEM images of the outer surface of m10 silicone-modified epoxy resin after 0/4/16/32 days hygrothermal aging is shown in
Figure 9. There were no obvious cracks on the resin surface at day 0 and day 4. At the end of the pseudo-saturation stage (day 16), obvious holes were observed on the resin surface. In the third stage (day 32), longer and deeper cracks due to water erosion could be observed.
Figure 10 shows the microstructure of the cross-section of the m0 sample after 0/4/16/32 d of hygrothermal aging. From the surface morphology of the cross-section, no obvious phase separation phenomenon was observed in the SEM images, indicating that the compatibility between the modified materials and epoxy resin was good. At day 0 and day 4, the SEM images of the m0 and m10 samples were similar. Similarly to
Figure 8b, black blotches also appeared in
Figure 10b at the end of the fast water absorption period. When the hygrothermal aging reached day 16, there were also obvious pores in
Figure 10c, but the size was relatively small compared with the pores in
Figure 8c. At the same time, it can be found from
Figure 10c that the pores were more likely to distribute at the interface between the cross-section and the outer surface, and there are signs of small holes merging into large holes, which proves that cracks develop and expand from the surface to the inside. By comparing the pore size in the cross-section of
Figure 10c,d, it can be observed that the pore size in
Figure 10d is significantly larger than that in
Figure 10c, which proves that the growth and merger of internal pores continued in the third stage of hygrothermal aging of Case II resin.
Comparing
Figure 7 and
Figure 9, it can be observed that at the same time point, the damage on the outer surface of the m0 resin sample was more serious than that of the m10 resin sample, and the generation and development speed of cracks and pores on the surface of the m0 epoxy resin sample was significantly higher than that of m10. There were already small cracks at the end of the fast water absorption period in m5, while obvious changes only appeared at the surface of m10 at the end of the pseudo-saturation period. In
Figure 7c, the pore diameter of the outer surface of the m0 sample was about 80 μm, while in
Figure 9c, the pore diameter of the outer surface of the m10 sample was only about 20 μm and only developed to about 80 μm at day 32.
Comparing
Figure 8 and
Figure 10, the micromorphology changes on sections of m0 and m10 were similar to the changes that happened on the external surface, and the deterioration rate of m0 was higher than that of m10. The inhomogeneous black blotches observed in
Figure 8b and
Figure 10b may be due to the inhomogeneous cross-linking density inside the epoxy resin material resulting in different diffusion rates of water in different regions of the epoxy resin [
20].
Comparisons between
Figure 7,
Figure 8,
Figure 9 and
Figure 10 reveal that silicone material slows down the generation and development rate of cracks and pores within the material. To better present the aging mechanism, the micromorphology of the cross-section at different filling contents of diphenylsilanediol at day 16 of aging is given in
Figure 11.
From
Figure 11, it can be observed that at day 16, the sizes of the cracks of m5, m10 and m15 are significantly smaller than that of m0. The size of the cracks of m5 is similar to that of m10, about 20 μm–30 μm, while in
Figure 11d, holes tend to merge into long cracks. This difference can be explained from chemical and physical perspectives. From the chemical perspective, the damage made by water is mainly through hydrolysis, which mainly occurs near the surface of the resin material. Hydrolysis can break the resin macromolecule chain and destroy the resin matrix. The chemical bonds in the diphenylsilanediol are more stable than those in the epoxy resin, making it more difficult to hydrolyze. From the physical point of view, the cracks may grow when free water molecules inside the pores change into vapor, causing a rise of internal stress inside the pores. It has been reported elsewhere that the addition of silicone materials can improve the mechanical properties of materials [
21], and the addition of diphenylsilanediol may improve the ability of the material to withstand internal stress by transferring internal stress and increasing the molecules’ flexibility [
22,
23]; this means that more energy may be needed to cause stress concentration that is severe enough to break the material, thus inhibiting the generation and development of cracks and pores. These observations prove the assumption in
Section 3.2 that the size of cracks and holes may play an important role in the changes of PDIV.
3.4. Analysis of Contact Angle
The surface contact angles of the four groups of samples with different aging times are shown in
Figure 12, and the photos of the samples are shown in
Figure 13.
When unaged, the contact angles of m0 and m10 samples were close, about 10° higher than those of the m0 and m15 samples. During 0 day–4 days of hydrothermal aging, the surface contact angles of the four groups of epoxy resin samples decreased significantly, with an average decrease of about 12°. It should be noted that the contact angles of m5 and m10 after 4 days of hygrothermal aging were still close to that of m0 and m15 before aging. After 4 days, the surface contact angles of the four groups of samples did not change significantly.
The difference in hydrophobicity may be due to the hydrophobic nature of the Si–O bond and the fact that silicon blocks tend to enrich on the surface of epoxy resin during the curing process [
24]. When the content of silicone exceeds 10 wt%, the silicone material on the surface tends to be saturated. Excessive silicone modifier may lead to worse compatibility, resulting in worse hydrophobicity.
3.5. FT-IR Spectra
The FT-IR spectra of the four groups of epoxy resin are shown in
Figure 14.
To minimize the error brought by the amount of sample used in the sample preparation process, the FT-IR spectra were normalized with ether bond. The 1100 cm−1–1000 cm−1 and 740 cm−1–870 cm−1 ranges contributed to the vibration of Si–O–Si and Si–C. There were dramatic increases in those two ranges after the modification, which confirm the grafting reaction. It is also noteworthy that while the Si–O–Si bond and Si–C bond increased with the content of the modifier under 10 wt%, they decreased after the content of the modifier exceeded 10 wt%. This may suggest that when the content exceeds 10 wt%, part of the modifier neither grafts on the main chain of the epoxy resin nor takes part in polymerization; instead, some modifier molecules exist in the system as impurities, resulting in the decrease in the anti-aging properties.
Figure 15 shows the change of the FT-IR spectra after the aging test. These spectra are modified with the phenyl group since it is the most stable group within the system and unlikely to change during the aging test. As shown in
Figure 15, in all four groups, it can be observed that the peak at around 1740 cm
−1 which is attributed to the carbonyl group dwindled during the aging process. This is because the carbonyl group is the most susceptible to hydrolysis. However, there were no obvious changes in the other peaks, indicating that the backbone of epoxy resin molecules is relatively stable. The changes in the peak around 1740 cm
−1 by finding the ratio of the peak height before and after the hygrothermal aging are compared using Equation (4):
The results are summarized in
Table 3. For all four groups, the ratios are almost the same, meaning the modifier did not change the hydrolysis rate of the carbonyl group. However, with the addition of the modifier, the content of the carbonyl group decreased, which could lead to better chemical stability.
3.6. FDS
Unaged samples and samples aged for 32 days were selected to perform the FDS test.
Figure 16 and
Figure 17 show the variation of the real and imaginary part of the complex permittivity with the frequency of the four groups of unaged samples.
Figure 18 shows the variation of the dissipation factor with the frequency of the four groups of unaged samples. The dissipation factor represents the ratio between active power and reactive power, and is defined by
The real part of the complex dielectric constant
reflects the polarization of molecules. From
Figure 16, it is obvious that
decreases as the frequency increases, which is because the relaxation of molecules cannot keep up with the changes of the electric field. Comparing the curves of different groups of samples, at the same frequency, with the increase in modifier, the
shows a decrease–increase trend. This is because the low polarity of diphenylsilanediol decreases the
of the insulating material, while excessive diphenylsilanediol leads to worse compatibility, intensifying the polarization of the insulating material under the electric field.
Figure 17 and
Figure 18 represent loss. At the same frequency, with the increase in modifier, the loss shows a decrease–increase–decrease trend. Under the electric field, the loss is mainly due to the polarization of dipoles and conductance. When the content of diphenylsilanediol is low, this modifier shows good compatibility with the epoxy resin. At 10 wt%, excessive diphenylsilanediol introduces more dipoles into the insulating material; thus, the loss increases. At 15 wt%, though dipoles continue to increase because of the fact that silicone materials have excellent insulation properties, the conductance of the insulating materials decrease; therefore, the material shows a lower
and dissipation factor.
Figure 19 and
Figure 20 show the real and imaginary parts of the complex dielectric constant of the four groups of aged samples.
Figure 21 shows the dissipation factor of the aged samples. During hygrothermal aging, hydrolysis may break the long chain and generate smaller molecules with stronger polarity; water molecules diffused into the system also have high polarity and relatively short relaxation time. Therefore, comparing
Figure 16 and
Figure 19, it can be observed that all curves moved upwards and became steeper under 0.1 Hz after hygrothermal aging. The comparison also reveals that during hygrothermal aging, the
of m0 increased faster than other modified epoxy resin insulating materials; this may be due to the fact that although FT-IR analysis showed those materials hydrolyze at about the same rate, modified insulating materials contain less carbonyl groups, thus containing less molecules with high polarity after aging. The fact that m5 had the smallest increase in
may be because it has the best compatibility, thus having fewer interfaces. Higher
means worse distortion of the electric field in micropores within the system, and this may help to explain why the PDIV of m0 and m15 are obviously lower than those of m5 and m10.
Comparing
Figure 17,
Figure 18,
Figure 20 and
Figure 21, after aging, the loss increased. This is because the water molecules that diffused into the material became carriers and increased the conductance of the insulating material. It can also be noticed that after aging, four curves in
Figure 20 and
Figure 21 almost overlap, which indicates that although diphenylsilanediol could decrease the conductance of unaged samples, it has a limited effect on the conductance during the hygrothermal aging process.