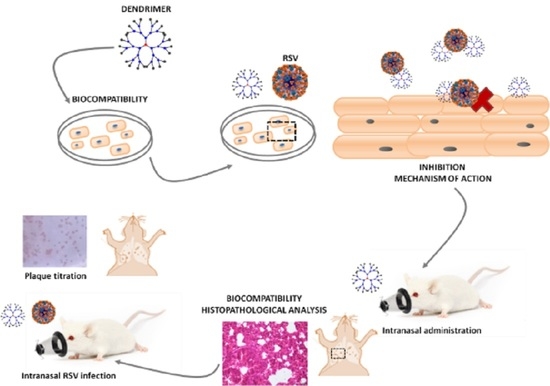
Role of G2-S16 Polyanionic Carbosilane Dendrimer in the Prevention of Respiratory Syncytial Virus Infection In Vitro and In Vivo in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dendrimers and Reagents
2.2. Cells and Viruses
2.3. Screening Procedure
2.4. Inhibition Assay
2.5. Inhibitions of RSV Attachment to Host Cells
2.6. G2-S16 Dendrimer–Cell Interaction Assay
2.7. Binding of G2-S16 Dendrimer to RSV
2.8. Syncytium Formation Assay
2.9. In Vivo Assays Statement
2.10. In Vivo RSV Challenge Assay
2.11. Statistical Analysis
3. Results
3.1. Biocompatibility of G2-S16 Polyanionic Carbosilane Dendrimer
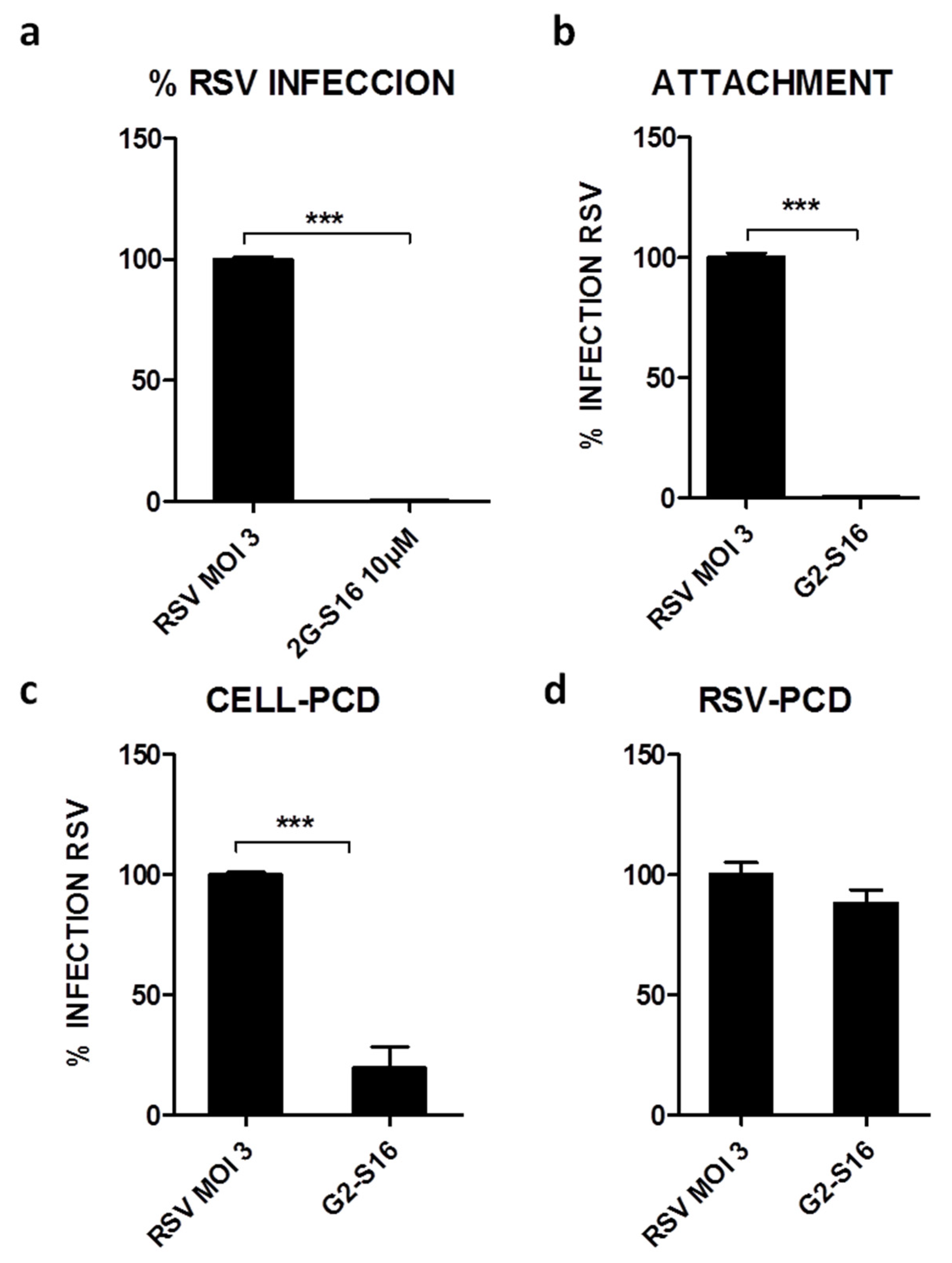
3.2. G2-S16 Polyanionic Carbosilane Dendrimer against Respiratory Syncytial Virus
3.3. Mechanism of Action of G2-S16 Polyanionic Carbosilane Dendrimer
3.4. Inhibition of Syncytium Formation
3.5. In Vivo Experiments: Murine Lung Model for Viability of G2-S16 Dendrimer and Respiratory Syncytial Virus Challenge
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Valdez, J.; Bawage, S.; Gomez, I.; Singh, S.R. Facile and rapid detection of respiratory syncytial virus using metallic nanoparticles. J. Nanobiotechnol. 2016, 14, 13. [Google Scholar] [CrossRef] [Green Version]
- Mazur, N.I.; Higgins, D.; Nunes, M.C.; Melero, J.A.; Langedijk, A.C.; Horsley, N.; Buchholz, U.J.; Openshaw, P.J.; McLellan, J.S.; Englund, J.A.; et al. The respiratory syncytial virus vaccine landscape: Lessons from the graveyard and promising candidates. Lancet Infect. Dis. 2018, 18, e295–e311. [Google Scholar] [CrossRef] [Green Version]
- Mejias, A.; Rodriguez-Fernandez, R.; Peeples, M.E.; Ramilo, O. Respiratory syncytial virus vaccines: Are we making progress? Pediatr. Infect. Dis. J. 2019, 38, e266–e269. [Google Scholar] [CrossRef]
- Mejias, A.; Rodriguez-Fernandez, R.; Oliva, S.; Peeples, M.E.; Ramilo, O. The journey to a respiratory syncytial virus vaccine. Ann. Allergy Asthma Immunol. 2020, 125, 36–46. [Google Scholar] [CrossRef]
- Santos, L.D.; Antunes, K.H.; Muraro, S.P.; de Souza, G.F.; da Silva, A.G.; de Souza Felipe, J.; Zanetti, L.C.; Czepielewski, R.S.; Magnus, K.; Scotta, M.; et al. Tnf-mediated alveolar macrophage necroptosis drives disease pathogenesis during respiratory syncytial virus infection. Eur. Respir. J. 2020, 57, 2003764. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, N.G.; Megremis, S.; Kitsioulis, N.A.; Vangelatou, O.; West, P.; Xepapadaki, P. Promising approaches for the treatment and prevention of viral respiratory illnesses. J. Allergy Clin. Immunol. 2017, 140, 921–932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Izquierdo, I.; Seramia, M.J.; Gomez, R.; de la Mata, F.J.; Bullido, M.J.; Munoz-Fernandez, M.A. Gold nanoparticles crossing blood-brain barrier prevent hsv-1 infection and reduce herpes associated amyloid-betasecretion. J. Clin. Med. 2020, 9, 155. [Google Scholar]
- Pissuwan, D.; Niidome, T. Polyelectrolyte-coated gold nanorods and their biomedical applications. Nanoscale 2015, 7, 59–65. [Google Scholar] [CrossRef]
- Bawage, S.S.; Tiwari, P.M.; Singh, A.; Dixit, S.; Pillai, S.R.; Dennis, V.A.; Singh, S.R. Gold nanorods inhibit respiratory syncytial virus by stimulating the innate immune response. Nanomedicine 2016, 12, 2299–2310. [Google Scholar] [CrossRef] [Green Version]
- Markoutsa, E.; McGill, A.R.; Singer, A.; Jadhav, H.; Mohapatra, S.; Mohapatra, S.S. A multifunctional nanoparticle as a prophylactic and therapeutic approach targeting respiratory syncytial virus. Nanomed. Nanotechnol. Biol. Med. 2020, 32, 102325. [Google Scholar] [CrossRef]
- Nikonova, A.; Shilovskiy, I.; Galitskaya, M.; Sokolova, A.; Sundukova, M.; Dmitrieva-Posocco, O.; Mitin, A.; Komogorova, V.; Litvina, M.; Sharova, N.; et al. Respiratory syncytial virus upregulates il-33 expression in mouse model of virus-induced inflammation exacerbation in ova-sensitized mice and in asthmatic subjects. Cytokine 2020, 138, 155349. [Google Scholar] [CrossRef]
- Kellar, G.G.; Barrow, K.A.; Rich, L.M.; Debley, J.S.; Wight, T.N.; Ziegler, S.F.; Reeves, S.R. Loss of versican and production of hyaluronan in lung epithelial cells are associated with airway inflammation during rsv infection. J. Biol. Chem. 2020, 296, 100076. [Google Scholar] [CrossRef]
- Bourgeois, C.; Bour, J.B.; Lidholt, K.; Gauthray, C.; Pothier, P. Heparin-like structures on respiratory syncytial virus are involved in its infectivity in vitro. J. Virol. 1998, 72, 7221–7227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escribano-Romero, E.; Rawling, J.; Garcia-Barreno, B.; Melero, J.A. The soluble form of human respiratory syncytial virus attachment protein differs from the membrane-bound form in its oligomeric state but is still capable of binding to cell surface proteoglycans. J. Virol. 2004, 78, 3524–3532. [Google Scholar] [CrossRef] [Green Version]
- Feldman, S.A.; Audet, S.; Beeler, J.A. The fusion glycoprotein of human respiratory syncytial virus facilitates virus attachment and infectivity via an interaction with cellular heparan sulfate. J. Virol. 2000, 74, 6442–6447. [Google Scholar] [CrossRef] [Green Version]
- Feldman, S.A.; Hendry, R.M.; Beeler, J.A. Identification of a linear heparin binding domain for human respiratory syncytial virus attachment glycoprotein g. J. Virol. 1999, 73, 6610–6617. [Google Scholar] [CrossRef] [Green Version]
- Harris, J.; Werling, D. Binding and entry of respiratory syncytial virus into host cells and initiation of the innate immune response. Cell. Microbiol. 2003, 5, 671–680. [Google Scholar] [CrossRef]
- Karger, A.; Schmidt, U.; Buchholz, U.J. Recombinant bovine respiratory syncytial virus with deletions of the g or sh genes: G and f proteins bind heparin. J. Gen. Virol. 2001, 82, 631–640. [Google Scholar] [CrossRef]
- Krusat, T.; Streckert, H.J. Heparin-dependent attachment of respiratory syncytial virus (rsv) to host cells. Arch. Virol. 1997, 142, 1247–1254. [Google Scholar] [CrossRef]
- Martinez, I.; Melero, J.A. Binding of human respiratory syncytial virus to cells: Implication of sulfated cell surface proteoglycans. J. Gen. Virol. 2000, 81, 2715–2722. [Google Scholar] [CrossRef]
- Arnaiz, E.; Doucede, L.I.; Garcia-Gallego, S.; Urbiola, K.; Gomez, R.; Tros de Ilarduya, C.; de la Mata, F.J. Synthesis of cationic carbosilane dendrimers via click chemistry and their use as effective carriers for DNA transfection into cancerous cells. Mol. Pharm. 2012, 9, 433–447. [Google Scholar] [CrossRef]
- Galan, M.; Sanchez Rodriguez, J.; Jimenez, J.L.; Relloso, M.; Maly, M.; de la Mata, F.J.; Munoz-Fernandez, M.A.; Gomez, R. Synthesis of new anionic carbosilane dendrimers via thiol-ene chemistry and their antiviral behaviour. Org. Biomol. Chem. 2014, 12, 3222–3237. [Google Scholar] [CrossRef]
- Rasines, B.; Sanchez-Nieves, J.; Maiolo, M.; Maly, M.; Chonco, L.; Jimenez, J.L.; Munoz-Fernandez, M.A.; de la Mata, F.J.; Gomez, R. Synthesis, structure and molecular modelling of anionic carbosilane dendrimers. Dalton Trans. 2012, 41, 12733–12748. [Google Scholar] [CrossRef]
- Martinez, I.; Dopazo, J.; Melero, J.A. Antigenic structure of the human respiratory syncytial virus g glycoprotein and relevance of hypermutation events for the generation of antigenic variants. J. Gen. Virol. 1997, 78 Pt 10, 2419–2429. [Google Scholar] [CrossRef]
- Mbiguino, A.; Menezes, J. Purification of human respiratory syncytial virus: Superiority of sucrose gradient over percoll, renografin, and metrizamide gradients. J. Virol. Methods 1991, 31, 161–170. [Google Scholar] [CrossRef]
- Guerrero-Beltran, C.; Rodriguez-Izquierdo, I.; Serramia, M.J.; Araya-Duran, I.; Marquez-Miranda, V.; Gomez, R.; de la Mata, F.J.; Leal, M.; Gonzalez-Nilo, F.; Munoz-Fernandez, M.A. Anionic carbosilane dendrimers destabilize the gp120-cd4 complex blocking hiv-1 entry and cell to cell fusion. Bioconjug. Chem. 2018, 29, 1584–1594. [Google Scholar] [CrossRef] [PubMed]
- Rusnati, M.; Vicenzi, E.; Donalisio, M.; Oreste, P.; Landolfo, S.; Lembo, D. Sulfated k5 escherichia coli polysaccharide derivatives: A novel class of candidate antiviral microbicides. Pharm. Ther. 2009, 123, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.; Mattila, J.M.; Lehtinen, P.; Vuorinen, T.; Waris, M.; Heikkinen, T. Burden of respiratory syncytial virus infection during the first year of life. J. Infect. Dis. 2020, 223, 811–817. [Google Scholar] [CrossRef]
- Pena, M.; Jara, C.; Flores, J.C.; Hoyos-Bachiloglu, R.; Iturriaga, C.; Medina, M.; Carcey, J.; Espinoza, J.; Bohmwald, K.; Kalergis, A.M.; et al. Severe respiratory disease caused by human respiratory syncytial virus impairs language learning during early infancy. Sci. Rep. 2020, 10, 22356. [Google Scholar] [CrossRef]
- Behzadi, M.A.; Leyva-Grado, V.H. Overview of current therapeutics and novel candidates against influenza, respiratory syncytial virus, and middle east respiratory syndrome coronavirus infections. Front. Microbiol. 2019, 10, 1327. [Google Scholar] [CrossRef] [Green Version]
- Dzmitruk, V.; Szulc, A.; Shcharbin, D.; Janaszewska, A.; Shcharbina, N.; Lazniewska, J.; Novopashina, D.; Buyanova, M.; Ionov, M.; Klajnert-Maculewicz, B.; et al. Anticancer sirna cocktails as a novel tool to treat cancer cells. Part (b). Efficiency of pharmacological action. Int. J. Pharm. 2015, 485, 288–294. [Google Scholar] [CrossRef]
- Perise-Barrios, A.J.; Gomez, R.; Corbi, A.L.; de la Mata, J.; Dominguez-Soto, A.; Munoz-Fernandez, M.A. Use of carbosilane dendrimer to switch macrophage polarization for the acquisition of antitumor functions. Nanoscale 2015, 7, 3857–3866. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Izquierdo, I.; Natalia, C.; Garcia, F.; los Ángeles Munoz-Fernandez, M. G2-s16 sulfonate dendrimer as new therapy for treatment failure in hiv-1 entry inhibitors. Nanomedicine 2019, 14, 1095–1107. [Google Scholar] [CrossRef] [PubMed]
- Cena-Diez, R.; Vacas-Cordoba, E.; Garcia-Broncano, P.; de la Mata, F.J.; Gomez, R.; Maly, M.; Munoz-Fernandez, M.A. Prevention of vaginal and rectal herpes simplex virus type 2 transmission in mice: Mechanism of antiviral action. Int J. Nanomed. 2016, 11, 2147–2162. [Google Scholar]
- Sepulveda-Crespo, D.; Sanchez-Rodriguez, J.; Serramia, M.J.; Gomez, R.; De La Mata, F.J.; Jimenez, J.L.; Munoz-Fernandez, M.A. Triple combination of carbosilane dendrimers, tenofovir and maraviroc as potential microbicide to prevent hiv-1 sexual transmission. Nanomedicine 2015, 10, 899–914. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Izquierdo, I.; Gasco, S.; Munoz-Fernandez, M.A. High preventive effect of g2-s16 anionic carbosilane dendrimer against sexually transmitted hsv-2 infection. Molecules 2020, 25, 2965. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Beltran, C.; Garcia-Heredia, I.; Cena-Diez, R.; Rodriguez-Izquierdo, I.; Serramia, M.J.; Martinez-Hernandez, F.; Lluesma-Gomez, M.; Martinez-Garcia, M.; Munoz-Fernandez, M.A. Cationic dendrimer g2-s16 inhibits herpes simplex type 2 infection and protects mice vaginal microbiome. Pharmaceutics 2020, 12, 515. [Google Scholar] [CrossRef]



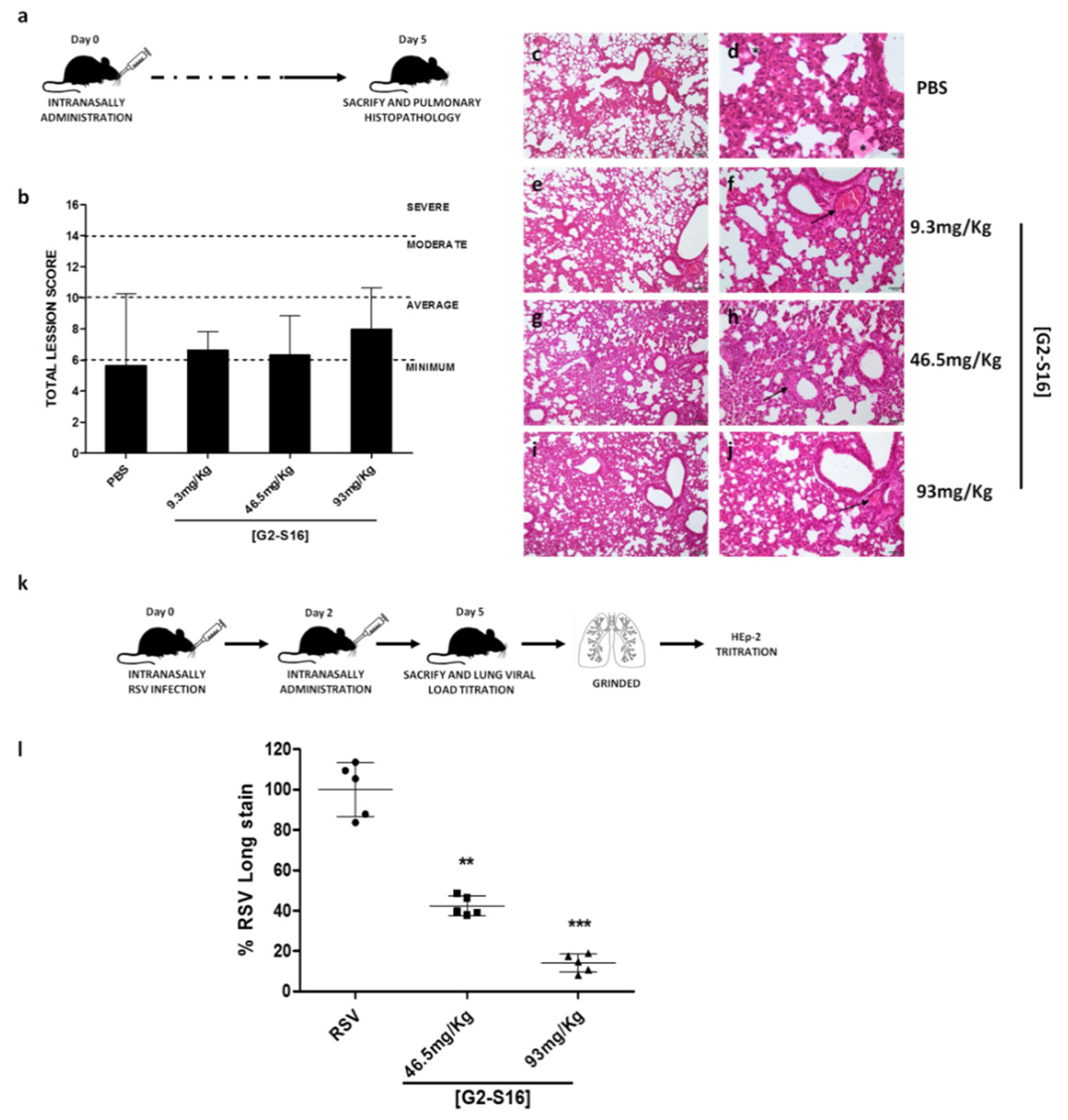
| Pulmonary Parenchyma | PBS | 9.3 mg/kg | 46.5 mg/kg | 93 mg/kg | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inflammation | 0 1 | 1 | 3 | 2 | 2 | 2 | 2 | 1 | 3 | 2 | 2 | 1 |
| Edema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Alveolar congestion | 0 | 0 | 3 | 1 | 1 | 2 | 1 | 2 | 3 | 2 | 1 | |
| Bleeding | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vascular thrombosis or congestion | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 2 | 1 | 2 | 3 | 2 |
| Atelectasis | 1 | 1 | 3 | 2 | 2 | 2 | 2 | 1 | 3 | 3 | 2 | 1 |
| Emphysema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pleura | PBS | 9.3 mg/kg | 46.5 mg/kg | 93 mg/kg | ||||||||
| Inflammatory infiltrate | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Edema | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Bleeding | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Neovasculation | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez-Izquierdo, I.; Ceña-Diez, R.; Serramia, M.J.; Rodriguez-Fernández, R.; Martínez, I.; Muñoz-Fernández, M. Role of G2-S16 Polyanionic Carbosilane Dendrimer in the Prevention of Respiratory Syncytial Virus Infection In Vitro and In Vivo in Mice. Polymers 2021, 13, 2141. https://doi.org/10.3390/polym13132141
Rodriguez-Izquierdo I, Ceña-Diez R, Serramia MJ, Rodriguez-Fernández R, Martínez I, Muñoz-Fernández M. Role of G2-S16 Polyanionic Carbosilane Dendrimer in the Prevention of Respiratory Syncytial Virus Infection In Vitro and In Vivo in Mice. Polymers. 2021; 13(13):2141. https://doi.org/10.3390/polym13132141
Chicago/Turabian StyleRodriguez-Izquierdo, Ignacio, Rafael Ceña-Diez, Maria Jesús Serramia, Rosa Rodriguez-Fernández, Isidoro Martínez, and Mariángeles Muñoz-Fernández. 2021. "Role of G2-S16 Polyanionic Carbosilane Dendrimer in the Prevention of Respiratory Syncytial Virus Infection In Vitro and In Vivo in Mice" Polymers 13, no. 13: 2141. https://doi.org/10.3390/polym13132141
APA StyleRodriguez-Izquierdo, I., Ceña-Diez, R., Serramia, M. J., Rodriguez-Fernández, R., Martínez, I., & Muñoz-Fernández, M. (2021). Role of G2-S16 Polyanionic Carbosilane Dendrimer in the Prevention of Respiratory Syncytial Virus Infection In Vitro and In Vivo in Mice. Polymers, 13(13), 2141. https://doi.org/10.3390/polym13132141