Ageing Effects in Mounting Media of Microscope Slide Samples from Natural History Collections: A Case Study with Canada Balsam and PermountTM
Abstract
:1. Introduction
2. Materials and Methods
2.1. Historical Microscope Slide Samples
2.2. Mounting Media and Sample Preparation for Accelerated Ageing Experiments
2.3. Accelerated Ageing Experiments
2.4. Spectroscopic Measurements and Microscopic Imaging
3. Results and Discussion
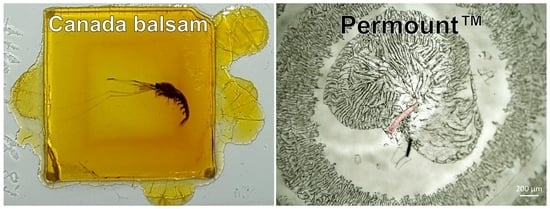
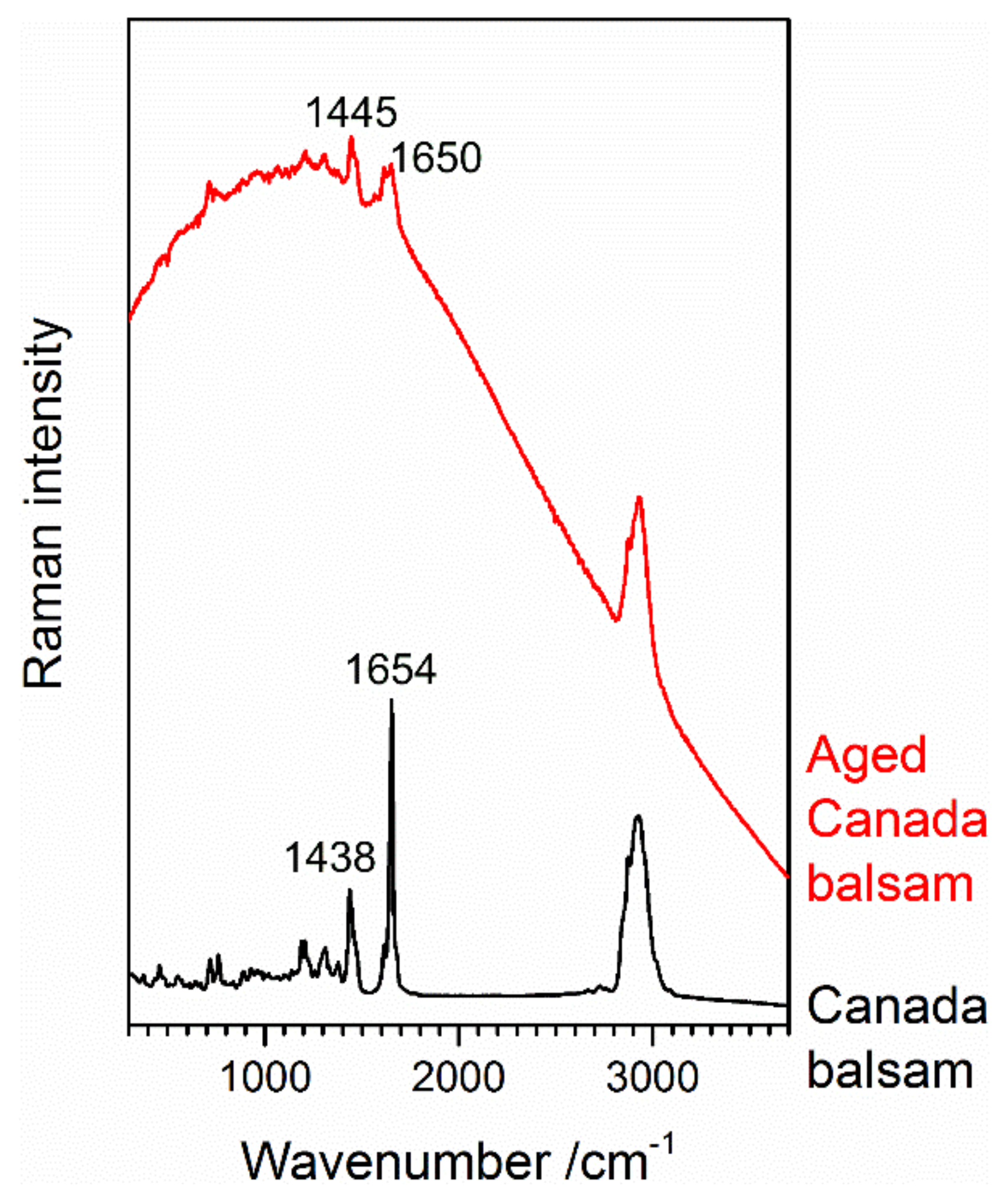
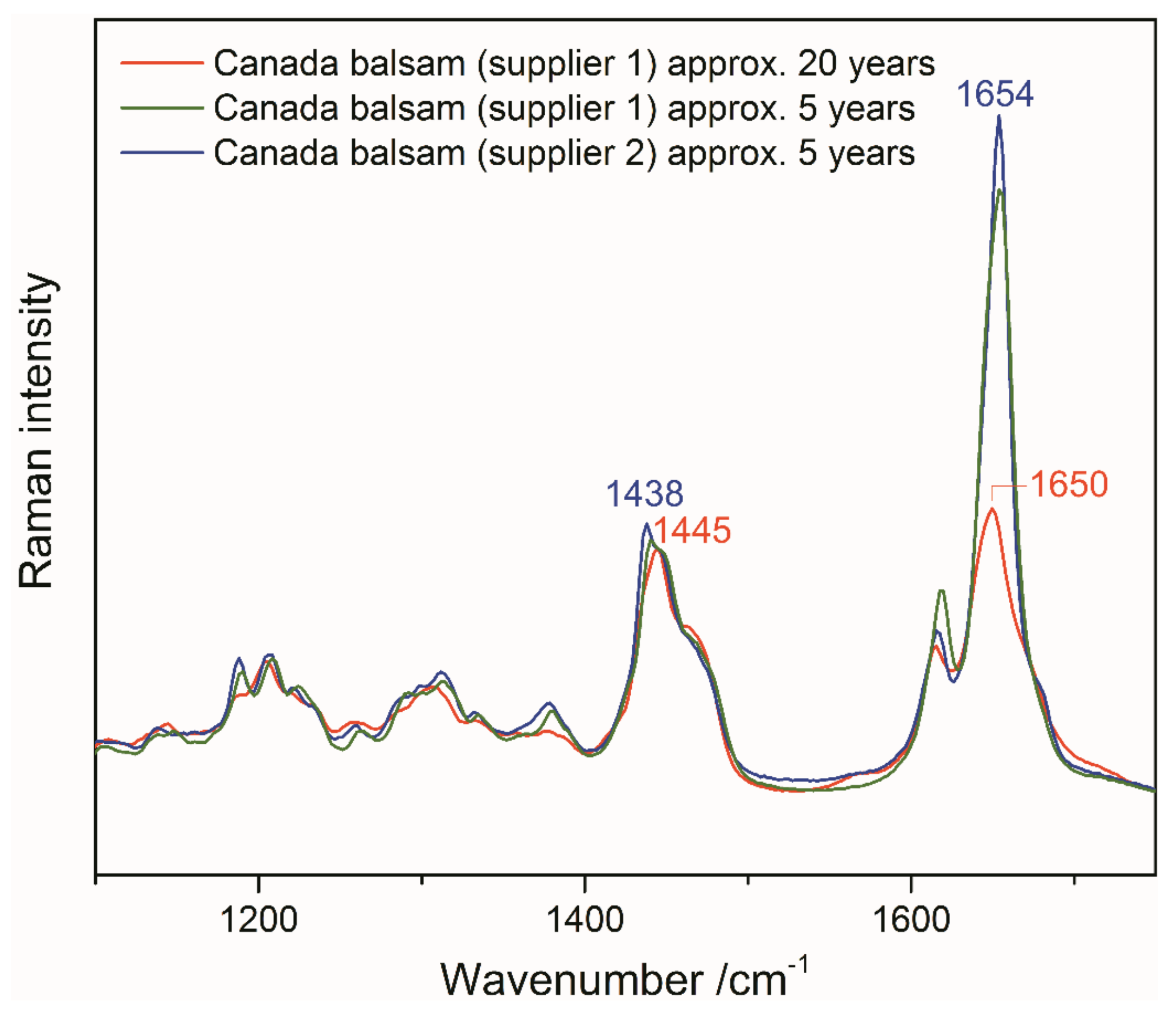
3.1. Identification of the Mounting Media of Historical Microscope Slide Samples
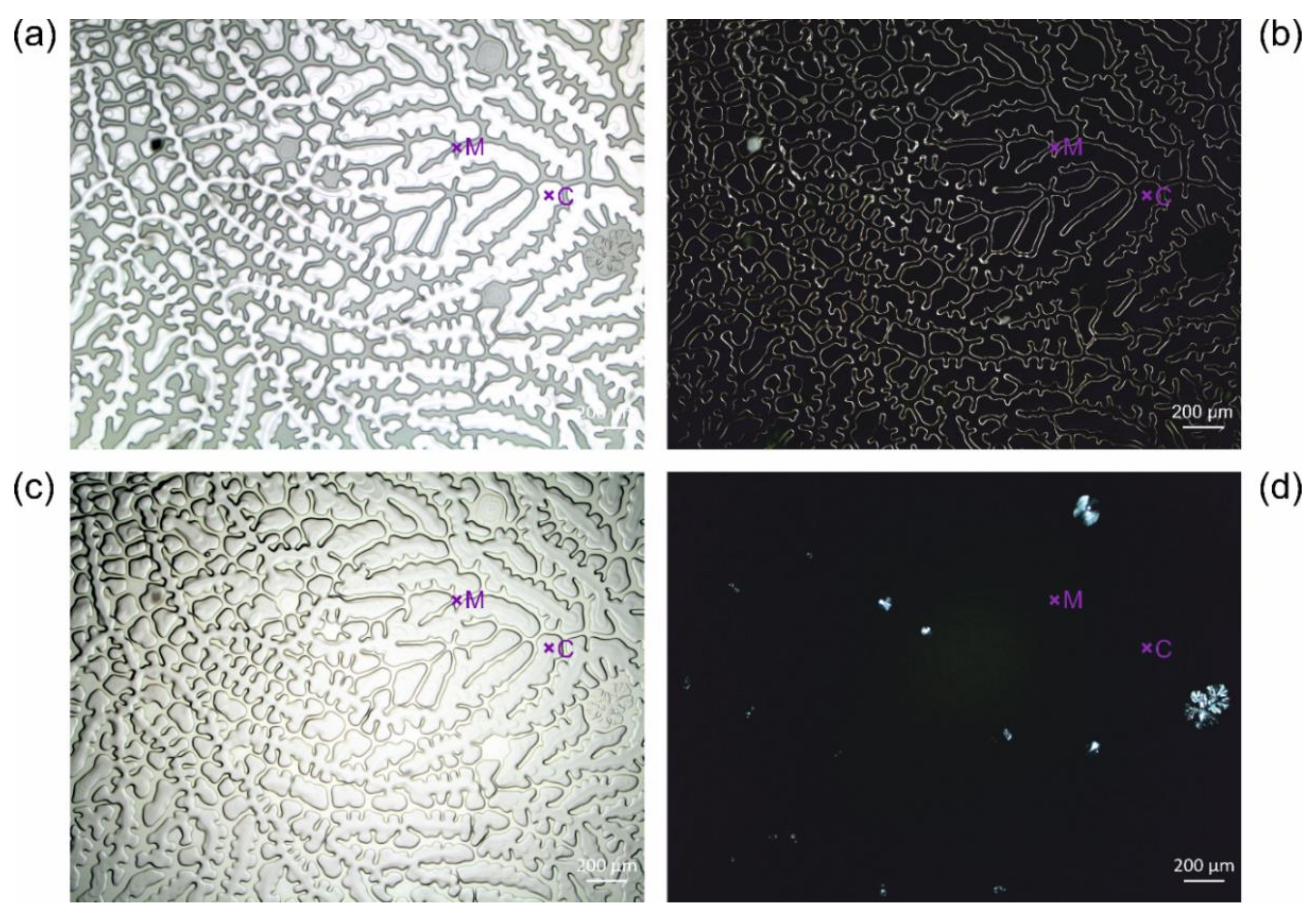
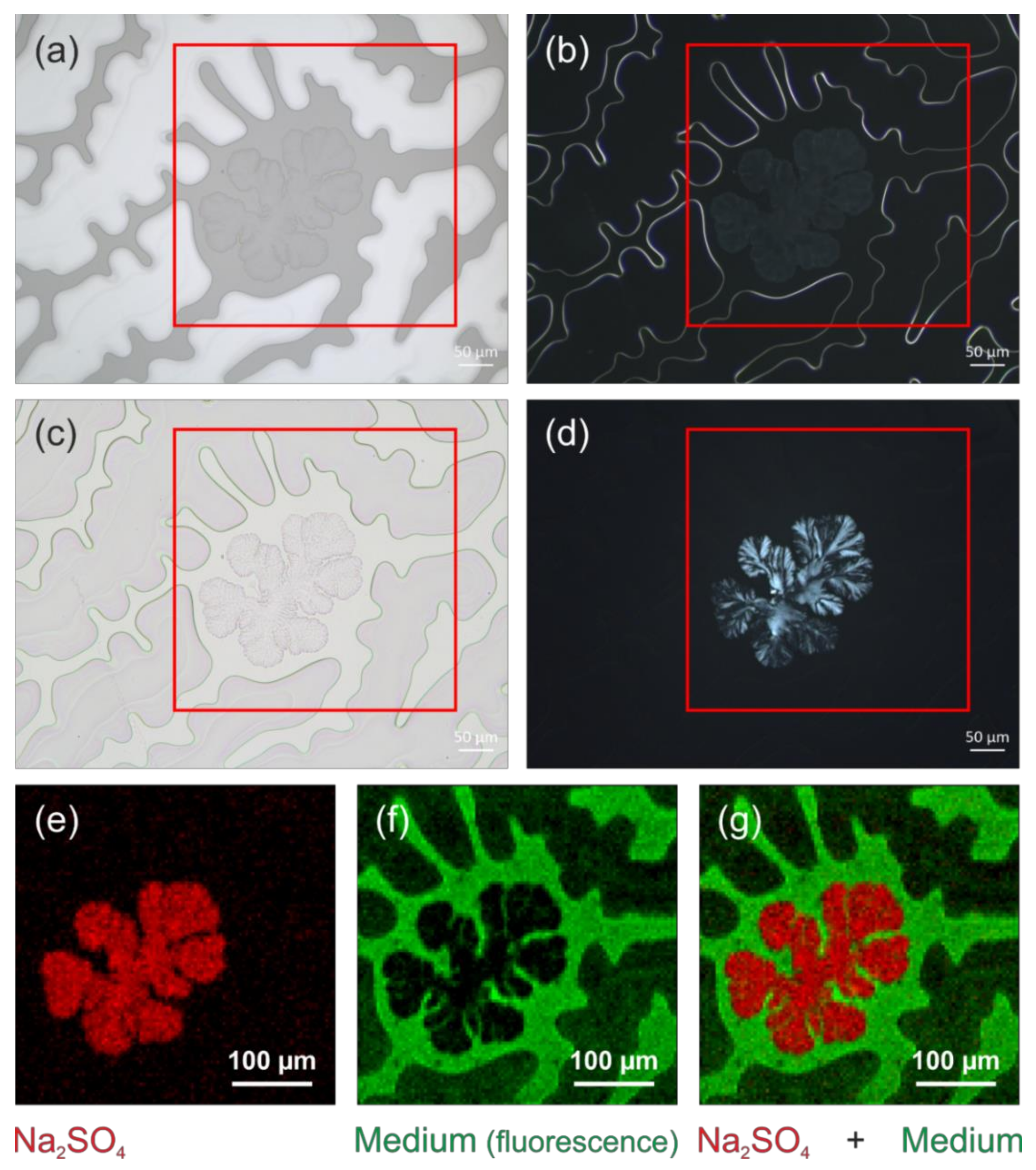
3.2. Typical Damages in Historical Microscope Slide Samples
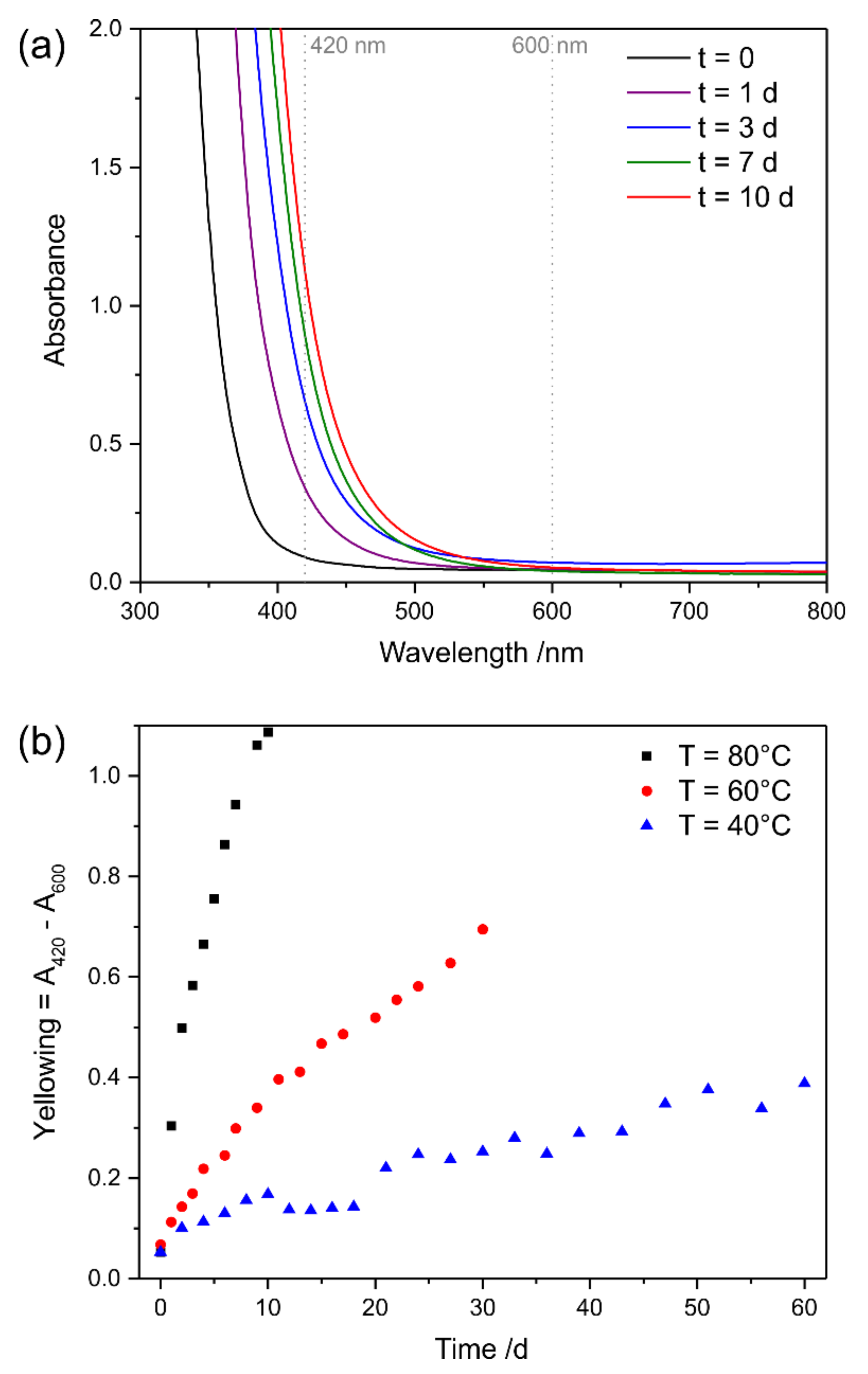
3.2.1. Yellowing
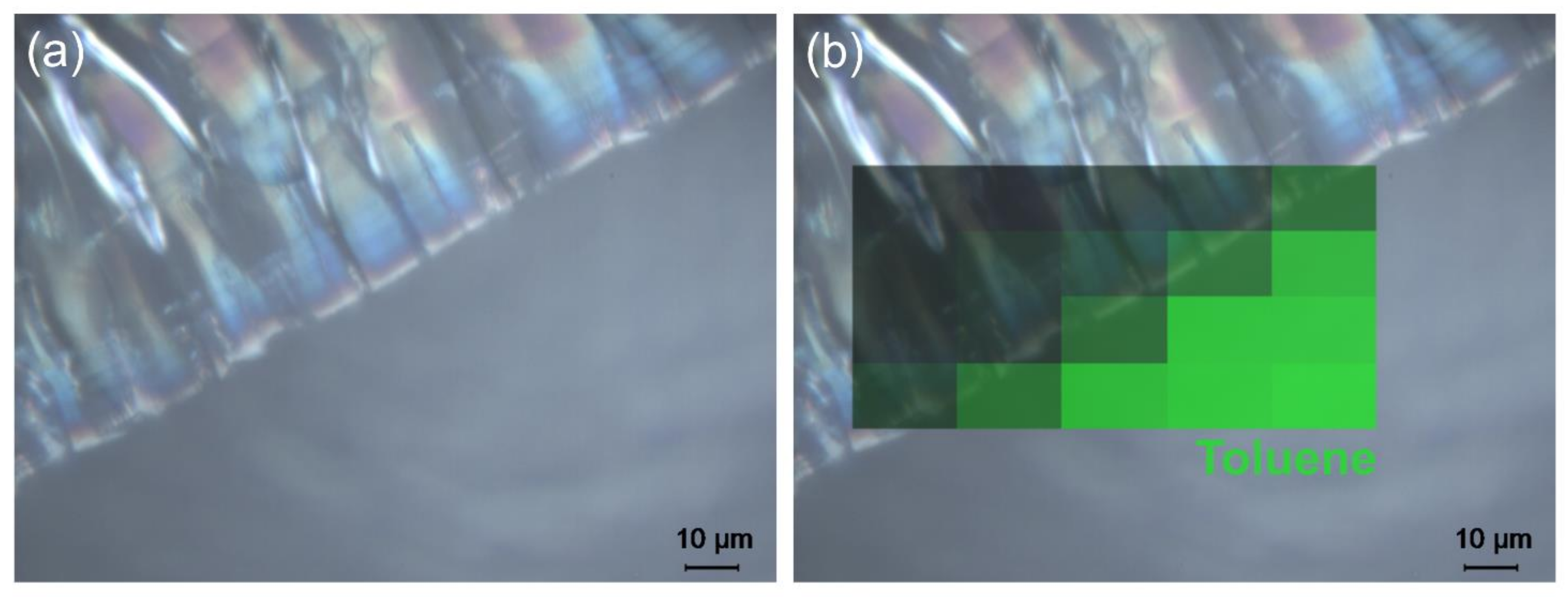
3.2.2. Crack Formation
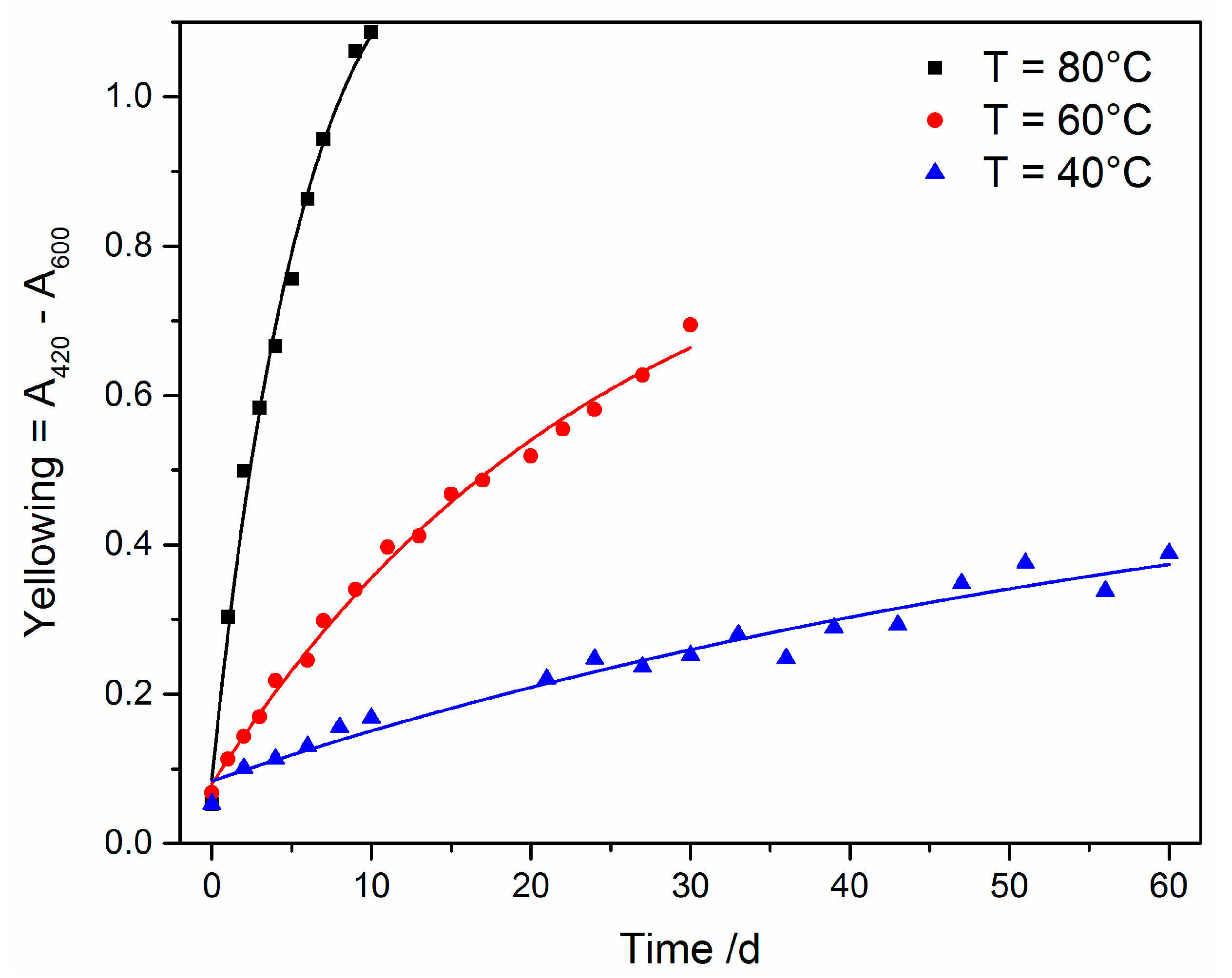
3.3. Accelerated Ageing of Mounting Media
3.3.1. UV Irradiation
3.3.2. Oxidative Ageing at Elevated Temperatures
4. Conclusions
5. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
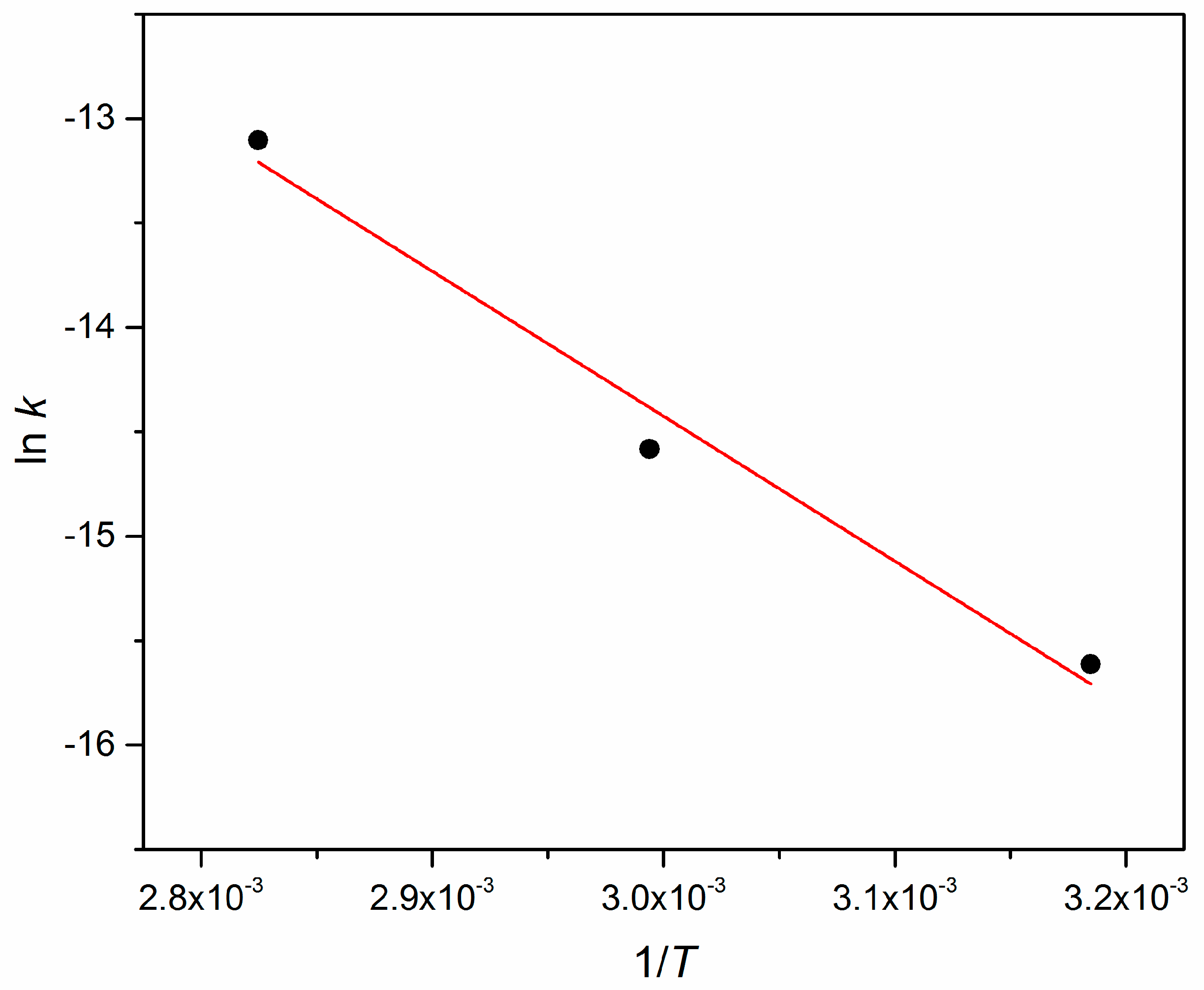
Appendix A. Arrhenius Plot
| T [°C] | k [s−1] | R2 |
|---|---|---|
| 40 | 1242 | 0.9571 |
| 60 | 3463 | 0.9938 |
| 80 | 15211 | 0.9907 |
References
- Brown, P.A. A review of techniques used in the preparation, curation and conservation of microscope slides at the Natural History Museum, London. Biol. Curator 1997, 10, 1–33. [Google Scholar]
- di Giacomo, M. Unhappy Couples: Degradation of Microscope Slides due to Their Mounting Media. In Abstract Book: 45th Annual Meeting of American Institute for Conservation of Historic and Artistic Works; American Institute for Conservation of Historic and Artistic Works: Washington, DC, USA, 2017; p. 32. [Google Scholar]
- Neuhaus, B.; Schmid, T.; Riedel, J. Collection management and study of microscope slides: Storage, profiling, deterioration, restoration procedures, and general recommendations. Zootaxa 2017, 4322, 1–173. [Google Scholar] [CrossRef]
- International Commission on Zoological Nomenclature. International Code of Zoological Nomenclature, 4th ed.; International Trust for Zoological Nomenclature, c/o The Natural History Museum: London, UK, 1999; 306p. [Google Scholar]
- Garner, R.; Horie, C.V. The conservation and restoration of microscope slides of mosses. Stud. Conserv. 1984, 29, 93–99. [Google Scholar]
- Valentine-Baars, A.; Kerbey, H. Historic Thin Sections—Crazy to Conserve? Poster at Geo-Material Sample Preparation for Microscopy Workshop, University of Oxford, Royal Microscopical Society: Oxford, UK. 2014. Available online: https://www.researchgate.net/publication/268683951 (accessed on 30 April 2021).
- Hollander, D.H.; Frost, J.K. Annual bands in synthetic-resin-mounted microscopic slides. Acta Cytol. 1970, 14, 142–144. [Google Scholar]
- Hollander, D.H.; Frost, J.K. Antioxidant inhibition of stain fading and mounting medium crazing. Acta Cytol. 1971, 15, 419. [Google Scholar]
- Hooper, D.J. Handling, Fixing and Mounting Nematodes. In Laboratory Methods for Work with Plant and Soil Nematodes, 5th ed.; Southey, J.F., Ed.; Ministry of Agriculture, Fisheries and Food, Technical Bulletin 2, Her Majesty’s Stationary Office: London, UK, 1970; pp. 39–54. [Google Scholar]
- Upton, M.S. Aqueous gum-chloral slide mounting media: An historical review. Bull. Entomol. Res. 1993, 83, 267–274. [Google Scholar] [CrossRef]
- Koomen, P.; von Vaupel Klein, J.C. The suitability of various mounting media for permanent mounts of small chitinous crustaceans, with special reference to the observation of integumental organs. Crustaceana 1995, 68, 428–437. [Google Scholar] [CrossRef]
- Wiggins, K.; Drummond, P. Identifying a suitable mounting medium for use in forensic fibre examination. Sci. Justice 2007, 47, 2–8. [Google Scholar] [CrossRef]
- de Lillo, E.; Craemer, C.; Amrine, J.W., Jr.; Nuzzaci, G. Recommended procedures and techniques for morphological studies of Eriophyoidea (Acari: Prostigmata). Exp. Appl. Acarol. 2010, 51, 283–307. [Google Scholar] [CrossRef]
- Jacinavicius, F.C.; Badari, J.C.; Ramirez, D.G.; Maraes, R.H.P.; Onofria, V.C.; Barros-Battesti, D.M. Techniques for restoration of mite (Acari) preparations in deteriorated Hoyer’s mounting medium. Neotrop. Entomol. 2013, 42, 328–329. [Google Scholar] [CrossRef]
- Neuhaus, B. Kinorhyncha (=Echinodera). In Volume 1 Nematomorpha, Priapulida, Kinorhyncha, Loricifera; Schmidt-Rhaesa, A., Ed.; Handbook of Zoology, Gastrotricha, Cycloneuralia and Gnathifera; Walter de Gruyter: Berlin, Germany, 2013; pp. 181–348. [Google Scholar]
- Neuhaus, B.; Sørensen, M.V. Populations of Campyloderes sp. (Kinorhyncha, Cyclorhagida): One species with significant morphological variation? Zool. Anz. 2013, 252, 48–75. [Google Scholar] [CrossRef] [Green Version]
- Neuhaus, B. Redescription of Tubulideres seminoli Sørensen et al., 2007 and notes on Wollunquaderes majkenae Sørensen & Thormar, 2010 (Kinorhyncha, Cyclorhagida): Morphology, postembryonic development, life cycle, and new characters. Zool. Anz. 2017, 270, 123–154. [Google Scholar]
- Neuhaus, B.; Kegel, A. Redescription of Cateria gerlachi (Kinorhyncha, Cyclorhagida) from Sri Lanka and of C. styx from Brazil, with notes on C. gerlachi from India and C. styx from Chile, and ground pattern of the genus. Zootaxa 2015, 3965, 1–77. [Google Scholar] [CrossRef] [Green Version]
- Ryss, A.Y. Express technique to prepare permanent collection slides of nematodes. Zoosystematica Ross. 2003, 11, 257–260. [Google Scholar]
- Ryss, A.Y. A simple express technique to process nematodes for collection slide mounts. J. Nematol. 2017, 49, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Loveland, R.P.; Centifanto, Y.M. Mounting media for microscopy. Microscope 1986, 34, 181–241. [Google Scholar]
- di Giacomo, M. Using FTIR to Identify Slide Mounting Media: A First Step towards Best Practices in the Care of Slide Mounted Specimens. In Abstract Book: 32nd Annual Meeting of the Society for the Preservation of Natural History Collections; Society for the Preservation of Natural History Collections: Chicago, IL, USA, 2017; pp. 49–50. [Google Scholar]
- Neuhaus, B.; dal Zotto, M.; Yamasaki, H.; Higgins, R.P. Revision of Condyloderes (Kinorhyncha, Cyclorhagida) including description of Condyloderes shirleyi sp. nov. Zootaxa 2019, 4561, 1–91. [Google Scholar] [CrossRef] [Green Version]
- Schmid, T.; Jungnickel, R.; Neuhaus, B.; Riedel, J.; Kneipp, J.; Lüter, L. Raman spectroscopy as a tool for the collection management of microscope slides. Zool. Anz. 2016, 265, 178–190. [Google Scholar] [CrossRef] [Green Version]
- Higgins, R.P. Taxonomy and postembryonic development of the Cryptorhagae, a new suborder for the mesopsammic kinorhynch genus Cateria. Transact. Am. Microsc. Soc. 1968, 87, 21–39. [Google Scholar] [CrossRef]
- de la Rie, E.R. Photochemical and thermal degradation of films of dammar resin. Stud. Conserv. 1988, 33, 53–70. [Google Scholar]
- Winkler, W.; Kirchner, E.C.; Asenbaum, A.; Musso, M. A Raman spectroscopic approach to the maturation process of fossil resins. J. Raman Spectrosc. 2001, 32, 59–63. [Google Scholar] [CrossRef]
- Naglik, B.; Mroczkowska-Szerszeń, M.; Dumańska-Słowik, M.; Natkaniec-Nowak, L.; Drzewicz, P.; Stach, P.; Żukowska, G. Fossil Resins–Constraints from Portable and Laboratory Near-infrared Raman Spectrometers. Minerals 2020, 10, 104. [Google Scholar] [CrossRef] [Green Version]
- Dietemann, P.; Kallin, M.; Zumbühl, S.; Knochenmuss, R.; Wüllfert, S.; Zenobi, R. A Mass Spectrometry and Electron Paramagnetic Resonance Study of Photochemical and Thermal Aging of Triterpenoid Varnishes. Anal. Chem. 2001, 73, 2087–2096. [Google Scholar] [CrossRef] [PubMed]
- Scalarone, D.; Duursma, M.C.; Boon, J.J.; Chiantore, O. MALDI-TOF mass spectrometry on cellulosic surfaces of fresh and photo-aged di- and triterpenoid varnish resins. J. Mass Spectrom. 2005, 40, 1527–1535. [Google Scholar] [CrossRef]
- Arrhenius, S. Über die Dissociationswärme und den Einfluss der Temperatur auf den Dissociationsgrad der Elektrolyte. Z. Phys. Chem. 1889, 4, 96–116. [Google Scholar] [CrossRef] [Green Version]
- Hemmerlich, K.J. General Aging Theory and Simplified Protocol for Accelerated Aging of Medical Devices. Medical Device and Diagnostics Industry News. 1998. Available online: https://www.mddionline.com/design-engineering/general-aging-theory-and-simplified-protocol-accelerated-aging-medical-devices (accessed on 30 April 2021).
- Hukins, D.W.L.; Mahomed, A.; Kukureka, S.N. Accelerated aging for testing polymeric biomaterials and medical devices. Med. Eng. Phys. 2008, 30, 1270–1274. [Google Scholar] [CrossRef]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. The Power of Databases: The Rruff Project. In Highlights in Mineralogical Crystallography; Armbruster, T., Danisi, R.M., Eds.; Walter de Gruyter: Berlin, Germany, 2015; pp. 1–30. [Google Scholar]





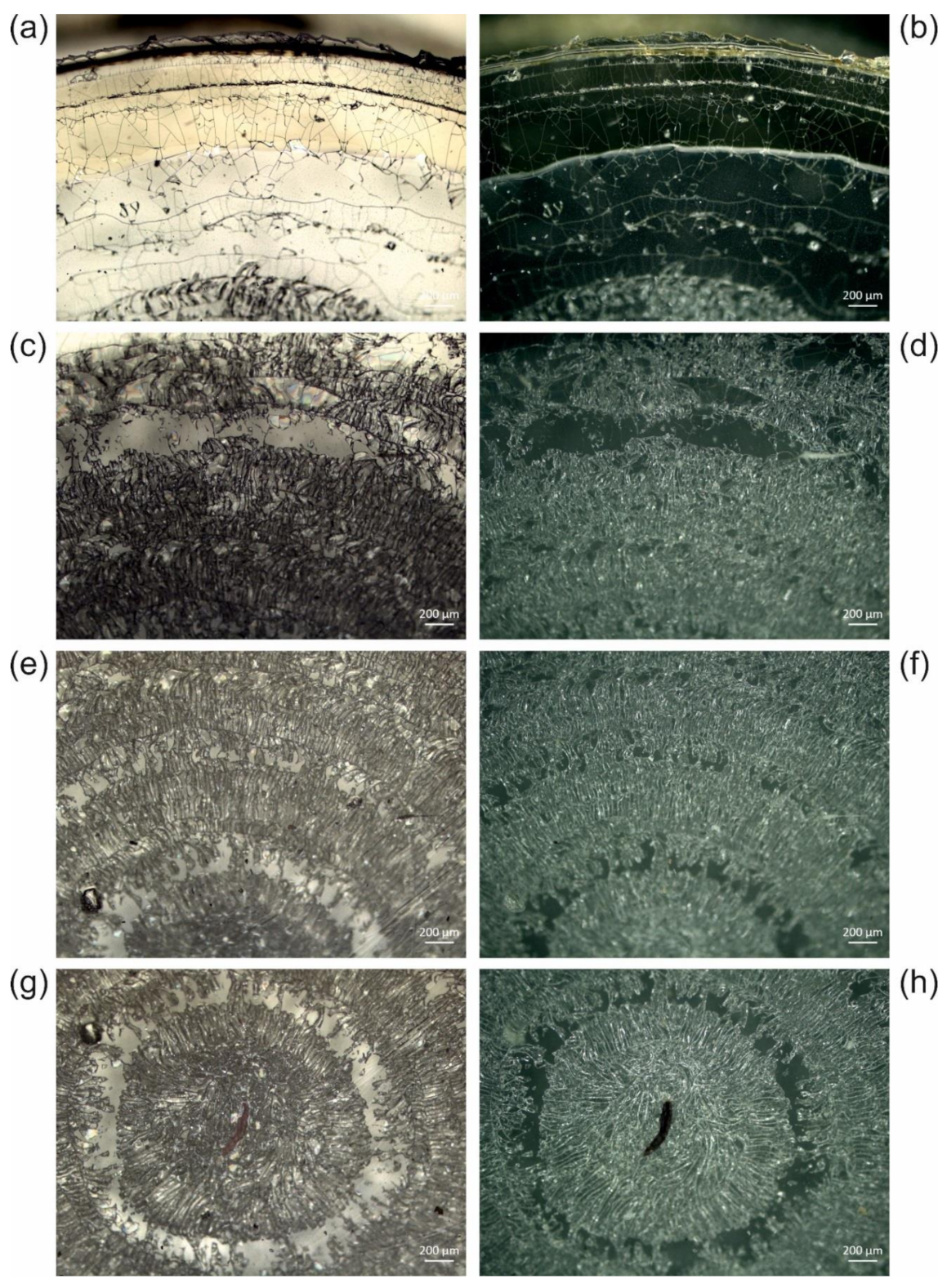
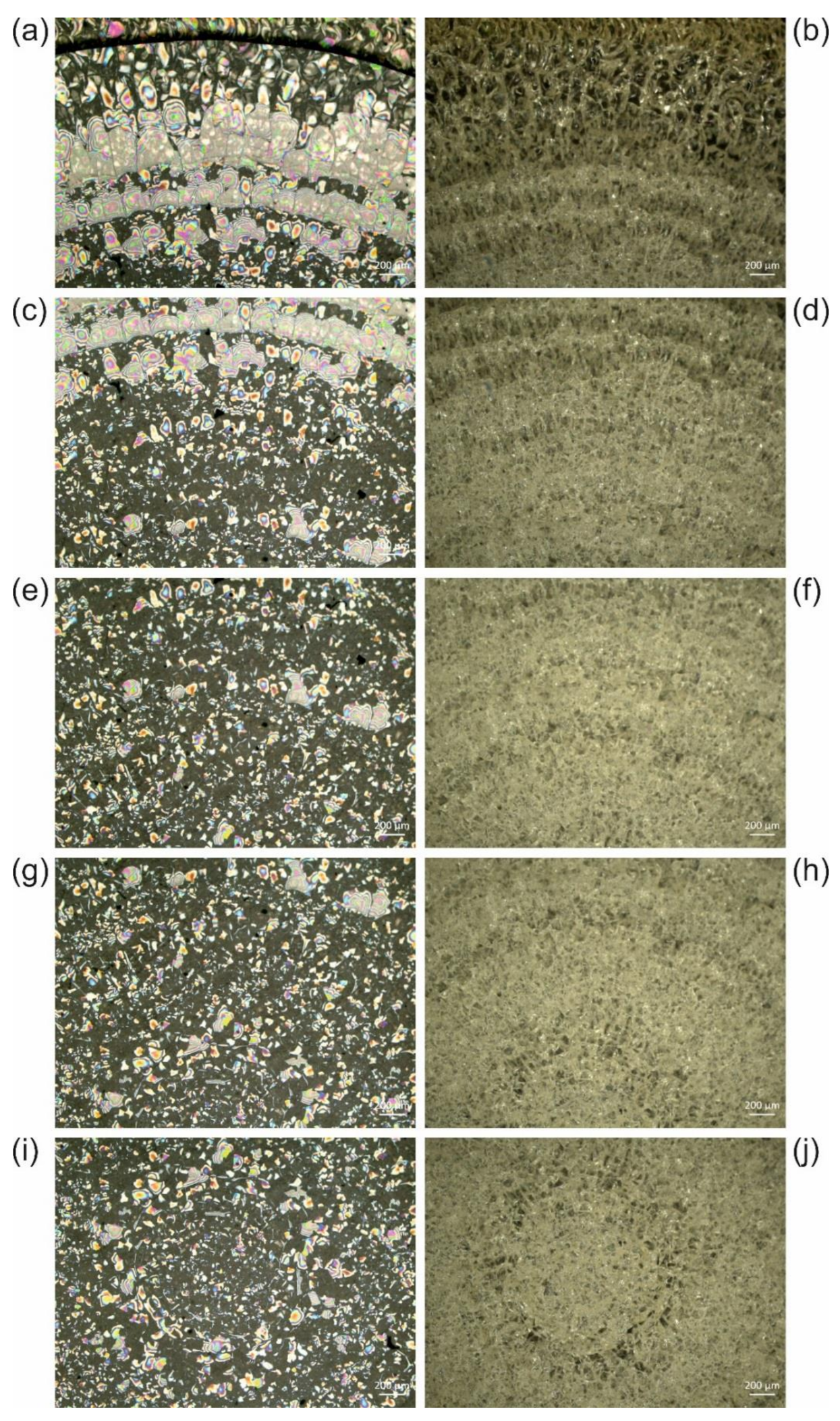





| Supraspecific Taxon | Species | Catalogue Number | Deposition Site | Location |
|---|---|---|---|---|
| Kinorhyncha | Cateria gerlachi Higgins, 1968 [25] | 1226524 | United States National Museum, Washington, D.C., Smithsonian Institution | North Indian Ocean, Bay of Bengal, India, Waltair, beach near Palm Beach Rocks |
| 1226531 | ||||
| 1226533 | ||||
| 1226536 | ||||
| 1226547 | ||||
| 1226549 | North Indian Ocean, Bay of Bengal, India, beach near Waltair, 17°50′ N, 083°15′ E | |||
| Insecta | Ceroplastes formicarius Hempel, 1900 | n. a. | Museum für Naturkunde Berlin | Brazil, Yparanga (=Ypiranga); mounted by T.K. Qin 1990 |
| Forda formicaria von Heyden, 1837 | n. a. | Germany, Saxony, Sächsische Schweiz, from grass roots; mounted by Heymons about or after 1907 | ||
| Crustacea | Stylocheiron longicorne Sars, 1883 (= S. mastigophorum G.O. Sars, 1883) | n. a. | Atlantic Ocean, 30°34.9′ S, 006°10.2′ E, 5108 m depth; mounted after 1899 | |
| undetermined species | n. a. | n. a. | ||
| Mammalia | Sorex Araneus Linnaeus, 1758 | 142b | Hubrecht Collection from Naturalis Biodiversity Center, Leiden, temporarily given on loan to Museum für Naturkunde Berlin | n. a. |
| Ctenodactylus gundi (Rothmann, 1776) | 208a | Utrecht; mounted by A. Weiss 1971 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmid, T.; Hidde, J.; Grünier, S.; Jungnickel, R.; Dariz, P.; Riedel, J.; Neuhaus, B. Ageing Effects in Mounting Media of Microscope Slide Samples from Natural History Collections: A Case Study with Canada Balsam and PermountTM. Polymers 2021, 13, 2112. https://doi.org/10.3390/polym13132112
Schmid T, Hidde J, Grünier S, Jungnickel R, Dariz P, Riedel J, Neuhaus B. Ageing Effects in Mounting Media of Microscope Slide Samples from Natural History Collections: A Case Study with Canada Balsam and PermountTM. Polymers. 2021; 13(13):2112. https://doi.org/10.3390/polym13132112
Chicago/Turabian StyleSchmid, Thomas, Julia Hidde, Sophie Grünier, Robert Jungnickel, Petra Dariz, Jens Riedel, and Birger Neuhaus. 2021. "Ageing Effects in Mounting Media of Microscope Slide Samples from Natural History Collections: A Case Study with Canada Balsam and PermountTM" Polymers 13, no. 13: 2112. https://doi.org/10.3390/polym13132112
APA StyleSchmid, T., Hidde, J., Grünier, S., Jungnickel, R., Dariz, P., Riedel, J., & Neuhaus, B. (2021). Ageing Effects in Mounting Media of Microscope Slide Samples from Natural History Collections: A Case Study with Canada Balsam and PermountTM. Polymers, 13(13), 2112. https://doi.org/10.3390/polym13132112