In Situ Characterization of Polycaprolactone Fiber Response to Quasi-Static Tensile Loading in Scanning Electron Microscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electrospun PCL Samples
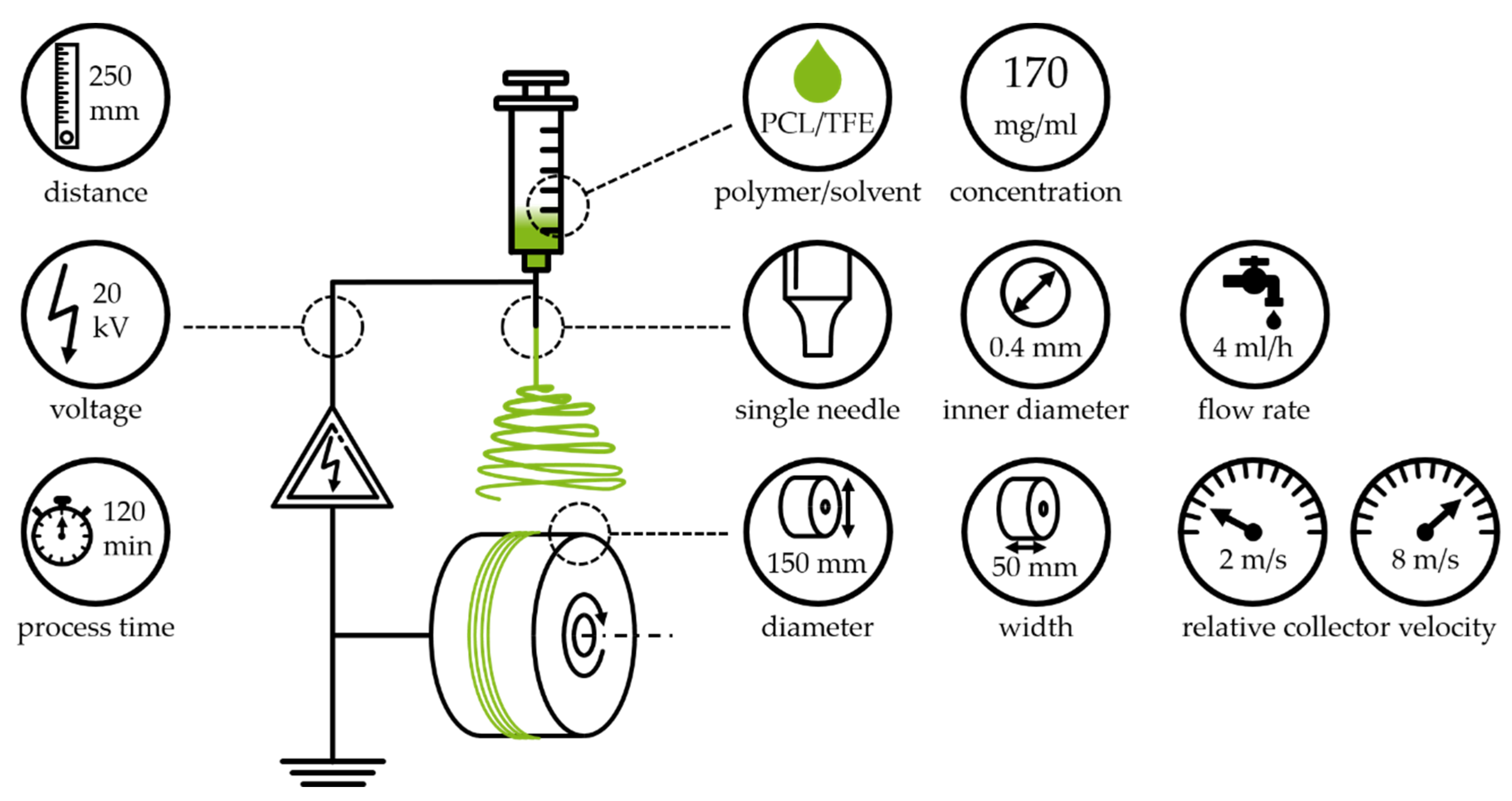
2.1.1. Electrospinning
2.1.2. Processing System
2.1.3. Experimental Procedure and Parameter Settings
2.1.4. SEM-Based Analysis of Fiber Diameter and Degree of Orientation
2.2. In Situ Tensile Testing of Polymer Fibers
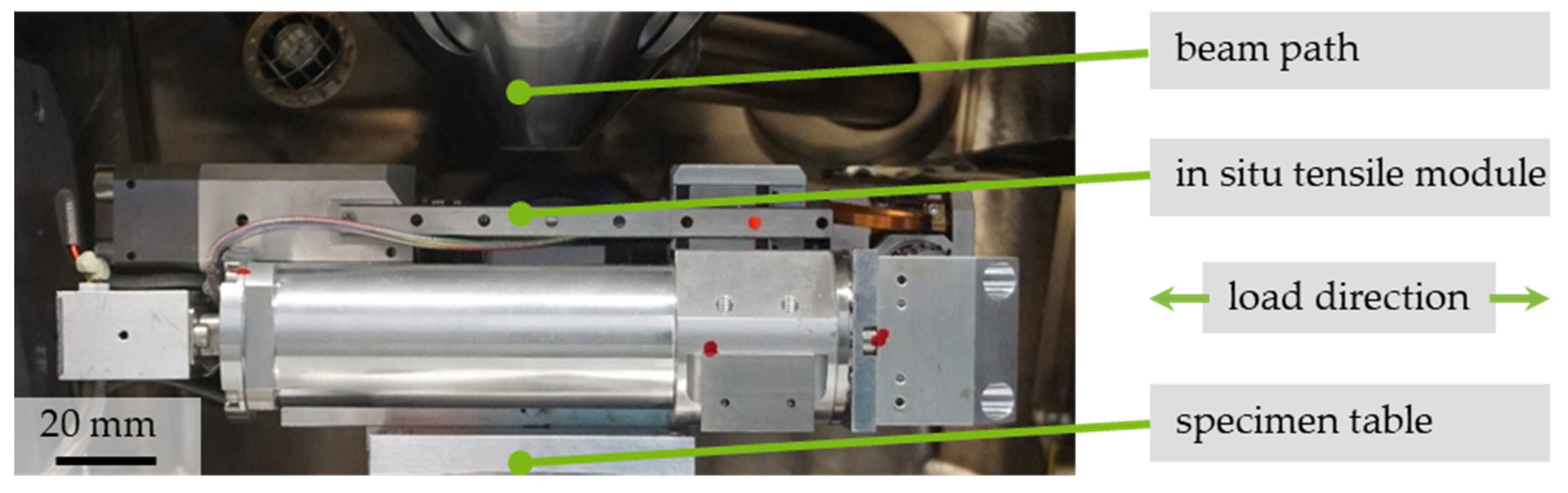
2.2.1. Test Setup for In Situ Testing
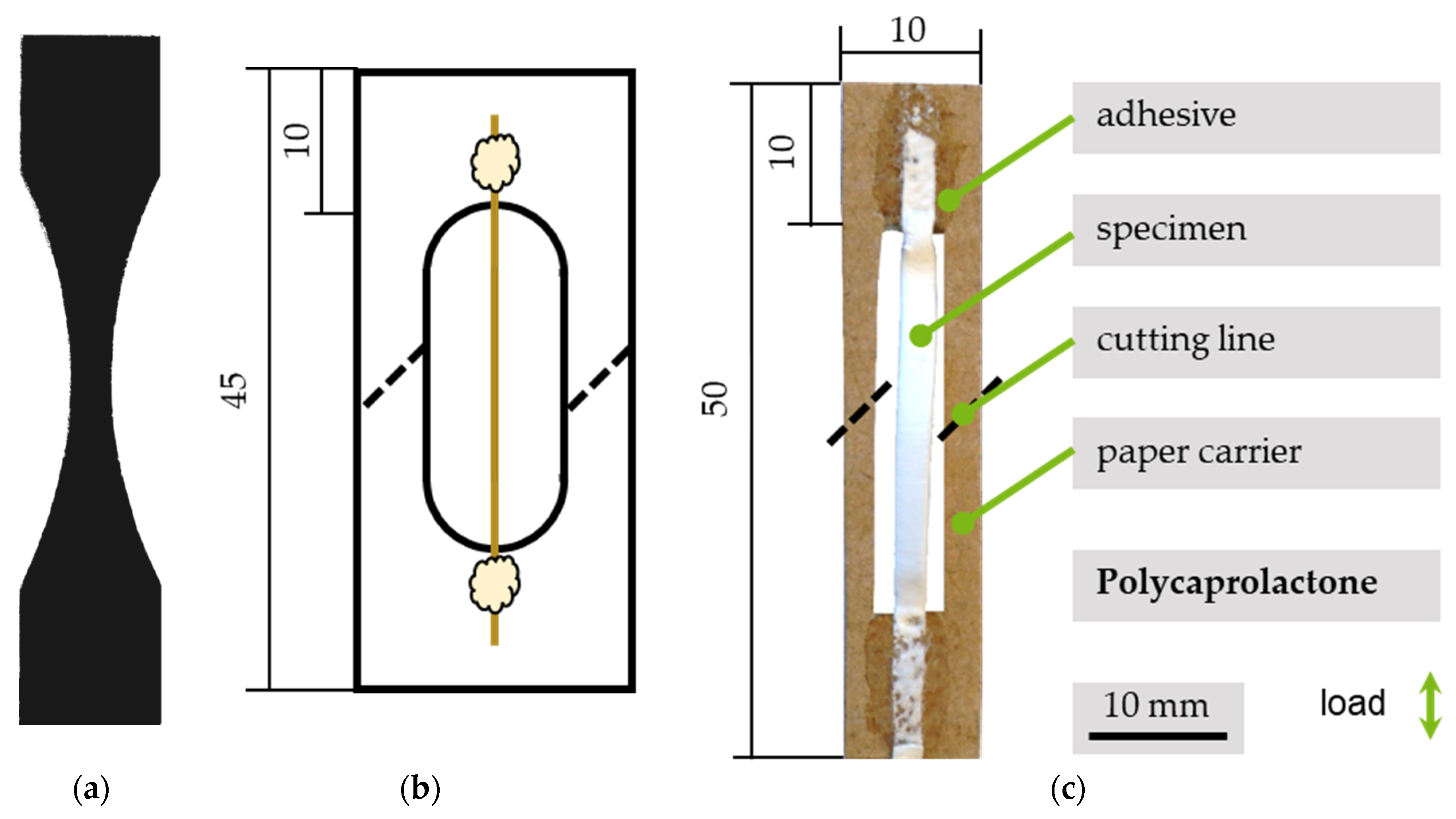
2.2.2. Sample Preparation
2.3. Qualitative and Quantitative Evaluation of Microscopic Images
2.4. Statistical Analysis
3. Results
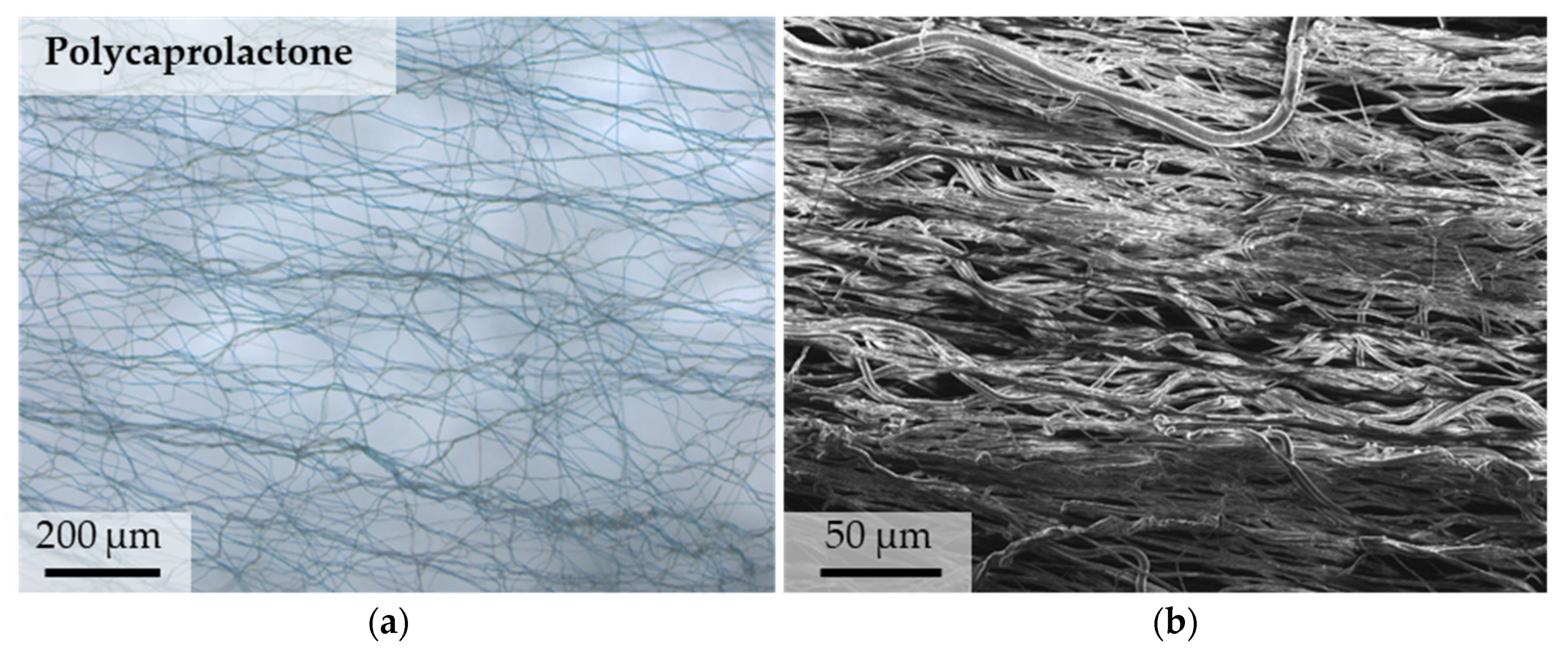
3.1. Microstructure of PCL
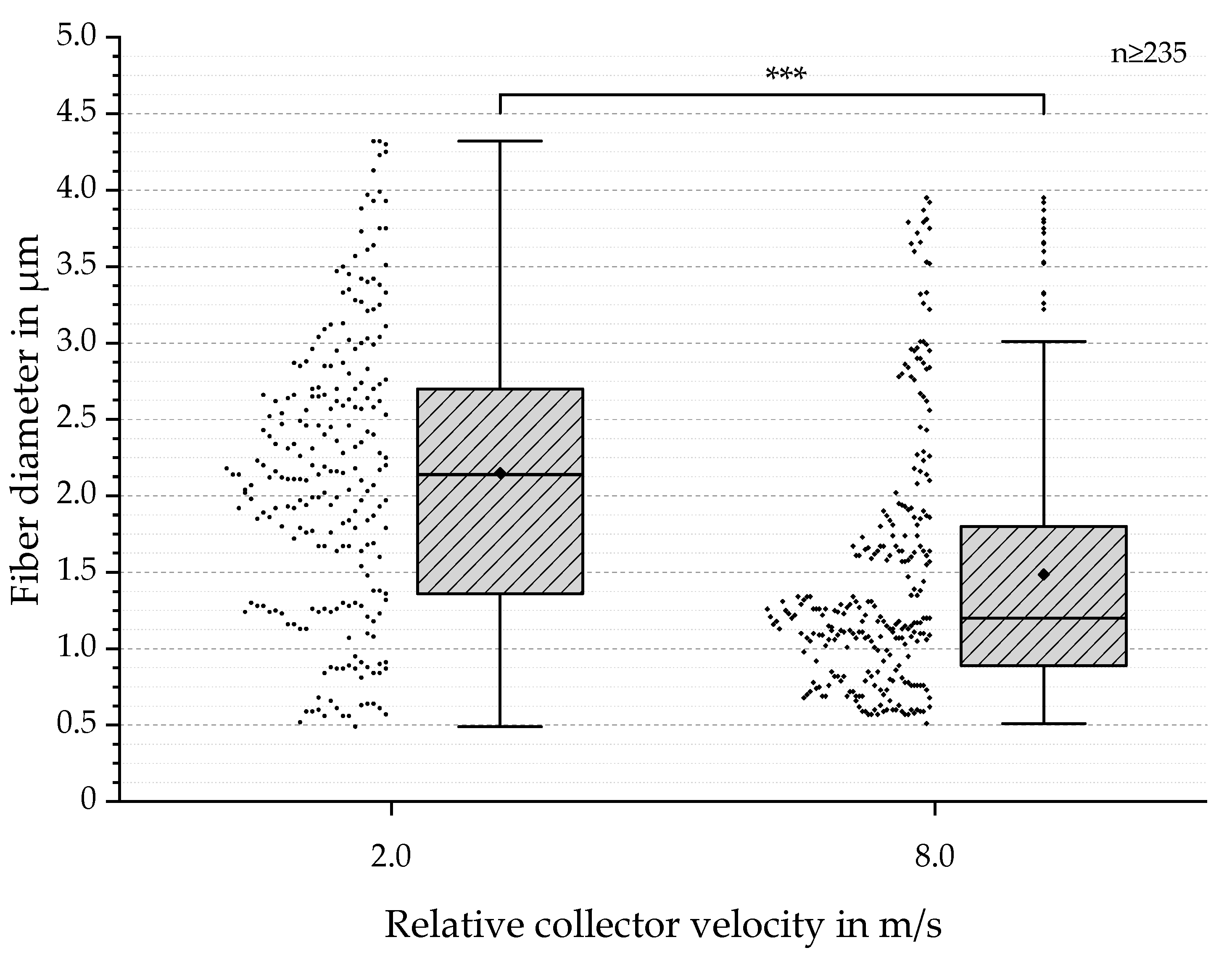
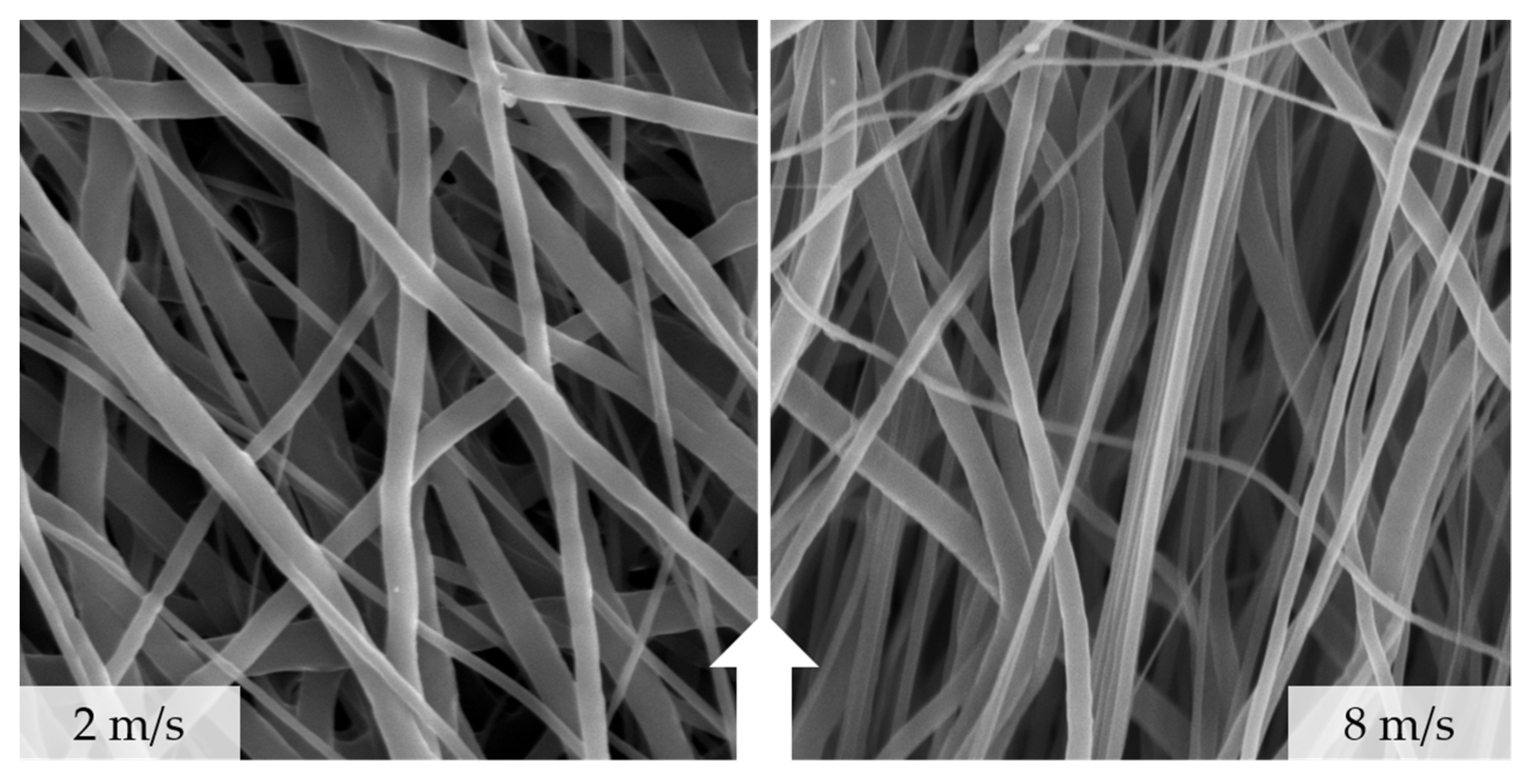
3.2. SEM-Based Analysis of Fiber Diameter and Degree of Orientation
3.3. Transversal Contraction under Tensile Load
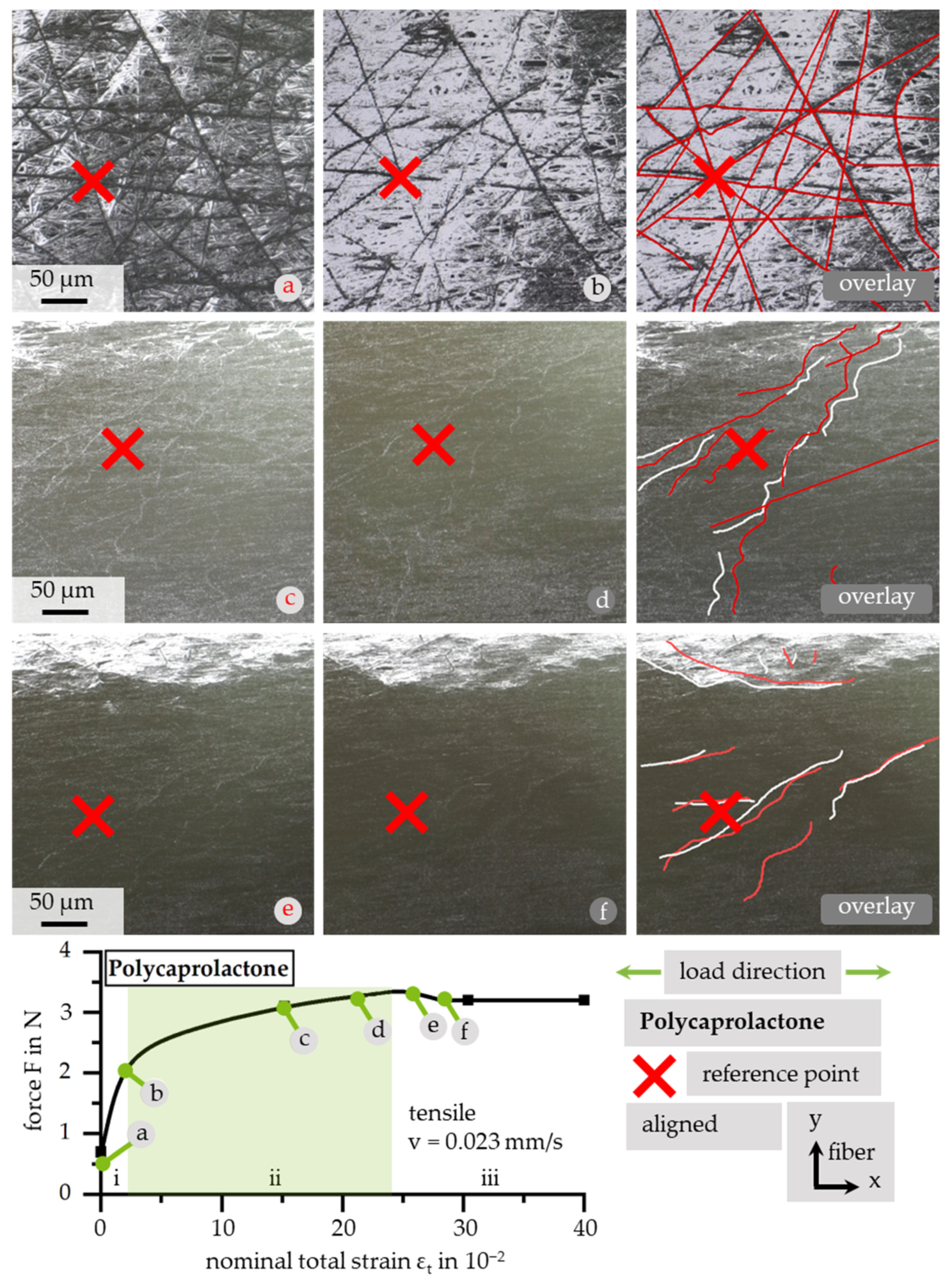
3.4. Response of Microstructure Due to In Situ Tensile Testing
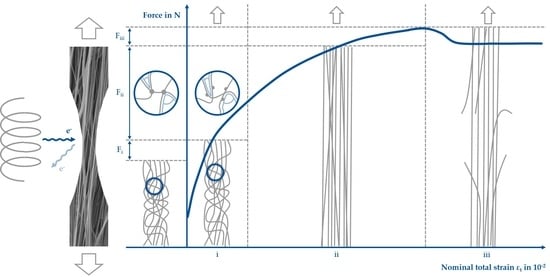
3.4.1. Stress–Strain Behavior of PCL under Tensile Loading
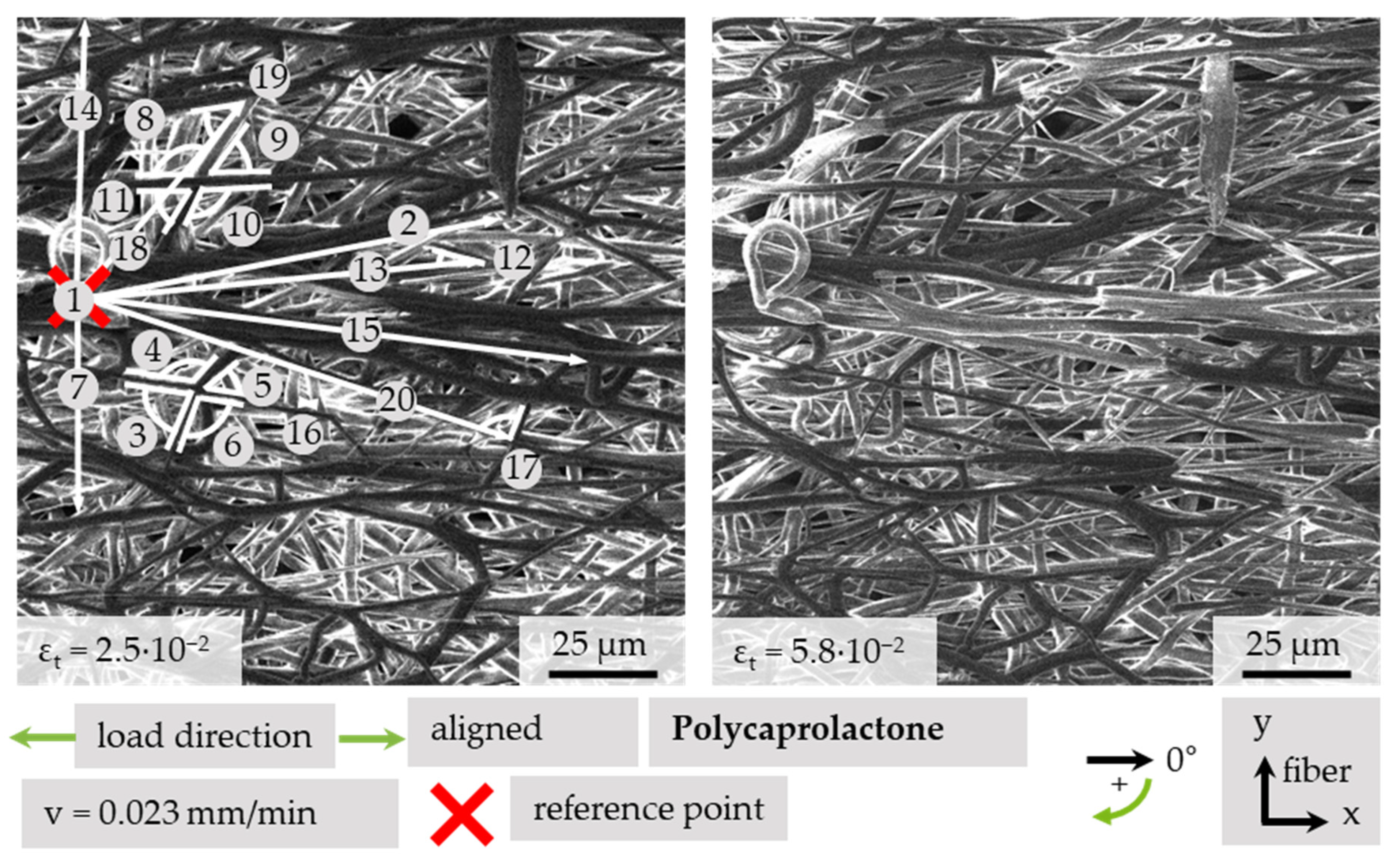
3.4.2. Quantitative Analysis of Fiber Movement
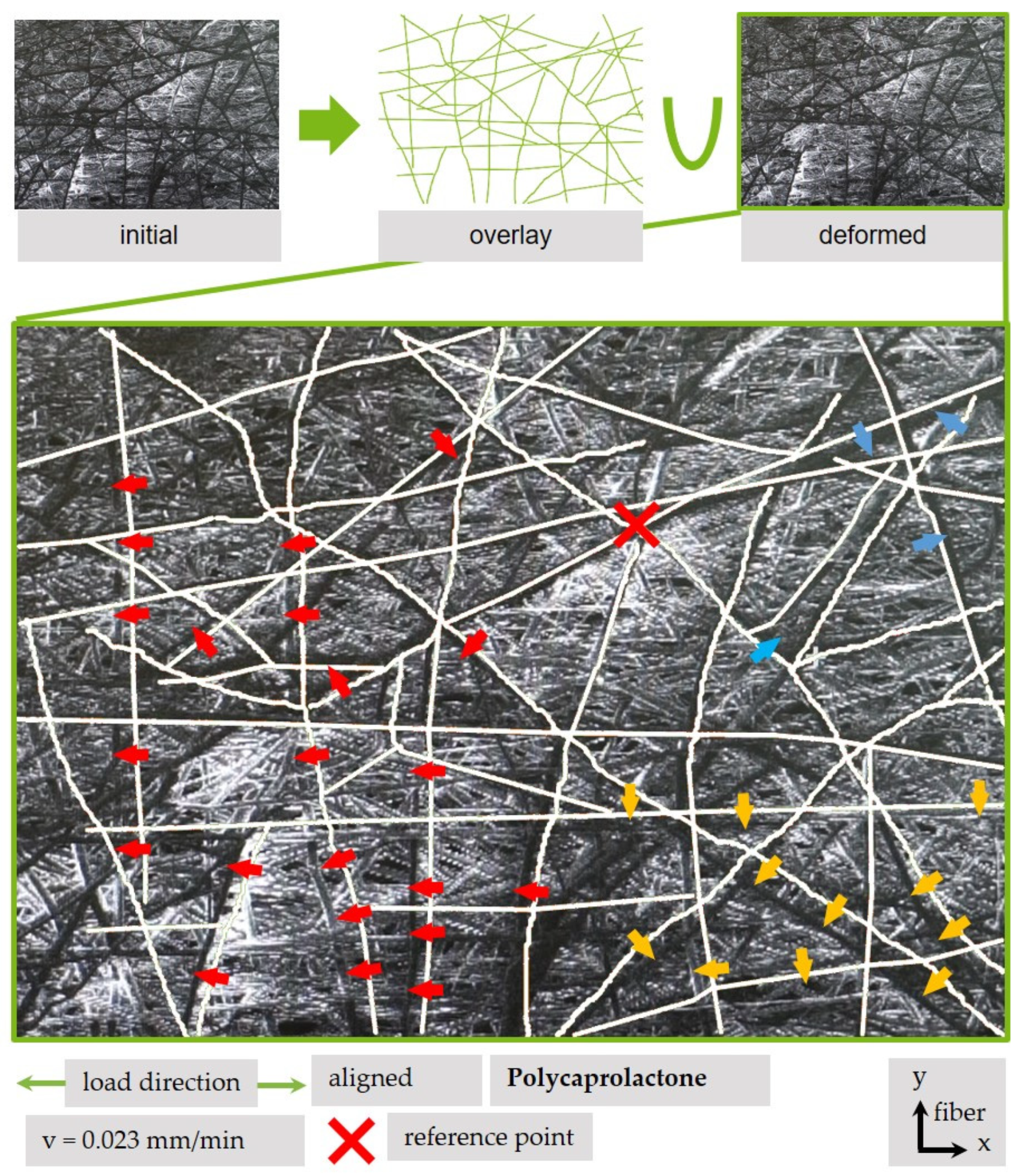
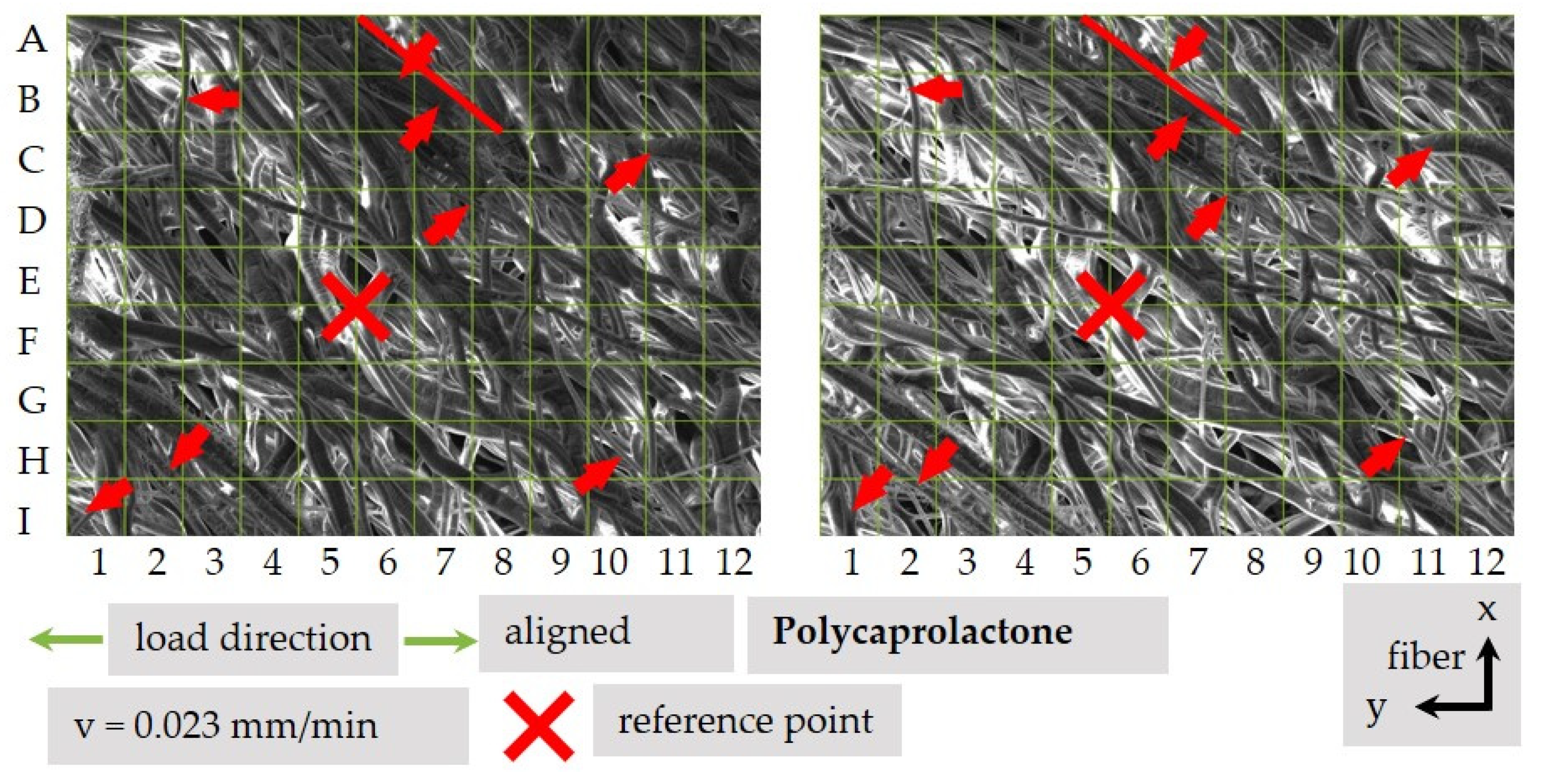
3.4.3. Qualitative Analysis of Fiber Movement
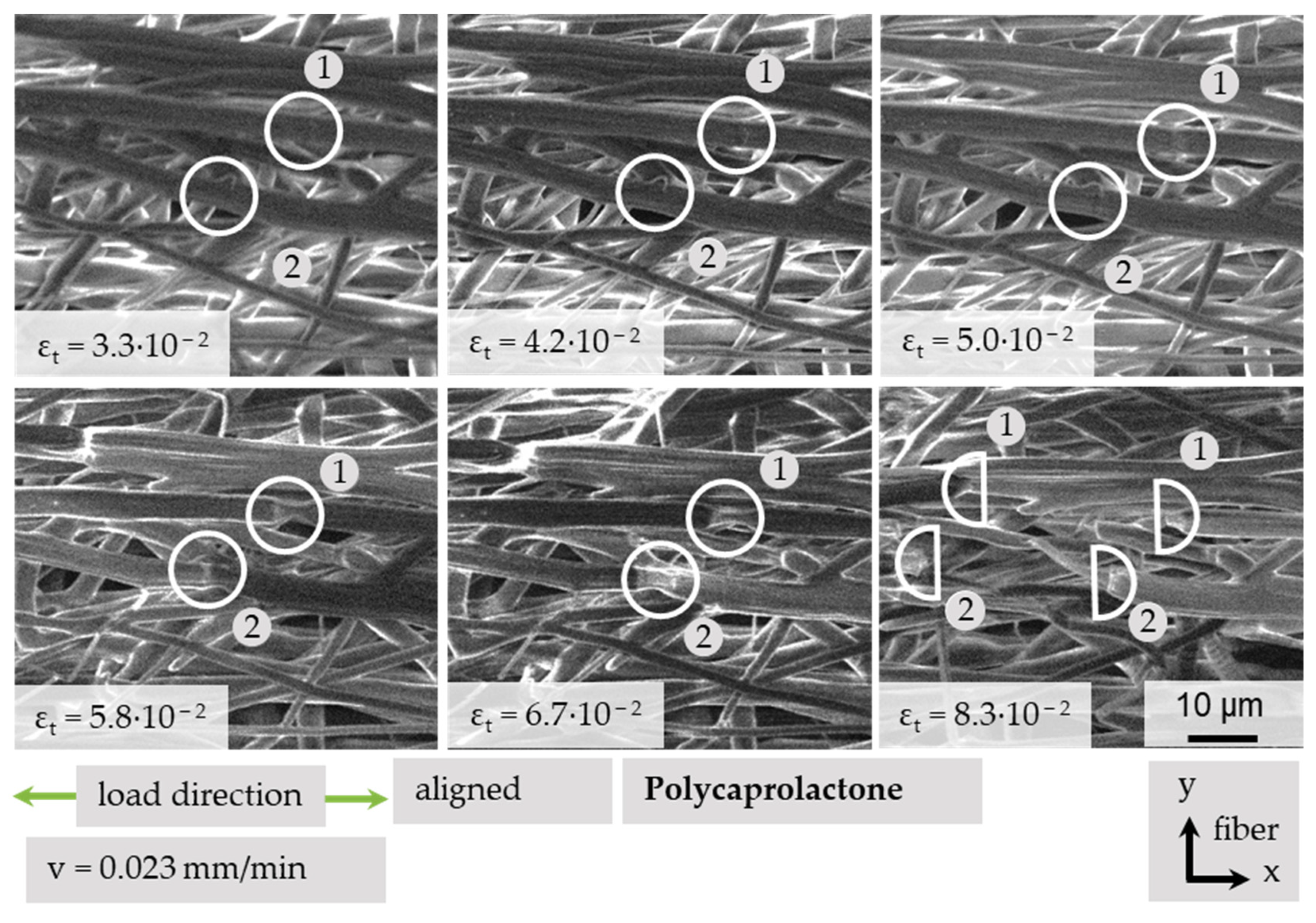
3.4.4. Local Fiber Fracture
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Platt, J.L.; Cascalho, M. New and old technologies for organ replacement. Curr. Opin. Organ Transplant. 2013, 18, 179–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunsberger, J.; Neubert, J.; Wertheim, J.A.; Allickson, J.; Atala, A. Bioengineering priorities on a path to ending organ shortage. Curr. Stem Cell Rep. 2016, 2, 118–127. [Google Scholar] [CrossRef] [Green Version]
- Cassan, D.; Becker, A.; Glasmacher, B.; Roger, Y.; Hoffmann, A.; Gengenbach, T.R.; Easton, C.D.; Hänsch, R.; Menzel, H. Blending chitosan-g-poly(caprolactone) with poly(caprolactone) by electrospinning to produce functional fiber mats for tissue engineering applications. J. Appl. Polym. Sci. 2020, 137, 48650. [Google Scholar] [CrossRef]
- Gryshkov, O.; Müller, M.; Leal-Marin, S.; Mutsenko, V.; Suresh, S.; Kapralova, V.M.; Glasmacher, B. Advances in the application of electrohydrodynamic fabrication for tissue engineering. J. Phys. Conf. Ser. 2019, 1236, 12024. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Deng, G.; Chen, W.; Ye, X.; Mo, X. A novel electrospun-aligned nanoyarn-reinforced nanofibrous scaffold for tendon tissue engineering. Colloids Surf. B Biointerfaces 2014, 122, 270–276. [Google Scholar] [CrossRef]
- Szentivanyi, A.; Chakradeo, T.; Zernetsch, H.; Glasmacher, B. Electrospun cellular microenvironments: Understanding controlled release and scaffold structure. Adv. Drug Deliv. Rev. 2011, 63, 209–220. [Google Scholar] [CrossRef]
- Bode, M.; Mueller, M.; Zernetsch, H.; Glasmacher, B. Electrospun vascular grafts with anti-kinking properties. Curr. Dir. Biomed. Eng. 2015, 1, 524–528. [Google Scholar] [CrossRef]
- Suresh, S.; Gryshkov, O.; Glasmacher, B. Impact of setup orientation on blend electrospinning of poly-ε-caprolactone-gelatin scaffolds for vascular tissue engineering. Int. J. Artif. Organs 2018, 41, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Fricke, D.; Becker, A.; Heratizadeh, A.; Knigge, S.; Jütte, L.; Wollweber, M.; Werfel, T.; Roth, B.W.; Glasmacher, B. Mueller Matrix analysis of collagen and gelatin containing samples towards more objective skin tissue diagnostics. Polymers 2020, 12, 1400. [Google Scholar] [CrossRef]
- Park, S.; Park, K.; Yoon, H.; Son, J.; Min, T.; Kim, G. Apparatus for preparing electrospun nanofibers: Designing an electrospinning process for nanofiber fabrication. Polym. Int. 2007, 56, 1361–1366. [Google Scholar] [CrossRef]
- Suresh, S.; Becker, A.; Glasmacher, B. Impact of apparatus orientation and gravity in electrospinning—A review of empirical evidence. Polymers 2020, 12, 2448. [Google Scholar] [CrossRef]
- Huang, Z.-M.; Zhang, Y.-Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Rogina, A. Electrospinning process: Versatile preparation method for biodegradable and natural polymers and biocomposite systems applied in tissue engineering and drug delivery. Appl. Surf. Sci. 2014, 296, 221–230. [Google Scholar] [CrossRef]
- Fricke, D.; Becker, A.; Jütte, L.; Bode, M.; de Cassan, D.; Wollweber, M.; Glasmacher, B.; Roth, B. Mueller matrix measurement of electrospun fiber scaffolds for tissue engineering. Polymers 2019, 11, 2062. [Google Scholar] [CrossRef] [Green Version]
- Gniesmer, S.; Brehm, R.; Hoffmann, A.; de Cassan, D.; Menzel, H.; Hoheisel, A.L.; Glasmacher, B.; Willbold, E.; Reifenrath, J.; Ludwig, N.; et al. Vascularization and biocompatibility of poly(ε-caprolactone) fiber mats for rotator cuff tear repair. PLoS ONE 2020, 15, e0227563. [Google Scholar] [CrossRef] [Green Version]
- Gniesmer, S.; Brehm, R.; Hoffmann, A.; de Cassan, D.; Menzel, H.; Hoheisel, A.-L.; Glasmacher, B.; Willbold, E.; Reifenrath, J.; Wellmann, M.; et al. In vivo analysis of vascularization and biocompatibility of electrospun polycaprolactone fibre mats in the rat femur chamber. J. Tissue Eng. Regen. Med. 2019, 13, 1190–1202. [Google Scholar] [CrossRef]
- Corduas, F.; Lamprou, D.A.; Mancuso, E. Next-generation surgical meshes for drug delivery and tissue engineering applications: Materials, design and emerging manufacturing technologies. Bio-Des. Manuf. 2021, 4, 278–310. [Google Scholar] [CrossRef]
- Can-Herrera, L.A.; Oliva, A.I.; Dzul-Cervantes, M.A.A.; Pacheco-Salazar, O.F.; Cervantes-Uc, J.M. Morphological and mechanical properties of electrospun polycaprolactone scaffolds: Effect of applied voltage. Polymers 2021, 13, 662. [Google Scholar] [CrossRef]
- Sowmya, B.; Hemavathi, A.B.; Panda, P.K. Poly (ε-caprolactone)-based electrospun nano-featured substrate for tissue engineering applications: A review. Prog. Biomater. 2021. [Google Scholar] [CrossRef]
- Scholz, R.; Delp, A.; Walther, F. In situ characterization of the damage initiation and evolution in sustainable cellulose-based cottonid. In TMS 2021 150th Annual Meeting & Exhibition Supplemental Proceedings; Springer International Publishing: Basel, Switzerland, 2021; pp. 867–878. [Google Scholar]
- Hülsbusch, D.; Helwing, R.; Mrzljak, S.; Walther, F. Comparison of the damage evolution in glass fiber-reinforced polyurethane and epoxy in the HCF and VHCF regimes investigated by intermittent in situ X-ray computed tomography. IOP Conf. Ser. Mater. Sci. Eng. 2020, 942, 12036. [Google Scholar] [CrossRef]
- Ambronn, H. Instructions for Using the Polarizing Microscope for Histological Examinations; J. H. Robolsky: Leipzig, Germany, 1892. (In German) [Google Scholar]
- Krönert, W.; Raith, F.A. Strenght investigation of single fibers in SEM (in german). Mater. Werkst. 1989, 20, 142–148. [Google Scholar] [CrossRef]
- Zernetsch, H.; Repanas, A.; Rittinghaus, T.; Mueller, M.; Alfred, I.; Glasmacher, B. Electrospinning and mechanical properties of polymeric fibers using a novel gap-spinning collector. Fibers Polym. 2016, 17, 1025–1032. [Google Scholar] [CrossRef]
- De Cassan, D.; Hoheisel, A.L.; Glasmacher, B.; Menzel, H. Impact of sterilization by electron beam, gamma radiation and X-rays on electrospun poly-(ε-caprolactone) fiber mats. J. Mater. Sci. Mater. Med. 2019, 30, 42. [Google Scholar] [CrossRef]
- Kumari, S.; Nithya, S.; Padmavathi, N.; Prasad, N.E.; Subrahmanyam, J. Tensile properties and fracture behaviour of carbon fibre filament materials. J. Mater. Sci. 2010, 45, 192–200. [Google Scholar] [CrossRef]
- Hülsbusch, D.; Mrzljak, S.; Walther, F. In situ computed tomography for the characterization of the fatigue damage development in glass fiber-reinforced polyurethane. Mater. Test. 2019, 61, 821–828. [Google Scholar] [CrossRef]
- Scholz, R.; Delp, A.; Walther, F. In situ characterization of damage development in cottonid due to quasi-static tensile loading. Materials 2020, 13, 2180. [Google Scholar] [CrossRef]
- Szentivanyi, A.L.; Zernetsch, H.; Menzel, H.; Glasmacher, B. A review of developments in electrospinning technology: New opportunities for the design of artificial tissue structures. Int. J. Artif. Organs 2011, 34, 986–997. [Google Scholar] [CrossRef]
- Zavan, B.; Gardin, C.; Guarino, V.; Rocca, T.; Maya, I.C.; Zanotti, F.; Ferroni, L.; Brunello, G.; Chachques, J.-C.; Ambrosio, L.; et al. Electrospun PCL-based vascular grafts: In vitro tests. Nanomaterials 2021, 11, 751. [Google Scholar] [CrossRef]
- Sundermann, J.; Oehmichen, S.; Sydow, S.; Burmeister, L.; Quaas, B.; Hänsch, R.; Rinas, U.; Hoffmann, A.; Menzel, H.; Bunjes, H. Varying the sustained release of BMP-2 from chitosan nanogel-functionalized polycaprolactone fiber mats by different polycaprolactone surface modifications. J. Biomed. Mater. Res. Part A 2020, 109, 600–614. [Google Scholar] [CrossRef]
- Cressie, N.A.C.; Whitford, H.J. How to use the two samplet-Test. Biom. J. 1986, 28, 131–148. [Google Scholar] [CrossRef]
- Mann, H.B.; Whitney, D.R. On a test of whether one of two random variables is stochastically larger than the other. Ann. Math. Statist. 1947, 18, 50–60. [Google Scholar] [CrossRef]
- Zuppolini, S.; Cruz-Maya, I.; Guarino, V.; Borriello, A. Optimization of polydopamine coatings onto Poly-ε-Caprolactone electrospun fibers for the fabrication of bio-rlectroconductive interfaces. J. Funct. Biomater. 2020, 11, 19. [Google Scholar] [CrossRef] [Green Version]


| No. | Strain, εt | Contraction, εcy | Force, F |
|---|---|---|---|
| i | 0 × 10−2 | 0 × 10−2 | 0.0 N |
| ii | 3 × 10−2 | −11 × 10−2 | 0.1 N |
| iii | 5 × 10−2 | −20 × 10−2 | 0.4 N |
| iv | 7 × 10−2 | −24 × 10−2 | 0.9 N |
| v | 10 × 10−2 | −32 × 10−2 | 1.8 N |
| No. | Length at εt | Angle at εt | Projected Strain | |||
|---|---|---|---|---|---|---|
| 2.5⋅10−2 | 5.8⋅10−2 | 2.5⋅10−2 | 5.8⋅10−2 | εlx | εly | |
| [µm] | [°] | [10−2] | [10−2] | |||
| 1 | Reference point | |||||
| 2 | 99.7 | 101.8 | 348.3 | 350.0 | 2.7 | −12.5 |
| 3 | --- | --- | 76.5 * | 70.1 * | --- | --- |
| 4 | --- | --- | 121.3 * | 125.7 * | --- | --- |
| 5 | --- | --- | 52.1 * | 42.4 * | --- | --- |
| 6 | --- | --- | 103.0 | 107.6 * | --- | --- |
| 7 | 47.1 | 42.7 | 90.0 | 90.0 | --- | −9.3 |
| 8 | --- | --- | 119.7 * | 125.2 * | --- | --- |
| 9 | --- | --- | 54.8 * | 51.2 * | --- | --- |
| 10 | --- | --- | 117.9 * | 117.5 * | --- | --- |
| 11 | --- | --- | 55.3 * | 51.2 * | --- | --- |
| 12 | --- | --- | 28.5 * | 18.5 * | --- | --- |
| 13 | 90.2 | 81.1 | 353.7 | 351.9 | −10.5 | 15.1 |
| 14 | 63.0 | 57.9 | 272.7 | 275.0 | --- | −8.3 |
| 15 | 115.6 | 118.6 | 6.6 | 4.5 | 3.0 | −29.6 |
| 16 | 3.2 | 4.0 | --- | --- | --- | --- |
| 17 | --- | --- | 90.0 * | 104.6 | --- | --- |
| 18 | 59.2 | 59.3 | 308.9 | 314.1 | 11.1 | −7.6 |
| 19 | --- | --- | 44.5 * | 27.0 * | --- | --- |
| 20 | 102.4 | 103.1 | 16.6 | 14.29 | 1.8 | −13.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delp, A.; Becker, A.; Hülsbusch, D.; Scholz, R.; Müller, M.; Glasmacher, B.; Walther, F. In Situ Characterization of Polycaprolactone Fiber Response to Quasi-Static Tensile Loading in Scanning Electron Microscopy. Polymers 2021, 13, 2090. https://doi.org/10.3390/polym13132090
Delp A, Becker A, Hülsbusch D, Scholz R, Müller M, Glasmacher B, Walther F. In Situ Characterization of Polycaprolactone Fiber Response to Quasi-Static Tensile Loading in Scanning Electron Microscopy. Polymers. 2021; 13(13):2090. https://doi.org/10.3390/polym13132090
Chicago/Turabian StyleDelp, Alexander, Alexander Becker, Daniel Hülsbusch, Ronja Scholz, Marc Müller, Birgit Glasmacher, and Frank Walther. 2021. "In Situ Characterization of Polycaprolactone Fiber Response to Quasi-Static Tensile Loading in Scanning Electron Microscopy" Polymers 13, no. 13: 2090. https://doi.org/10.3390/polym13132090
APA StyleDelp, A., Becker, A., Hülsbusch, D., Scholz, R., Müller, M., Glasmacher, B., & Walther, F. (2021). In Situ Characterization of Polycaprolactone Fiber Response to Quasi-Static Tensile Loading in Scanning Electron Microscopy. Polymers, 13(13), 2090. https://doi.org/10.3390/polym13132090