Feasibility Assessment of Parathyroid Hormone Adsorption by Using Polysaccharide-Based Multilayer Film Systems
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
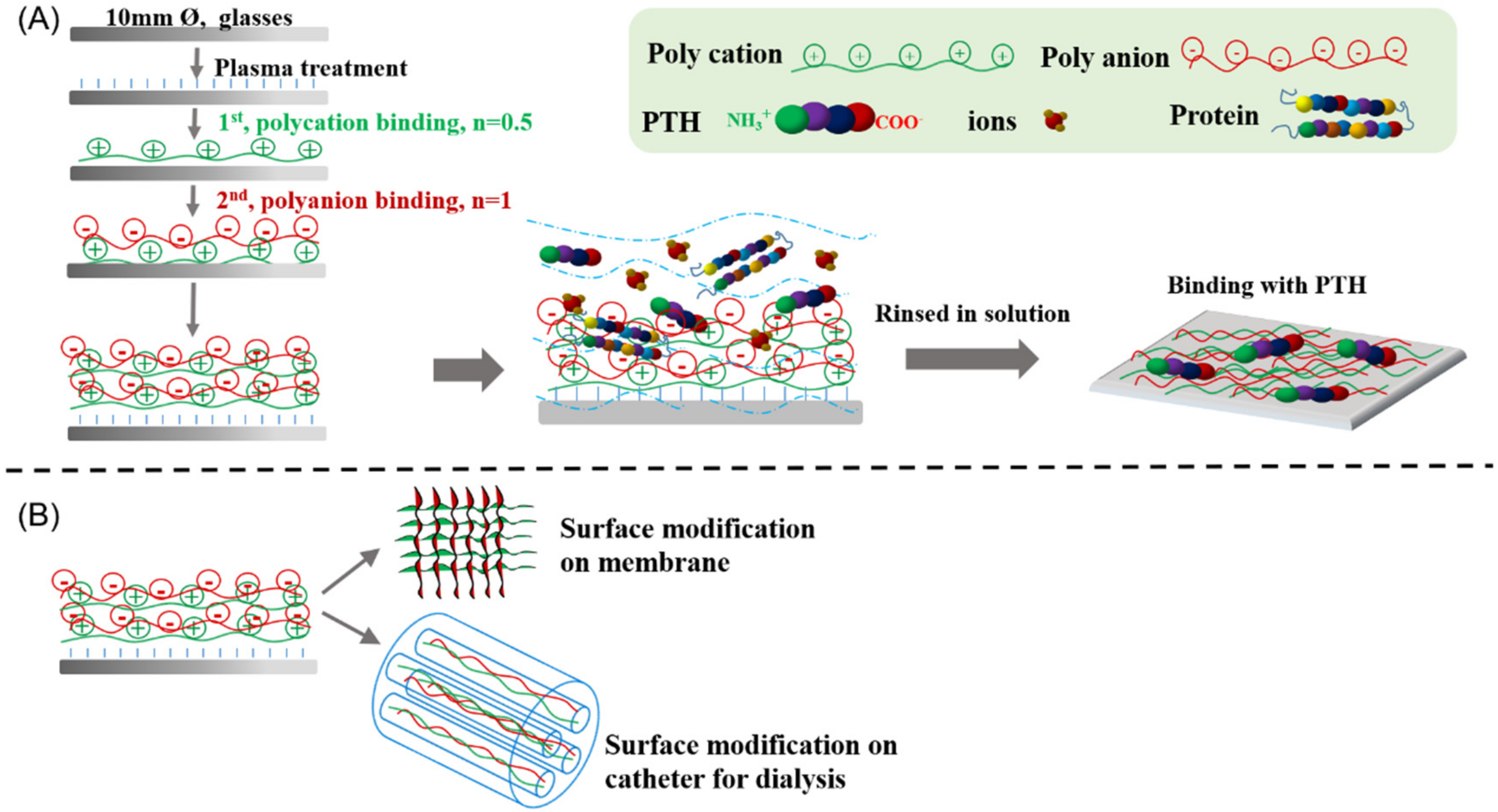
2.2. Preparation of Polysaccharide-Based Multilayer Films
2.3. Analysis of Thickness, Contact Angle and Surface Electrostatic Potential of Polysaccharide-Based Multilayer Films
2.4. Adsorption of PTH on Polysaccharide-Based Multilayer Films
2.5. BSA Adsorption
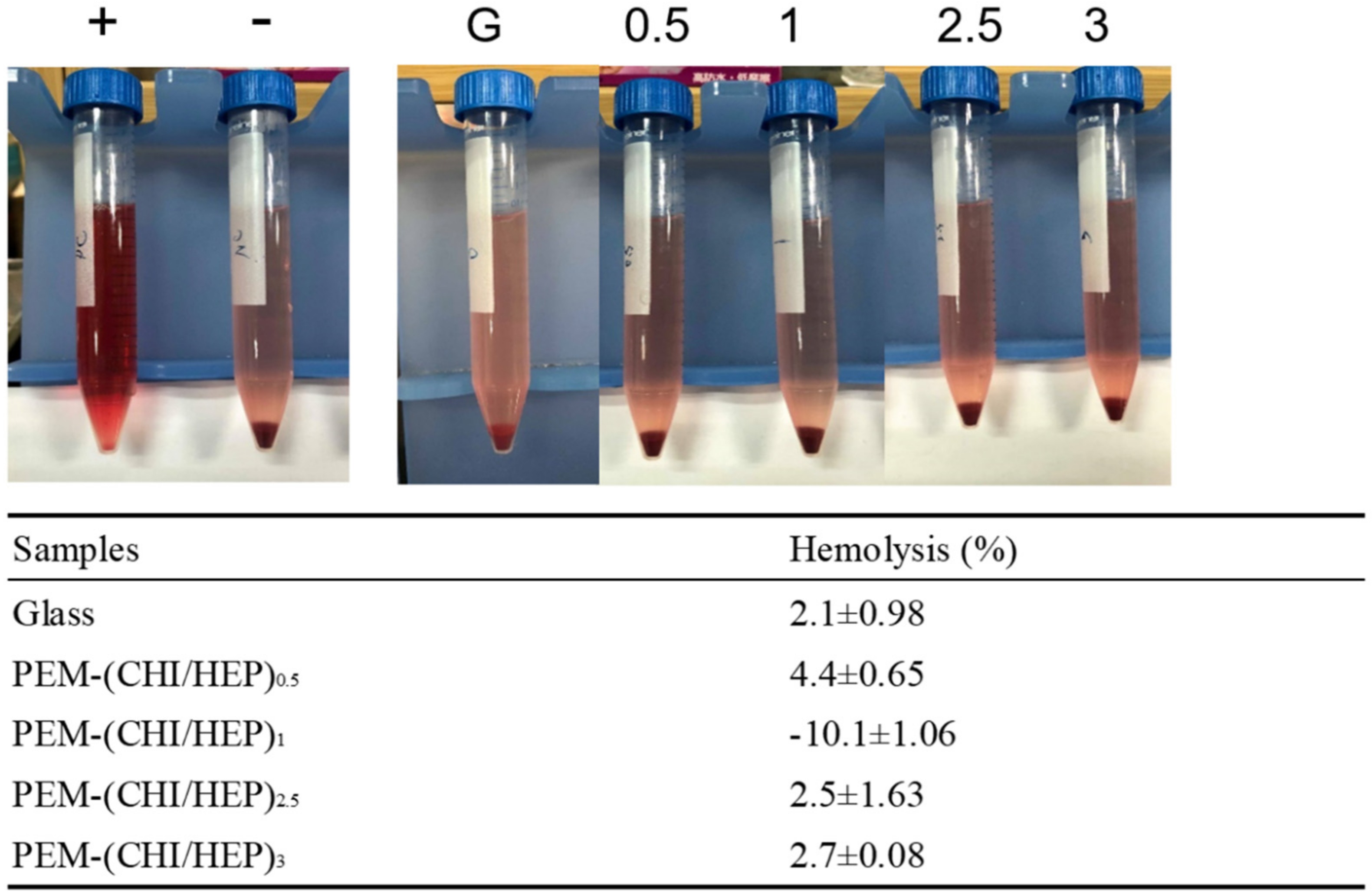
2.6. Hemolysis Test
2.7. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of Polysaccharide-Based Multilayer Films
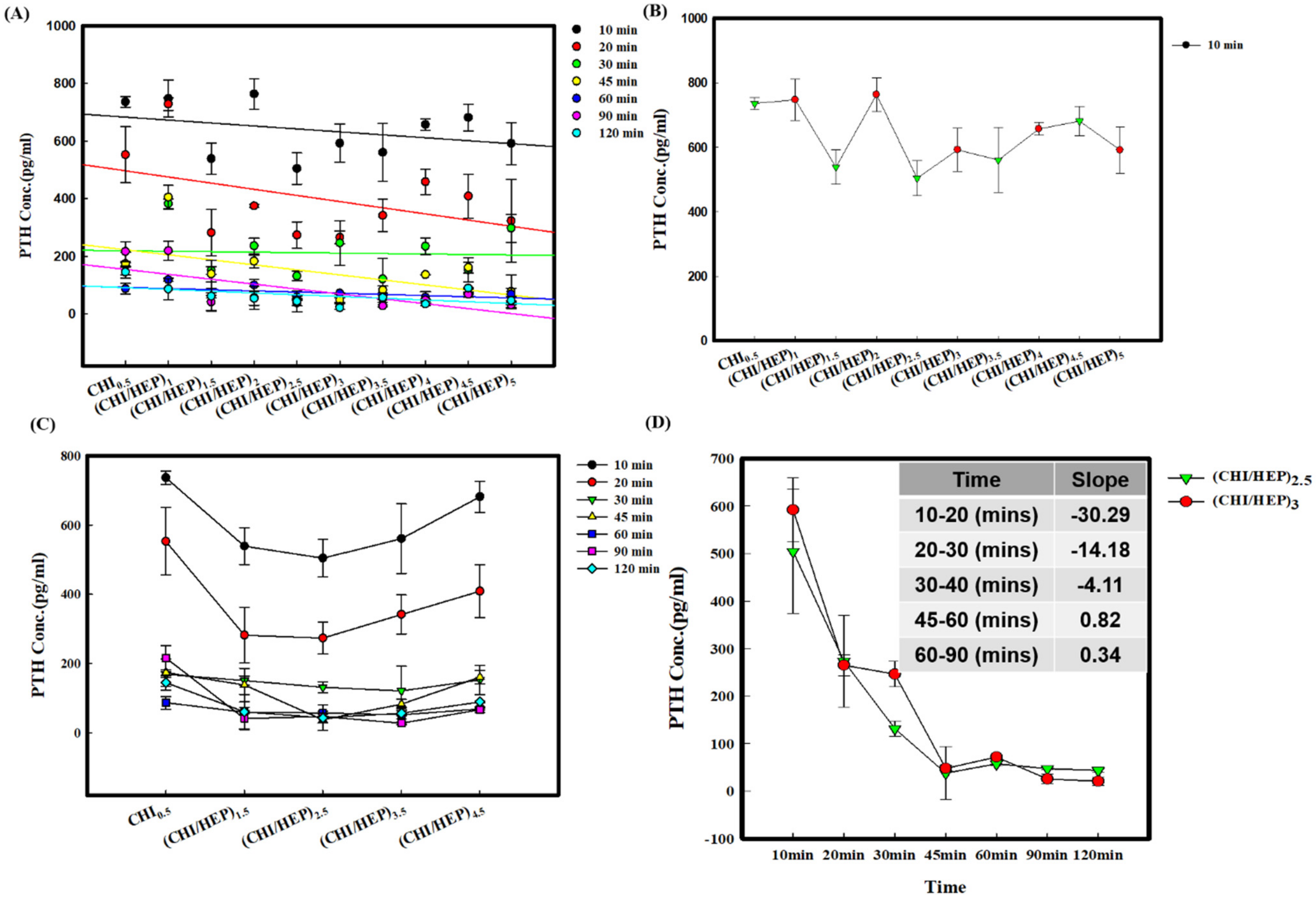
3.2. Adsorption of PTH on a Series of Polysaccharide-Based Multilayer Films after Different Incubation Times
3.3. Adsorption of PTH on a Series of Polysaccharide-Based Multilayer Films in the Solution with BSA
3.4. Blood Compatibility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Evenepoel, P.; Bover, J.; Torres, P.U. Parathyroid hormone metabolism and signaling in health and chronic kidney disease. Kidney Int. 2016, 90, 1184–1190. [Google Scholar] [CrossRef]
- Llach, F.; Forero, F.V. Secondary hyperparathyroidism in chronic renal failure: Pathogenic and clinical aspects. Am. J. Kidney Dis. 2001, 38, S20–S33. [Google Scholar] [CrossRef] [PubMed]
- El Arbagy, A.R.; Koura, M.A.E.A.; Nasr, A.E.S.S.A.E.; Elbarbary, H.S. Study of Effect of High-Flux Versus Low-Flux Dialysis Membranes on Parathyroid Hormone. Am. J. Clin. Med. Res. 2014, 2, 36–42. [Google Scholar] [CrossRef] [Green Version]
- Block, G.A.; Klassen, P.S.; Lazarus, J.M.; Ofsthun, N.; Lowrie, E.G.; Chertow, G.M. Mineral Metabolism, Mortality, and Morbidity in Maintenance Hemodialysis. J. Am. Soc. Nephrol. 2004, 15, 2208–2218. [Google Scholar] [CrossRef] [Green Version]
- Evenepoel, P. Calcimimetics in chronic kidney disease: Evidence, opportunities and challenges. Kidney Int. 2008, 74, 265–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, M.; Lorenzo, V. Parathyroid Hormone, A Uremic Toxin. Semin. Dial. 2009, 22, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Duque, E.J.; Elias, R.M.; Moysés, R.M.A. Parathyroid Hormone: A Uremic Toxin. Toxins 2020, 12, 189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, S.; Hodge, N.; Laski, M.; Wiesner, T.F. Middle-Molecule Uremic Toxin Removal via Hemodialysis Augmented with an Immunosorbent Packed Bed. Ind. Eng. Chem. Res. 2010, 49, 1359–1369. [Google Scholar] [CrossRef]
- Vanholder, R.; De Smet, R.; Glorieux, G.; Argilés, A.; Baurmeister, U.; Brunet, P.; Clark, W.F.; Cohen, G.M.; De Deyn, P.P.; Deppisch, R.; et al. Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int. 2003, 63, 1934–1943. [Google Scholar] [CrossRef] [Green Version]
- Ishihara, M.; Kishimoto, S.; Nakamura, S.; Sato, Y.; Hattori, H. Polyelectrolyte Complexes of Natural Polymers and Their Biomedical Applications. Polymers 2019, 11, 672. [Google Scholar] [CrossRef] [Green Version]
- Sakiyama-Elbert, S.E. Incorporation of heparin into biomaterials. Acta Biomater. 2014, 10, 1581–1587. [Google Scholar] [CrossRef] [Green Version]
- Ben-Ounis, W.; Gauthier, S.F.; Turgeon, S.L.; Roufik, S.; Pouliot, Y. Separation of minor protein components from whey protein isolates by heparin affinity chromatography. Int. Dairy J. 2008, 18, 1043–1050. [Google Scholar] [CrossRef]
- Kamerzell, T.J.; Joshi, S.B.; McClean, D.; Peplinskie, L.; Toney, K.; Papac, D.; Li, M.; Middaugh, C.R. Parathyroid hormone is a heparin/polyanion binding protein: Binding energetics and structure modification. Protein Sci. 2007, 16, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Gangadharappa, H.; Mruthunjaya, K. Phytosome complexed with chitosan for gingerol delivery in the treatment of respiratory infection: In vitro and in vivo evaluation. Eur. J. Pharm. Sci. 2018, 122, 214–229. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.V.; Su, C.-H.; Velusamy, P. Ciprofloxacin loaded genipin cross-linked chitosan/heparin nanoparticles for drug delivery application. Mater. Lett. 2016, 180, 119–122. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, Y.; Pan, X.; Chen, S.; Zhuang, H.; Wang, S. A composite hydrogel of chitosan/heparin/poly (γ-glutamic acid) loaded with superoxide dismutase for wound healing. Carbohydr. Polym. 2018, 180, 168–174. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, D.; Jian, S.; He, L.; Fu, L.; Zhao, Y.; Wang, Y.; Mo, H.; Shen, J. Multistructured vascular patches constructed via layer-by-layer self-assembly of heparin and chitosan for vascular tissue engineering applications. Chem. Eng. J. 2019, 370, 1057–1067. [Google Scholar] [CrossRef]
- Fu, J.; Ji, J.; Yuan, W.; Shen, J. Construction of anti-adhesive and antibacterial multilayer films via layer-by-layer assembly of heparin and chitosan. Biomaterials 2005, 26, 6684–6692. [Google Scholar] [CrossRef] [PubMed]
- Abri, S.; Ghatpande, A.A.; Ress, J.; Barton, H.A.; Leipzig, N.D. Polyionic Complexed Antibacterial Heparin–Chitosan Particles for Antibiotic Delivery. ACS Appl. Bio Mater. 2019, 2, 5848–5858. [Google Scholar] [CrossRef]
- Yuan, W.; Fu, J.; Su, K.; Ji, J. Self-assembled chitosan/heparin multilayer film as a novel template for in situ synthesis of silver nanoparticles. Colloids Surf. B Biointerfaces 2010, 76, 549–555. [Google Scholar] [CrossRef]
- Jin, Y.; Zhu, Z.; Liang, L.; Lan, K.; Zheng, Q.; Wang, Y.; Guo, Y.; Zhu, K.; Mehmood, R.; Wang, B. A facile heparin/carboxymethyl chitosan coating mediated by polydopamine on implants for hemocompatibility and antibacterial properties. Appl. Surf. Sci. 2020, 528, 146539. [Google Scholar] [CrossRef]
- Lin, W.-C.; Liu, T.-Y.; Yang, M.-C. Hemocompatibility of polyacrylonitrile dialysis membrane immobilized with chitosan and heparin conjugate. Biomaterials 2004, 25, 1947–1957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, G.; Zhang, H.; Li, J.; Yao, X.; Tang, B. Surface immobilization of heparin and chitosan on titanium to improve hemocompatibility and antibacterial activities. Colloids Surf. B Biointerfaces 2018, 172, 338–345. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juang, R.-S.; Su, X.; Lee, I.-C. Feasibility Assessment of Parathyroid Hormone Adsorption by Using Polysaccharide-Based Multilayer Film Systems. Polymers 2021, 13, 2070. https://doi.org/10.3390/polym13132070
Juang R-S, Su X, Lee I-C. Feasibility Assessment of Parathyroid Hormone Adsorption by Using Polysaccharide-Based Multilayer Film Systems. Polymers. 2021; 13(13):2070. https://doi.org/10.3390/polym13132070
Chicago/Turabian StyleJuang, Ruey-Shin, Xing Su, and I-Chi Lee. 2021. "Feasibility Assessment of Parathyroid Hormone Adsorption by Using Polysaccharide-Based Multilayer Film Systems" Polymers 13, no. 13: 2070. https://doi.org/10.3390/polym13132070
APA StyleJuang, R.-S., Su, X., & Lee, I.-C. (2021). Feasibility Assessment of Parathyroid Hormone Adsorption by Using Polysaccharide-Based Multilayer Film Systems. Polymers, 13(13), 2070. https://doi.org/10.3390/polym13132070







