Printability Study of a Conductive Polyaniline/Acrylic Formulation for 3D Printing
Abstract
:1. Introduction
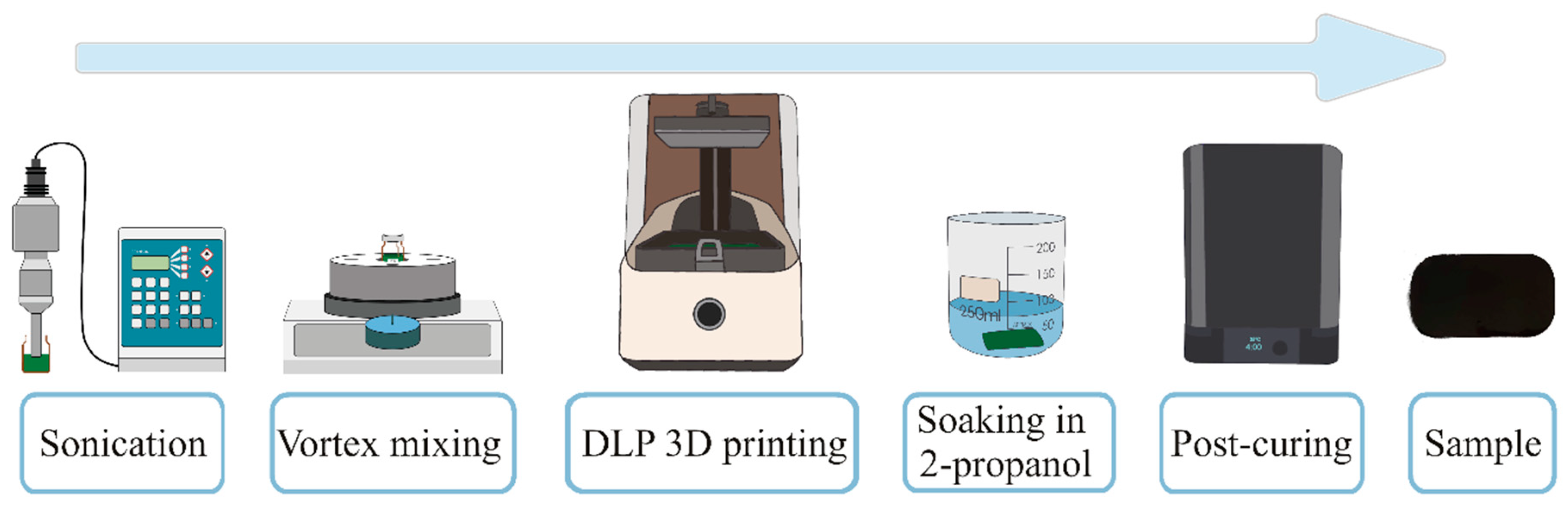
2. Experimental Section
2.1. Materials
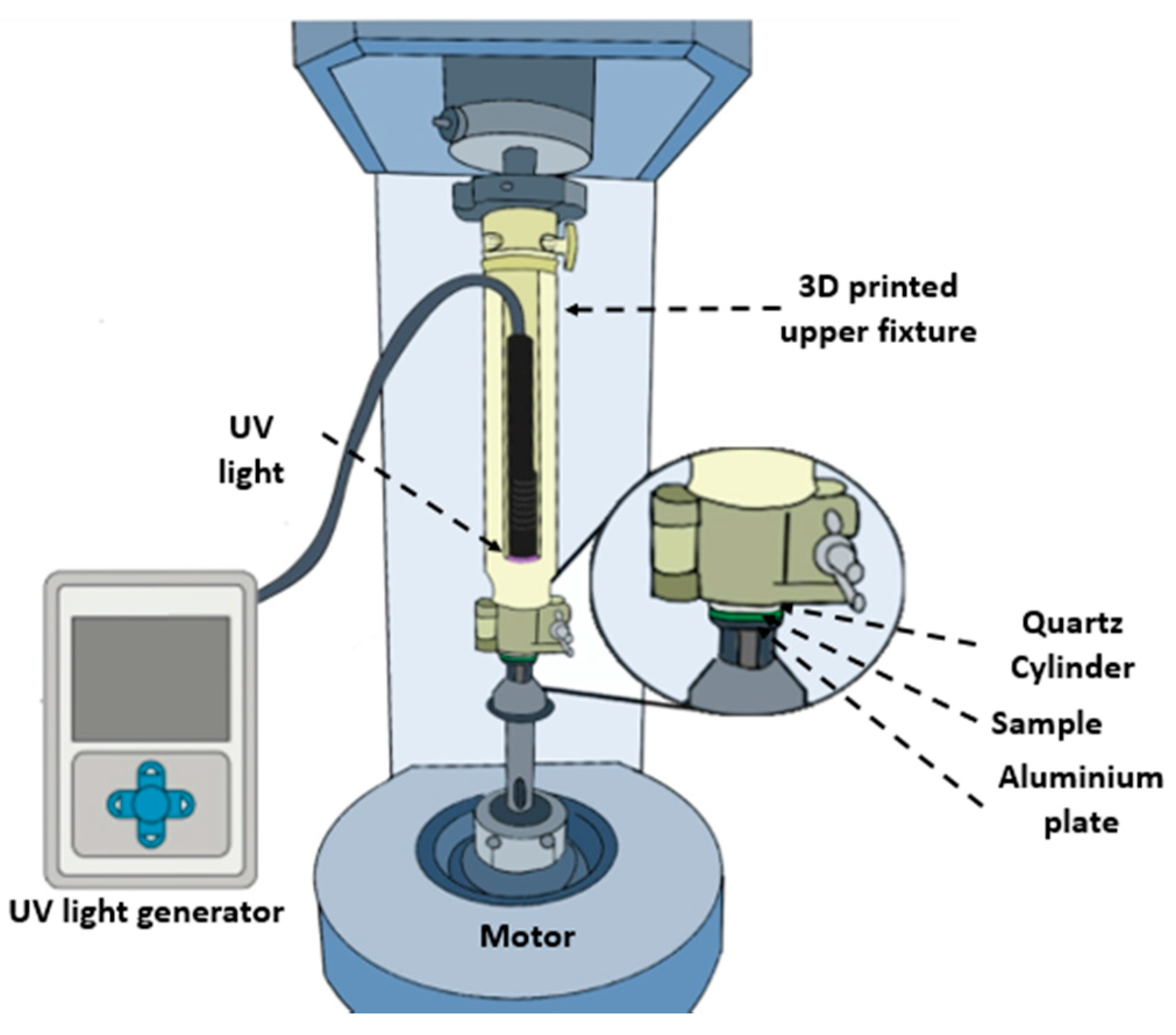
2.2. Materials Characterization
3. Results
3.1. Rheological Results
3.1.1. Viscosity of Liquid Formulations
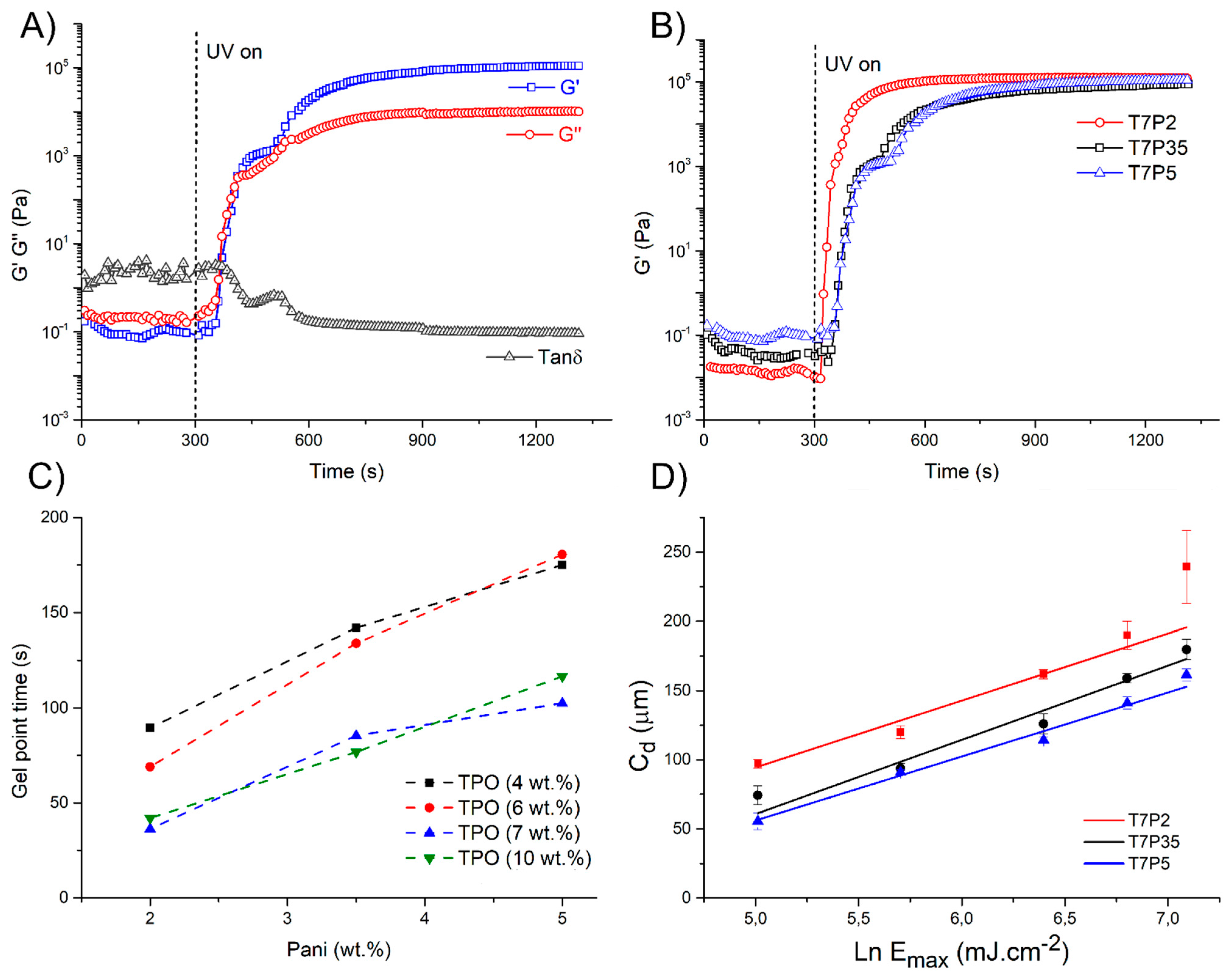
3.1.2. Photorheology
3.1.3. Cure Depth
3.1.4. Printer Tests
3.1.5. Characterization of PANI and the Printed Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, J.; Xiao, P. 3D printing of photopolymers. Polym. Chem. 2018, 9, 1530–1540. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Huang, D.; Liu, S.; Dong, X.; Li, Y.; Zhang, H.; Yang, Z.; Su, Q.; Huang, W.; Zheng, W.; et al. Zirconia toughened hydroxyapatite biocomposite formed by a DLP 3D printing process for potential bone tissue engineering. Mater. Sci. Eng. C 2019, 105, 110054. [Google Scholar] [CrossRef] [PubMed]
- Cooperstein, I.; Sachyani-Keneth, E.; Shukrun-Farrell, E.; Rosental, T.; Wang, X.; Kamyshny, A.; Magdassi, S. Hybrid Materials for Functional 3D Printing. Adv. Mater. Interfaces 2018, 5, 1–15. [Google Scholar] [CrossRef]
- Hamzah, H.H.; Shafiee, S.A.; Abdalla, A.; Patel, B.A. 3D printable conductive materials for the fabrication of electrochemical sensors: A mini review. Electrochem. Commun. 2018, 96, 27–31. [Google Scholar] [CrossRef]
- Komissarenko, D.A.; Sokolov, P.S.; Evstigneeva, A.D.; Slyusar, I.V.; Nartov, A.S.; Volkov, P.A.; Lyskov, N.V.; Evdokimov, P.V.; Putlayev, V.I.; Dosovitsky, A.E. Journal of the European Ceramic Society DLP 3D printing of scandia-stabilized zirconia ceramics. J. Eur. Ceram. Soc. 2020. [Google Scholar] [CrossRef]
- Gonzalez, G.; Chiappone, A.; Roppolo, I.; Fantino, E.; Bertana, V.; Perrucci, F.; Scaltrito, L.; Pirri, F.; Sangermano, M. Development of 3D printable formulations containing CNT with enhanced electrical properties. Polymer 2017, 109, 246–253. [Google Scholar] [CrossRef]
- Mendes-Felipe, C.; Oliveira, J.; Costa, P.; Ruiz-Rubio, L.; Iregui, A.; González, A.; Vilas, J.L.; Lanceros-Mendez, S. Stimuli responsive UV cured polyurethane acrylated/carbon nanotube composites for piezoresistive sensing. Eur. Polym. J. 2019, 120, 109226. [Google Scholar] [CrossRef]
- Mu, Q.; Wang, L.; Dunn, C.K.; Kuang, X.; Duan, F.; Zhang, Z.; Qi, H.J.; Wang, T. Digital light processing 3D printing of conductive complex structures. Addit. Manuf. 2017, 18, 74–83. [Google Scholar] [CrossRef]
- Paul, D.R.; Robeson, L.M. Polymer nanotechnology: Nanocomposites. Polymer 2008, 49, 3187–3204. [Google Scholar] [CrossRef] [Green Version]
- Horta-Romarís, L.; Abad, M.-J.; González-Rodríguez, M.V.; Lasagabáster, A.; Costa, P.; Lanceros-Méndez, S. Cyclic temperature dependence of electrical conductivity in polyanilines as a function of the dopant and synthesis method. Mater. Des. 2017, 114, 288–296. [Google Scholar] [CrossRef]
- Jia, R.; Shen, G.; Qu, F.; Chen, D. Flexible on-chip micro-supercapacitors: Efficient power units for wearable electronics. Energy Storage Mater. 2020, 27, 169–186. [Google Scholar] [CrossRef]
- Stejskal, J. Conducting polymers are not just conducting: A perspective for emerging technology. Polym. Int. 2019. [Google Scholar] [CrossRef]
- Bhadra, J.; Al-Thani, N.J.; Madi, N.K.; Al-Maadeed, M.A. Effects of aniline concentrations on the electrical and mechanical properties of polyaniline polyvinyl alcohol blends. Arab. J. Chem. 2017, 10, 664–672. [Google Scholar] [CrossRef] [Green Version]
- Jordan, R.S.; Wang, Y. 3D printing of conjugated polymers. J. Polym. Sci. Part B Polym. Phys. 2019, 57, 1592–1605. [Google Scholar] [CrossRef]
- Joo, H.; Cho, S. Comparative studies on polyurethane composites filled with polyaniline and graphene for DLP-type 3D printing. Polymers 2020, 12. [Google Scholar] [CrossRef] [Green Version]
- Han, H.; Cho, S. Fabrication of Conducting Polyacrylate Resin Solution with Polyaniline Nanofiber and Graphene for Conductive 3D Printing Application. Polymers 2018, 10, 1003. [Google Scholar] [CrossRef] [Green Version]
- Kowsari, K.; Zhang, B.; Panjwani, S.; Chen, Z.; Hingorani, H.; Akbari, S.; Fang, N.X.; Ge, Q. Photopolymer formulation to minimize feature size, surface roughness, and stair-stepping in digital light processing-based three-dimensional printing. Addit. Manuf. 2018, 24, 627–638. [Google Scholar] [CrossRef]
- Gojzewski, H.; Guo, Z.; Grzelachowska, W.; Ridwan, M.G.; Hempenius, M.A.; Grijpma, D.W.; Vancso, G.J. Layer-by-Layer Printing of Photopolymers in 3D: How Weak is the Interface? ACS Appl. Mater. Interfaces 2020, 12, 8908–8914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofstetter, C.; Orman, S.; Baudis, S.; Stampfl, J. Combining cure depth and cure degree, a new way to fully characterize novel photopolymers. Addit. Manuf. 2018, 24, 166–172. [Google Scholar] [CrossRef]
- Bennett, J. Measuring UV curing parameters of commercial photopolymers used in additive manufacturing. Addit. Manuf. 2017, 18, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, P.F. Rapid Prototyping & Manufacturing. Fundamentals of StereoLithography, 1st ed.; Society of Manufacturing Engineers: Southfield, MI, USA, 1992; ISBN 0-87263-425-6. [Google Scholar]
- Zhakeyev, A.; Zhang, L.; Xuan, J. Photoactive Resin Formulations and Composites for Optical 3D and 4D Printing of Functional Materials and Devices; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128168059. [Google Scholar]
- Arias-Ferreiro, G.; Ares-Pernas, A.; Dopico-García, M.S.; Lasagabáster-Latorre, A.; Abad, M.J. Photocured conductive PANI/acrylate composites for digital light processing. Influence of HDODA crosslinker in rheological and physicochemical properties. Eur. Polym. J. 2020, 136, 109887. [Google Scholar] [CrossRef]
- Park, H.-W.; Kim, T.; Huh, J.; Kang, M.; Lee, J.E.; Yoon, H. Anisotropic growth control of polyaniline nanostructures and their morphology-dependent electrochemical characteristics. ACS Nano 2012, 6, 7624–7633. [Google Scholar] [CrossRef]
- Schmidt, L.E.; Leterrier, Y.; Vesin, J.M.; Wilhelm, M.; Månson, J.A.E. Photorheology of fast UV-curing multifunctional acrylates. Macromol. Mater. Eng. 2005, 290, 1115–1124. [Google Scholar] [CrossRef]
- Zhang, X.; Keck, S.; Qi, Y.; Baudis, S.; Zhao, Y. Study on Modified Dealkaline Lignin as Visible Light Macromolecular Photoinitiator for 3D Printing. ACS Sustain. Chem. Eng. 2020, 8, 10959–10970. [Google Scholar] [CrossRef]
- Sirrine, J.M.; Meenakshisundaram, V.; Moon, N.G.; Scott, P.J.; Mondschein, R.J.; Weiseman, T.F.; Williams, C.B.; Long, T.E. Functional siloxanes with photo-activated, simultaneous chain extension and crosslinking for lithography-based 3D printing. Polymer 2018, 152, 25–34. [Google Scholar] [CrossRef]
- Gorsche, C.; Harikrishna, R.; Baudis, S.; Knaack, P.; Husar, B.; Laeuger, J.; Hoffmann, H.; Liska, R. Real Time-NIR/MIR-Photorheology: A Versatile Tool for the in Situ Characterization of Photopolymerization Reactions. Anal. Chem. 2017, 89, 4958–4968. [Google Scholar] [CrossRef]
- Verney, V.; Commereuc, S. Molecular evolution of polymers through photoageing: A new UV in situ viscoelastic technique. Macromol. Rapid Commun. 2005, 26, 868–873. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Johansson, M.; Sundell, P.E.; Claudino, M.; Pan, J.; Claesson, P.M. UV-curable acrylate-based nanocomposites: Effect of polyaniline additives on the curing performance. Polym. Adv. Technol. 2013, 24, 668–678. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Panda, S.S.; Raju, K.V.S.N. Thermal and mechanical properties of epoxy acrylate/methacrylates UV cured coatings. Prog. Org. Coatings 2005, 54, 10–19. [Google Scholar] [CrossRef]
- Moon, J.I.; Lee, Y.H.; Kim, H.J. Synthesis and characterization of acrylic-grafted polyester coatings for automotive pre-coated metal. Prog. Org. Coatings 2012, 75, 162–169. [Google Scholar] [CrossRef]
- Qin, X.H.; Torgersen, J.; Saf, R.; Mühleder, S.; Pucher, N.; Ligon, S.C.; Holnthoner, W.; Redl, H.; Ovsianikov, A.; Stampfl, J.; et al. Three-dimensional microfabrication of protein hydrogels via two-photon-excited thiol-vinyl ester photopolymerization. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 4799–4810. [Google Scholar] [CrossRef] [Green Version]
- Oesterreicher, A.; Gorsche, C.; Ayalur-Karunakaran, S.; Moser, A.; Edler, M.; Pinter, G.; Schlögl, S.; Liska, R.; Griesser, T. Exploring Network Formation of Tough and Biocompatible Thiol-yne Based Photopolymers. Macromol. Rapid Commun. 2016, 37, 1701–1706. [Google Scholar] [CrossRef]
- Winter, H.H. Can the gel point of a cross-linking polymer be detected by the G′ – G″ crossover? Polym. Eng. Sci. 1987, 27, 1698–1702. [Google Scholar] [CrossRef]
- Higham, A.K.; Garber, L.A.; Latshaw, D.C.; Hall, C.K.; Pojman, J.A.; Khan, S.A. Gelation and cross-linking in multifunctional thiol and multifunctional acrylate systems involving an in situ comonomer catalyst. Macromolecules 2014, 47, 821–829. [Google Scholar] [CrossRef]
- Ligon, S.C.; Seidler, K.; Gorsche, C.; Griesser, M.; Moszner, N.; Liska, R. Allyl sulfides and α-substituted acrylates as addition-fragmentation chain transfer agents for methacrylate polymer networks. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 394–406. [Google Scholar] [CrossRef]
- Catel, Y.; Fässler, P.; Fischer, U.; Gorsche, C.; Schörpf, S.; Tauscher, S.; Liska, R.; Moszner, N. Evaluation of Difunctional Vinylcyclopropanes as Reactive Diluents for the Development of Low-Shrinkage Composites. Macromol. Mater. Eng. 2017, 302, 1–10. [Google Scholar] [CrossRef]
- Raponi, O.d.A.; Barbosa, L.C.M.; de Souza, B.R.; Ancelotti Junior, A.C. Study of the influence of initiator content in the polymerization reaction of a thermoplastic liquid resin for advanced composite manufacturing. Adv. Polym. Technol. 2018, 37, 3579–3587. [Google Scholar] [CrossRef] [Green Version]
- Barkane, A.; Platnieks, O.; Jurinovs, M.; Kasetaite, S.; Ostrauskaite, J.; Gaidukovs, S.; Habibi, Y. Uv-light curing of 3d printing inks from vegetable oils for stereolithography. Polymers 2021, 13, 1–16. [Google Scholar] [CrossRef]
- Sutton, J.T.; Rajan, K.; Harper, D.P.; Chmely, S.C. Lignin-Containing Photoactive Resins for 3D Printing by Stereolithography. ACS Appl. Mater. Interfaces 2018, 10, 36456–36463. [Google Scholar] [CrossRef]
- Lee, J.H.; Prud’homme, R.K.; Aksay, I.A. Cure depth in photopolymerization: Experiments and theory. J. Mater. Res. 2001, 16, 3536–3544. [Google Scholar] [CrossRef] [Green Version]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef] [Green Version]
- Dizon, J.R.C.; Espera, A.H.; Chen, Q.; Advincula, R.C. Mechanical characterization of 3D-printed polymers. Addit. Manuf. 2018, 20, 44–67. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, W. A study on the synthesis, characterization and properties of polyaniline using acrylic acid as a primary dopant. I: Polymerization and polymer. Polym. Adv. Technol. 2005, 16, 24–31. [Google Scholar] [CrossRef]
- Rebers, L.; Reichsöllner, R.; Regett, S.; Tovar, G.E.M.; Borchers, K.; Baudis, S.; Southan, A. Differentiation of physical and chemical cross-linking in gelatin methacryloyl hydrogels. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Valentová, H.; Stejskal, J. Mechanical properties of polyaniline. Synth. Met. 2010, 160, 832–834. [Google Scholar] [CrossRef]
- Borrello, J.; Nasser, P.; Iatridis, J.C.; Costa, K.D. 3D printing a mechanically-tunable acrylate resin on a commercial DLP-SLA printer. Addit. Manuf. 2018, 23, 374–380. [Google Scholar] [CrossRef]
- Feng, Z.; Li, Y.; Xin, C.; Tang, D.; Xiong, W.; Zhang, H. Fabrication of Graphene-Reinforced Nanocomposites with Improved Fracture Toughness in Net Shape for Complex 3D Structures via Digital Light Processing. C J. Carbon Res. 2019, 5, 25. [Google Scholar] [CrossRef] [Green Version]
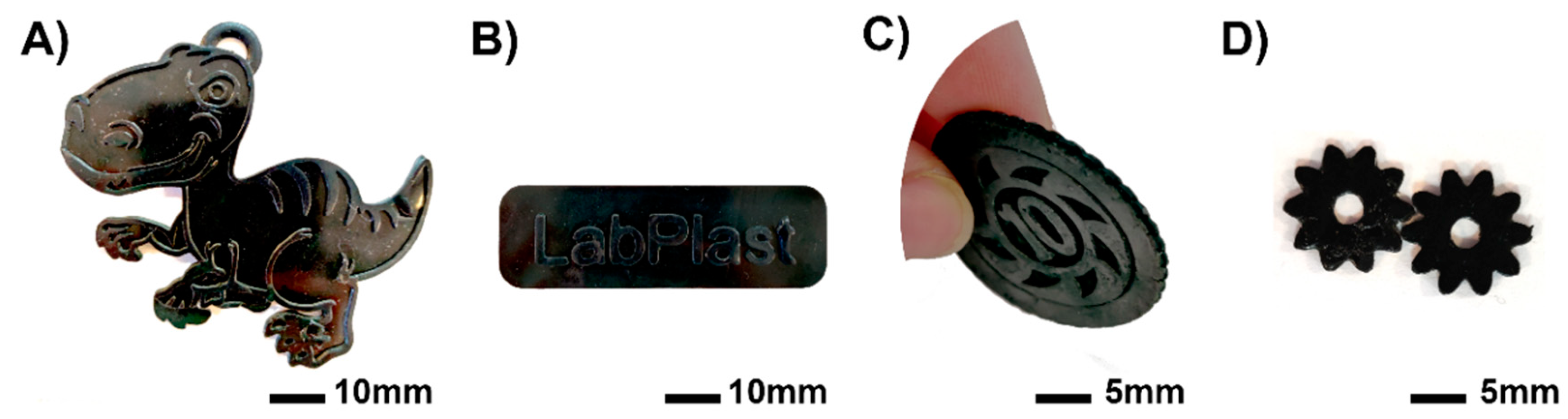
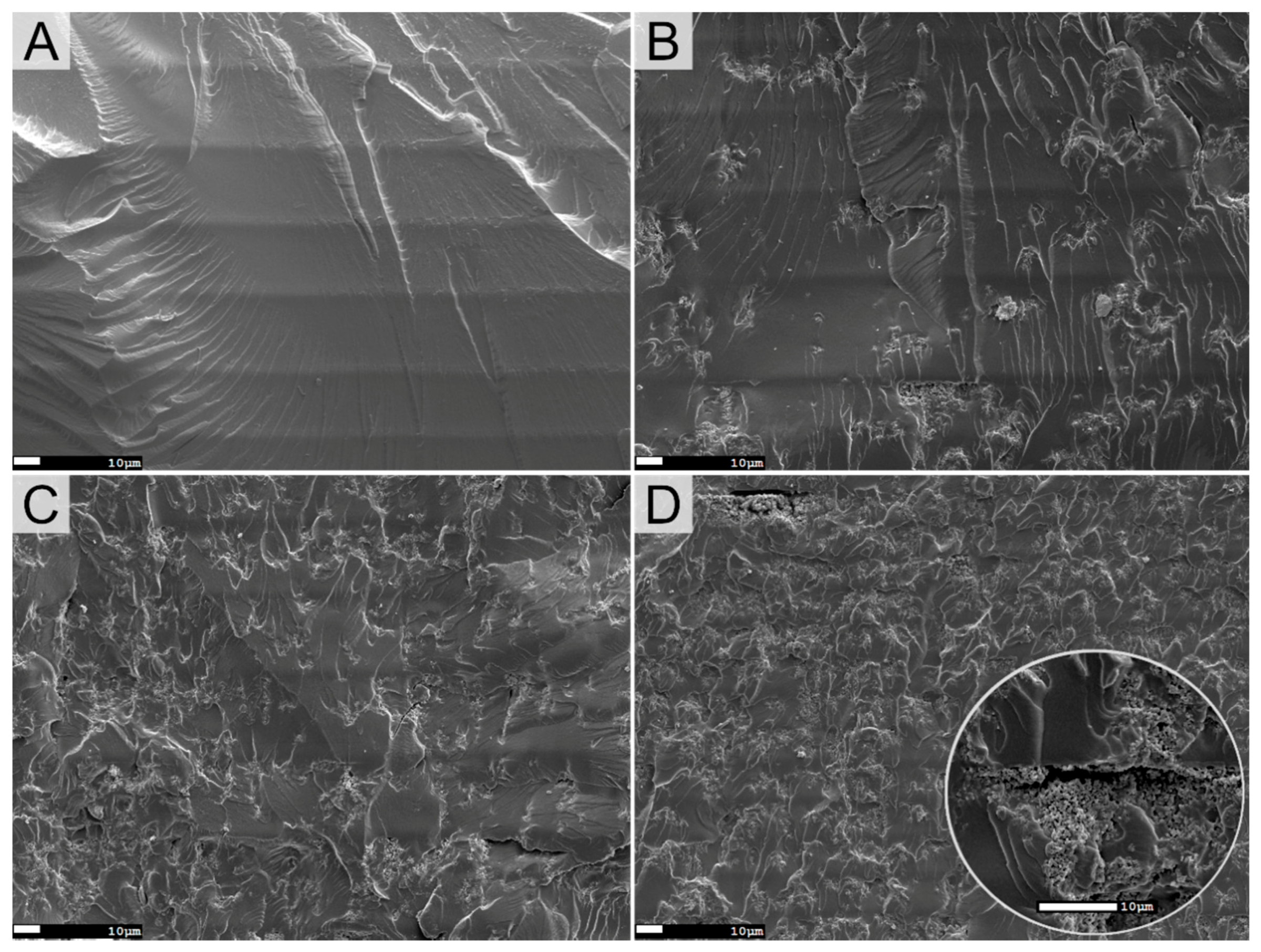
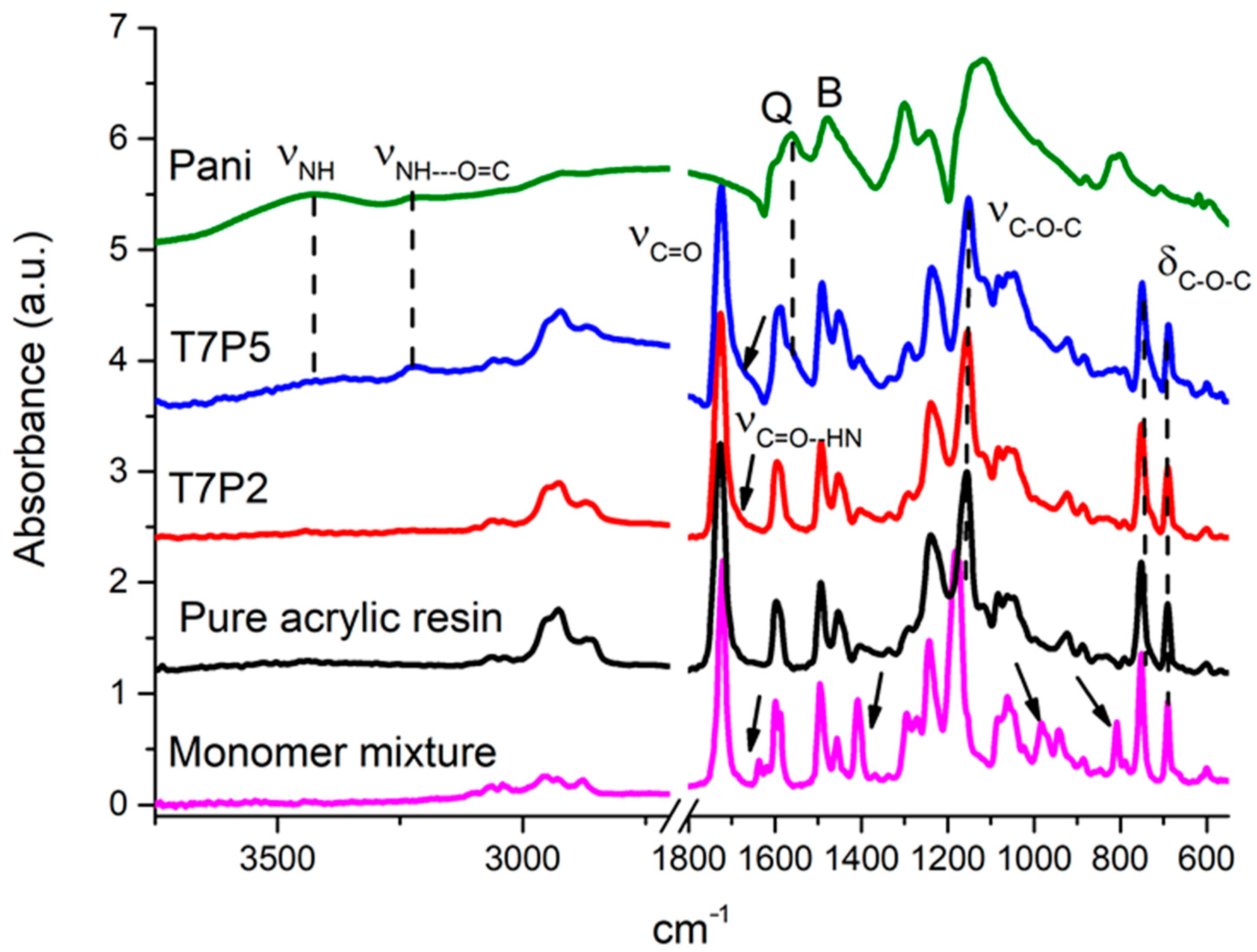

| Sample | TPO (wt%) | Theoretical PANI (wt%) | Dp (µm) | Ec (mJ cm−2) | Cd (µm) | Viscosity (Pa.s) 1 s−1 |
|---|---|---|---|---|---|---|
| T4P2 | 4 | 2 | 71 | 42 | 43 | 0.047 |
| T4P35 | 4 | 3.5 | 55 | 42 | 34 | 0.072 |
| T4P5 | 4 | 5 | 59 | 65 | 10 | 0.159 |
| T6P2 | 6 | 2 | 63 | 34 | 50 | 0.051 |
| T6P35 | 6 | 3.5 | 48 | 30 | 45 | 0.059 |
| T6P5 | 6 | 5 | 52 | 51 | 21 | 0.153 |
| T7P2 | 7 | 2 | 48 | 21 | 62 | 0.071 |
| T7P35 | 7 | 3.5 | 54 | 48 | 25 | 0.078 |
| T7P5 | 7 | 5 | 46 | 44 | 25 | 0.155 |
| T10P2 | 10 | 2 | 43 | 21 | 56 | 0.025 |
| T10P35 | 10 | 3.5 | 49 | 45 | 26 | 0.078 |
| T10P5 | 10 | 5 | 49 | 69 | 5 | 0.151 |
| TPO (wt%) | |||||
|---|---|---|---|---|---|
| 4 | 6 | 7 | 10 | ||
| PANI (wt%) | 2 | ✔ ✔ | ✔ ✔ ✔ | ✔ ✔ ✔ | ✔ ✔ ✔ |
| 3.5 | ✔ ✔ | ✔ ✔ | ✔ ✔ | ✔ ✔ | |
| 5 | x | x | ✔ | ✔ | |
| Sample Formulation | PANI (T) (wt%) | PANI (EA) (wt%) | Conductivity (S cm−1) | DBC% |
|---|---|---|---|---|
| Reference | 0 | <0.05 | ≤10−8 * | 97.9 ± 0.7 |
| T4P2 | 2 | 2.10 ± 0.11 | (3.46 ± 0.50)·10−6 | 96.3 ± 1.4 |
| T4P35 | 3.5 | 3.06 ± 0.09 | (1.68 ± 0.25)·10−6 | 93.7 ± 1.5 |
| T6P2 | 2 | 1.71 ± 0.03 | (1.76 ± 0.51)·10−6 | 95.4 ± 0.5 |
| T6P35 | 3.5 | 2.86 ± 0.19 | (1.39 ± 0.22)·10−6 | 94.9 ± 1.0 |
| T7P2 | 2 | 1.60 ± 0.05 | (1.59 ± 0.06)·10−6 | 95.7 ± 1.5 |
| T7P35 | 3.5 | 3.03 ± 0.05 | (2.20 ± 0.37)·10−5 | 94.4 ± 2.2 |
| T7P5 | 5 | 3.03 ± 0.01 | (1.96 ± 0.96)·10−5 | 95.7 ± 1.5 |
| T10P2 | 2 | 1.55 ± 0.07 | (3.43 ± 0.56)·10−6 | 96.4 ± 1.5 |
| T10P35 | 3.5 | 2.64 ± 0.11 | (3.88 ± 0.85)·10−6 | 96.1 ± 1.3 |
| T10P5 | 5 | 3.60 ± 0.20 | (1.72 ± 0.65)·10−5 | 93.0 ± 2.5 |
| Sample Formulation | υc(·10−3) (mol.cm−3) | Mc (g·mol) | Tg (°C) |
|---|---|---|---|
| Reference | 1.58 ± 0.12 | 757 ± 59 | 27.1 ± 0.5 |
| T4P2 | 1.89 ± 0.21 | 636 ± 72 | 25.5 ± 0.6 |
| T4P35 | 2.00 ± 0.07 | 589 ± 19 | 24.4 ± 1.3 |
| T6P2 | 1.40 ± 0.39 | 895 ± 248 | 25.5 ± 0.8 |
| T6P35 | 1.96 ± 0.18 | 602 ± 55 | 25.4 ± 0.1 |
| T7P2 | 1.75 ± 0.01 | 687 ± 2 | 26.1 ± 0.1 |
| T7P35 | 2.04 ± 0.09 | 588 ± 26 | 25.7 ± 0.6 |
| T7P5 | 2.21 ± 0.01 | 543 ± 4 | 24.7 ± 0.7 |
| T10P2 | 1.34 ± 0.17 | 910 ± 116 | 25.0 ± 0.2 |
| T10P35 | 1.72 ± 0.22 | 705 ± 92 | 25.9 ± 0.2 |
| T10P5 | 2.11 ± 0.10 | 562 ± 26 | 25.2 ± 0.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arias-Ferreiro, G.; Ares-Pernas, A.; Lasagabáster-Latorre, A.; Aranburu, N.; Guerrica-Echevarria, G.; Dopico-García, M.S.; Abad, M.-J. Printability Study of a Conductive Polyaniline/Acrylic Formulation for 3D Printing. Polymers 2021, 13, 2068. https://doi.org/10.3390/polym13132068
Arias-Ferreiro G, Ares-Pernas A, Lasagabáster-Latorre A, Aranburu N, Guerrica-Echevarria G, Dopico-García MS, Abad M-J. Printability Study of a Conductive Polyaniline/Acrylic Formulation for 3D Printing. Polymers. 2021; 13(13):2068. https://doi.org/10.3390/polym13132068
Chicago/Turabian StyleArias-Ferreiro, Goretti, Ana Ares-Pernas, Aurora Lasagabáster-Latorre, Nora Aranburu, Gonzalo Guerrica-Echevarria, M. Sonia Dopico-García, and María-José Abad. 2021. "Printability Study of a Conductive Polyaniline/Acrylic Formulation for 3D Printing" Polymers 13, no. 13: 2068. https://doi.org/10.3390/polym13132068
APA StyleArias-Ferreiro, G., Ares-Pernas, A., Lasagabáster-Latorre, A., Aranburu, N., Guerrica-Echevarria, G., Dopico-García, M. S., & Abad, M.-J. (2021). Printability Study of a Conductive Polyaniline/Acrylic Formulation for 3D Printing. Polymers, 13(13), 2068. https://doi.org/10.3390/polym13132068