Influence of the Alkali Treatment of Flax and Hemp Fibers on the Properties of PHBV Based Biocomposites
Abstract
1. Introduction
- – Purity of cellulose: Raw cellulosic material, such as unwashed sisal fibers, absorbs at least twice as much water as washed fibers due to a 24% pectin content.
- – Degree of crystallinity: All the OH groups in the amorphous phase are accessible to water, while only a small amount of water interacts with the surface OH groups of the crystalline phase.
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Methods
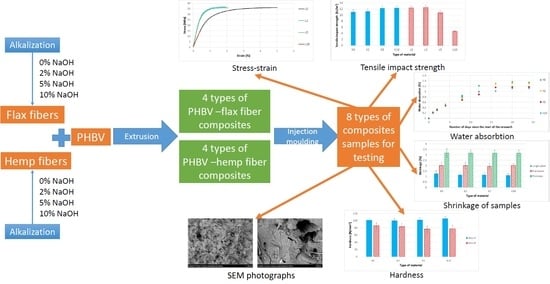
3. Results
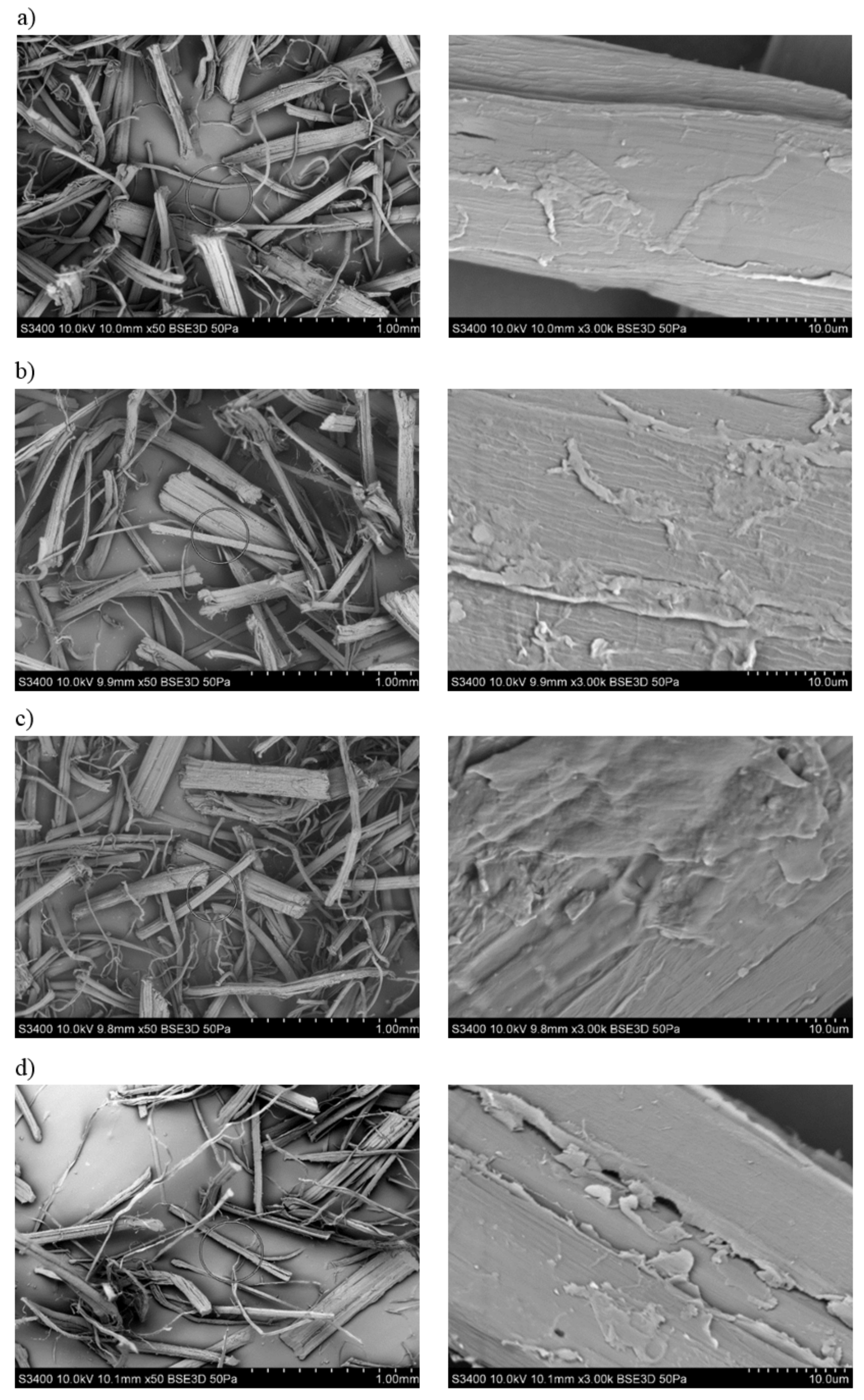
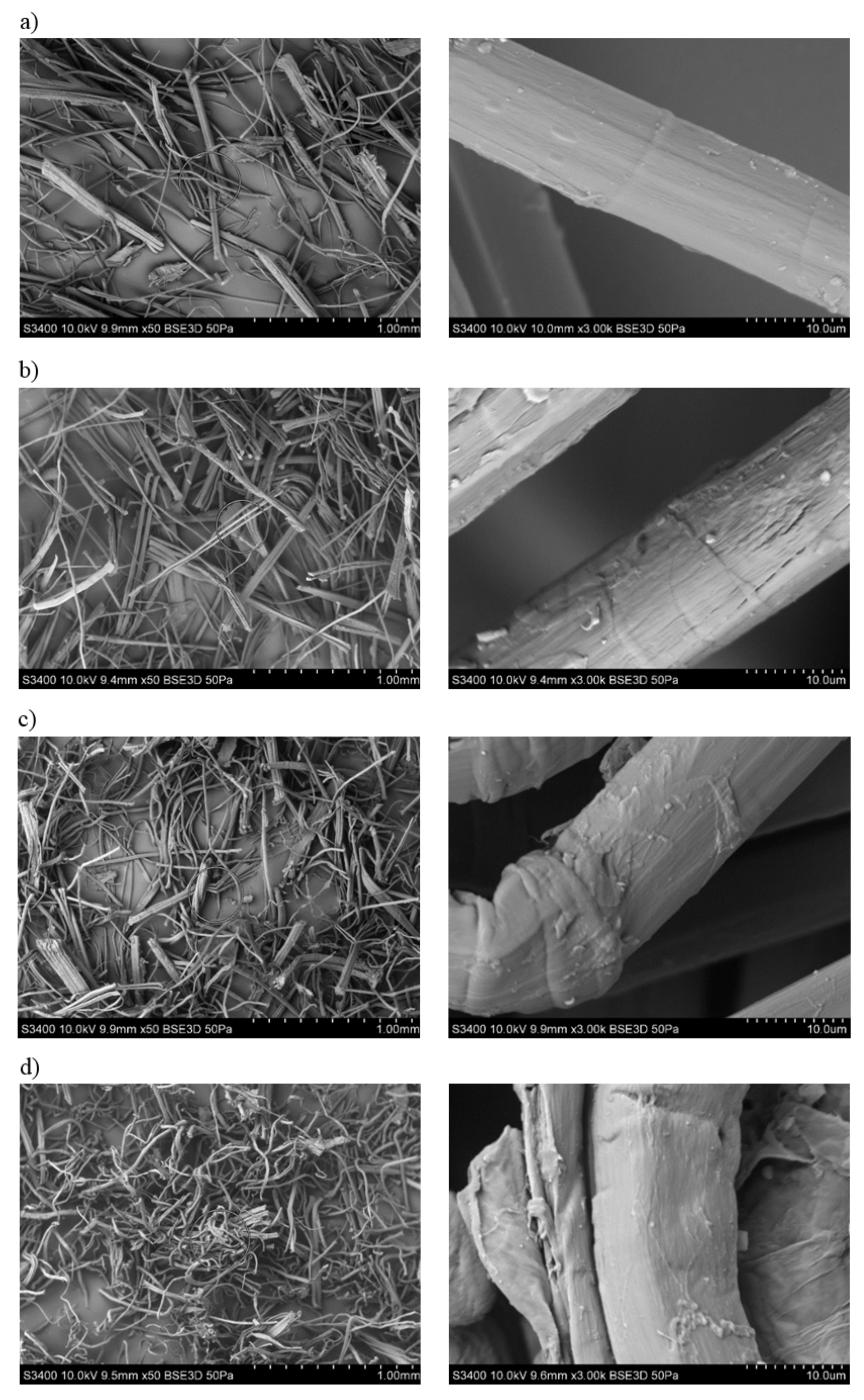
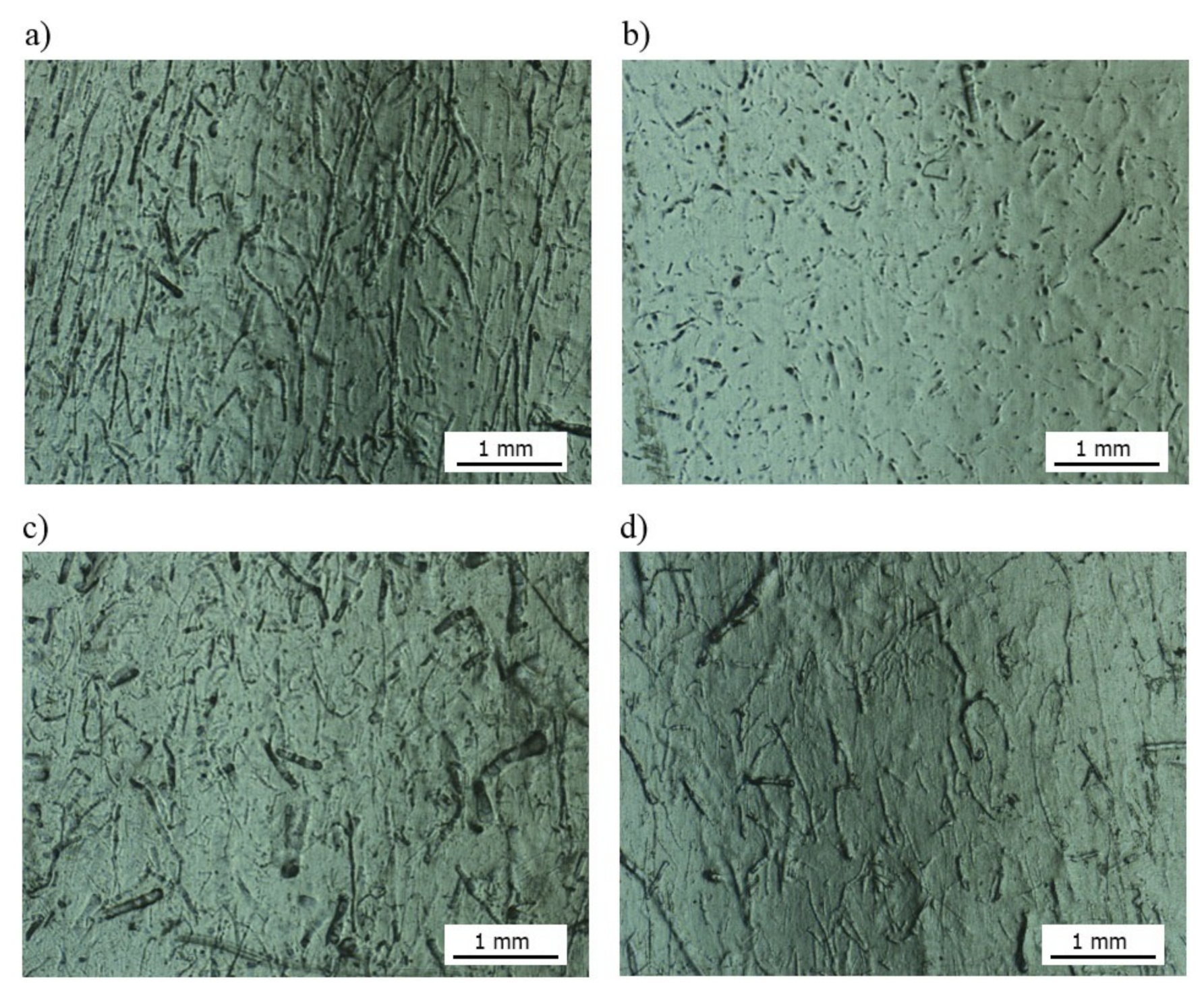
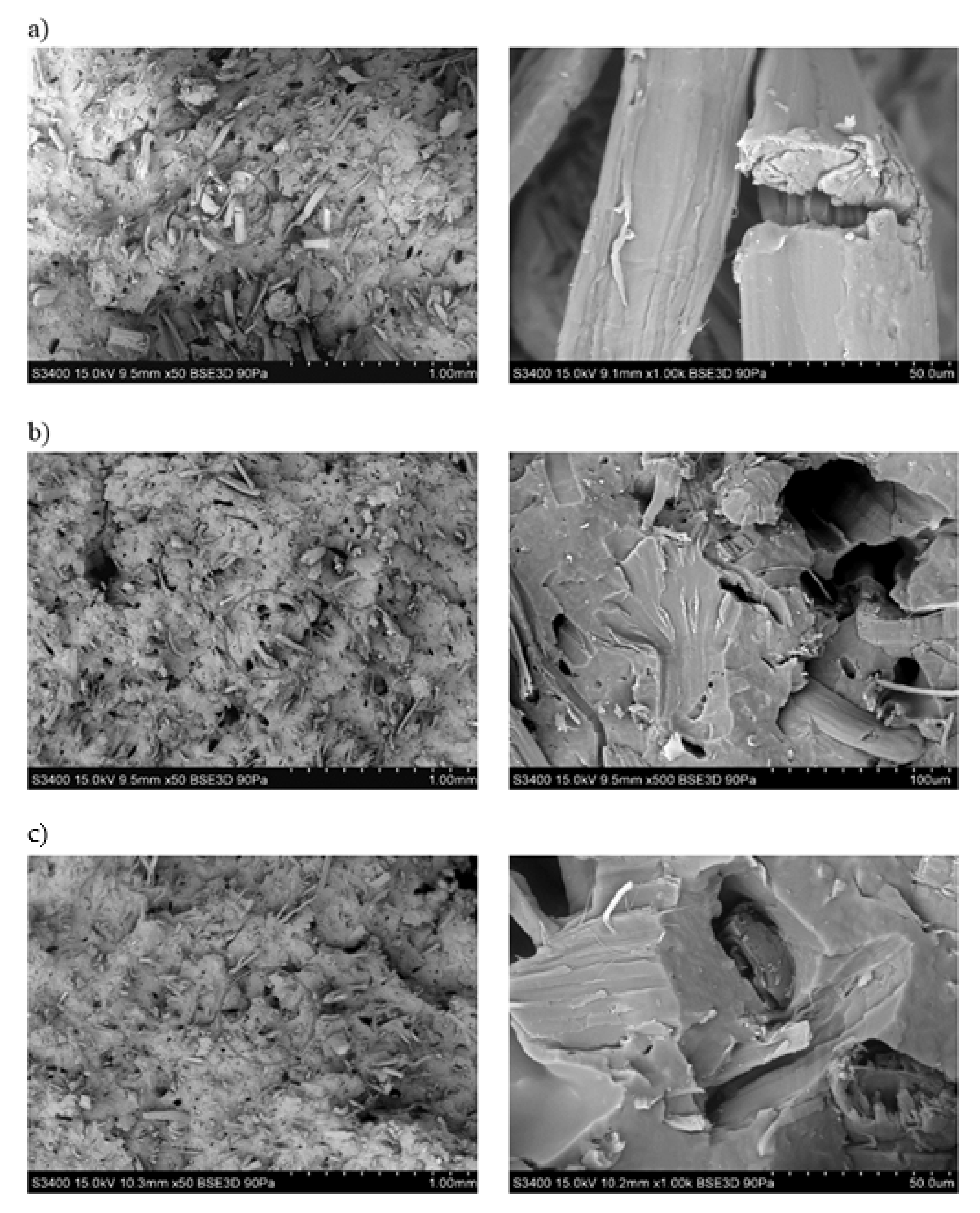
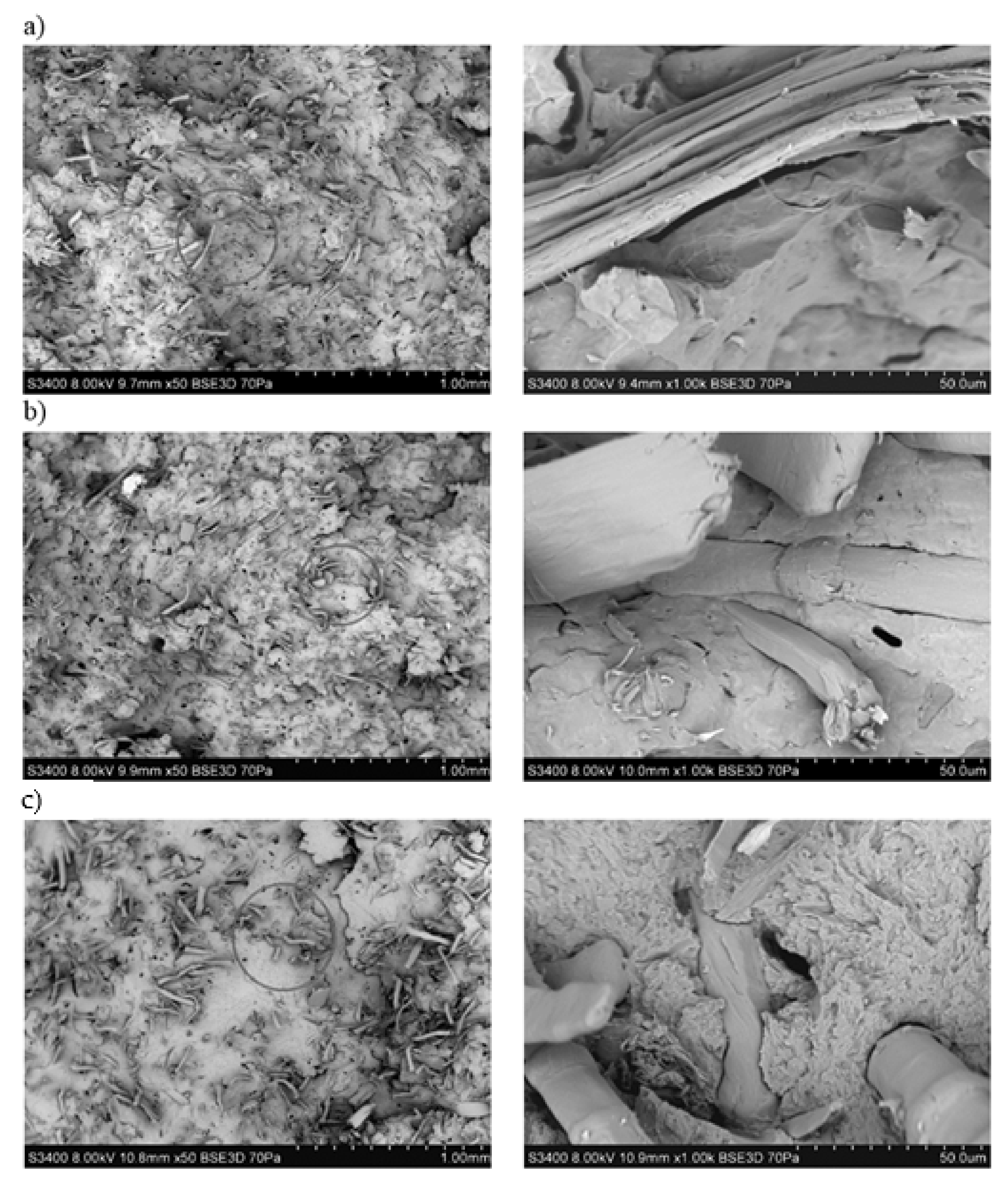
3.1. Assessment of the Surface Microstructure of Composites
3.2. The Fibers Shape Assessment of Factor Using Microscopic Examination
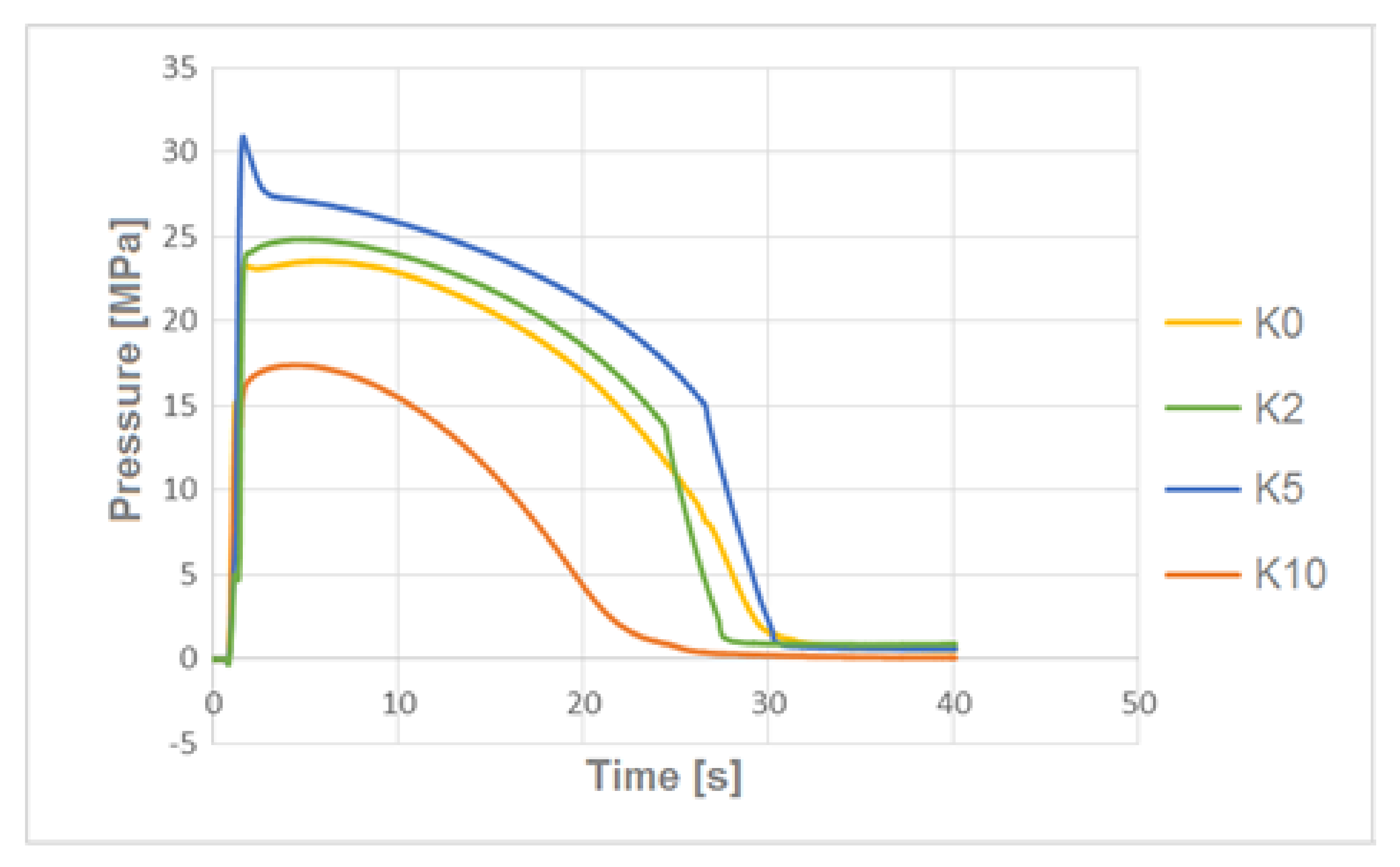
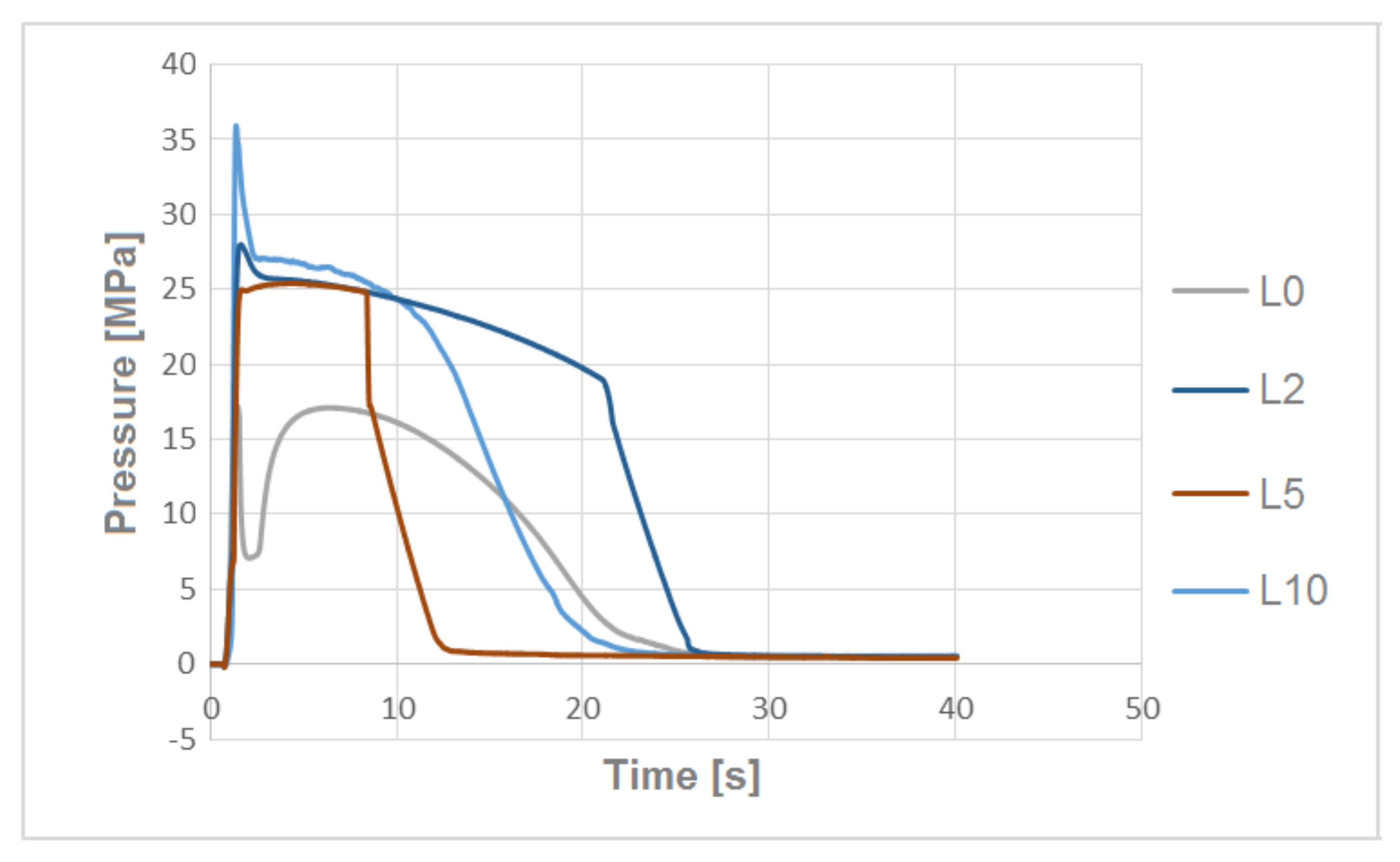
3.3. The Pressure Change Profile Analysis in the Mold Cavity
3.4. The Surface Quality Assessment of the Molded Piece Using Optical Microscopy
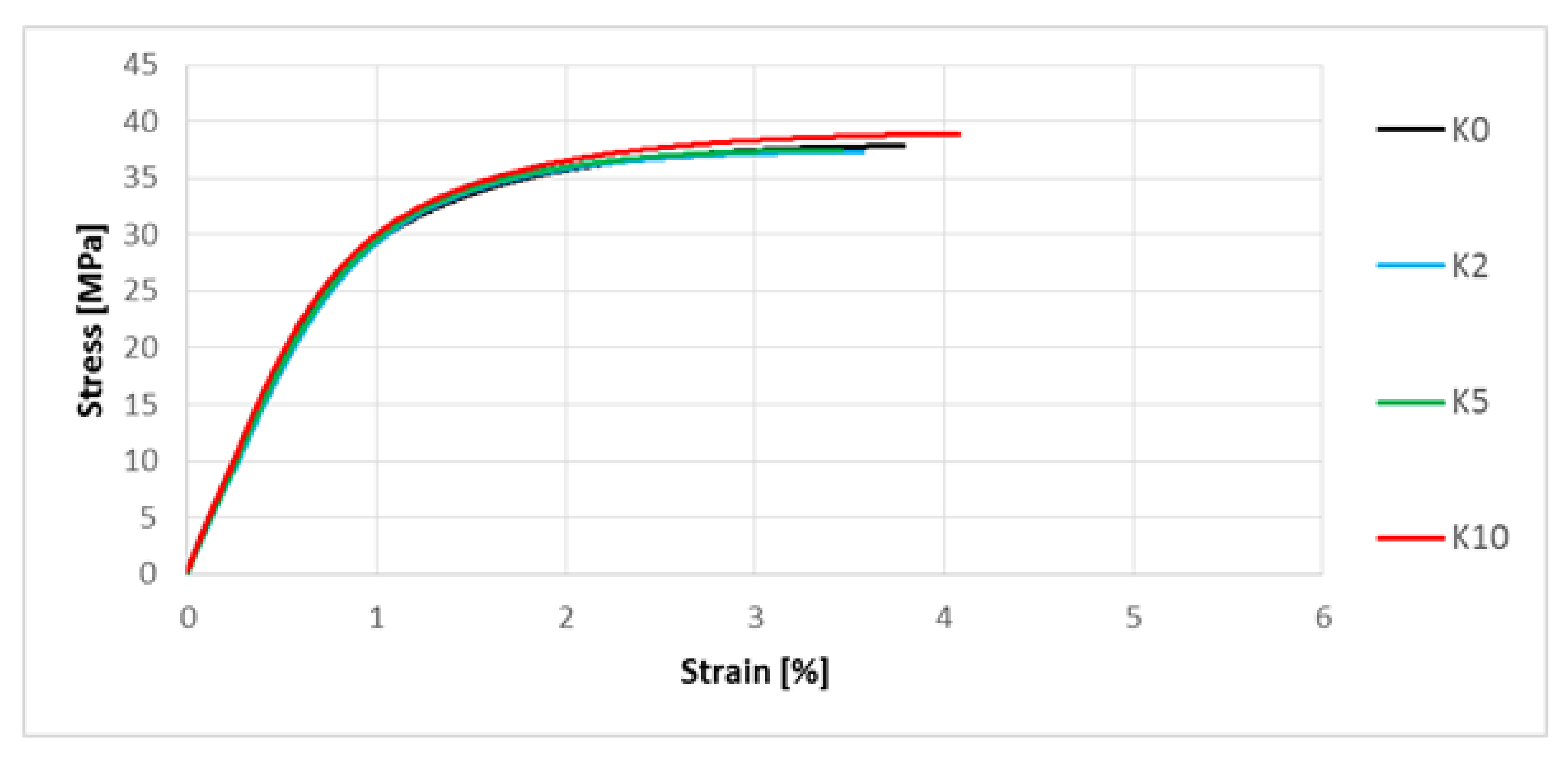
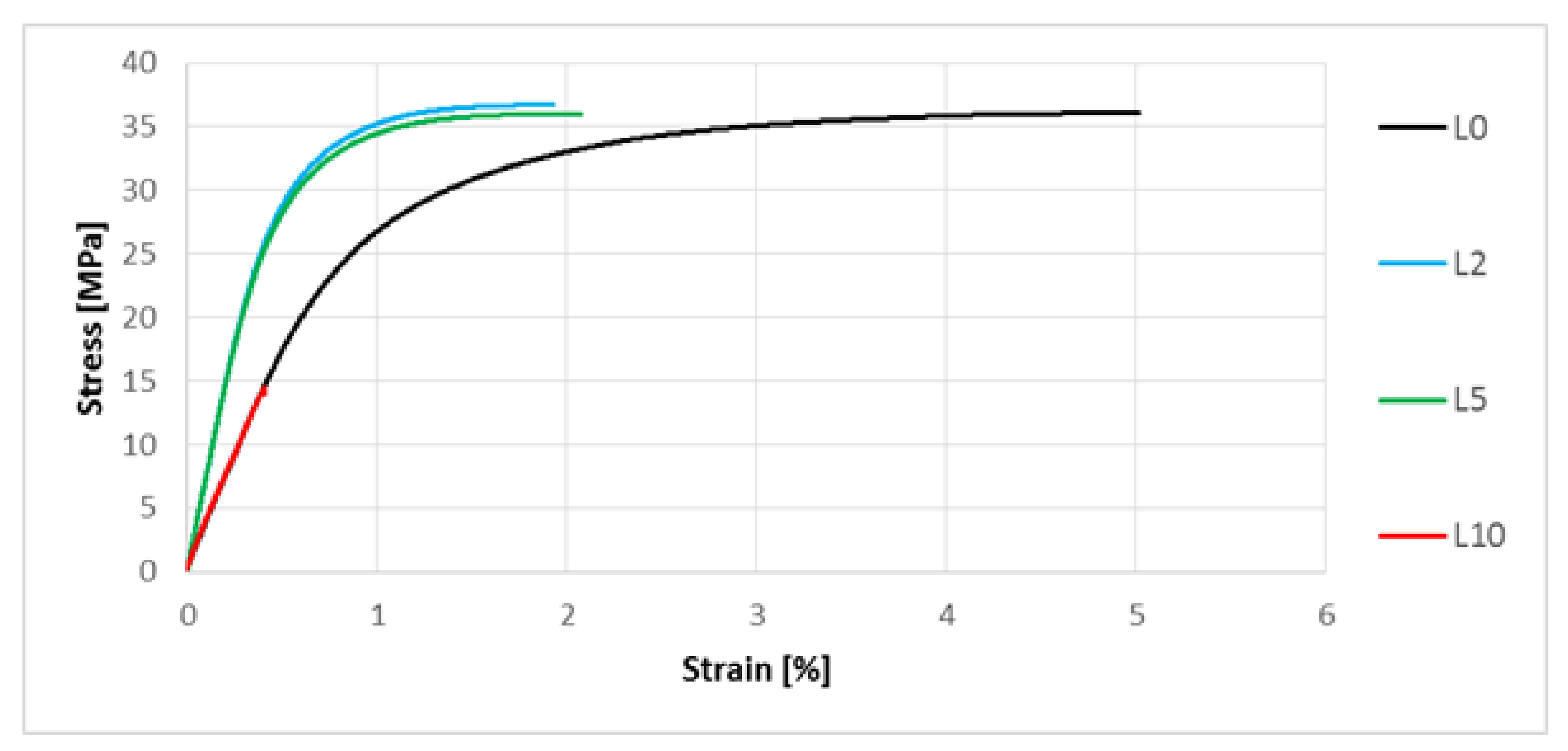
3.5. Determination of Mechanical Properties by Means of the Uniaxial Tensile Test
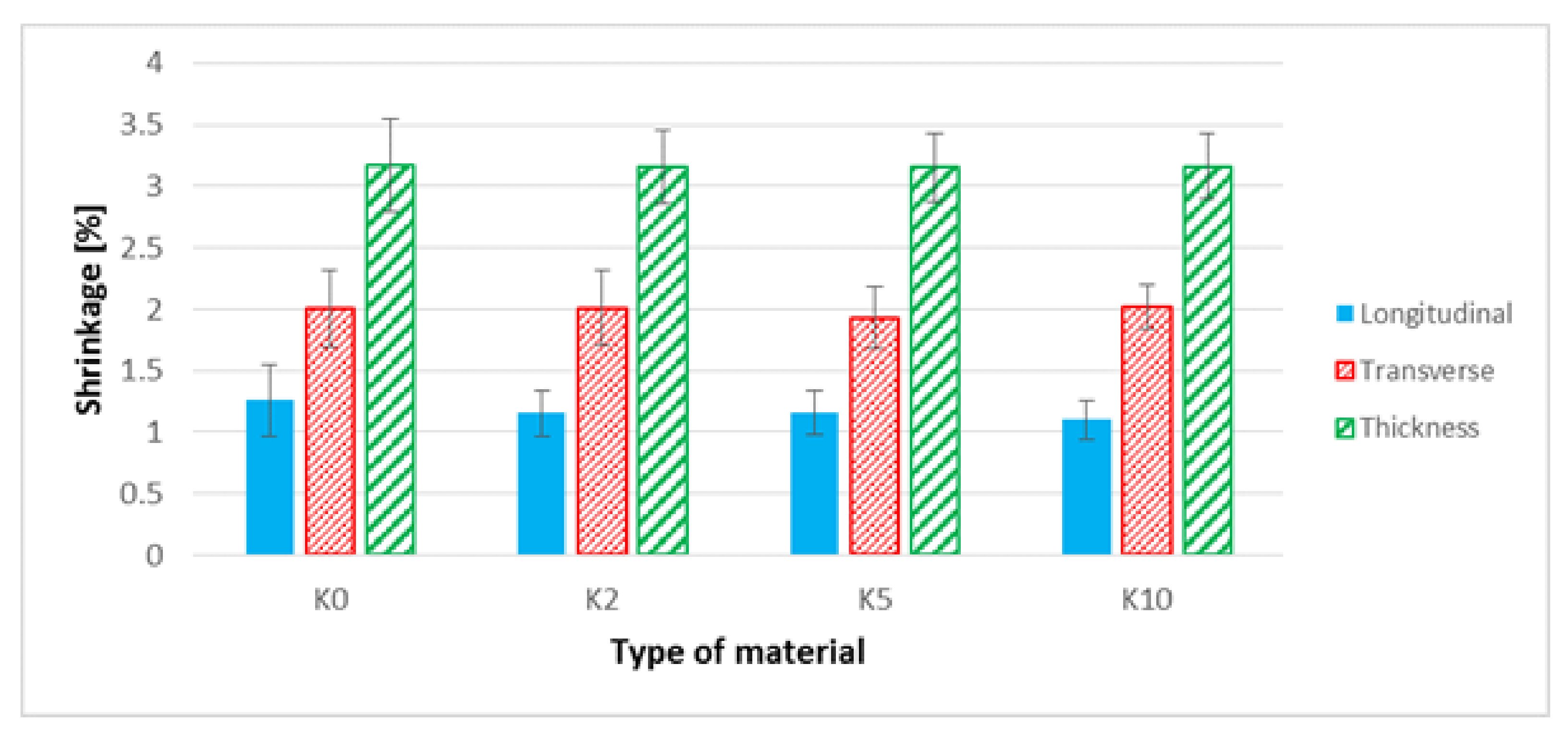
3.6. The Shrinkage Biocomposites Assessment
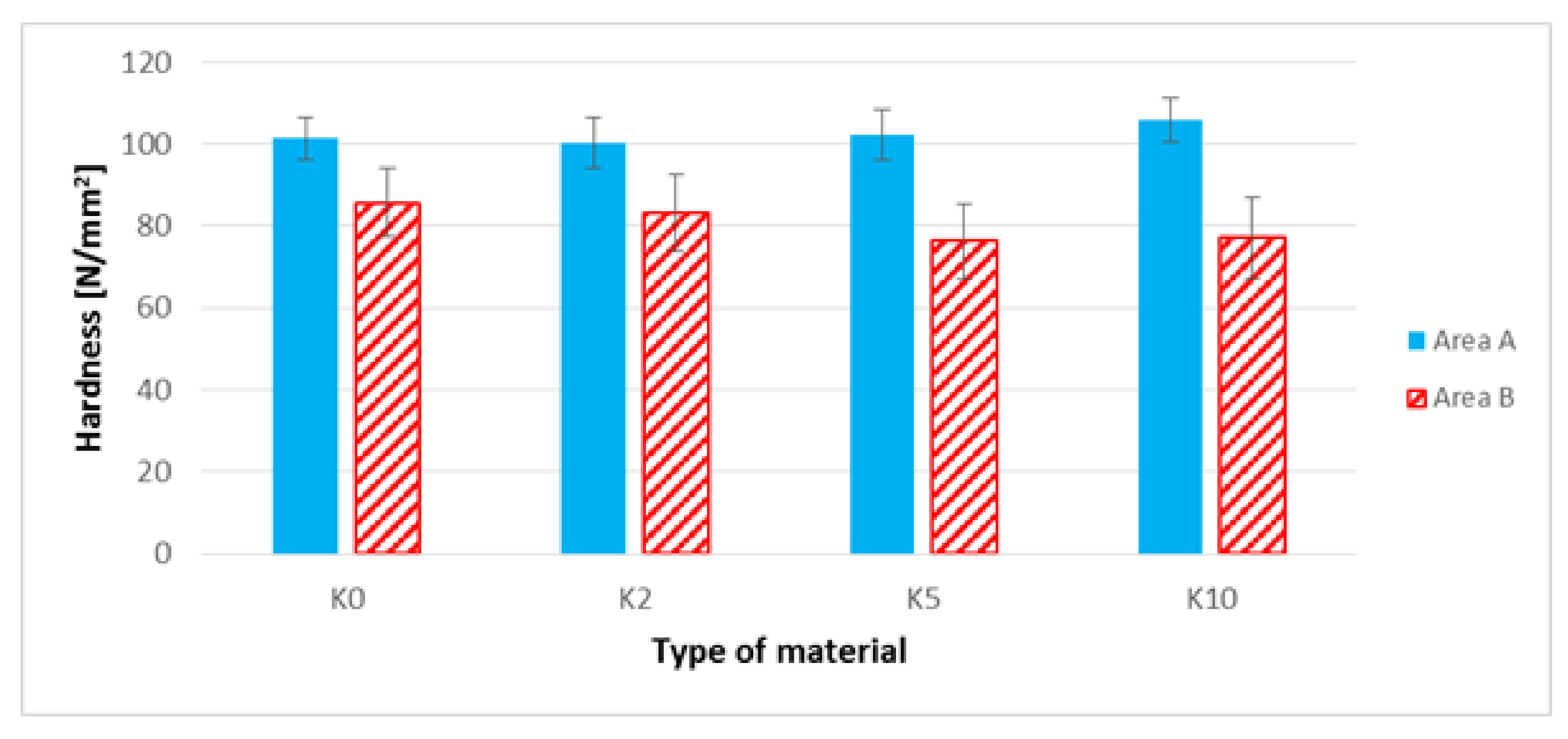
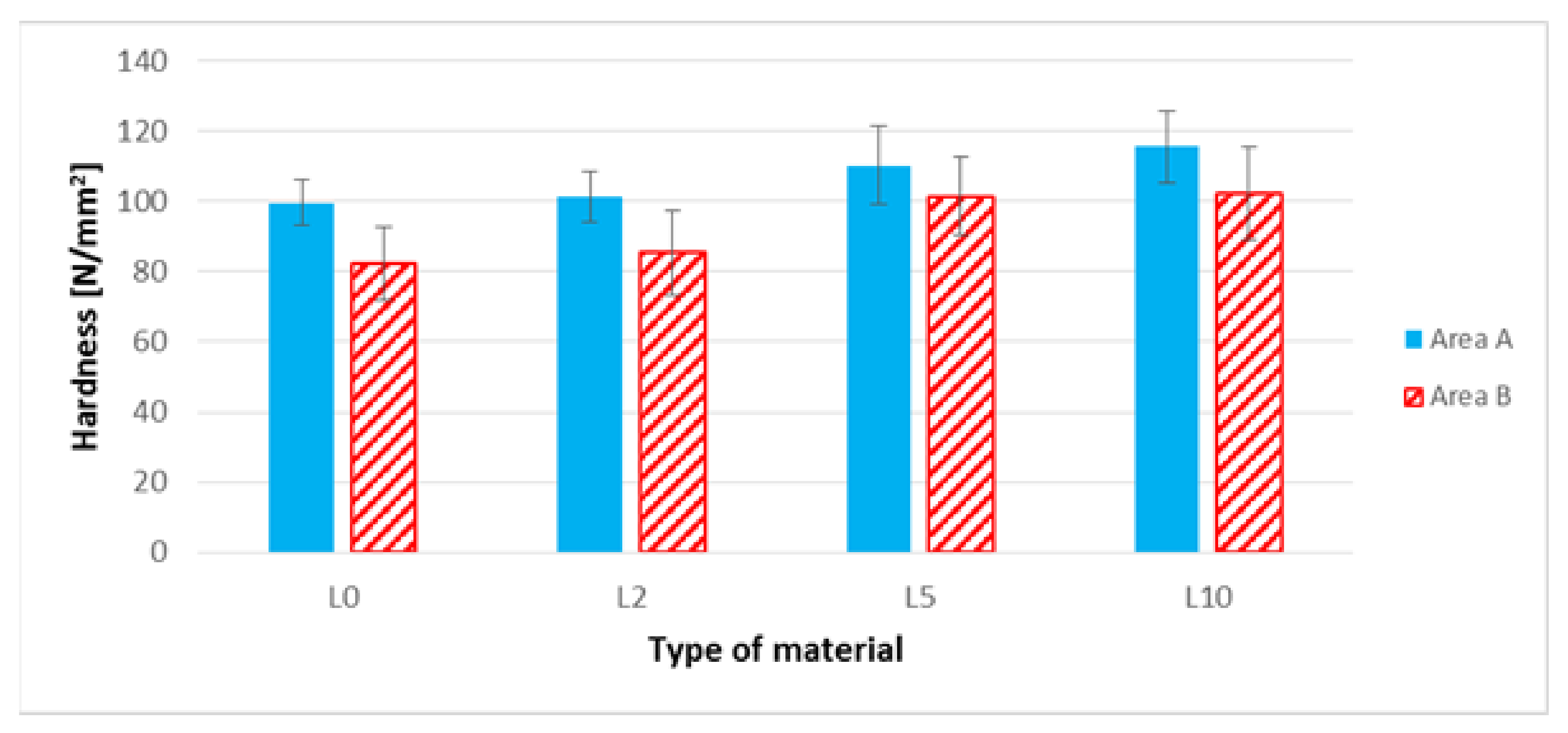
3.7. The Brinell Hardness Test
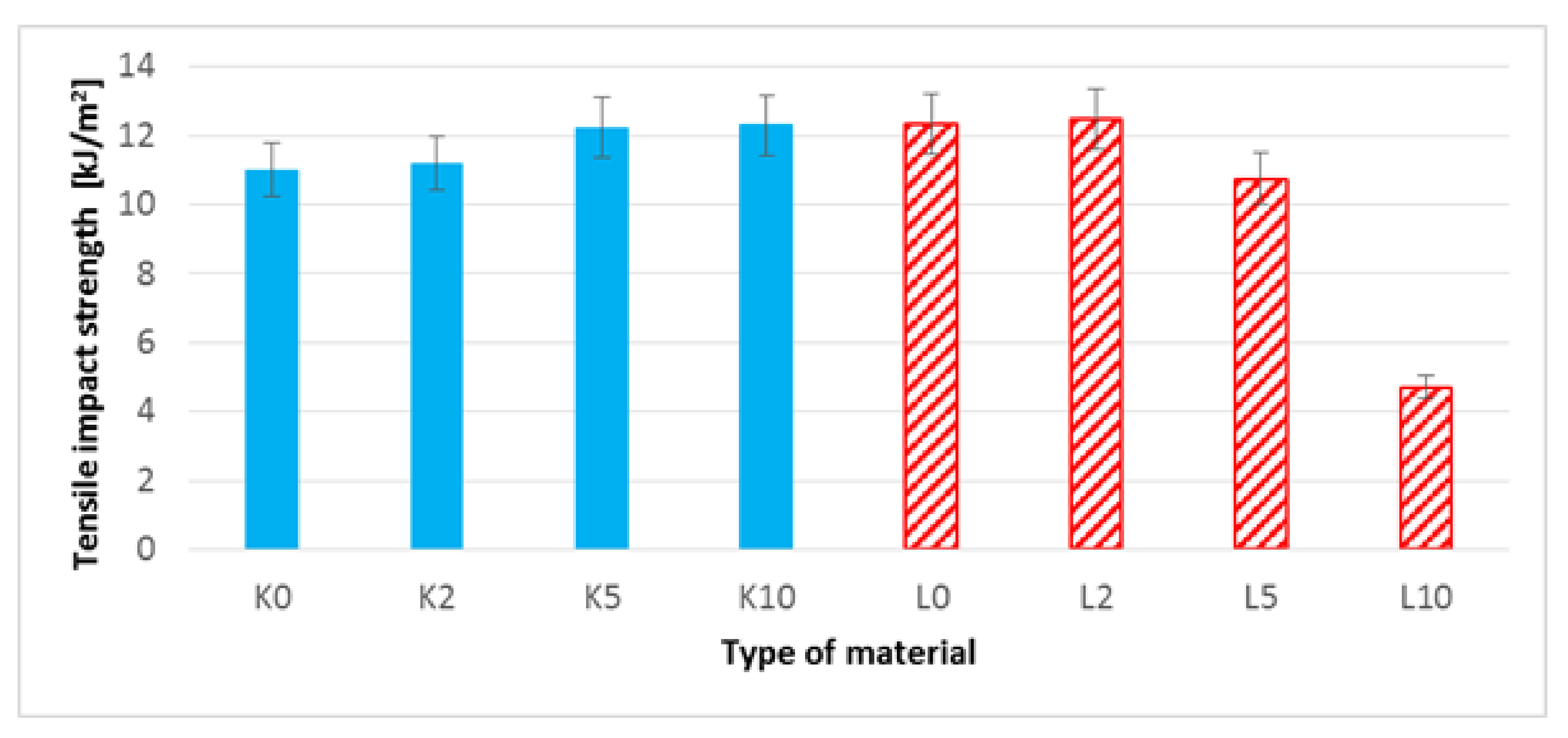
3.8. Impact Tensile Strength Test
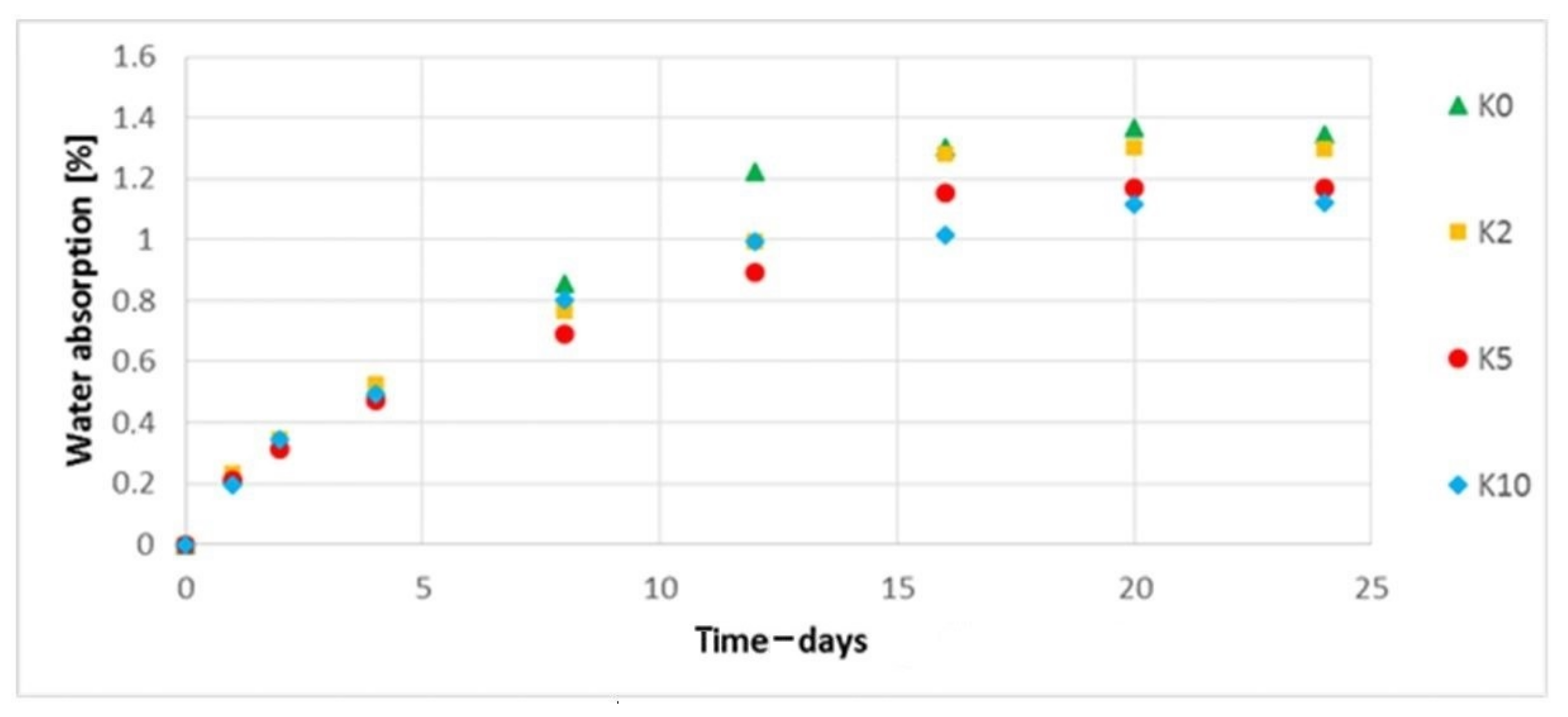
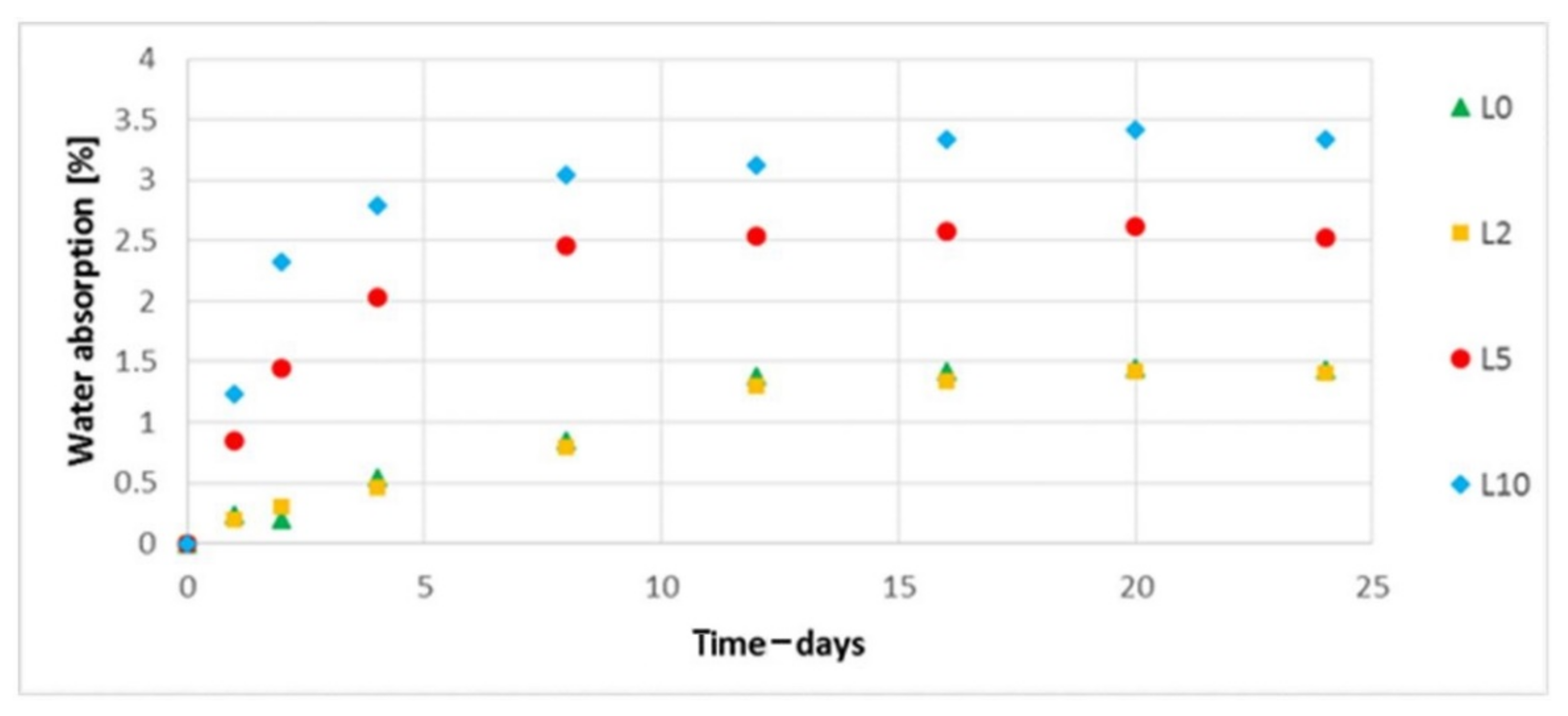
3.9. The Water Absorption Assessment
3.10. Assessment of the Composite Surface Microstructure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalia, S.; Kaith, B.S.; Kaur, I. Cellulose Fibers: Bio- and Nano-Polymer Composites: Green Chemistry and Technology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Kim, J.K.; Pal, K. Recent Advances in The Processing of Wood-Plastic Composites; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Klyosov, A.A. Wood-Plastic; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Rouchi, A.M. Lignin and Lignan Biosynthesis. Chem. Eng. News 2000, 78, 29–32. [Google Scholar] [CrossRef]
- Väisänen, T.; Haapala, A.; Lappalainen, R.; Tomppo, L. Utilization of agricultural and forest industry waste and residues in natural fiber-polymer composites: A review. Waste Manag. 2016, 54, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Stamman, A.J. Wood and Cellulose Science; Ronald Press: New York, NY, USA, 1964. [Google Scholar]
- Tarkow, H.; Turner, H.D. The swelling pressure of wood. For. Prod. J. 1958, 8, 193–197. [Google Scholar]
- Kondo, T. Hydrogen bonds in regioselectively substituted cellulose derivatives. J. Polym. Sci. Part B Polym. Phys. 1994, 32, 1229–1236. [Google Scholar] [CrossRef]
- Hearle, J.W.; Morton, W.E. Physical Properties of Textile Fibres; Woodhead Publishing: Manchester, UK, 2008. [Google Scholar]
- Smole, M.S.; Hribernik, S.; Kurečič, M.; Krajnc, A.U.; Kreže, T.; Kleinschek, K.S. Surface Properties of Non-Conventional Cellulose Fibres; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar]
- Nair, K.M.; Diwan, S.M.; Thomas, S. Tensile properties of short sisal fiber reinforced polystyrene composites. J. Appl. Polym. Sci. 1996, 60, 1483–1497. [Google Scholar] [CrossRef]
- George, J.; Bhagawan, S.S.; Prabhakaran, N.; Thomas, S. Short pineapple-leaf-fiber- reinforced low-density polyethylene composites. J. Appl. Polym. Sci. 1995, 57, 843–854. [Google Scholar] [CrossRef]
- Joseph, K.; Varghese, S.; Kalaprasad, G.; Thomas, S.; Prasannakumari, L.; Koshy, P.; Pavithran, C. Influence of interfacial adhesion on the mechanical properties and fracture behaviour of short sisal fibre reinforced polymer composites. Eur. Polym. J. 1996, 32, 1243–1250. [Google Scholar] [CrossRef]
- Joseph, P.V.; Mathew, G.; Joseph, K.; Thomas, S.; Pradeep, P. Mechanical properties of short sisal fiber-reinforced polypropylene composites: Comparison of experimental data with theoretical predictions. J. Appl. Polym. Sci. 2003, 88, 602–611. [Google Scholar] [CrossRef]
- Faruk, O.; Bledzki, A.K.; Fink, H.P.; Sain, M. Biocomposites reinforced with natural fibers: 2000–2010. Prog. Polym. Sci. 2012, 37, 1552–1596. [Google Scholar] [CrossRef]
- Pach, J.; Kaczmar, J.W. Wpływ chemicznej modyfikacji włókien konopnych na wybrane właściwości mechaniczne kompozytów na osnowie polipropylenowej. Polimery 2011, 56, 385–389. [Google Scholar] [CrossRef]
- Nevell, T.P.; Zeronian, S.H. Cellulose Chemistry and Its Applications; Halsted Press: New York, NY, USA, 1985. [Google Scholar]
- Happey, F. Applied Fibre Science; Academic Press: London, UK, 1979. [Google Scholar]
- Nguyen, T.; Zavarin, E.; Barrall, E.M. Thermal Analysis of lignocellulosic materials: Part I. Unmodified materials. J. Macromol. Sci. Rev. Macromol. Chem. 1981, 20, 1–65. [Google Scholar] [CrossRef]
- Ouajai, S.; Shanks, R.A. Composition, structure and thermal degradation of hemp cellulose after chemical treatments. Polym. Degrad. Stab. 2005, 89, 327–335. [Google Scholar] [CrossRef]
- Mwaikambo, L.Y.; Ansell, M.P. Chemical modification of hemp, sisal, jute, and kapok fibers by alkalization. J. Appl. Polym. Sci. 2002, 84, 2222–2234. [Google Scholar] [CrossRef]
- Suardana, N.P.G.; Piao, Y.; Lim, J.K. Mechanical properties of hemp fibers and hemp/pp composites: Effects of chemical surface treatment. Mater. Phys. Mech. 2011, 11, 1–8. [Google Scholar]
- Hu, R.; Lim, J.K. Fabrication and mechanical properties of completely biodegradable hemp fiber reinforced polylactic acid composites. J. Compos. Mater. 2007, 41, 1655–1669. [Google Scholar] [CrossRef]
- Pickering, K.L.; Sawpan, M.A.; Jayaraman, J.; Fernyhough, A. Influence of loading rate, alkali fibre treatment and crystallinity on fracture toughness of random short hemp fibre reinforced polylactide biocomposites. Compos. Part A Appl. Sci. Manuf. 2011, 42, 1148–1156. [Google Scholar] [CrossRef]
- Zafeiropoulos, N.E.; Williams, D.R.; Baillie, C.A.; Matthews, F.L. Engineering and characterisation of the interface in flax fibre/polypropylene composite materials. Part I. Development and investigation of surface treatments. Compos. Part A Appl. Sci. Manuf. 2002, 33, 1083–1093. [Google Scholar] [CrossRef]
- Van de Weyenberg, I.; Ivens, J.; De Coster, A.; Kino, B.; Baetens, E.; Verpoest, I. Influence of processing and chemical treatment of flax fibres on their composites. Compos. Sci. Technol. 2003, 63, 1241–1246. [Google Scholar] [CrossRef]
- Li, X.; Panigrahi, S.; Tabil, L.G. A study on flax fiber-reinforced polyethylene biocomposites. Appl. Eng. Agric. 2009, 25, 525–531. [Google Scholar] [CrossRef]
- Sikora, J.; Gajdoš, I.; Puszka, A. Polyethylene-Matrix Composites with Halloysite Nanotubes with Enhanced Physical/Thermal Properties. Polymers 2019, 11, 787. [Google Scholar] [CrossRef]
- Zini, E.; Focarete, M.L.; Noda, I.; Scandola, M. Bio-composite of bacterial poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) reinforced with vegetable fibers. Compos. Sci. Technol. 2007, 67, 2085–2094. [Google Scholar] [CrossRef]
- Avella, M.; La Rota, G.; Martuscelli, E.; Raimo, M.; Sadocco, P.; Elegir, G.; Riva, R. Poly (3-hydroxybutyrate-co-3-hydroxyvalerate) and wheat straw fibre composites: Thermal, mechanical properties and biodegradation behaviour. J. Mater. Sci. 2000, 35, 829–836. [Google Scholar] [CrossRef]
- Roy, I.; Visakh, P.M. Polyhydroxyalkanoate (PHA) Based Blends, Composites and Nano-Composites; Royal Society of Chemistry: Cambridge, UK, 2014. [Google Scholar]
- Luo, S.; Netravali, A.N. Interfacial and mechanical properties of environment-friendly “green” composites made from pineapple fibers and poly (hydroxybutyrate-co-valerate) resin. J. Mater. Sci. 1999, 34, 3709–3719. [Google Scholar] [CrossRef]
- Bhardwaj, R.; Mohanty, A.K.; Drzal, L.T.; Pourboghrat, F.; Misra, M. Renewable resource-based green composites from recycled cellulose fiber and poly (3-hydroxybutyrate-co-3-hydroxyvalerate) bioplastic. Biomacromolecules 2006, 7, 2044–2051. [Google Scholar] [CrossRef]
- Torres-Giner, S.; Hilliou, L.; Melendez-Rodriguez, B.; Figueroa-Lopez, K.J.; Madalena, L.; Cabedo, D.; Covas, J.A.; Vicente, A.A.; Lagaron, J.M. Melt processability, characterization, and antibacterial activity of compression-molded green composite sheets made of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) reinforced with coconut fibers impregnated with oregano essential oil. Food Packag. Shelf Life 2018, 17, 39–49. [Google Scholar] [CrossRef]
- Hosokawa, M.N.; Darros, A.B.; Moris, V.A.D.S.; Paiva, J.M.F.D. Polyhydroxybutyrate Composites with Random Mats of Sisal and Coconut Fibers. Mater. Res. 2017, 20, 279–290. [Google Scholar] [CrossRef]
- Javadi, A.; Srithep, Y.; Pilla, S.; Lee, J.; Gong, S.; Turng, L.S. Processing and characterization of solid and microcellular PHBV/coir fiber composites. Mater. Sci. Eng. C 2010, 30, 749–757. [Google Scholar] [CrossRef]
- Macedo, J.D.S.; Costa, M.F.; Tavares, M.I.; Thiré, R.M. Preparation and characterization of composites based on polyhydroxybutyrate and waste powder from coconut fibers processing. Polym. Eng. Sci. 2010, 50, 1466–1475. [Google Scholar] [CrossRef]
- Corradini, E.; Ferreira, F.C.; Agnelli, J.A.; Marconcini, J.M.; Mattoso, L.H.; Rosa, M.F. Water uptake, water solubility, mechanical and morphological properties of corn gluten meal and poly (hydroxybutyrate-co-hydroxyvalerate) composites reinforced with green coconut fibers. Polímeros 2013, 23, 807–813. [Google Scholar] [CrossRef][Green Version]
- Qian, S.; Dai, X.; Qi, Y.; Ren, H. Preparation and characterization of polyhydroxybutyrate-bamboo lignophenol biocomposite films. BioResources 2015, 10, 3169–3180. [Google Scholar] [CrossRef]
- Liu, D.; Song, J.; Anderson, D.P.; Chang, P.R.; Hua, Y. Bamboo fiber and its reinforced composites: Structure and properties. Cellulose 2012, 19, 1449–1480. [Google Scholar] [CrossRef]
- Krishnaprasad, R.; Veena, N.R.; Maria, H.J.; Rajan, R.; Skrifvars, M.; Joseph, K. Mechanical and thermal properties of bamboo microfibril reinforced polyhydroxybutyrate biocomposites. J. Polym. Environ. 2009, 17, 109–114. [Google Scholar] [CrossRef]
- Rajan, K.P.; Veena, N.R.; Maria, H.J.; Rajan, R.; Skrifvars, M.; Joseph, K. Extraction of bamboo microfibrils and development of biocomposites based on polyhydroxybutyrate and bamboo microfibrils. J. Compos. Mater. 2011, 45, 1325–1329. [Google Scholar] [CrossRef]
- Liu, L.; Qin, L.; Ming, Z.; Peng, C.; Zhi-hui, W.; Qun, G. Effect of Bamboo Flour Size on Properties of Bamboo/PHBV Bio-Composites. J. Zhejiang For. Sci. Technol. 2011, 4, 1–3. [Google Scholar]
- Jiang, L.; Chen, F.; Qian, J.; Huang, J.; Wolcott, M.; Liu, L.; Zhang, J. Reinforcing and toughening effects of bamboo pulp fiber on poly (3-hydroxybutyrate-co-3-hydroxyvalerate) fiber composites. Ind. Eng. Chem. Res. 2009, 49, 572–577. [Google Scholar] [CrossRef]
- Jiang, L.; Huang, J.; Qian, J.; Chen, F.; Zhang, J.; Wolcott, M.P.; Zhu, Y. Study of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV)/bamboo pulp fiber composites: Effects of nucleation agent and compatibilizer. J. Polym. Environ. 2008, 16, 83–93. [Google Scholar] [CrossRef]
- Yu, Z. Study on Mechanical Properties of The Bamboo Fiber/PHBV Composites. J. Text. Res. 2004, 6, 1–14. [Google Scholar]
- Shibata, M.; Takachiyo, K.I.; Ozawa, K.; Yosomiya, R.; Takeishi, H. Biodegradable polyester composites reinforced with short abaca fiber. J. Appl. Polym. Sci. 2002, 85, 129–138. [Google Scholar] [CrossRef]
- Teramoto, N.; Urata, K.; Ozawa, K.; Shibata, M. Biodegradation of aliphatic polyester composites reinforced by abaca fiber. Polym. Degrad. Stab. 2004, 86, 401–409. [Google Scholar] [CrossRef]
- Luo, S.; Netravali, A.N. Mechanical and thermal properties of environment-friendly “green” composites made from pineapple leaf fibers and poly (hydroxybutyrate-co-valerate) resin. Polym. Compos. 1999, 20, 367–378. [Google Scholar] [CrossRef]
- Dangtungee, R.; Tengsuthiwat, J.; Boonyasopon, P.; Siengchin, S. Sisal natural fiber/clay-reinforced poly (hydroxybutyate-co-hydroxyvalerate) hybrid composites. J. Thermoplast. Compos. Mater. 2015, 28, 879–895. [Google Scholar] [CrossRef]
- Badia, J.D.; Kittikorn, T.; Strömberg, E.; Santonja-Blasco, L.; Martínez-Felipe, A.; Ribes-Greus, A.; Karlsson, S. Water absorption and hydrothermal performance of PHBV/sisal biocomposites. Polym. Degrad. Stab. 2014, 108, 166–174. [Google Scholar] [CrossRef]
- Tengsuthiwat, J.; Boonyasopon, P.; Dangtungee, R.; Siengchin, S. Characterization of poly (hydroxybutyate-co-hydroxyvalerate)/Sisal Fiber/Clay bio-composites Prepared by Casting Technique. Period. Polytech. Eng. Mech. Eng. 2016, 60, 103–112. [Google Scholar] [CrossRef]
- Keller, A. Compounding and mechanical properties of biodegradable hemp fibre composites. Compos. Sci. Technol. 2003, 63, 1307–1316. [Google Scholar] [CrossRef]
- Barkoula, N.M.; Garkhail, S.K.; Peijs, T. Biodegradable composites based on flax/polyhydroxybutyrate and its copolymer with hydroxyvalerate. Ind. Crops Prod. 2010, 31, 34–42. [Google Scholar] [CrossRef]
- Lu, N.; Oza, S.; Ferguson, I. Effect of alkali and silane treatment on the thermal stability of hemp fibers as reinforcement in composite structures. Adv. Mater. Res. 2012, 415, 666–670. [Google Scholar] [CrossRef]
- Shahzad, A. Hemp fiber and its composites–A review. J. Compos. Mater. 2012, 46, 973–986. [Google Scholar] [CrossRef]
- Sair, S.; Oushabi, A.; Kammouni, A.; Tanane, O.; Abboud, Y.; Hassani, F.O.; El Bouari, A. Effect of surface modification on morphological, mechanical and thermal conductivity of hemp fiber: Characterization of the interface of hemp–Polyurethane composite. Case Stud. Therm. Eng. 2017, 10, 550–559. [Google Scholar] [CrossRef]
- Gassan, J.; Mildner, I.; Bledzki, A.K. Influence of fiber structure modification on the mechanical properties of flax fiber-epoxy composites. Mech. Compos. Mater. 1999, 35, 435–440. [Google Scholar] [CrossRef]
- Aly, M.; Hashmi, M.S.J.; Olabi, A.G.; Benyounis, K.Y.; Messeiry, M.; Hussain, A.I.; Abadir, E.F. Optimization of alkaline treatment conditions of flax fiber using Box–Behnken method. J. Nat. Fibers 2012, 9, 256–276. [Google Scholar] [CrossRef]
- Gopalakrishnan, P.; Saiah, R.; Gattin, R.; Saiter, J.M. Effect of mercerization of flax fibers on wheat flour/flax fiber biocomposite with respect to thermal and tensile properties. Compos. Interfaces 2008, 15, 759–770. [Google Scholar] [CrossRef]
- ISO 527-1, Plastics—Determination of tensile properties. In Part 1: General principles; ISO Copyright Office: Geneva, Switzerland, 2012.
- ISO 2039-1, Plastics—Determination of hardness. In Part 1: Ball indentation method; ISO Copyright Office: Geneva, Switzerland, 2001.
- ISO 8256, Plastics—Determination of Tensile-Impact Strength; ISO Copyright Office: Geneva, Switzerland, 2004.
- ISO 62, Plastics—Determination of Water Absorption; ISO Copyright Office: Geneva, Switzerland, 2008.
- ISO 294-4, Plastics—Injection moulding of test specimens of thermoplastic materials. In Part 4: Determination of Moulding Shrinkage; ISO Copyright Office: Geneva, Switzerland, 2001.
- Czerniecka-Kubicka, A.; Janowski, G.; Pyda, M.; Frącz, W. Biocomposites based on the poly (3-hydroxybutyrate-co-3-hydroxyvalerate) matrix with the hemp fibers: Thermal and mechanical properties. J. Therm. Anal. Calorim. 2021, 112, 1–13. [Google Scholar]



| Type of Biocomposite | Head | Zones 3–7 | Zone 2 | Zone 1 | Charge |
|---|---|---|---|---|---|
| K0, K2, K5, K10, L0, L2, L5 | 160 °C | 160 °C | 155 °C | 145 °C | 50 °C |
| L10 | 140 °C | 140 °C | 135 °C | 125 °C | 50 °C |
| Parameter | K0, K2, K5, K10, L0, L2, L5 | L10 |
|---|---|---|
| Mold temperature (°C) | 60 | 60 |
| Melt temperature (°C) | 167 | 147 |
| Cooling time (s) | 25 | 25 |
| Packing time (s) | 25 | 25 |
| Packing pressure (MPa) | 30 | 30 |
| Flow rate (cm3/s) | 35 | 35 |
| Mold temperature (°C) | 60 | 60 |
| Type of Biocomposite | L (mm) | SDL | CVL | d (mm) | SDd | CVd | L/d |
|---|---|---|---|---|---|---|---|
| L0 | 1.002 | 0.114 | 11.38 | 0.095 | 0.058 | 61.05 | 10.55 |
| L2 | 0.994 | 0.113 | 11.37 | 0.090 | 0.055 | 61.11 | 11.04 |
| L5 | 1.001 | 0.118 | 11.79 | 0.065 | 0.038 | 58.46 | 15.40 |
| L10 | 0.998 | 0.121 | 12.12 | 0.047 | 0.029 | 61.70 | 21.23 |
| K0 | 0.987 | 0.112 | 11.35 | 0.138 | 0.051 | 36.96 | 7.15 |
| K2 | 1.003 | 0.102 | 10.17 | 0.134 | 0.050 | 37.31 | 7.49 |
| K5 | 0.997 | 0.099 | 9.93 | 0.121 | 0.038 | 31.40 | 8.24 |
| K10 | 0.991 | 0.095 | 9.59 | 0.113 | 0.042 | 37.17 | 8.77 |
| Type of Biocomposite | Statistics | E (MPa) | σM (MPa) | εM (%) |
|---|---|---|---|---|
| K0 | AM | 3815.78 | 37.58 | 3.83 |
| SD | 24.00 | 0.23 | 0.06 | |
| CV | 0.63 | 0.62 | 1.66 | |
| K2 | AM | 3855.64 | 37.17 | 3.57 |
| SD | 45.64 | 0.84 | 0.07 | |
| CV | 1.18 | 2.25 | 2.02 | |
| K5 | AM | 3885.91 | 37.65 | 3.73 |
| SD | 47.73 | 0.52 | 0.08 | |
| CV | 1.23 | 1.42 | 2.32 | |
| K10 | AM | 3992.55 | 38.11 | 4.05 |
| SD | 43.10 | 1.08 | 0.14 | |
| CV | 1.12 | 2.84 | 3.45 |
| Type of Material | Statistics | E (MPa) | σM (MPa) | εM (%) |
|---|---|---|---|---|
| L0 | AM | 3495.11 | 36.29 | 5.07 |
| SD | 41.71 | 0.53 | 0.25 | |
| CV | 1.19 | 1.45 | 4.90 | |
| L2 | AM | 3860.63 | 36.94 | 4.21 |
| SD | 45.22 | 0.34 | 0.12 | |
| CV | 1.17 | 0.98 | 3.38 | |
| L5 | AM | 3762.51 | 35.78 | 4.00 |
| SD | 55.10 | 0.82 | 0.07 | |
| CV | 1.46 | 2.28 | 1.81 | |
| L10 | AM | 3352.45 | 15.35 | 0.46 |
| SD | 143.21 | 1.29 | 0.07 | |
| CV | 4.27 | 8.42 | 16.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frącz, W.; Janowski, G.; Bąk, Ł. Influence of the Alkali Treatment of Flax and Hemp Fibers on the Properties of PHBV Based Biocomposites. Polymers 2021, 13, 1965. https://doi.org/10.3390/polym13121965
Frącz W, Janowski G, Bąk Ł. Influence of the Alkali Treatment of Flax and Hemp Fibers on the Properties of PHBV Based Biocomposites. Polymers. 2021; 13(12):1965. https://doi.org/10.3390/polym13121965
Chicago/Turabian StyleFrącz, Wiesław, Grzegorz Janowski, and Łukasz Bąk. 2021. "Influence of the Alkali Treatment of Flax and Hemp Fibers on the Properties of PHBV Based Biocomposites" Polymers 13, no. 12: 1965. https://doi.org/10.3390/polym13121965
APA StyleFrącz, W., Janowski, G., & Bąk, Ł. (2021). Influence of the Alkali Treatment of Flax and Hemp Fibers on the Properties of PHBV Based Biocomposites. Polymers, 13(12), 1965. https://doi.org/10.3390/polym13121965