Biopolymer and Synthetic Polymer-Based Nanocomposites in Wound Dressing Applications: A Review
Abstract
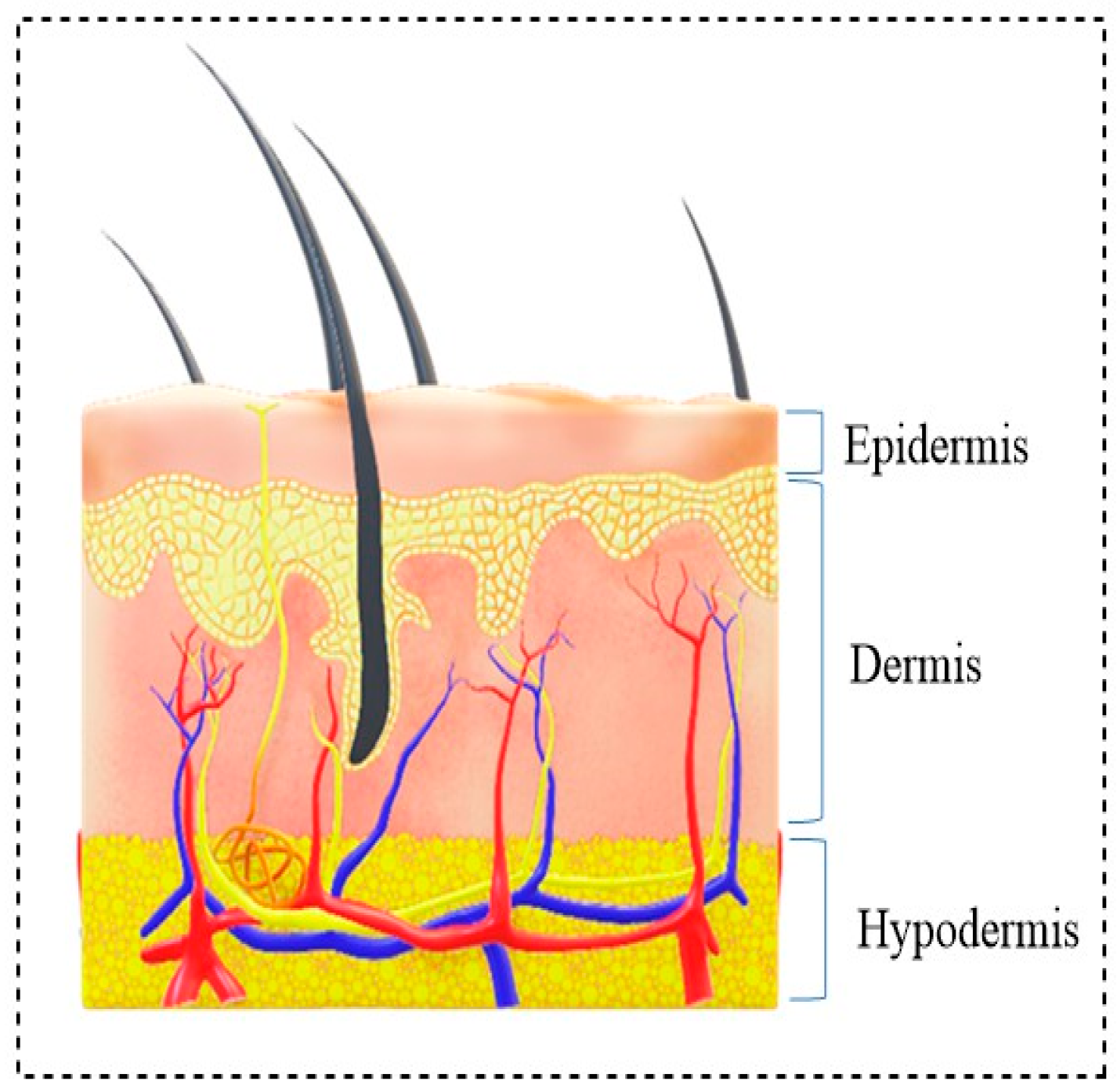
1. Introduction
- Hemostasis (immediately after injury);
- Inflammation (shortly after damage to tissue), during which swelling occurs;
- Proliferation, where blood vessels and new tissues form;
2. Biopolymer and Synthetic Polymer-Based Wound Dressing Agents
2.1. Biopolymer
2.2. Synthetic Polymer
3. Polymeric Nanocomposites in Wound Dressing
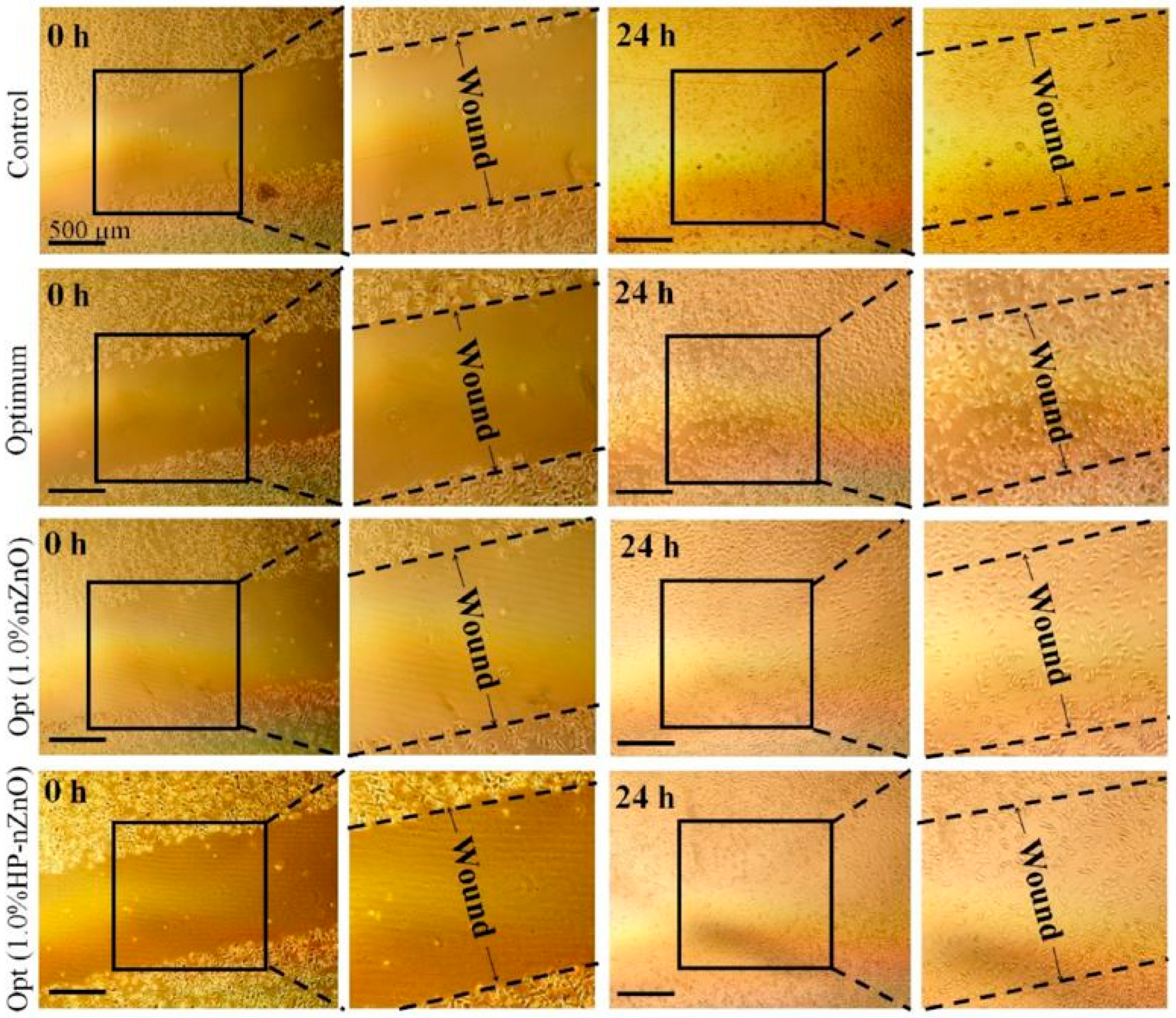
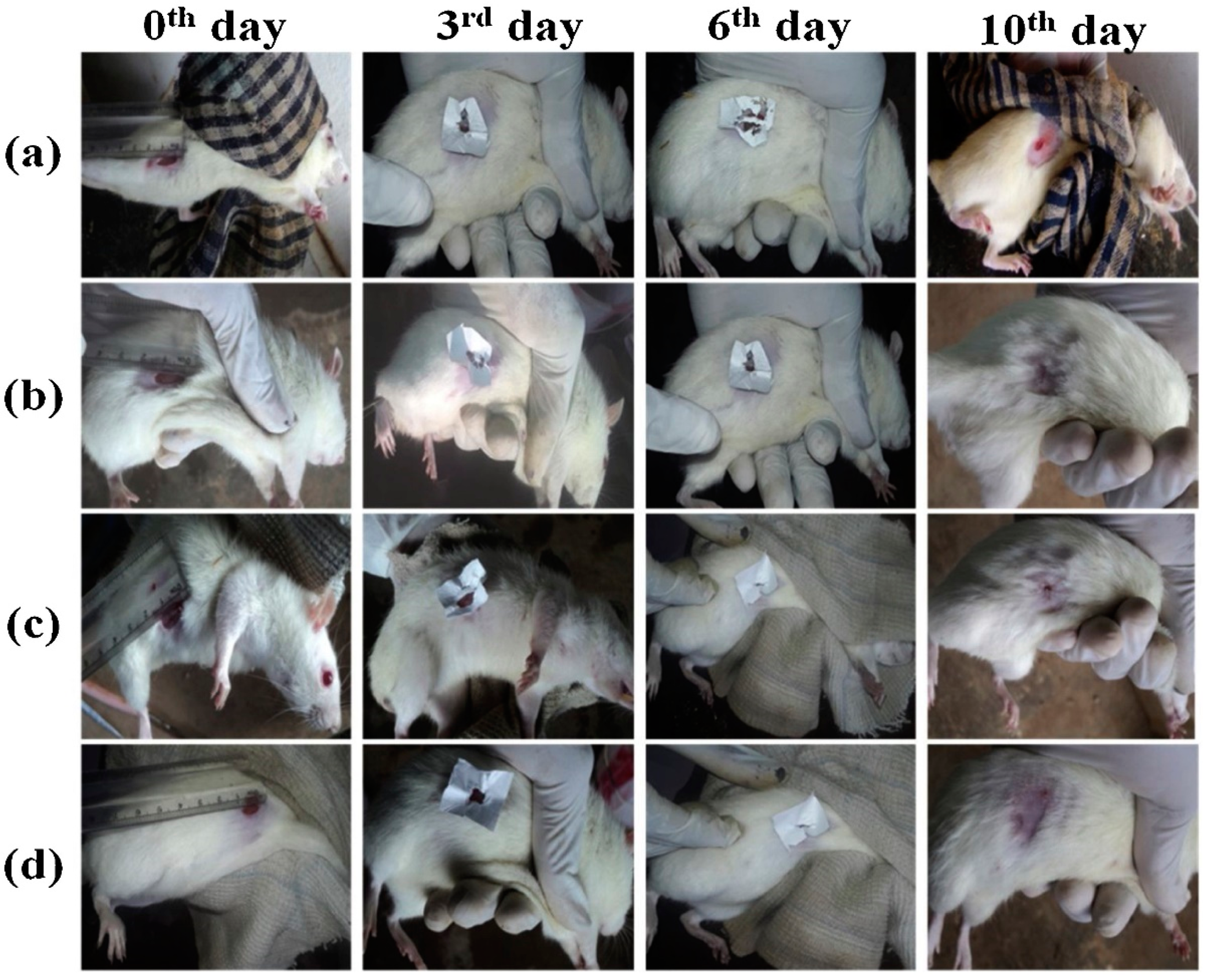
3.1. Zinc Oxide Embedded in Wound Dressings
3.2. Cerium Oxide Embedded in Wound Dressings
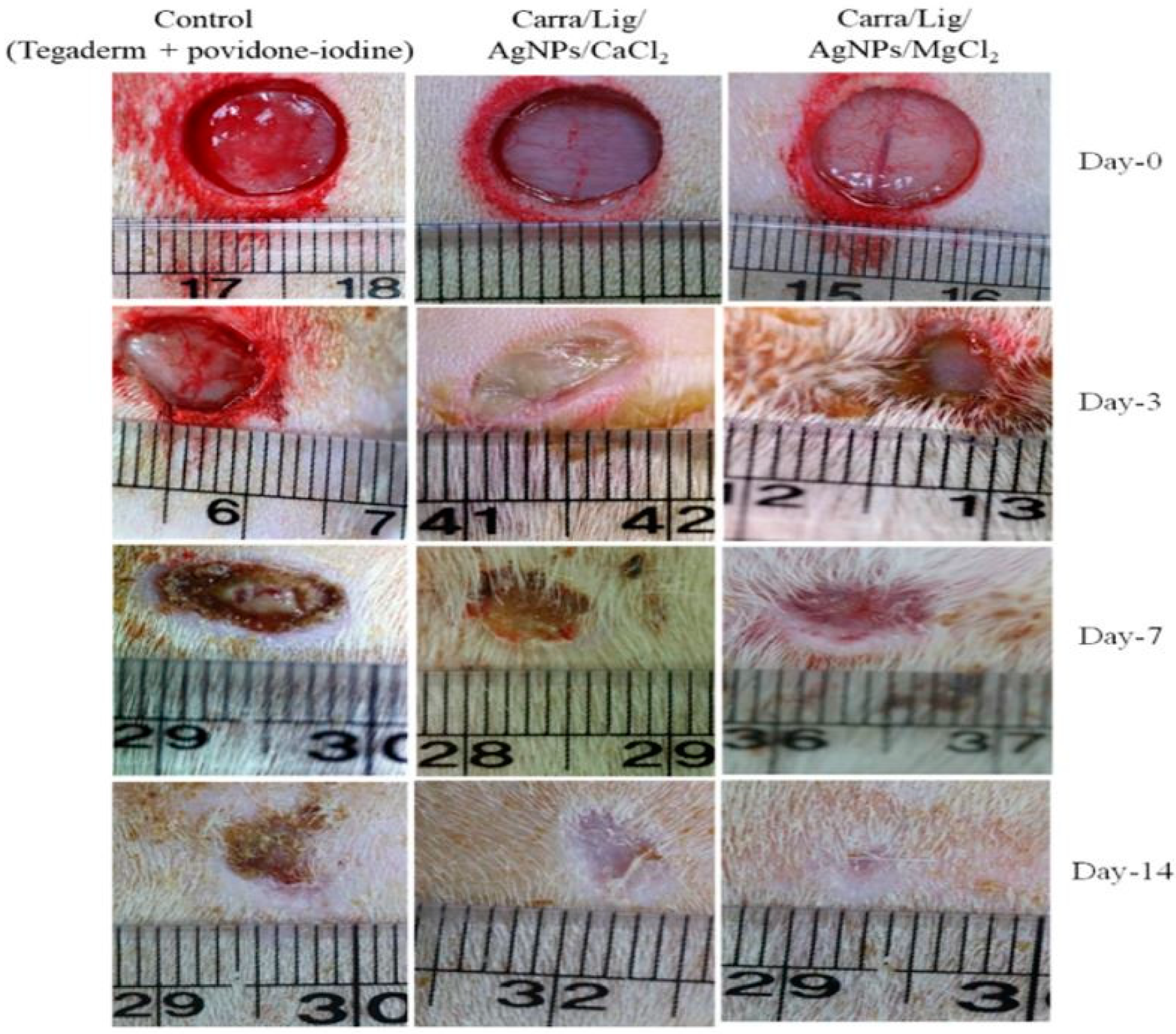
3.3. Silver Embedded in Wound Dressings
3.4. Titanium Dioxide Embedded in Wound Dressings
3.5. Iron Oxides Embedded in Wound Dressings
3.6. Graphene Oxides Embedded in Wound Dressings
3.7. Mesoporous Silica and Carbon Nanotubes Embedded in Wound Dressings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morgado, P.I.; Aguiar-Ricardo, A.; Correia, I.J. Asymmetric membranes as ideal wound dressings: An overview on produc-tion methods, structure, properties and performance relationship. J. Membr. Sci. 2015, 490, 139–151. [Google Scholar] [CrossRef]
- Manimalha, B.; Ravi, K.T.; Mary, B. Skin substitutes: A review. Burns 2001, 27, 534–544. [Google Scholar]
- Biedermann, T.; Boettcher-Haberzeth, S.; Reichmann, E. Tissue Engineering of Skin for Wound Coverage. Eur. J. Pediatr. Surg. 2013, 23, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, M.; Boroujeni, S.M.; Omidvarkordshouli, N.; Soleimani, M. Advances in skin regeneration: Application of electro-spun scaffolds. Adv. Healthc. Mater. 2015, 8, 1114–1133. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, A.A.; Vig, K.; Baganizi, D.R.; Sahu, R.; Dixit, S.; Dennis, V.; Pillai, S.R. Future prospects for scaffolding methods and biomaterials in skin tissue engineering: A review. Int. J. Mol. Sci. 2016, 17, 1974. [Google Scholar] [CrossRef]
- Capanema, N.S.; Mansur, A.A.; de Jesus, A.C.; Carvalho, S.M.; de Oliveira, L.C.; Mansur, H.S. Superabsorbent cross-linked carboxymethyl cellulose-PEG hydrogels for potential wound dressing applications. Int. J. Biol. Macromol. 2018, 106, 1218–1234. [Google Scholar] [CrossRef]
- Madaghiele, M.; Demitri, C.; Sannino, A.; Ambrosio, L. Polymeric hydrogels for burn wound care: Advanced skin wound dressings and regenerative templates. Burns Trauma 2014, 2, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Ravichandiran, P.; Masłyk, M.; Sheet, S.; Janeczko, M.; Premnath, D.; Kim, A.R.; Park, B.; Han, M.; Yoo, D.J. Synthesis and Antimicrobial Evaluation of 1,4-Naphthoquinone Derivatives as Potential Antibacterial Agents. ChemistryOpen 2019, 8, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Ravichandiran, P.; Sheet, S.; Premnath, D.; Kim, A.R.; Yoo, D.J. 1,4-Naphthoquinone Analogues: Potent Antibacterial Agents and Mode of Action Evaluation. Molecules 2019, 24, 1437. [Google Scholar] [CrossRef]
- Ravichandiran, P.; Athinarayanan, J.; Premnath, D.; Periasamy, V.S.; Alshatwi, A.A.; Vasanthkumar, S. Synthesis, molecu-lar docking and biological evaluation of novel 6-(4-(4-aminophenylsulfonyl) phenylamino)-5H-benzo [a] phenothiazin-5-one derivatives. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 139, 477–487. [Google Scholar] [CrossRef]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. BioMedicine 2015, 5, 22. [Google Scholar] [CrossRef]
- Parani, M.; Lokhande, G.; Singh, A.; Gaharwar, A. Engineered Nanomaterials for Infection Control and Healing Acute and Chronic Wounds. ACS Appl. Mater. Interfaces 2016, 8, 10049–10069. [Google Scholar] [CrossRef]
- Stefanov, I.; Pérez-Rafael, S.; Hoyo, J.; Cailloux, J.; Pérez, O.O.S.; Hinojosa-Caballero, D.; Tzanov, T. Multifunctional Enzymatically Generated Hydrogels for Chronic Wound Application. Biomacromolecules 2017, 18, 1544–1555. [Google Scholar] [CrossRef] [PubMed]
- Pinho, E.; Soares, G. Functionalization of cotton cellulose for improved wound healing. J. Mater. Chem. B 2018, 6, 1887–1898. [Google Scholar] [CrossRef]
- Varaprasad, K.; Jayaramudu, T.; Kanikireddy, V.; Toro, C.; Sadiku, E.R. Alginate-based composite materials for wound dressing application:A mini review. Carbohydr. Polym. 2020, 236, 116025. [Google Scholar] [CrossRef] [PubMed]
- Shitole, A.A.; Raut, P.W.; Khandwekar, A.; Sharma, N.; Baruah, M. Design and engineering of polyvinyl alcohol based bi-omimetic hydrogels for wound healing and repair. J. Polym. Res. 2019, 26, 201. [Google Scholar] [CrossRef]
- Kenawy, E.; Omer, A.M.; Tamer, T.M.; Elmeligy, M.A.; Eldin, M.M. Fabrication of biodegradable gela-tin/chitosan/cinnamaldehyde crosslinked membranes for antibacterial wound dressing applications. Int. J. Biol. Macromol. 2019, 139, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Poonguzhali, R.; Basha, S.K.; Kumari, V.S. Novel asymmetric chitosan/PVP/nanocellulose wound dressing: In vitro and in vivo evaluation. Int. J. Biol. Macromol. 2018, 112, 1300–1309. [Google Scholar] [CrossRef] [PubMed]
- Morgado, P.I.; Lisboa, P.F.; Ribeiro, M.P.; Miguel, S.P.; Simões, P.C.; Correia, I.J.; Aguiar-Ricardo, A. Poly (vinyl alco-hol)/chitosan asymmetrical membranes: Highly controlled morphology toward the ideal wound dressing. J. Membr. Sci. 2014, 469, 262–271. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Kumar, P.S.; Nair, S.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef]
- Hiranpattanakul, P.; Jongjitpissamai, T.; Aungwerojanawit, S.; Tachaboonyakiat, W. Fabrication of a chitin/chitosan hy-drocolloid wound dressing and evaluation of its bioactive properties. Res. Chem. Intermed. 2018, 44, 4913–4928. [Google Scholar] [CrossRef]
- Sahana, T.G.; Rekha, P. Biopolymers: Applications in wound healing and skin tissue engineering. Mol. Biol. Rep. 2018, 45, 2857–2867. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A. Current research on the blends of natural and synthetic polymers as new biomaterials. Prog. Polym. Sci. 2011, 36, 1254–1276. [Google Scholar] [CrossRef]
- Mogosanu, G.D.; Grumezescu, A.M. Natural and synthetic polymers for wounds and burns dressing. Int. J. Pharm. 2014, 463, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.M.; Fan, C.S.; Wang, N.C.; Chang, Y.H. Evaluation of membrane preparation method on the performance of alka-line polymer electrolyte: Comparison between poly (vinyl alcohol)/chitosan blended membrane and poly (vinyl alco-hol)/chitosan electrospun nanofiber composite membranes. Electrochim. Acta 2018, 266, 332–340. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Wound Healing Bionanocomposites Based on Castor Oil Polymeric Films Reinforced with Chitosan-Modified ZnO Nanoparticles. Biomacromolecules 2015, 16, 2631–2644. [Google Scholar] [CrossRef]
- Liu, J.; Willför, S.; Xu, C. A review of bioactive plant polysaccharides: Biological activities, functionalization, and biomedical applications. Bioact. Carbohydrates Diet. Fibre 2015, 5, 31–61. [Google Scholar] [CrossRef]
- Hemamalini, T.; Dev, V.R.G. Comprehensive review on electrospinning of starch polymer for biomedical applications. Int. J. Biol. Macromol. 2018, 106, 712–718. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.-R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021, 28, 1893–1931. [Google Scholar] [CrossRef]
- Del Valle, L.J.; Díaz, A.; Puiggalí, J. Hydrogels for biomedical applications: Cellulose, chitosan, and protein/peptide derivatives. Gels 2017, 3, 27. [Google Scholar] [CrossRef]
- Lin, W.-C.; Lien, C.-C.; Yeh, H.-J.; Yu, C.-M.; Hsu, S.-H. Bacterial cellulose and bacterial cellulose–Chitosan membranes for wound dressing applications. Carbohydr. Polym. 2013, 94, 603–611. [Google Scholar] [CrossRef]
- Wen, X.; Zheng, Y.; Wu, J.; Yue, L.; Wang, C.; Luan, J.; Wu, Z.; Wang, K. In vitro and in vivo investigation of bacterial cellulose dressing containing uniform silver sulfadiazine nanoparticles for burn wound healing. Prog. Nat. Sci. 2015, 25, 197–203. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, Y.; Song, W.; Luan, J.; Wen, X.; Wu, Z.; Chen, X.; Wang, Q.; Guo, S. In situ synthesis of silver-nanoparticles/bacterial cellulose composites for slow-released antimicrobial wound dressing. Carbohydr. Polym. 2014, 102, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Schumann, D.; Udhardt, U.; Marsch, S. Bacterial synthesized cellulose—Artificial blood vessels for microsurgery. Prog. Polym. Sci. 2001, 26, 1561–1603. [Google Scholar] [CrossRef]
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Brown, R.M. The Future Prospects of Microbial Cellulose in Biomedical Applications. Biomacromolecules 2007, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Dicker, K.T.; Gurski, L.A.; Pradhan-Bhatt, S.; Witt, R.L.; Farach-Carson, M.; Jia, X. Hyaluronan: A simple polysaccharide with diverse biological functions. Acta Biomater. 2014, 10, 1558–1570. [Google Scholar] [CrossRef]
- Karbownik, M.S.; Nowak, J.Z. Hyaluronan: Towards novel anti-cancer therapeutics. Pharmacol. Rep. 2013, 65, 1056–1074. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Wu, H.; Wang, X.; Yu, W.; Han, F. Biochemical characterization of a thermophilic hyaluronate lyase TcH-ly8C from Thermasporomyces composti DSM22891. Int. J. Biol. Macromol. 2020, 165, 1211–1218. [Google Scholar] [CrossRef]
- Goodarzi, N.; Varshochian, R.; Kamalinia, G.; Atyabi, F.; Dinarvand, R. A review of polysaccharide cytotoxic drug conju-gates for cancer therapy. Carbohydr. Polym. 2013, 92, 1280–1293. [Google Scholar] [CrossRef]
- Stern, R. Hyaluronidases in Cancer Biology. Semin. Cancer. Biol. 2008, 18, 275–280. [Google Scholar] [CrossRef]
- Weber, G.C.; Buhren, B.A.; Schrumpf, H.; Wohlrab, J.; Gerber, P.A. Clinical Applications of Hyaluronidase. Adv. Exp. Med. Biol. 2019, 1148, 255–277. [Google Scholar]
- Kim, J.K.; Kim, H.J.; Chung, J.-Y.; Lee, J.-H.; Young, S.-B.; Kim, Y.-H. Natural and synthetic biomaterials for controlled drug delivery. Arch. Pharmacal Res. 2014, 37, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S. Alginate dressings in surgery and wound management: Part 3. J. Wound Care 2000, 9, 163–166. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Chen, X.; Eldin, M.M.; Kenawy, E.-R. Crosslinked poly(vinyl alcohol) hydrogels for wound dressing applications: A review of remarkably blended polymers. Arab. J. Chem. 2015, 8, 1–14. [Google Scholar] [CrossRef]
- Andersen, T.; Strand, B.L.; Formo, K.; Alsberg, E.; Christensen, B.E. Chapter 9. Alginates as biomaterials in tissue engineering. Carbohydr. Chem. 2011, 37, 227–258. [Google Scholar] [CrossRef]
- Murakami, K.; Aoki, H.; Nakamura, S.; Nakamura, S.-I.; Takikawa, M.; Hanzawa, M.; Kishimoto, S.; Hattori, H.; Tanaka, Y.; Kiyosawa, T.; et al. Hydrogel blends of chitin/chitosan, fucoidan and alginate as healing-impaired wound dressings. Biomaterials 2010, 31, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, G. Collagen/alginate scaffolds comprising core (PCL)–shell (collagen/alginate) struts for hard tissue regenera-tion: Fabrication, characterisation, and cellular activities. J. Materi. Chem. B 2013, 1, 3185–3194. [Google Scholar] [CrossRef]
- Mir, M.; Ali, M.N.; Barakullah, A.; Gulzar, A.; Arshad, M.; Fatima, S.; Asad, M. Synthetic polymeric biomaterials for wound healing: A review. Prog. Biomater. 2018, 7, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Kariduraganavar, M.Y.; Kittur, A.A.; Kamble, R.R. Polymer Synthesis and Processing. In Natural and Synthetic Biomedical Polymers; Elsevier Science: Amsterdam, The Netherlands, 2014; pp. 1–31. [Google Scholar]
- Chen, E.Y.; Liu, W.F.; Megido, L.; Díez, P.; Fuentes, M.; Fager, C.; Mathur, S. Understanding and utilizing the biomole-cule/nanosystems interface. In Nanotechnologies in Preventive and Regenerative Medicine, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2018; pp. 207–297. [Google Scholar]
- Gyles, D.A.; Castro, L.D.; Silva, J.O.C., Jr.; Ribeiro-Costa, R.M. A review of the designs and prominent biomedical advances of natural and synthetic hydrogel formulations. Eur. Polym. J. 2017, 88, 373–392. [Google Scholar] [CrossRef]
- Norouzi, M.A.; Montazer, M.; Harifi, T.; Karimi, P. Flower buds like PVA/ZnO composite nanofibers assembly: Antibacteri-al, in vivo wound healing, cytotoxicity and histological studies. Polym. Test. 2021, 93, 106914. [Google Scholar] [CrossRef]
- Venkataprasanna, K.; Prakash, J.; Vignesh, S.; Bharath, G.; Venkatesan, M.; Banat, F.; Sahabudeen, S.; Ramachandran, S.; Venkatasubbu, G.D. Fabrication of Chitosan/PVA/GO/CuO patch for potential wound healing application. Int. J. Biol. Macromol. 2020, 143, 744–762. [Google Scholar] [CrossRef] [PubMed]
- Abdelghany, A.M.; Menazea, A.A.; Ismail, A.M. Synthesis, characterization and antimicrobial activity of Chi-tosan/Polyvinyl Alcohol blend doped with Hibiscus sabdariffa L. extract. J. Mol. Struct. 2019, 1197, 603–609. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Khalili, F.; Aliabadi, H.A.M.; Maleki, A.; Madanchi, H.; Ziabari, E.Z.; Bani, M.S. Alginate hydro-gel-polyvinyl alcohol/silk fibroin/magnesium hydroxide nanorods: A novel scaffold with biological and antibacterial activity and improved mechanical properties. Int. J. Biol. Macromol. 2020, 162, 1959–1971. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.; Niazi, M.B.K.; Hussain, A.; Farrukh, S.; Ahmad, T. Development of Anti-bacterial PVA/Starch Based Hydrogel Membrane for Wound Dressing. J. Polym. Environ. 2017, 26, 235–243. [Google Scholar] [CrossRef]
- Shahverdi, S.; Hajimiri, M.; Esfandiari, M.A.; Larijani, B.; Atyabi, F.; Rajabiani, A.; Dehpour, A.R.; Gharehaghaji, A.A.; Dinarvand, R. Fabrication and structure analysis of poly(lactide-co-glycolic acid)/silk fibroin hybrid scaffold for wound dressing applications. Int. J. Pharm. 2014, 473, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Lumbreras-Aguayo, A.; Meléndez-Ortiz, H.I.; Puente-Urbina, B.; Alvarado-Canché, C.; Ledezma, A.; Romero-García, J.; Betancourt-Galindo, R. Poly (methacrylic acid)-modified medical cotton gauzes with antimicrobial and drug delivery proper-ties for their use as wound dressings. Carbohydr. Polym. 2019, 205, 203–210. [Google Scholar] [CrossRef]
- Anjum, S.; Gupta, A.; Sharma, D.; Gautam, D.; Bhan, S.; Sharma, A.; Kapil, A.; Gupta, B. Development of novel wound care systems based on nanosilver nanohydrogels of polymethacrylic acid with Aloe vera and curcumin. Mater. Sci. Eng. C 2016, 64, 157–166. [Google Scholar] [CrossRef]
- Qi, X.; Wei, W.; Li, J.; Liu, Y.; Hu, X.; Zhang, J.; Dong, W. Fabrication and characterization of a novel anticancer drug deliv-ery system: Salecan/poly (methacrylic acid) semi-interpenetrating polymer network hydrogel. ACS Biomater. Sci. Eng. 2015, 1, 1287–1299. [Google Scholar] [CrossRef]
- Kausar, A. Poly(methyl methacrylate) nanocomposite reinforced with graphene, graphene oxide, and graphite: A review. Polym. Technol. Mater. 2018, 58, 821–842. [Google Scholar] [CrossRef]
- Kumar, M.; Mishra, R.K.; Banthia, A.K. Development of pectin based hydrogel membranes for biomedical applications. Int. J. Plast. Technol. 2010, 14, 213–223. [Google Scholar] [CrossRef]
- Yeh, J.-T.; Chen, C.-L.; Huang, K.S.; Nien, Y.H.; Chen, J.L.; Huang, P.Z. Synthesis, characterization, and application of PVP/chitosan blended polymers. J. Appl. Polym. Sci. 2006, 101, 885–891. [Google Scholar] [CrossRef]
- Aragón, J.; Costa, C.; Coelhoso, I.; Mendoza, G.; Aguiar-Ricardo, A.; Irusta, S. Electrospun asymmetric membranes for wound dressing applications. Mater. Sci. Eng. C 2019, 103, 109822. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Yusoh, K. A review on the recent research of polycaprolactone (PCL). Adv. Mater. Res. 2016, 1134, 249–255. [Google Scholar] [CrossRef]
- Chavalitpanya, K.; Phattanarudee, S. Poly(Lactic Acid)/Polycaprolactone Blends Compatibilized with Block Copolymer. Energy Procedia 2013, 34, 542–548. [Google Scholar] [CrossRef]
- Santoro, M.; Shah, S.R.; Walker, J.L.; Mikos, A.G. Poly(lactic acid) nanofibrous scaffolds for tissue engineering. Adv. Drug Deliv. Rev. 2016, 107, 206–212. [Google Scholar] [CrossRef]
- Garlotta, D. A Literature Review of Poly(Lactic Acid). J. Polym. Environ. 2001, 9, 63–84. [Google Scholar] [CrossRef]
- Honarbakhsh, S.; Pourdeyhimi, B. Scaffolds for drug delivery, part I: Electrospun porous poly(lactic acid) and poly(lactic acid)/poly(ethylene oxide) hybrid scaffolds. J. Mater. Sci. 2010, 46, 2874–2881. [Google Scholar] [CrossRef]
- Khil, M.-S.; Cha, D.-I.; Kim, H.Y.; Kim, I.-S.; Bhattarai, N. Electrospun nanofibrous polyurethane membrane as wound dressing. J. Biomed. Mater. Res. 2003, 67, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.; Hassanajili, S.; Karimi, M.B.; Emami, A.; Ghaffari, F.; Azarpira, N. Effect of organic/inorganic nanoparticles on performance of polyurethane nanocomposites for potential wound dressing applications. J. Mech. Behav. Biomed. Mater. 2018, 88, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Nethi, S.K.; Das, S.; Patra, C.R.; Mukherjee, S. Recent advances in inorganic nanomaterials for wound-healing applications. Biomater. Sci. 2019, 7, 2652–2674. [Google Scholar] [CrossRef] [PubMed]
- Parham, S.; Wicaksono, D.H.; Bagherbaigi, S.; Lee, S.L.; Nur, H. Antimicrobial treatment of different metal oxide nanoparti-cles: A critical review. J. Chin. Chem. Soc. 2016, 63, 385–393. [Google Scholar] [CrossRef]
- Nikolova, M.P.; Chavali, M.S. Metal Oxide Nanoparticles as Biomedical Materials. Biomimetics 2020, 5, 27. [Google Scholar] [CrossRef] [PubMed]
- Stoica, A.E.; Chircov, C.; Grumezescu, A.M. Nanomaterials for Wound Dressings: An Up-to-Date Overview. Molecules 2020, 25, 2699. [Google Scholar] [CrossRef]
- Kalashnikova, I.; Das, S.; Seal, S. Nanomaterials for wound healing: Scope and advancement. Nanomedicine 2015, 10, 2593–2612. [Google Scholar] [CrossRef]
- Wells, A.; Nuschke, A.; Yates, C.C. Skin tissue repair: Matrix microenvironmental influences. Matrix Biol. 2016, 49, 25–36. [Google Scholar] [CrossRef]
- Dumville, J.C.; Gray, T.A.; Walter, C.J.; Sharp, C.A.; Page, T.; Macefield, R.; Blazeby, J. Dressings for the prevention of surgical site infection. Cochrane Database Syst. Rev. 2016, 12, CD003091. [Google Scholar] [CrossRef] [PubMed]
- Rakhshaei, R.; Namazi, H. A potential bioactive wound dressing based on carboxymethyl cellulose/ZnO impregnated MCM-41 nanocomposite hydrogel. Mater. Sci. Eng. C 2017, 73, 456–464. [Google Scholar] [CrossRef]
- Khorasani, M.T.; Joorabloo, A.; Moghaddam, A.; Shamsi, H.; MansooriMoghadam, Z. Incorporation of ZnO nanoparticles into heparinised polyvinyl alcohol/chitosan hydrogels for wound dressing application. Int. J. Biol. Macromol. 2018, 114, 1203–1215. [Google Scholar] [CrossRef]
- Joorabloo, A.; Khorasani, M.T.; Adeli, H.; Mansoori-Moghadam, Z.; Moghaddam, A. Fabrication of heparinized nano ZnO/poly(vinylalcohol)/carboxymethyl cellulose bionanocomposite hydrogels using artificial neural network for wound dressing application. J. Ind. Eng. Chem. 2019, 70, 253–263. [Google Scholar] [CrossRef]
- Rao, K.M.; Suneetha, M.; Zo, S.; Duck, K.H.; Han, S.S. One-pot synthesis of ZnO nanobelt-like structures in hyaluronan hydrogels for wound dressing applications. Carbohydr. Polym. 2019, 223, 115124. [Google Scholar] [CrossRef]
- Jatoi, A.W. Polyurethane nanofibers incorporated with ZnAg composite nanoparticles for antibacterial wound dressing ap-plications. Compos. Commun. 2020, 19, 103–107. [Google Scholar] [CrossRef]
- Majumder, S.; Dahiya, U.R.; Yadav, S.; Sharma, P.; Ghosh, D.; Rao, G.K.; Rawat, V.; Kumar, G.; Kumar, A.; Srivastava, C.M. Zinc Oxide Nanoparticles Functionalized on Hydrogel Grafted Silk Fibroin Fabrics as Efficient Composite Dressing. Biomolecules 2020, 10, 710. [Google Scholar] [CrossRef] [PubMed]
- Naseri-Nosar, M.; Farzamfar, S.; Sahrapeyma, H.; Ghorbani, S.; Bastami, F.; Vaez, A.; Salehi, M. Cerium oxide nanoparti-cle-containing poly (ε-caprolactone)/gelatin electrospun film as a potential wound dressing material: In vitro and in vivo evaluation. Mater. Sci. Eng. C 2017, 81, 366–372. [Google Scholar] [CrossRef]
- Bharathi, B.S.; Stalin, T. Cerium oxide and peppermint oil loaded polyethylene oxide/graphene oxide electrospun nano-fibrous mats as antibacterial wound dressings. Mater. Today Commun. 2019, 21, 100664. [Google Scholar] [CrossRef]
- Kalaycıoğlu, Z.; Kahya, N.; Adımcılar, V.; Kaygusuz, H.; Torlak, E.; Akın-Evingür, G.; Erim, F.B. Antibacterial nano cerium oxide/chitosan/cellulose acetate composite films as potential wound dressing. Eur. Polym. J. 2020, 133, 109777. [Google Scholar] [CrossRef]
- Kalantari, K.; Mostafavi, E.; Saleh, B.; Soltantabar, P.; Webster, T.J. Chitosan/PVA hydrogels incorporated with green syn-thesized cerium oxide nanoparticles for wound healing applications. Eur. Polym. J. 2020, 134, 109853. [Google Scholar] [CrossRef]
- Augustine, R.; Kalarikkal, N.; Thomas, S. Electrospun PCL membranes incorporated with biosynthesized silver nanoparticles as antibacterial wound dressings. Appl. Nanosci. 2016, 6, 337–344. [Google Scholar] [CrossRef]
- Hiep, N.T.; Khon, H.C.; Niem, V.V.T.; Van Toi, V.; Quyen, T.N.; Hai, N.D.; Anh, M.N.T. Microwave-Assisted Synthesis of Chitosan/Polyvinyl Alcohol Silver Nanoparticles Gel for Wound Dressing Applications. Int. J. Polym. Sci. 2016, 2016, 1–11. [Google Scholar] [CrossRef]
- Alippilakkotte, S.; Kumar, S.; Sreejith, L. Fabrication of PLA/Ag nanofibers by green synthesis method using Momordica charantia fruit extract for wound dressing applications. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 771–782. [Google Scholar] [CrossRef]
- Mehrabani, M.G.; Karimian, R.; Mehramouz, B.; Rahimi, M.; Kafil, H.S. Preparation of biocompatible and biodegradable silk fibroin/chitin/silver nanoparticles 3D scaffolds as a bandage for antimicrobial wound dressing. Int. J. Biol. Macromol. 2018, 114, 961–971. [Google Scholar] [CrossRef]
- Sofi, H.S.; Akram, T.; Tamboli, A.H.; Majeed, A.; Shabir, N.; Sheikh, F.A. Novel lavender oil and silver nanoparticles sim-ultaneously loaded onto polyurethane nanofibers for wound-healing applications. Int. J. Pharm. 2019, 569, 118590. [Google Scholar] [CrossRef]
- Li, X.; Cai, Z.; Ahn, D.U.; Huang, X. Development of an antibacterial nanobiomaterial for wound-care based on the absorp-tion of AgNPs on the eggshell membrane. Colloids Surf. B Biointerfaces 2019, 183, 110449. [Google Scholar] [CrossRef]
- Nasef, S.; Khozemy, E.E.; Kamoun, E.A.; El-Gendi, H. Gamma radiation-induced crosslinked composite membranes based on polyvinyl alcohol/chitosan/AgNO3/vitamin E for biomedical applications. Int. J. Biol. Macromol. 2019, 137, 878–885. [Google Scholar] [CrossRef]
- Ahmed, A.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Hussain, A.; Pervaiz, E.; Hussain, Z. In-vitro and in-vivo study of super-absorbent PVA/Starch/g-C3N4/Ag@TiO2 NPs hydrogel membranes for wound dressing. Eur. Polym. J. 2020, 130, 109650. [Google Scholar] [CrossRef]
- Jaiswal, L.; Shankar, S.; Rhim, J.-W.; Hahm, D.-H. Lignin-mediated green synthesis of AgNPs in carrageenan matrix for wound dressing applications. Int. J. Biol. Macromol. 2020, 159, 859–869. [Google Scholar] [CrossRef]
- Archana, D.; Singh, B.K.; Dutta, J.; Dutta, P. In vivo evaluation of chitosan–PVP–titanium dioxide nanocomposite as wound dressing material. Carbohydr. Polym. 2013, 95, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Montaser, A.; Wassel, A.; Al-Shaye’A, O.N. Synthesis, characterization and antimicrobial activity of Schiff bases from chitosan and salicylaldehyde/TiO2 nanocomposite membrane. Int. J. Biol. Macromol. 2019, 124, 802–809. [Google Scholar] [CrossRef] [PubMed]
- Archana, D.; Dutta, J.; Dutta, P. Evaluation of chitosan nano dressing for wound healing: Characterization, in vitro and in vivo studies. Int. J. Biol. Macromol. 2013, 57, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Ulu, A.; Birhanlı, E.; Köytepe, S.; Ateş, B. Chitosan/polypropylene glycol hydrogel composite film designed with TiO2 nano-particles: A promising scaffold of biomedical applications. Int. J. Biol. Macromol. 2020, 163, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Cai, N.; Li, C.; Han, C.; Luo, X.; Shen, L.; Xue, Y.; Yu, F. Tailoring mechanical and antibacterial properties of chitosan/gelatin nanofiber membranes with Fe3O4 nanoparticles for potential wound dressing application. Appl. Surf. Sci. 2016, 369, 492–500. [Google Scholar] [CrossRef]
- Cao, Z.; Shen, Z.; Luo, X.; Zhang, H.; Liu, Y.; Cai, N.; Yu, F. Citrate-modified maghemite enhanced binding of chitosan coat-ing on cellulose porous membranes for potential application as wound dressing. Carbohydr. Polym. 2017, 166, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Sathiyaseelan, A.; Saravanakumar, K.; Mariadoss, A.; Wang, M.-H. Antimicrobial and Wound Healing Properties of FeO Fabricated Chitosan/PVA Nanocomposite Sponge. Antibiotics 2021, 10, 524. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Zayed, M.; El-Dek, S.; Hady, M.A.; El Sherbiny, D.H.; Uskoković, V. Nanofibrous ε-polycaprolactone scaffolds containing Ag-doped magnetite nanoparticles: Physicochemical characterization and biological testing for wound dressing applications in vitro and in vivo. Bioact. Mater. 2021, 6, 2070–2088. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, H.; Jian, Z.; Fei, G.; Qian, W.; Luo, G.; Wang, Z.; Xia, H. Novel Poly(vinyl alcohol)/Chitosan/Modified Graphene Oxide Biocomposite for Wound Dressing Application. Macromol. Biosci. 2020, 20, e1900385. [Google Scholar] [CrossRef]
- Ma, R.; Wang, Y.; Qi, H.; Shi, C.; Wei, G.; Xiao, L.; Guo, Z. Nanocomposite sponges of sodium alginate/graphene ox-ide/polyvinyl alcohol as potential wound dressing: In vitro and in vivo evaluation. Compos. Part B-Eng. 2019, 167, 396–405. [Google Scholar] [CrossRef]
- Lin, C.-M.; Chang, Y.-C.; Cheng, L.-C.; Liu, C.-H.; Chang, S.C.; Hsien, T.-Y.; Wang, D.-M.; Hsieh, H.-J. Preparation of graphene-embedded hydroxypropyl cellulose/chitosan/polyethylene oxide nanofiber membranes as wound dressings with enhanced antibacterial properties. Cellulose 2020, 27, 2651–2667. [Google Scholar] [CrossRef]
- Sadeghianmaryan, A.; Sardroud, H.A.; Allafasghari, S.; Yazdanpanah, Z.; Naghieh, S.; Gorji, M.; Chen, X. Electrospinning of polyurethane/graphene oxide for skin wound dressing and its in vitro characterization. J. Biomater. Appl. 2020, 35, 135–145. [Google Scholar] [CrossRef]
- Wang, Y.; Yin, M.; Zheng, X.; Li, W.; Ren, X. Chitosan/mesoporous silica hybrid aerogel with bactericidal properties as hemo-static material. Eur. Polym. J. 2021, 142, 110132. [Google Scholar] [CrossRef]
- Shen, Z.; Cai, N.; Xue, Y.; Yu, B.; Wang, J.; Song, H.; Deng, H.; Yu, F. Porous SBA-15/cellulose membrane with prolonged anti-microbial drug release characteristics for potential wound dressing application. Cellulose 2020, 27, 2737–2756. [Google Scholar] [CrossRef]
- Liao, J.L.; Zhong, S.; Wang, S.H.; Liu, J.Y.; Chen, J.; He, G.; Zhou, J.D. Preparation and properties of a novel carbon nano-tubes/poly (vinyl alcohol)/epidermal growth factor composite biological dressing. Exp. Ther. Med. 2017, 14, 2341–2348. [Google Scholar] [CrossRef] [PubMed]


| Biopolymers/Synthetic Polymers | Chemical Structures | Properties | Biomedical Applications | Ref. |
|---|---|---|---|---|
| Chitin and Chitosan | 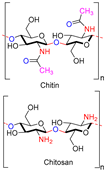 | Non-toxic, biodegradable, biocompatible, antibacterial, anti-inflammatory | Wound dressing, drug delivery, bone regeneration, food packing, tissue engineering, cosmetics, etc. | [20,21,22,23,24] |
| Starch |  | Biocompatible, non-toxic, biodegradable, non-carcinogenic, low immunogenicity | Wound dressing, artificial organs, drug delivery, tissue engineering, biosensor, etc. | [28,29,30] |
| Cellulose |  | Biocompatible, biodegradable, non-toxic, high exudate ability, thermal stability | Artificial skin, blood vessels, drug delivery, tissue engineering, bone regeneration, etc. | [33,34,35,36] |
| Hyaluronic acid | 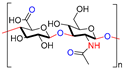 | Non-toxic, biodegradable, biocompatible, non-immunogenicity, non-inflammatory | Wound healing, drug delivery, tissue engineering, cosmetics, etc. | [40,41,42] |
| Alginate | 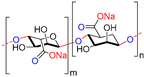 | Non-toxic, biocompatible, biodegradable, bio-stable | Drug delivery, wound dressings, tissue engineering, blood vessels, bone regeneration, etc. | [47,48,49] |
| Poly(vinyl alcohol) (PVA) | 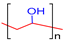 | Non-toxic, biocompatible, biodegradable, mechanical strength, good hydrophilicity, thermal stability, excellent film forming | Wound dressing, tissue engineering, contact lenses, drug delivery, water filtration, implantable devices, food packaging, etc. | [55,56,57] |
| Poly(ethylene glycol) (PEG) | 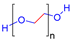 | Non-toxic, bio-inert, non-immunogenic, biocompatible | Wound healing, tissue engineering, drug delivery, etc. | [52,58,59] |
| Poly (methacrylic acid) (PMAA) | 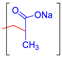 | Biocompatible, non-toxic, high swelling capacity | Drug delivery, wound dressing, tissue engineering, etc. | [60,61,62,63] |
| Polyvinylpyrrolidone (PVP) |  | Non-toxic, biocompatible, excellent film forming | Tissue engineering, wound dressings, artificial pancreas, cardiovascular device, artificial skin, food packaging, etc. | [28,64,65] |
| Polycaprolactone (PCL) |  | Biodegradable, biocompatibility, good mechanical properties | Wound dressing, tissue engineering, drug delivery systems, packaging, etc. | [66,67,68] |
| Poly (lactic acid) (PLA) | 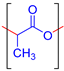 | Biodegradability, biocompatibility, mechanical strength, thermal stability. | Tissue engineering, wound dressing, drug delivery, packaging, etc. | [69,70,71] |
| Polyurethane (PU) |  | Non-toxic, biodegradable, excellent mechanical property, excellent film forming, adhesive. | Biosensor devices, drug delivery, wound dressing, tissue regeneration, etc. | [72,73] |
| Membrane Composition | Method of Preparation | Antibacterial Strain | Cell Line Used | Ref. |
|---|---|---|---|---|
| ZnO/MCM-41 | Solution casting | S. aureus and E. coli | - | [81] |
| Heparinized ZnO/Poly(vinylalcohol)/Carboxymethyl Cellulose | Freeze–thaw | S. aureus and E. coli | L-929 and Human dermal fibroblasts | [82] |
| ZnO/Poly(vinyl alcohol)/Chitosan | Freeze–thaw | S. aureus and E. coli | Mouse fibroblast cells (L-929) | [83] |
| ZnO/Hyaluronic acid | One-pot synthesis | S. aureus, B. subtilis, E. coli, P. aeruginosa, and V. cholerae | Human skin Fibroblasts | [84] |
| Polyurethane/ZnAg | Electrospun | E. coli, S. aureus and B. subtilis | - | [85] |
| ZnO -coated silk fibroin fabric | - | E. coli | L929 Fibroblast cells | [86] |
| CeO2/Poly (ε-caprolactone)/Gelatin | Electrospun | - | L929 Murine fibroblast cell | [87] |
| CeO2/Peppermint oil on polyethylene oxide/Graphene oxide | Electrospun | S. aureus and E. coli | - | [88] |
| CeO2/Chitosan/cellulose acetate | Solution Casting | S. aureus and E. coli | - | [89] |
| Chitosan/Poly(vinyl alcohol)/CeO2 | Freeze-thaw | S. aureus and E. coli | Human dermal fibroblast cells | [90] |
| Polycaprolactone/Ag | Electrospun | S. aureus and E. coli | [91] | |
| Chitosan/Poly(vinyl lcohol)/Ag | Freeze dryer | P. aeruginosa and S. aureus | Fibroblast cells | [92] |
| Polylactide/Ag | Electrospun | S. aureus and E. coli | Fibroblast cells | [93] |
| Silkfibroin/chitin/Ag | Freeze dryer | E. coli, S. aureus and C. albicans | - | [94] |
| Polyurethane/lavender oil/Ag | Electrospun | S. aureus and E. coli | - | [95] |
| Ag/Eggshell membrane | Chemical reduction | S. aureus and E. coli | - | [96] |
| Chitosan/Poly(vinyl alcohol)/AgNO3 and vitamin E | Solution Casting | Salmonella typhimurium | - | [97] |
| Poly(vinyl alcohol)/Starch/g-C3N4/Ag@TiO2 | Solution casting | S. aureus and E. coli | - | [98] |
| Carra/Lig/Ag (Carra/Lig/Ag/CaCl2, Carra/Lig/Ag/CuCl2 and Carra/Lig/Ag/MgCl2) | Solution Casting | S. aureus and E. coli | Mouse fibroblast cell line | [99] |
| Chitosan/poly(N-vinylpyrrolidone)/TiO2 | Solution Casting | E. coli, S. aureus, B. subtilis and P. aeruginosa | NIH3T3 and L929 fibroblast cells | [100] |
| Salicylimine-Chitosan/TiO2 | Solution Casting | S. Aureus, P. aeruginosa | - | [101] |
| Chitosan/pectin/TiO2 | Solution Casting | E. coli, S. aereus, P. aeruginosa, A. niger, B. subtilis | NIH3T3 and L929 fibroblast cells | [102] |
| Chitosan/poly (propylene glycol)/TiO2 | Solution Casting | E. coli, S.aureus, and C.lipolytica | - | [103] |
| Fe3O4/Chitosan/Gelatin | Electrospun | S. aureus and E. coli | - | [104] |
| Fe2O3, γ/Chitosan/porous cellulose membranes | Freeze dryer | S. aureus and E. coli | - | [105] |
| Chitosan/Poly(vinyl alcohol)/FeO | Freeze dryer | S. aureus and E. coli | HEK-293 cells | [106] |
| Ag doped Fe3O4/Poly caprolactone | Electrospun | S. aureus and E. coli | Human melanocyte cell line, HFB4 | [107] |
| Poly(vinyl alcohol)/Chitosan/mGO | Solution casting | S. aureus and E. coli | Human keratinocyte cells | [108] |
| Sodium alginate/Graphene oxide/Poly(vinyl alcohol) | Freeze dryer | S. aureus and E. coli | NIH 3T3 cells | [109] |
| Hydroxypropyl cellulose/Chitosan/polyethylene oxide/graphene (HCPG) | Electrospun | S. aureus and E. coli | Human fetal skin fibroblast cells | [110] |
| Polyurethane/graphene oxide (GO) | Electrospun | S. aureus and E. coli | Human dermal fibroblast | [111] |
| Chitosan/Mesoporous silica | Freeze dryer | S. aureus and E. coli | - | [112] |
| Mesoporous silica/cellulose | Solution casting | S. aureus and E. coli | - | [113] |
| Carbon nanotubes/Poly(vinyl alcohol)/epidermal growth factor (EGF) | Electrospun | - | L929 mouse fibroblast | [114] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gobi, R.; Ravichandiran, P.; Babu, R.S.; Yoo, D.J. Biopolymer and Synthetic Polymer-Based Nanocomposites in Wound Dressing Applications: A Review. Polymers 2021, 13, 1962. https://doi.org/10.3390/polym13121962
Gobi R, Ravichandiran P, Babu RS, Yoo DJ. Biopolymer and Synthetic Polymer-Based Nanocomposites in Wound Dressing Applications: A Review. Polymers. 2021; 13(12):1962. https://doi.org/10.3390/polym13121962
Chicago/Turabian StyleGobi, Ravichandran, Palanisamy Ravichandiran, Ravi Shanker Babu, and Dong Jin Yoo. 2021. "Biopolymer and Synthetic Polymer-Based Nanocomposites in Wound Dressing Applications: A Review" Polymers 13, no. 12: 1962. https://doi.org/10.3390/polym13121962
APA StyleGobi, R., Ravichandiran, P., Babu, R. S., & Yoo, D. J. (2021). Biopolymer and Synthetic Polymer-Based Nanocomposites in Wound Dressing Applications: A Review. Polymers, 13(12), 1962. https://doi.org/10.3390/polym13121962










