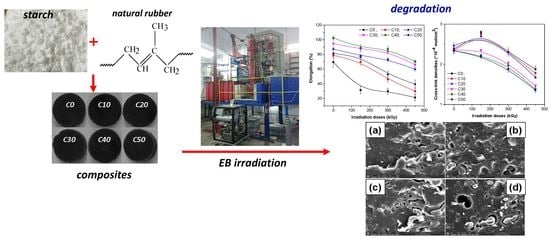
Electron Beam Irradiation: A Method for Degradation of Composites Based on Natural Rubber and Plasticized Starch
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Samples Preparation
2.2. Laboratory Tests
2.2.1. Mechanical Characteristics Evaluation
2.2.2. Sol-Gel and Cross-Link Density Analysis
2.2.3. Cross-Link and Chain Scission Yields Evaluation
2.2.4. Water Uptake Tests
2.2.5. Weight Loss in Toluene and Water
2.2.6. Structural Investigations through Fourier Transform Infrared Spectroscopy
2.2.7. Morphological Investigations through Scanning Electron Microscopy
3. Results and Discussion
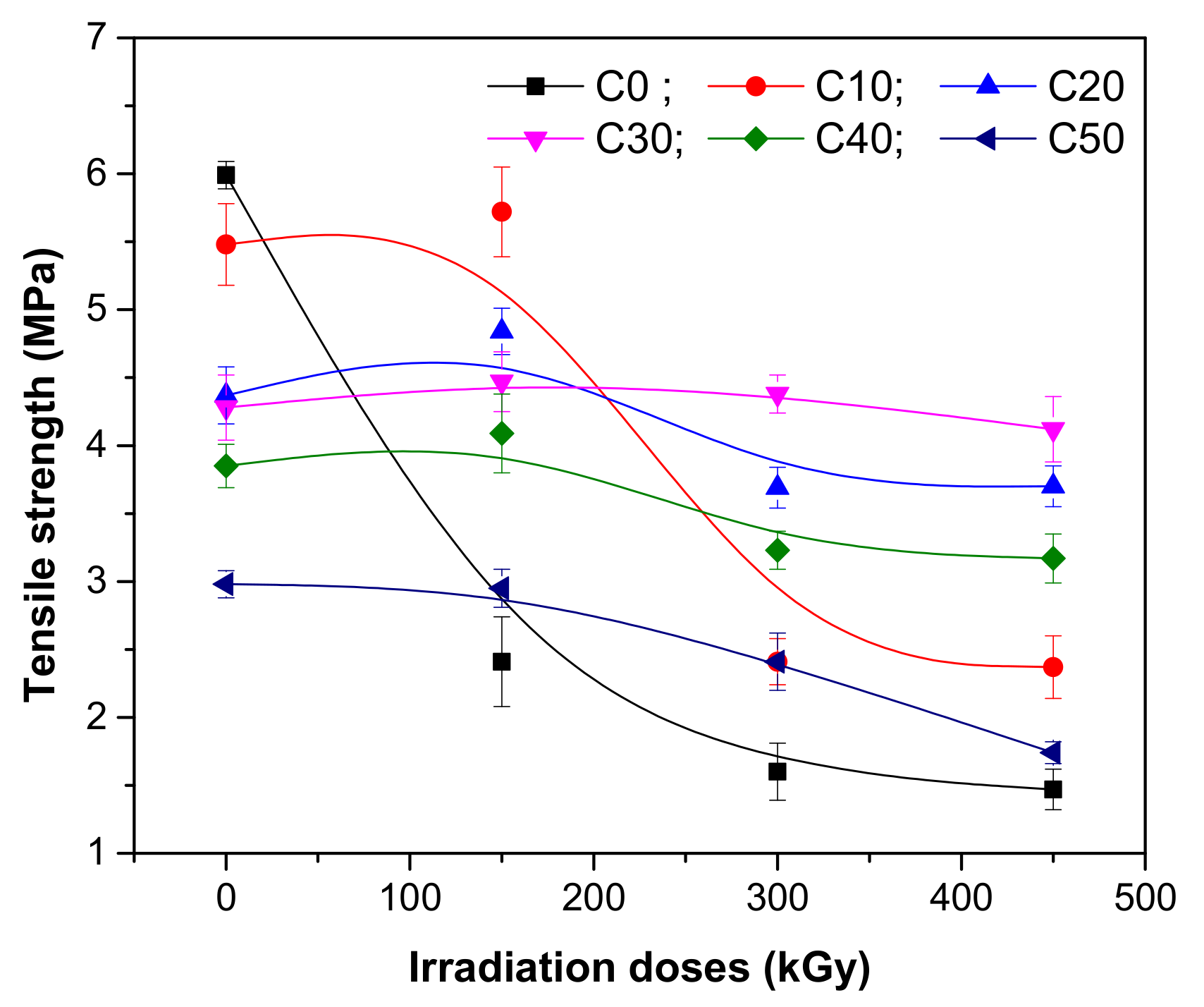
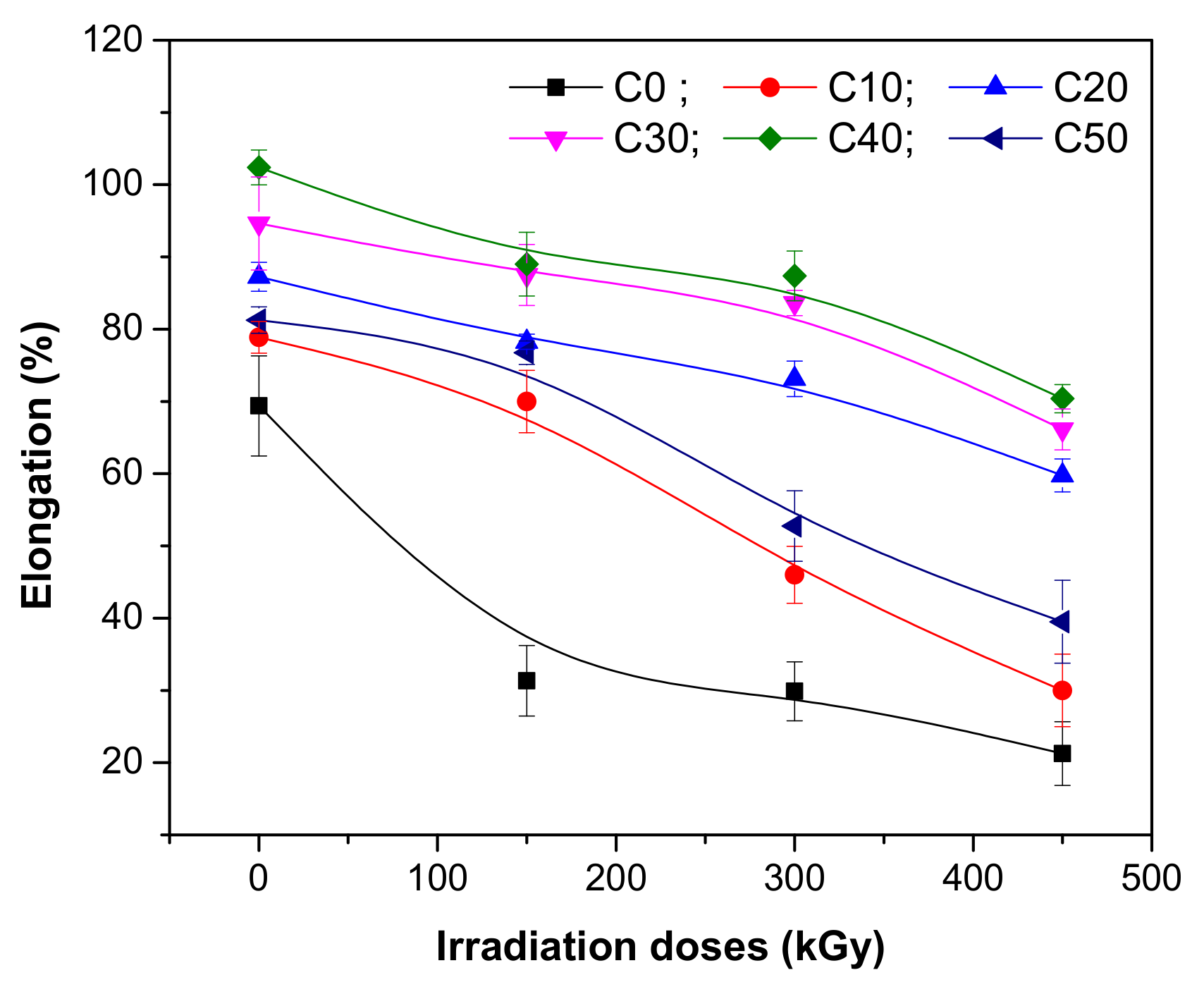
3.1. Mechanical Characteristics
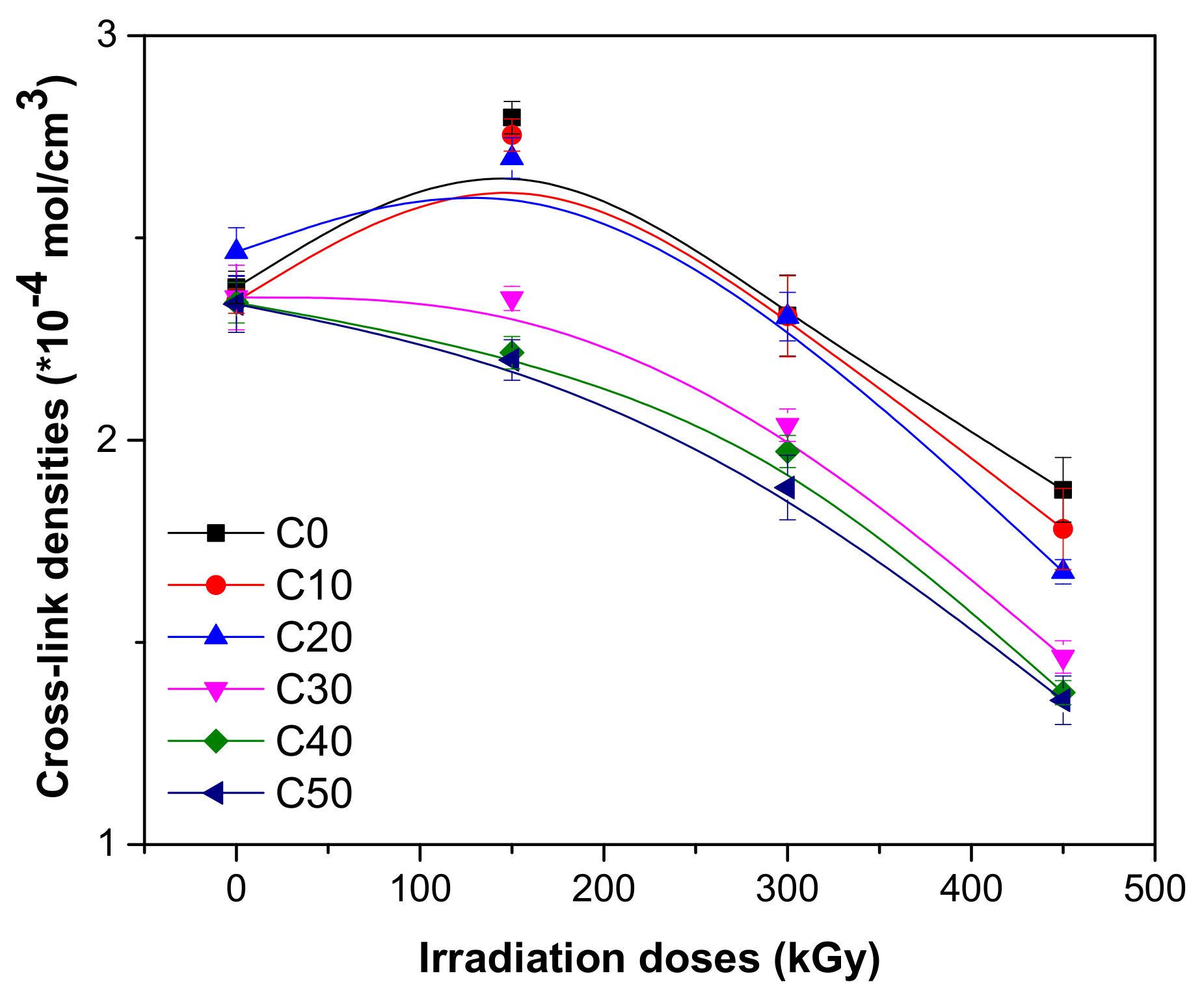
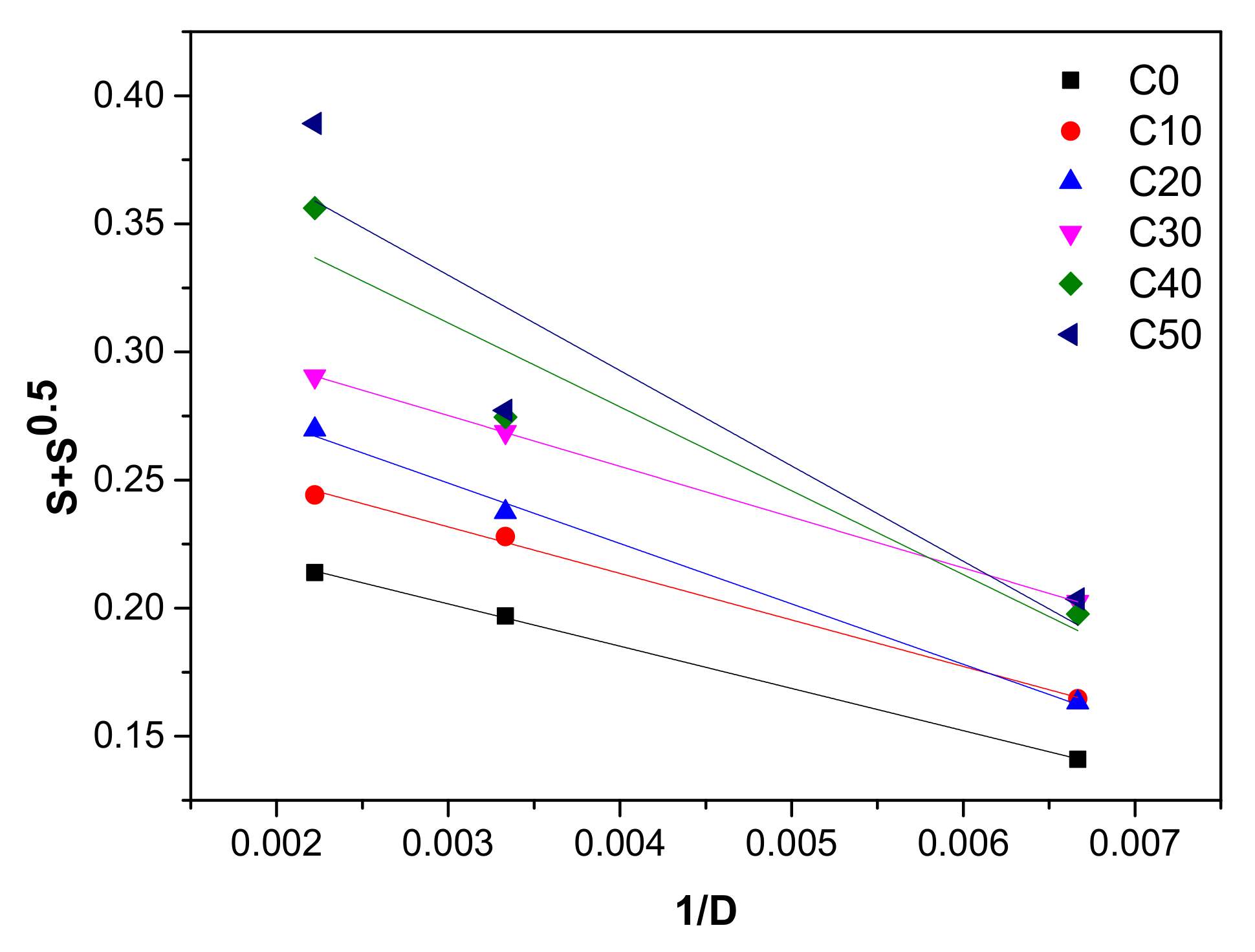
3.2. Sol-Gel and Cross-Link Density
3.3. The Cross-Link and Chain Scission Yields
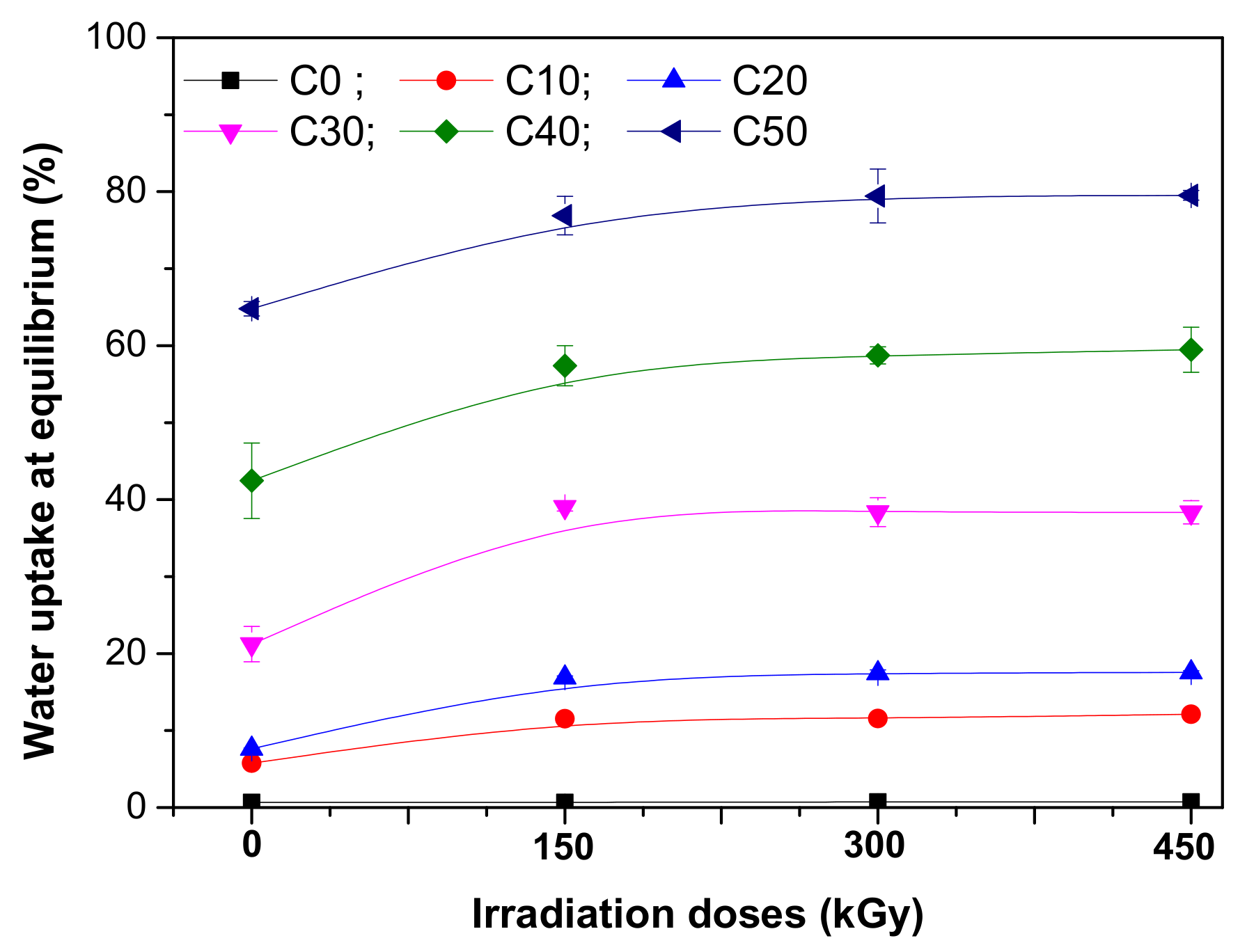
3.4. Water Uptake
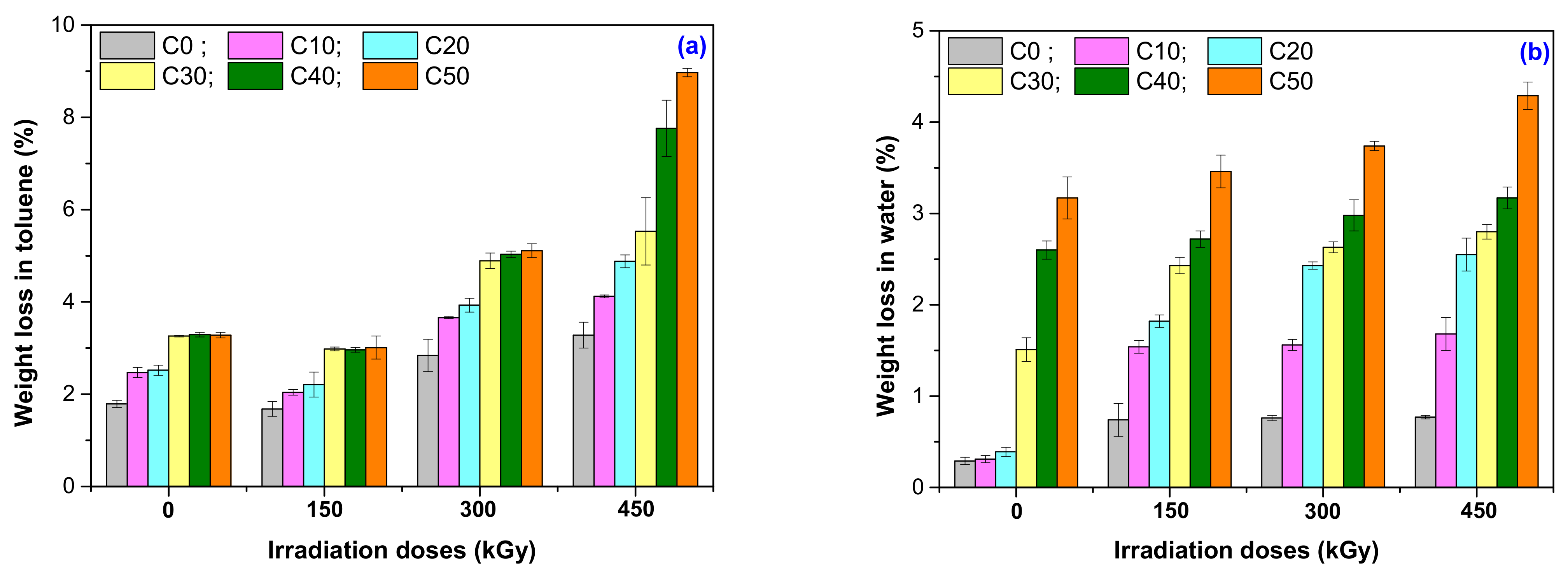
3.5. Weight Loss in Solvents (Toluene and Water)
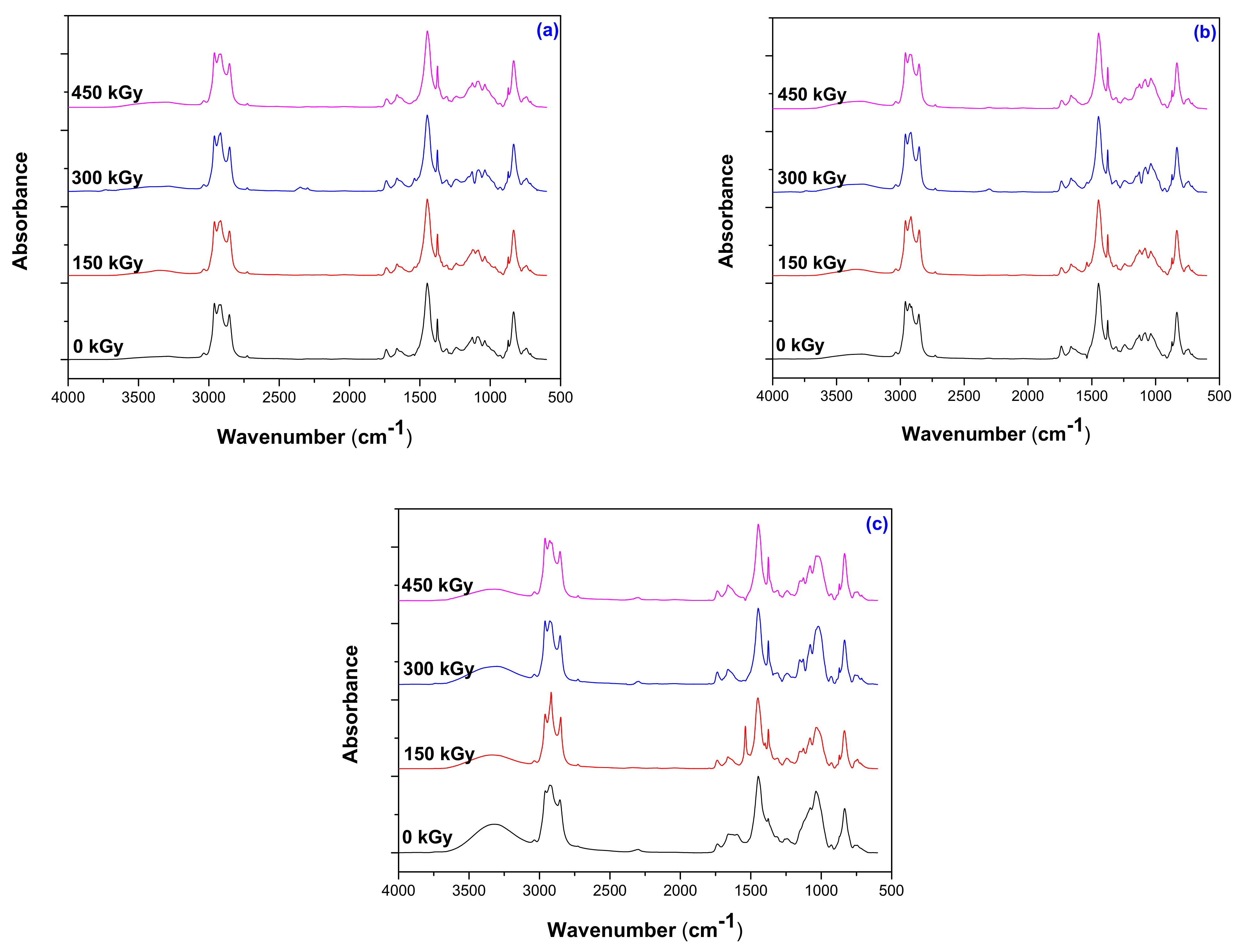
3.6. Structural Investigations by Fourier Transform Infrared Spectroscopy (FTIR) Technique
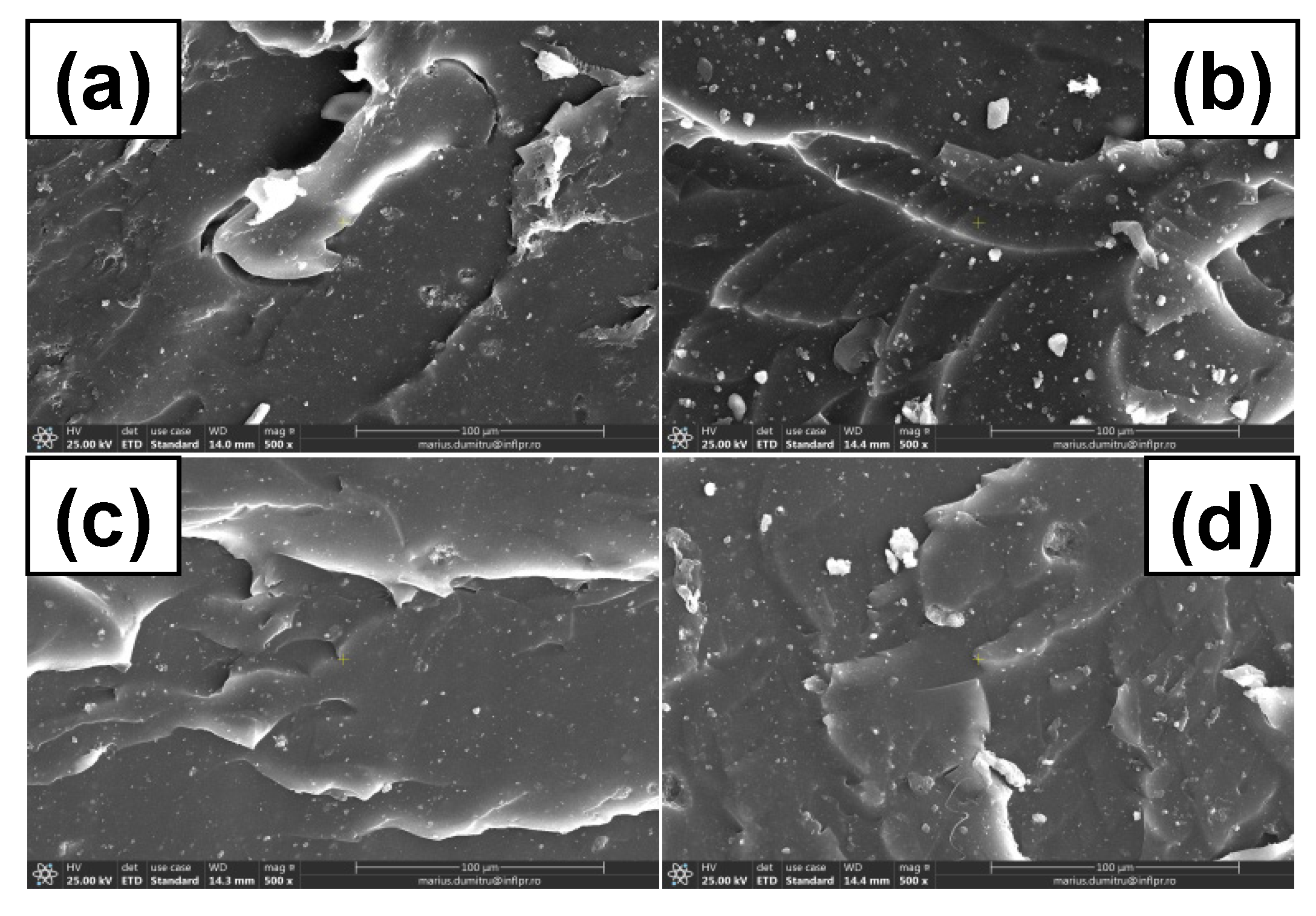
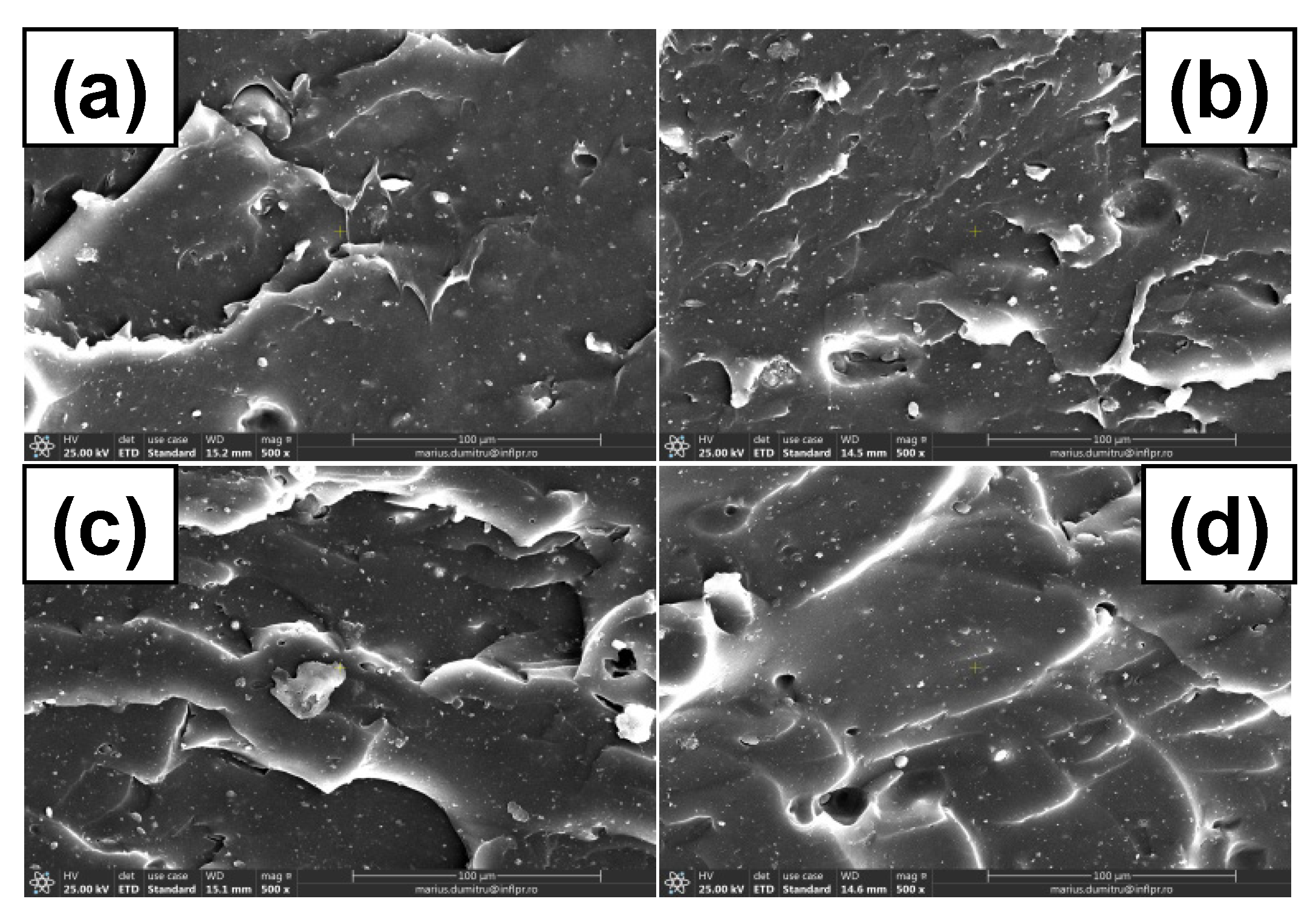
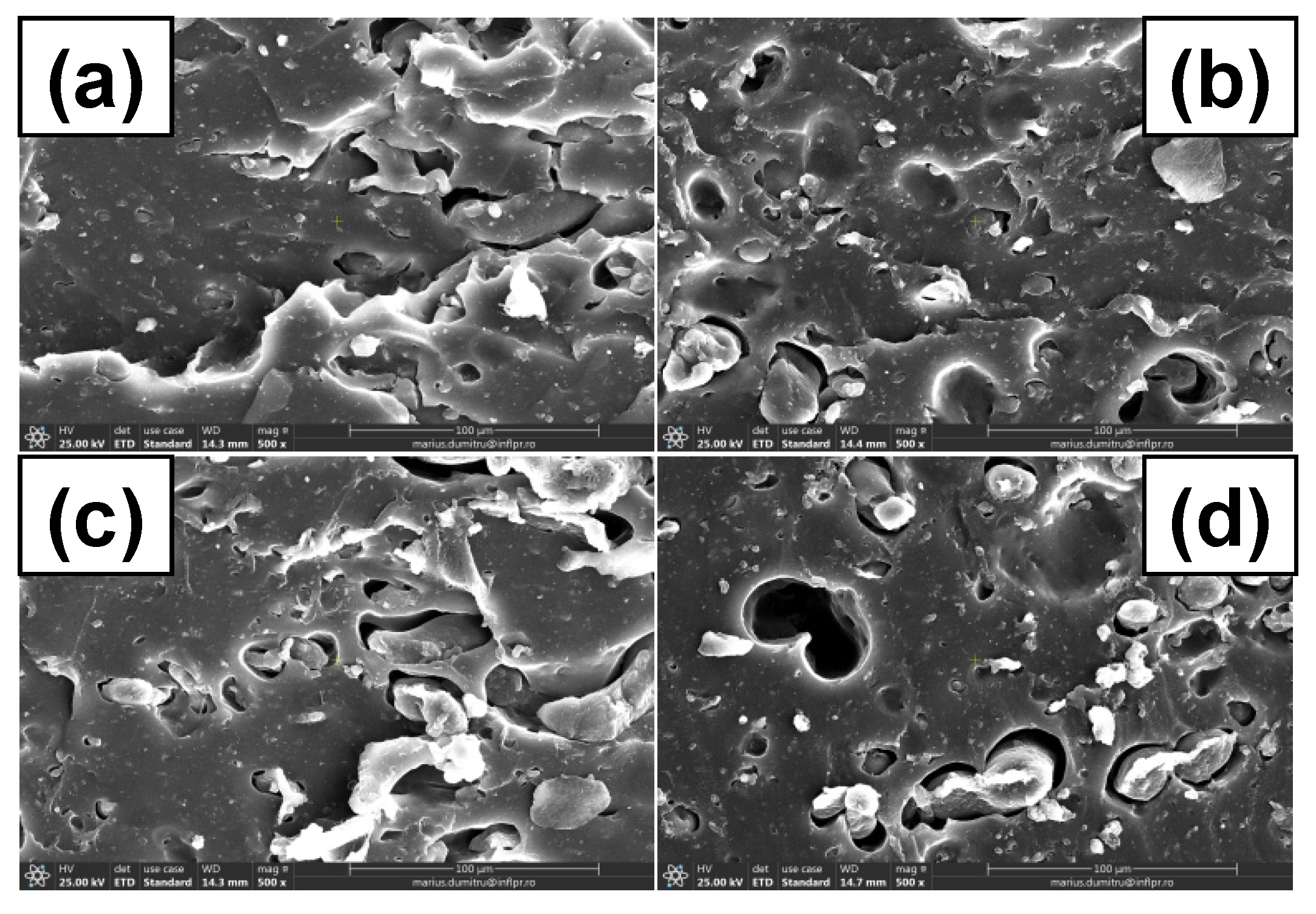
3.7. Morphological Investigations by Scanning Electron Microscopy (SEM) Technique
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, M. Polycyclic aromatic hydrocarbons, elemental and organic carbon emissions from tire-wear. Sci. Total Environ. 2010, 408, 4563–4568. [Google Scholar] [CrossRef]
- Adhikari, B.; De, D.; Maiti, S. Reclamation and recycling of waste rubber. Prog. Polym. Sci. 2000, 25, 909–948. [Google Scholar] [CrossRef]
- Artinano, B.; Gomez-Moreno, F.J.; Diaz, E.; Amato, F.; Pandolfi, M.; Alonso-Blanco, E.; Coz, E.; Garcia-Alonso, S.; Becerril-Valle, M.; Querol, X.; et al. Outdoor and indoor particle characterization from a large and uncontrolled combustion of a tire landfill. Sci. Total Environ. 2017, 593, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Chapiro, A. General consideration of the radiation chemistry of polymers. Nucl. Instrum. Meth. B 1995, 105, 5–7. [Google Scholar] [CrossRef]
- Kornacka, E.M. Radiation-induced oxidation of polymers. In Applications of Ionizing Radiation in Materials Processing; Yongxia, S., Chmielewski, A.G., Eds.; Institute of Nuclear Chemistry and Technology: Warszawa, Poland, 2017; Volume 1, pp. 183–192. [Google Scholar]
- Spadaro, G.; Alessi, S.; Dispenza, C. Ionizing radiation-induced crosslinking and degradation of polymers. In Applications of Ionizing Radiation in Materials Processing, 1st ed.; Yongxia, S., Chmielewski, A.G., Eds.; Institute of Nuclear Chemistry and Technology: Warszawa, Poland, 2017; Volume 1, pp. 167–182. [Google Scholar]
- De Coster, N.; Magg, H. NBR in contact with food, potable water, pharmaceutical and cosmetic applications. Kaut Gummi Kunstst 2003, 56, 405–411, WOS:000184855200007. [Google Scholar]
- Basfar, A.A.; Abdel-Aziz, M.M.; Mofti, S. Influence of different curing systems on the physico-mechanical properties and stability of SBR and NR rubbers. Radiat. Phys. Chem. 2002, 63, 81–87. [Google Scholar] [CrossRef]
- Dluzneski, P.R. Peroxide vulcanization of elastomers. Rubber Chem. Technol. 2001, 74, 451–492. [Google Scholar] [CrossRef]
- Dikland, H.G.; Ruardy, T.; Vanderdoes, L.; Bantjes, A. New coagents in peroxide vulcanization of EPM. Rubber Chem. Technol. 1993, 66, 693–711. [Google Scholar] [CrossRef]
- Liu, C.; Shao, Y.; Jia, D. Chemically modified starch reinforced natural rubber composites. Polymer 2008, 49, 2176–2181. [Google Scholar] [CrossRef]
- Gaspar, M.; Benko, Z.; Dogossy, G.; Reczey, K.; Czigany, T. Reducing water absorption in compostable starch-based plastics. Polym. Degrad. Stabil. 2005, 90, 563–569. [Google Scholar] [CrossRef]
- Manaila, E.; Stelescu, M.D.; Craciun, G. Degradation Studies Realized on Natural Rubber and Plasticized Potato Starch Based Eco-Composites Obtained by Peroxide Cross-Linking. Int. J. Mol. Sci. 2018, 19, 2862. [Google Scholar] [CrossRef]
- Brașoveanu, M.; Nemțanu, M.R. Aspects on Starches Modified by Ionizing Radiation Processing. In Applications of Modified Starches; Huicochea, E.F., Rendon, R., Eds.; IntechOpen: Rijeka, Croatia, 2018; Chapter 5; pp. 49–68. [Google Scholar] [CrossRef]
- Craciun, G.; Manaila, E.; Ighigeanu, D.; Stelescu, M.D. A method to improve the characteristics of EPDM rubber based eco-composites with electron beam. Polymers 2020, 12, 215. [Google Scholar] [CrossRef]
- Manaila, E.; Stelescu, M.D.; Craciun, G.; Ighigeanu, D. Wood Sawdust/Natural Rubber Ecocomposites Cross-Linked by Electron Beam Irradiation. Materials 2016, 9, 503. [Google Scholar] [CrossRef]
- Craciun, G.; Manaila, E.; Stelescu, M.D. New Elastomeric Materials Based on Natural Rubber Obtained by Electron Beam Irradiation for Food and Pharmaceutical Use. Materials 2016, 9, 999. [Google Scholar] [CrossRef] [PubMed]
- Fiti, M. Dozimetria Chimica a Radiatiilor Ionizante (Ionizing Radiation Chemical Dosimetry), 1st ed.; Editura Academiei Republicii Socialiste Romania: Bucuresti, Romania, 1973; pp. 24–70. [Google Scholar]
- Cleland, M.R. Industrial Applications of Electron Accelerators—Ion Beam Applications. Presented at the CERN Accelerator School/Small Accelerator Course, Zeegse, The Netherlands, 24 May–2 June 2005; Available online: https://cds.cern.ch/record/1005393/files/p383.pdf (accessed on 15 January 2021).
- Stelescu, M.D.; Airinei, A.; Manaila, E.; Craciun, G.; Fifere, N.; Varganici, C.; Pamfil, D.; Doroftei, F. Effects of Electron Beam Irradiation on the Mechanical, Thermal, and Surface Properties of Some EPDM/Butyl Rubber Composites. Polymers 2018, 10, 1206. [Google Scholar] [CrossRef]
- Lopez-Manchado, M.A.; Herrero, B.; Arroyo, M. Preparation and characterization of organoclay nanocomposites based on natural rubber. Polym. Int. 2003, 52, 1070–1077. [Google Scholar] [CrossRef]
- Chenal, J.M.; Chazeau, L.; Guy, L.; Bomal, Y.; Gauthier, C. Molecular weight between physical entanglements in natural rubber: A critical parameter during strain-induced crystallization. Polymer 2007, 48, 1042–1046. [Google Scholar] [CrossRef]
- Arroyo, M.; Lopez-Manchado, M.A.; Herrero, B. Organo-montmorillonite as substitute of carbon black in natural rubber compounds. Polymer 2003, 44, 2447–2453. [Google Scholar] [CrossRef]
- Dubey, K.A.; Bhardwaj, Y.K.; Chaudhari, C.V.; Kumar, V.; Goel, N.K.; Sabharwal, S. Radiation processed ethylene vinyl acetate-multiple walled carbon nanotube nano-composites: Effect of MWNT addition on the gel content and crosslinking density. Express. Polym. Lett. 2009, 3, 492–500. [Google Scholar] [CrossRef]
- Charlesby, A.; Pinner, S.H. Analysis of the solubility behaviour of irradiated polyethylene and other polymers. Proc. R. Soc. Lon. Ser. A 1959, 249, 367–386. [Google Scholar] [CrossRef]
- Eyssa, H.M.; Mohamed, W.S.; El-Zayat, M.M. Irradiated rubber composite with nano and micro fillers for mining rock application. Radiochim. Acta 2019, 107, 737–753. [Google Scholar] [CrossRef]
- Ahmed, K.; Nizami, S.S.; Raza, N.Z.; Mahmood, K. Effect of micro-sized marble sludge on physical properties of natural rubber composites. Chem. Ind. Chem. Eng. Q. 2013, 19, 281–293. [Google Scholar] [CrossRef]
- Chen, R.S.; Ahmad, S.; Ab Ghani, M.H.; Salleh, M.N. Optimization of High Filler Loading on Tensile Properties of Recycled HDPE/PET Blends Filled with Rice Husk. AIP Conf. Proc. 2014, 1614, 46–51. [Google Scholar] [CrossRef]
- Nourbakhsh, A.; Baghlani, F.F.; Ashori, A. Nano-SiO2 filled rice husk/polypropylene composites: Physico-mechanical properties. Ind. Crop. Prod. 2011, 33, 183–187. [Google Scholar] [CrossRef]
- Ismail, H.; Shuhelmy, S.; Edgham, M.R. The effect of a silane coupling agent on curing characteristics and mechanical properties of bamboo fibre filled NR composites. Eur. Polym. J. 2002, 38, 39–45. [Google Scholar] [CrossRef]
- Kohls, D.J.; Beauage, G. Rational design of reinforced rubber. Curr. Opin. Solid 2002, 6, 183–194. [Google Scholar] [CrossRef]
- Dong, Z.X.; Liu, M.X.; Jia, D.M.; Zhou, Y.H. Synthesis of natural rubber-g-maleic anhydride and its use as a compatibilizer in natural rubber/short nylon fiber composites. Chin. J. Polym. Sci. 2013, 31, 1127–1138. [Google Scholar] [CrossRef]
- Ahmed, K. Hybrid composites prepared from Industrial waste: Mechanical and swelling behavior. J. Adv. Res. 2015, 6, 225–232. [Google Scholar] [CrossRef]
- Kukle, S.; Gravitis, J.; Putnina, A.; Stikute, A. The effect of steam explosion treatment on technical hemp fibres. In Proceedings of the 8th International Scientific and Practical Conference on Environment, Technology, Resources, Rezekne, Latvia, 20–22 June 2011; pp. 230–237. [Google Scholar] [CrossRef]
- Valodkar, M.; Thakore, S. Thermal and mechanical properties of natural rubber and starch nanocomposites. Int. J. Polym Anal. Ch 2010, 15, 387–395. [Google Scholar] [CrossRef]
- Martins, A.F.; Visconte, L.L.Y.; Schuster, R.H.; Boller, F.; Nunes, R.C.R. Ageing Effect on Dynamic and Mechanical Properties of NR/Cel II Nanocomposites. Kaut Gummi Kunstst 2004, 57, 446–451, WOS:000223950700006. [Google Scholar]
- Somers, A.E.; Bastow, T.J.; Burgar, M.I.; Forsyth, M.; Hill, A.J. Quantifying rubber degradation using NMR. Polym. Degrad. Stabil. 2000, 70, 31–37. [Google Scholar] [CrossRef]
- Mănăilă, E.; Craciun, G.; Stelescu, M.D.; Dinca, C.L.; Surdu, L.; Gurau, D. Polymeric composites based on flax wastes and natural rubber. Ind. Textila 2014, 65, 53–60, WOS:000333065000009. [Google Scholar]
- Qu, P.; Gao, Y.; Wu, G.F.; Zhang, L.P. Nanocomposites of poly (lactic acid) reinforced with cellulose nanofibrils. Bioresources 2010, 5, 1811–1823. [Google Scholar] [CrossRef]
- Luo, S.P.; Cao, J.Z.; Wang, X. Investigation of the interfacial compatibility of PEG and thermally modified wood flour/polypropylene composites using the stress relaxation approach. Bioresources 2013, 8, 2064–2073. [Google Scholar] [CrossRef]
- Luo, S.P.; Cao, J.Z.; Wang, X. Properties of PEG/thermally modified wood flour/polypropylene (PP) composites. For. Stud. China 2012, 14, 307–314. [Google Scholar] [CrossRef]
- Riyajan, S.-A.; Sasithornsonti, Y.; Phinyocheep, P. Green natural rubber-g-modified starch for controlling urea release. Carbohydr. Polym. 2012, 89, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.M.; Fowler, P.A.; Tomkinson, J.; Hill, C.A.S. The preparation and characterization of a series of chemically modified potato starches. Carbohydr. Polym. 2002, 47, 245–252. [Google Scholar] [CrossRef]
- Mu, T.-H.; Zhang, M.; Raad, L.; Sun, H.-N.; Wang, C. Effect of α-Amylase Degradation on Physicochemical Properties of Pre-High Hydrostatic Pressure-Treated Potato Starch. PLoS ONE 2015, 10, e0143620. [Google Scholar] [CrossRef] [PubMed]
- Eng, A.H.; Tanaka, Y.; Gan, S.N. FTIR studies on amino groups in purified Hevea rubber. J. Nat. Rubber Res. 1992, 7, 152–155. Available online: https://www.researchgate.net/publication/236170151 (accessed on 29 January 2021).
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry, 1st ed.; Meyers, R.A., Ed.; John Wiley & Sons Ltd: Chichester, UK, 2000; pp. 10815–10837. Available online: https://pdfs.semanticscholar.org/9203/59b562f615d9f68f8e57b6b6b505aa213174.pdf (accessed on 4 February 2021).
- Shyichuk, A.; Tokaryk, G. A comparison of methods to determination of macromolecule crosslinking yield from gel fraction data. Polimery 2005, 50, 219–221. Available online: https://studyres.com/doc/22401524/a-comparison-of-methods-to-determination-of-macromolecule (accessed on 10 February 2021). [CrossRef][Green Version]
- Rohana Yahya, Y.S.; Azura, A.R.; Ahmad, Z. Effect of Curing Systems on Thermal Degradation Behaviour of Natural Rubber (SMR CV 60). J. Phys. Sci. 2011, 22, 1–14. Available online: http://web.usm.my/jps/22-2-11/22.2.1.pdf (accessed on 26 March 2021).
- Azura, A.R.; Muhr, A.H.; Thomas, A.G. Diffusion and reactions of oxygen during ageing for conventionally cured natural rubber vulcanisate. Polym. Plast. Technol. 2006, 45, 893–896. [Google Scholar] [CrossRef]
- Pimolsiriphol, V.; Saeoui, P.; Sirisinha, C. Relationship Among Thermal Ageing Degradation, Dynamic Properties, Cure Systems, and Antioxidants in Natural Rubber Vulcanisates. Polym. Plast. Technol. 2007, 46, 113–121. [Google Scholar] [CrossRef]
- Kamal, H.; Sabry, G.M.; Lotfy, S.; Abdallah, N.M.; Ulanski, P.; Rosiak, J.; Hegazy, E.S.A. Controlling of Degradation Effects in Radiation Processing of Starch. J. Macromol. Sci. A 2007, 44, 865–875. [Google Scholar] [CrossRef]
- Gani, A.; Gazanfar, T.; Jan, R.; Wani, S.M.; Masoodi, F.A. Effect of gamma irradiation on the physicochemical and morphological properties of starch extracted from lotus stem harvested from Dal lake of Jammu and Kashmir, India. J. Saudi Soc. Agric. Sci. 2013, 12, 109–115. [Google Scholar] [CrossRef]
- Nemtanu, M.R.; Brasoveanu, M. Radio-sensitivity of some starches treated with, accelerated electron beam. Starch-Starke 2012, 64, 435–440. [Google Scholar] [CrossRef]
- Saputra, A.H.; Johan, J.; Sari, T.I.; Cifriadi, A.; Maspanger, D.R.; Bismo, S. Degradation Characteristics of Vulcanized Natural Rubber by Dimethyl Ether through Filler and Plasticizer Composition Variations. Int. J. Technol. 2016, 7, 616–624. [Google Scholar] [CrossRef][Green Version]
- Horikx, M.M. Chain Scissions in a Polymer Network. Rubber Chem. Technol. 1956, 29, 1166–1173. [Google Scholar] [CrossRef]
- Tangboriboon, N.; Phudkrachang, P.; Mulsow, L.; Kunchornsup, W.; Sirivat, A. Removal of water extractable proteins from concentrated natural rubber latex by eggshells. J. Elastom. Plast. 2012, 45, 253–269. [Google Scholar] [CrossRef]
- Nemtanu, M.; Brasoveanu, M.; Iovu, H. Degradation rate of some electron beam irradiated starches. U.P.B. Sci. Bull. Ser. B 2010, 72, 69–74. Available online: https://www.scientificbulletin.upb.ro/rev_docs_arhiva/full8377.pdf (accessed on 15 February 2021).
- Bhat, R.; Karim, A.A. Impact of Radiation Processing on Starch. Compr. Rev. Food Sci. Food Saf. 2009, 8, 44–58. [Google Scholar] [CrossRef]
- Kim, I.S.; Lee, B.W.; Sohn, K.S.; Yoon, J.; Lee, J.H. Characterization of the UV Oxidation of Raw Natural Rubber Thin Film Using Image and FT-IR Analysis. Elastom. Compos. 2016, 51, 1–9. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; de Paoli, M.A. The Chemical effects of Photo-oxidation on Isoprene Rubber. Eur. Polym. J. 1985, 21, 15–23. [Google Scholar] [CrossRef]
- Pages, P. Characterization of polymer materials using FT-IR and DSC techniques. In Thermal Analysis. Fundamentals and Applications to Material Characterization; Artiaga, R., Ed.; Universidade da Coruña, Servizo de Publicacións: Galicia, Spain, 2005; pp. 124–140. ISBN1 84-9749-100-9. Available online: https://ruc.udc.es/dspace/bitstream/handle/2183/11499/CC-80%20art%208.pdf (accessed on 12 February 2021)ISBN2 84-9749-100-9.
- Ferreira-Villadiego, J.; García-Echeverri, J.; Vidal, M.V.; Pasqualino, J.; Meza-Castellar, P.; Lambis-Miranda, H.A. Chemical Modification and Characterization of Starch Derived from Plantain (Musa paradisiaca) Peel Waste, as a Source of Biodegradable Material. Chem. Eng. Trans. 2018, 65, 763–768. [Google Scholar] [CrossRef]
- Munajad, A.; Subroto, C. Suwarno. Fourier Transform Infrared (FTIR) Spectroscopy Analysis of Transformer Paper in Mineral Oil-Paper Composite Insulation under Accelerated Thermal Aging. Energies 2018, 11, 364. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S. Introduction to Spectroscopy: A Guide for Students of Organic Chemistry; W.B. Saunders Co.: Philadelphia, PA, USA, 1979; Available online: https://www.hdki.hr/_download/repository/Pavia-Introduction-to-Spectroscopy%5B1%5D.pdf (accessed on 11 February 2021).
- Pires, R.V.; Pessoa, L.M.B.; Sant’Anna, M.D.A.D.; Fainleib, A.; Nunes, R.D.C.P.; Lucas, E.F. Synthesis and characterization of isoprene oligomers to compare different production chemical processes. Polím. Ciênc. Tecnol. 2019, 29, e2019015. [Google Scholar] [CrossRef]
- Poh, B.T.; Lee, K.S. FTIR study of thermal oxidation of ENR. Eur. Polym. J. 1994, 30, 17–23. [Google Scholar] [CrossRef]
- Huang, Y.; Gohs, U.; Müller, M.T.; Zschech, C.; Wießner, S. Evaluation of Electron Induced Crosslinking of Masticated Natural Rubber at Different Temperatures. Polymers 2019, 11, 1279. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, G. Starch content and physicochemical properties of green wheat starch. Int. J. Food Prop. 2019, 22, 1463–1474. [Google Scholar] [CrossRef]
- Gaber, M.H. Effect of gamma-irradiation on the molecular properties of bovine serum albumin. J. Biosci. Bioeng. 2005, 100, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Lomelí Ramírez, M.G.; Satyanarayana, K.G.; Iwakiri, S.; Bolzon de Muniz, G.; Tanobe, V.; Sydenstricker Flores-Sahagun, T. Study of the properties of biocomposites. Part I. Cassava starch-green coir fibers from Brazil. Carbohydr. Polym. 2011, 86, 1712–1722. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.X.; Cho, U. Study on Improvement of Properties for Epoxidized Natural Rubber by Addition of Starch and Molybdenum Disulfide. Polymer 2018, 42, 1085–1090. [Google Scholar] [CrossRef]
- Wang, Z.F.; Peng, Z.; Li, S.D.; Lin, H.; Zhang, K.X.; She, X.D.; Fu, X. The impact of esterification on the properties of starch/natural rubber composite. Compos. Sci. Technol. 2009, 69, 1797–1803. [Google Scholar] [CrossRef]
- Lee, H.K.; Kim, D.S.; Won, J.S.; Jin, D.Y.; Lee, H.J.; Lee, S.G. Effects of Thermal and Humidity aging on the Interfacial Adhesion of Polyketone Fiber Reinforced Natural Rubber Composites. Adv. Mater. Sci. Eng. 2016, 4159072. [Google Scholar] [CrossRef]


| Material | Properties |
|---|---|
| Natural rubber Crepe 1X, from Sangtvon Rubber Ltd., Nakhon Si Thammarat, Thailand | Mooney viscosity: 67.64 ML1+4 at 100 °C, volatile materials content: 0.5%, nitrogen content: 0.4%, ash: 0.25%, impurities content: 0.026% |
| Soluble potato Starch, from Lach-Ner, Neratovice, Czech Republic | Water insoluble substances: 0.28%, loss on drying: 16.9%, easily biodegradable: BOD5—0.6 g/g and COD—1.2 mg/g |
| Glycerine, from SC Chimreactiv SRL, Bucharest, Romania | Density: 1.26 g/cm3, purity: 99.5%, free acidity: 0.02% Used for plasticized starch obtaining |
| IPPD antioxidant (4010 NA) N-isopropyl-N-phenyl-phenylene diamine, from Dalian Richon Chem Co. Ltd., Dalian, China | Molecular mass: 493.6374 g/mol, purity: 98%, Used as antioxidant |
| Perkadox 40 dibenzoyl peroxide, from AkzoNobel Chemicals, Deventer, Netherlands | Density: 1.60 g/cm3, active oxygen content: 3.8%, peroxide content: 40%, pH 7 Used as cross-linking agent |
| TMPT DL 75 Luvomaxx-trimethylolpropane trimethacrylate, from Lehmann & Voss & Co Hamburg, Germany | Density 1.36 g/cm3, ash: 22%, pH 9.2, active ingredient: 75 ± 3%. Used as co-agent curing |
| Ingredients (phr) | Mixtures Codes | |||||
|---|---|---|---|---|---|---|
| NR | NR-10 | NR-20 | NR-30 | NR-40 | NR-50 | |
| Natural rubber (NR) | 100 | 100 | 100 | 100 | 100 | 100 |
| Starch | 0 | 10 | 20 | 30 | 40 | 50 |
| Glycerine | 0 | 6 | 12 | 18 | 24 | 30 |
| Dibenzoyl Peroxyde | 8 | 8 | 8 | 8 | 8 | 8 |
| TMPT | 3 | 3 | 3 | 3 | 3 | 3 |
| IPPD Antioxidant | 1 | 1 | 1 | 1 | 1 | 1 |
| PS Amount in NR/PS Composites | C0 | C10 | C20 | C30 | C40 | C50 |
|---|---|---|---|---|---|---|
| p0/q0 ratio | 0.2514 | 0.2862 | 0.3195 | 0.3347 | 0.4096 | 0.4412 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manaila, E.; Craciun, G.; Ighigeanu, D.; Lungu, I.B.; Dumitru, M.; Stelescu, M.D. Electron Beam Irradiation: A Method for Degradation of Composites Based on Natural Rubber and Plasticized Starch. Polymers 2021, 13, 1950. https://doi.org/10.3390/polym13121950
Manaila E, Craciun G, Ighigeanu D, Lungu IB, Dumitru M, Stelescu MD. Electron Beam Irradiation: A Method for Degradation of Composites Based on Natural Rubber and Plasticized Starch. Polymers. 2021; 13(12):1950. https://doi.org/10.3390/polym13121950
Chicago/Turabian StyleManaila, Elena, Gabriela Craciun, Daniel Ighigeanu, Ion Bogdan Lungu, Marius Dumitru, and Maria Daniela Stelescu. 2021. "Electron Beam Irradiation: A Method for Degradation of Composites Based on Natural Rubber and Plasticized Starch" Polymers 13, no. 12: 1950. https://doi.org/10.3390/polym13121950
APA StyleManaila, E., Craciun, G., Ighigeanu, D., Lungu, I. B., Dumitru, M., & Stelescu, M. D. (2021). Electron Beam Irradiation: A Method for Degradation of Composites Based on Natural Rubber and Plasticized Starch. Polymers, 13(12), 1950. https://doi.org/10.3390/polym13121950