Abstract
In this work, copper-mediated reversible deactivation radical polymerization (RDRP) of homo-polyacrylamides was conducted in aqueous solutions at 0.0 °C. Various degrees of polymerization (DP = 20, 40, 60, and 80) of well-defined water-soluble homopolymers were targeted. In the absence of any significant undesirable side reactions, the dispersity of polydiethylacrylamide (PDEA) and polydimethylacrylamide (PDMA) was narrow under controlled polymerization conditions. To accelerate the polymerization rate, disproportionation of copper bromide in the presence of a suitable ligand was performed prior to polymerization. Full conversion of the monomer was confirmed by nuclear magnetic resonance (NMR) analysis. Additionally, the linear evolution of the polymeric chains was established by narrow molecular weight distributions (MWDs). The values of theoretical and experimental number average molecular weights (Mn) were calculated, revealing a good matching and robustness of the system. The effect of decreasing the reaction temperature on the rate of polymerization was also investigated. At temperatures lower than 0.0 °C, the controlled polymerization and the rate of the process were not affected.
1. Introduction
Hydrophilic monomers, such as acrylamides, as well as their homopolymers and copolymers, have been utilized in oil recovery, wastewater treatment, and environmental systems [1,2]. The conventional acrylamide polymerization techniques have been widely investigated; however, only few methods utilizing controlled polymerization have been reported. Despite significant advances in controlled polymerization technologies, the reversible deactivation radical polymerization (RDRP) of water-soluble acrylamides remains a challenge [3]. Controlled polymerization in the presence of aqueous media or below ambient temperature is difficult. The fast RDRP in water often leads to undesirable side reactions. To suppress such reactions, controlled polymerization approaches have been employed previously [4,5,6,7,8,9].
The recently proposed mechanism of copper-mediated RDRP involved an equilibrium between activating and deactivating species, which was mediated by Cu–ligand complexes [10,11,12,13,14,15]. It has been determined that controlled polymerization of water-soluble acrylamides is a challenging task due to undesirable termination reactions, which lead to the loss of the end group fidelity [16,17,18,19,20,21,22,23]. In the single electron transfer living radical polymerization (SET-LRP) method, which is the latest technique classed as RDRP, the disproportionation of the copper bromide complex in the presence of nitrogen-based ligands occurs to provide the system with Cu(0) catalyst and a CuBr2 disruptor species prior to the addition of the initiator and monomer [10]. The disproportionation of Cu(Ι)Br/tris[2-(dimethylamino)ethyl]amine (Me6TREN) in an aqueous solution was also examined to prepare multiblock polyacrylamides [20,21]. Equal polymer chain growth (Ð < 1.10) and full monomer conversion were achieved using this approach, which was confirmed by nuclear magnetic resonance (NMR) and gel permeation chromatography (GPC) analyses.
Polydimethylacrylamide (PDMA) is a useful polymer with intriguing properties. It contains a hydrophilic acrylamide group; therefore, it is fully soluble in water and exhibits excellent biocompatibility. It has been extensively used in different industrial applications, e.g., as an associating polymer for controlling the physical behavior of paints and coatings as well as for enhanced oil recovery [24]. The biological applications of PDMA include the preparation of proteins, two-phase catalysts, and drug delivery systems [25,26].
Matyjaszewski et al. [17,18] reported on the first polymerization of N,N-dimethylacrylamide (DMA) using the aqueous atom transfer radical polymerization (ATRP) method; however, the polymerization was not controlled. Similar results were obtained by Brittain and co-workers [19]. Both groups concluded that controlling the aqueous ATRP of DMA was challenging when only water was used as the solvent at room temperature. Recently, Haddleton and coworkers [21] found that PDMA could be successfully polymerized by aqueous SET-LRP. Although controlled polymerization was conducted, the resulting PDMA displayed a low molecular weight (Mn ~3100 and 5100 g mol−1), which could limit the applications of the material in cases where polymeric materials with high Mn are required.
Reversible deactivation radical polymerization techniques have been developed to become powerful tools for the preparation of well-controlled polymers with predetermined molecular weight and narrow molecular weight distribution. However, mostly, they have still proved problematic in aqueous media. Given the increased interest in water-soluble and biocompatible polymers in advanced applications, this issue in the synthetic toolbox needs to be addressed. In light of the abovementioned issues, the work on them needs more attention. In this work, a facile Cu(0)-mediated RDRP method was utilized to synthesize two well-defined homopolymers containing DMA and diethylacrylamide (DEA) units. Water was used as the solvent to produce the homopolymers below ambient temperature. The polymerization reactions were conducted in a controlled manner to achieve low molecular weight distributions (MWDs). Full monomer consumption was obtained within 1 h, which was evidenced by 1H NMR and size-exclusion chromatography (SEC) analyses. In situ CuBr disproportionation was utilized to achieve a rapid synthesis of two different acrylamides. Homopolymerization of both DMA and DEA monomers was performed to afford narrow MWDs. Moreover, we investigated the effect of reducing the temperature of the reaction on the rate of polymerization. Ultimately, the described approach using water as the solvent could be applied both in academic and industrial settings to provide valuable polymers at temperatures below ambient temperature.
2. Materials and Methods
2.1. Materials
DEA (99%) and DMA (99%) were supplied by Sigma-Aldrich, Darmstadt, Germany. Prior to use, the monomers were filtered by injection over a column filled with alumina to eliminate inhibitors. High-performance liquid chromatography (HPLC) grade H2O (VWR international, LLC, Radnor, PA, USA) was used as the solvent for disproportionation and polymerization processes.
The water-soluble initiator (WSI), namely dihydroxypropane-3-oxy-(2-bromo-2-methylpropionyl), and Me6TREN were prepared according to previously reported procedures [27,28]. The latter was stored under nitrogen prior to any experiential work. CuBr (98%, Sigma-Aldrich, St. Louis, MO, USA) was successively washed with acetic acid and ethanol, and then dried under vacuum.
2.2. Instruments and Analysis
1H NMR spectra were obtained on a 300 and 600 MHz JEOL NMR spectrometer using deuterated solvents (D2O) as locking agents. The monomer conversion for the homopolymerization of DMA and DEA was measured by evaluating the integrals of the peaks corresponding to the vinyl protons as well as those attributed to dimethyl and diethyl protons, respectively.
SEC was performed employing the Agilent N29812 system using dimethylformamide (DMF) containing 5 mM NH4BF4 as the mobile phase at 45 °C. The system was equipped with ultraviolet, refractive index, and viscometer detectors as well as an autosampler, a PLgel 5 μm MIXED-D column (300 × 7.5 mm), and a PLgel 5 μm guard column (50 × 7.5 mm). In addition, an Agilent 1260 infinity ii isocratic pump with a maximum pressure of 600 bar was used.
Linear PMMA standards in the range of 200 to 1.0 × 106 g mol−1 were used to calibrate the system of reactions. All samples were passed through a 0.45 μm polytetrafluoroethylene (PTFE) filter. All reactions were conducted under a N2 atmosphere employing a typical Schlenk technique.
2.3. Synthesis of PDEA and PDMA Homopolymers by Aqueous Cu(0)-Mediated Polymerization at 0 °C
H2O (2 mL), a small magnetic stirrer, and Me6TREN-ligand (0.026 mL, 0.0001 mol) were transfer to a Schlenk tube and the solution was bubbled with nitrogen for 15 min (Scheme 1). The degassed solution was transferred to another deoxygenated Schlenk tube containing CuBr (0.014 g, 0.0001 mol) and a small magnetic stirrer via a cannula. The reaction mixture turned blue and red copper particles formed in the solution. The reaction mixture was stirred at 20 °C for 25 min under a N2 blanket. Subsequently, the Schlenk tube was placed in an ice-water bath.
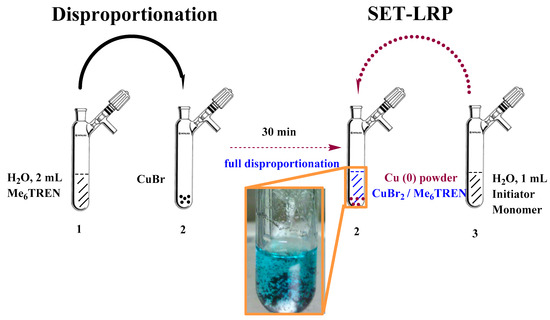
Scheme 1.
Aqueous Cu(0)-mediated polymerization of acrylamide monomers following a disproportionation reaction.
Water (1 mL), dihydroxypropane-3-oxy-(2-bromo-2-methylpropionyl) (0.60 g, 0.00025 mol), and acrylamide monomer were added to a new Schlenk tube equipped with a small magnetic stirrer. The amount of monomer was based on the individual degree of polymerization (DP) of DMA or DEA. The solution was deoxygenated by bubbling with nitrogen for 15 min. The deoxygenated solution was subsequently transferred to a Schlenk tube placed in an ice-water bath via a cannula. The reaction solution was stirred at 0.0 °C for different times.
Samples of the reaction solution were carefully removed by a degassed syringe, for 1H NMR and SEC analysis. Then, the taken sample (50 μL) was exposed to air which stopped catalyst from being active. For 1H NMR evaluation, the samples were simply diluted with D2O. However, prior to SEC analysis, the samples were passed through an aluminum oxide column to eliminate any unreactive catalyst.
3. Results and Discussion
Well-defined homopolymers of two acrylamide derivatives were synthesized via Cu(0)-mediated RDRP in pure water at 0 °C (Scheme 2).
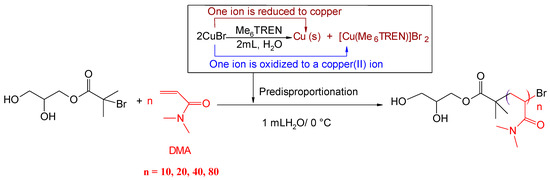
Scheme 2.
Synthesis of polydimethylacrylamide (PDMA) (degree of polymerization (DP) = 10, 20, 40, and 80) by Cu(0)-mediated polymerization in water at 0.0 °C via prior disproportionation of copper bromide. Conditions: [WSI]:[CuBr]:[Me6TREN] = 1:0.4:0.4.
Homopolymerization of DMA in aqueous solutions was exploited employing the Cu(0)-mediated polymerization approach. Initially, the polymerization of DMA was investigated using the aforementioned conditions: [DMA]:[WSI]:[CuBr]:[Me6TREN] = [10 or 20]:[1]:[0.4]:[0.4]. 1H NMR analysis confirmed that 100% monomer conversion was achieved in 8 min for each DP = 10 and 20. SEC traces revealed one symmetrical peak distribution with narrow MWDs (DP = 10, Đ ~1.09 and DP = 20, 1.28), demonstrating the controlled nature of the system (Table 1 and Figure 1).

Table 1.
Summary of characteristics of different DPs of dimethylacrylamide (DMA) by Cu(0)-mediated polymerization under various conditions.
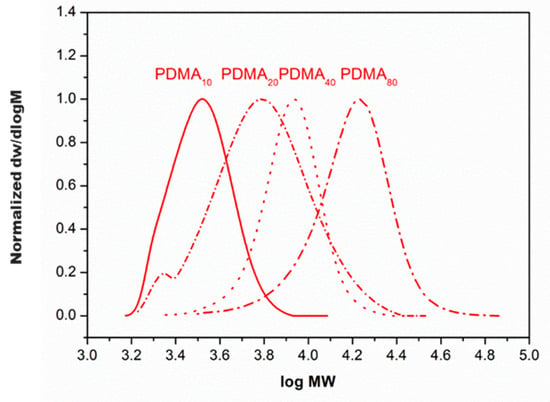
Figure 1.
The evolution of molecular weights of PDMA (DP = 10–80) catalyzed by the generated Cu(0) particles/CuBr2/Me6TREN complex in water determined by gel permeation chromatography (GPC) using dimethylformamide (DMF) as the mobile phase.
Nonetheless, the appearance of a small low molecular weight shoulder was detected in the case of DP = 20, indicating the lack of a catalyst. When the target was DP = 40, the synthesis was primarily conducted utilizing previous conditions; however, a broad dispersity (Đ) was observed. Thus, the initial catalytic ratio was increased to [copper]:[ligand] = [2]:[1]. The polymerization then reached full conversion within the same reaction time (8 min). Initially, our approach was to fix the initial monomer concentration at 10% v/v as a workable solution viscosity. Due to the increase in the solution viscosity for the case of DP 80, DMA monomer was dissolved in 4 mL of water instead of 1 mL. Notably, narrow dispersity (Đ ~ 1.10) over MWDs was achieved, demonstrating that the polymerization was fast and controlled.
The optimized reaction conditions were used to obtain polymers with high molecular weights (DP = 80). Pleasingly, quantitative conversions and narrow MWDs (~1.19) were achieved within 8 min, clearly demonstrating the remarkable potential of the polymerization processes. A well-defined molar mass polymer (size = 14 K g mol−1) was obtained in an ultrafast reaction time (Figure 2 and Table 1).
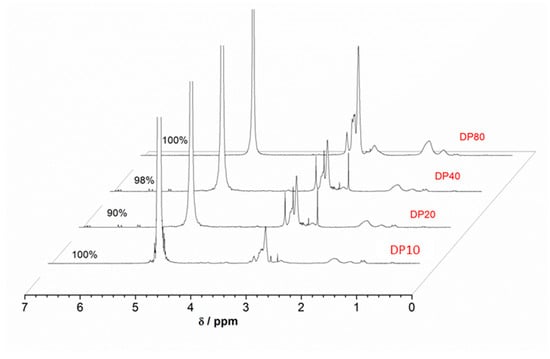
Figure 2.
1H nuclear magnetic resonance (NMR) spectra of the Cu(0)-mediated polymerization of PDMA (DP = 10, 20, 40 and 80) in D2O.
We were also interested in the homopolymerization of DEA, which is another intriguing hydrophilic acrylamide. Although PDEA is considered to be biocompatible [29,30] and less toxic than poly(N-isopropylacrylamide) (PNIPAM) [31,32], it is considerably less well studied. Both PDEA and PNIPAM exhibit a low critical solution temperature (LCST) in aqueous media (approximately 25–33 °C). Notably, this feature of the polymers can be exploited for specific applications. However, the phase behavior, hydration, and volume transition of PDEA are broader than those of PNIPAM due to the absence of the N–H group [33,34]. This phenomenon is particularly valuable for achieving a progressive release system for encapsulated drugs.
PDEA is less utilized in advanced applications, which can be attributed to the lack of attempts to prepare well-defined PDEA, especially in aqueous media. Delaittre and Charleux [34] were the first to report nitroxide-mediated polymerization (NMP) of DEA in toluene. Gody et al. [22,23] employed reversible addition−fragmentation chain-transfer polymerization (RAFT) to synthesize PDEA; however, the use of a high temperature was required (70 °C).
To investigate the potential of the aqueous copper-mediated RDRP to control the reaction system, four polymerizations, i.e., DP = 20, 40, 60, and 80, were targeted (Scheme 3). Utilizing the previously optimized conditions for PDMA, controlled polymerizations were effectively performed with full monomer consumption. As summarized in Table 2, narrow dispersities were achieved. This was further confirmed by NMR and GPC analyses (Figure 3 and Figure 4).
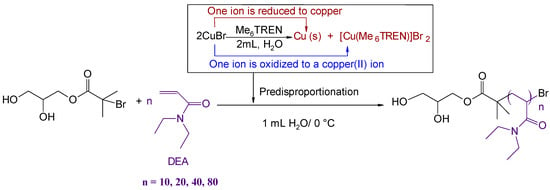
Scheme 3.
Synthesis of PDEA (DP = 10, 20, 40, 80) by Cu(0)-mediated polymerization in water at 0 °C via prior disproportionation of copper bromide. Conditions: [WSI]:[copper]:[ligand] = 1:0.4:0.4.

Table 2.
Summary of characteristics of different DPs of diethylacrylamide (DEA) by Cu(0)-mediated polymerization under various conditions.
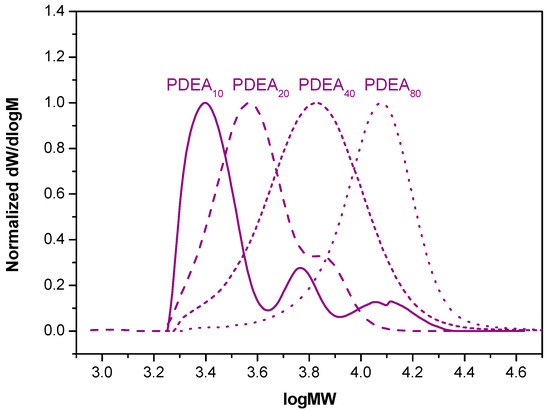
Figure 3.
The evolution of molecular weights of PDEA (DP = 10–80) catalyzed by the generated Cu(0) particles/CuBr2/Me6TREN complex in water determined by GPC using DMF as the mobile phase.
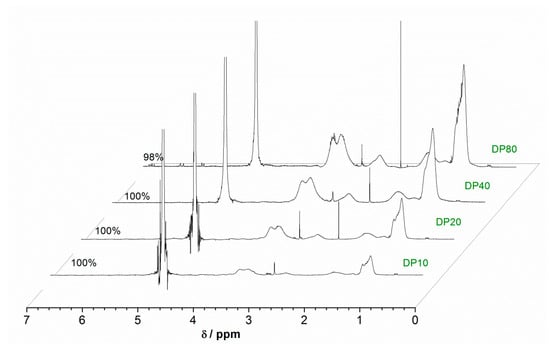
Figure 4.
1H NMR spectra for Cu(0)-mediated polymerization of PDEA (DP = 10, 20, 40, and 80) in D2O.
Intriguingly, although the reactivity of DEA was similar to that of DMA [35,36,37], compared to the same DP of PDMA, the full conversion of DEA was achieved after a longer reaction time. The GPC evaluation revealed some high molecular weight shoulders for DP = 10 and 20; however, the MWDs were within the controlled limit of less than 1.5.
Effect of Decreasing the Reaction Temperature on the Rate of Polymerization
To investigate the effect of decreasing the reaction temperature on the rate of polymerization, the procedure for the preparation of PDEA was employed. The disproportionation and polymerization conditions were identical to those utilized in experiments involving the acrylamide monomers. DEA (2.72 mL, 20 mmol (DP 80)) was used as a monomer and was allowed to polymerize at 0.0 °C and −10 °C. Two systems were used to decrease the temperature of the reaction. The first consisted of an ice-water bath (0.0 °C), while the second was a mixture of salt and ice (−10 °C). The temperature was monitored at different times using a thermometer. Subsequently, reaction samples taken at specific times were analyzed by 1H NMR and SEC. The time and conversion of each polymerization reaction are summarized in Table 3 and Figure 5 and Figure 6.

Table 3.
Summary of characteristics of polymers of DEA obtained by Cu(0)-mediated polymerization. Conditions: [WSI]:[copper]:[ligand] = 1:0.4:0.4.
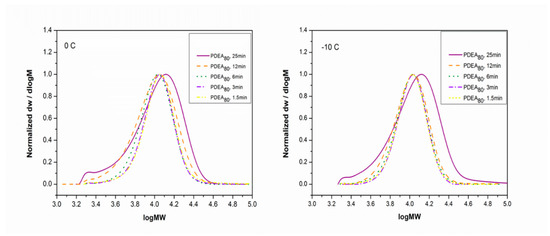
Figure 5.
Gel permeation chromatography (GPC) traces showing the molecular weight evolutions for Cu(0)-mediated polymerization of DEA in H2O at 0.0 °C and −10 °C.
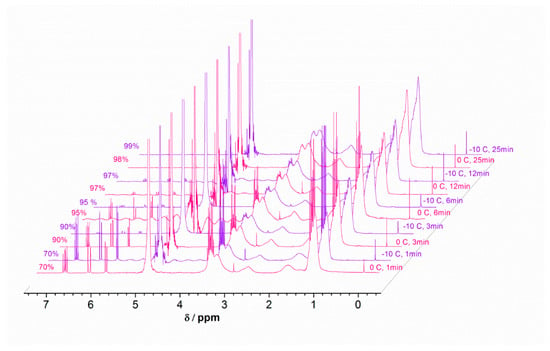
Figure 6.
1H NMR spectra for Cu(0)-mediated polymerization of DEA in H2O at 0.0 °C and −10 °C using the previously mentioned conditions: [WSI]:[copper]:[ligand] in D2O.
Notably, 70% conversion was achieved after just 1.5 min, as illustrated by Table 3. The polymerization reaction was controlled and nearly all of the monomer was converted into the polymer after 25 min, demonstrating the effectiveness of the system. Changing the reaction temperature from 0.0 °C to −10 °C did not affect the rate of polymerization, indicating the potential of applying the system in a biological environment. GPC analysis, shown in Figure 5, confirms the occurrence of controlled polymerization. Figure 6 shows the monitoring of monomer conversion by 1H NMR.
4. Conclusions
In this work, syntheses of hydrophilic homopolymers of two acrylamide-based monomers in aqueous media at temperatures below room temperature were successfully conducted. In addition, a facile route for the preparation of discreet PDEA and PDMA in water was developed. Rapid polymerization was achieved after full disproportionation of copper bromide. The occurrence of controlled polymerization was confirmed by GPC analysis, while the monomer conversion was monitored by 1H NMR. Employing Cu(0)-mediated RDRP, several PDMA and PDEA polymers (DP = 10, 20, 40, and 80) with excellent MWDs were obtained. Moreover, the results illustrated that the polymerization could be performed at a low temperature (−10 °C) without any decrease in the reaction rate, demonstrating the possible biocompatibility of the developed system. It is confirmed that this versatile technique involving low temperatures can be successfully used to prepare more water-soluble monomers. The synthesis of temperature-responsive copolyacrylamides in aqueous solutions is the main objective of an ongoing study.
Author Contributions
Data curation, F.M.A., B.A.A., and H.F.; Formal analysis, F.M.A.; Funding acquisition, O.Y.A.; Methodology, F.M.A., B.A.A., and H.F.; Resources, O.Y.A.; Writing—original draft, F.M.A.; Writing—review and editing, O.Y.A., B.A.A., and H.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Deanship of Scientific Research at King Saud University, through research group no. RGP-133.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group no. RGP-133. We gratefully thank King Abdulaziz City for Science and Technology (KACST) for technical support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wever, D.A.Z.; Picchioni, F.; Broekhuis, A.A. Polymers for enhanced oil recovery: A paradigm for structure-property relationship in aqueous solution. Prog. Polym. Sci. 2011, 36, 1558–1628. [Google Scholar] [CrossRef]
- Xiong, B.; Loss, R.D.; Shields, D.; Pawlik, T.; Hochreiter, R.; Zydney, A.L.; Kumar, M. Polyacrylamide degradation and its implications in environmental systems. NPJ Clean Water 2018, 1, 17. [Google Scholar] [CrossRef]
- Jones, G.; Anastasaki, A.; Whitfield, R.; Engelis, N.; Liarou, E.; Haddleton, D. Copper-mediated reversible deactivation radical polymerization in aqueous media. Angew. Chem. 2018, 33, 10468–10482. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Narain, R. Water-Assisted Atom Transfer Radical Polymerization of N-Isopropylacrylamide: Nature of Solvent and Temperature. J. Phys. Chem. B 2009, 113, 676–681. [Google Scholar] [CrossRef]
- Corrigan, N.; Jung, K.; Moad, G.; Hawker, C.J.; Matyjaszewski, K.; Boyer, C. Reversible-deactivation radical polymerization (Controlled/living radical polymerization): From discovery to materials design and applications. Prog. Polym. Sci. 2020, 111, 101311. [Google Scholar] [CrossRef]
- Wever, D.A.Z.; Raffa, P.; Picchioni, F.; Broekhuis, A.A. Acrylamide Homopolymers and Acrylamide–N-Isopropylacrylamide Block Copolymers by Atomic Transfer Radical Polymerization in Water. Macromolecules 2012, 45, 4040–4045. [Google Scholar] [CrossRef][Green Version]
- Parkatzidis, K.; Wang, H.S.; Truong, N.P.; Anastasaki, A. Recent developments and future challenges in controlled radical polymerization: A 2020 update. Chem 2020, 6, 1575–1588. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Rosen, B.M.; Percec, V.J. SET-LRP of N,N-dimethylacrylamide and of N-isopropylacrylamide at 25 °C in protic and in dipolar aprotic solvents. Polym. Sci. A Polym. Chem. 2010, 48, 1752–1763. [Google Scholar] [CrossRef]
- Matyjaszewski, K. Atom transfer radical polymerization (ATRP): Current status and future perspectives. Macromolecules 2012, 45, 4015–4039. [Google Scholar] [CrossRef]
- Percec, V.; Guliashvili, T.; Ladislaw, J.S.; Wistrand, A.; Stjerndahl, A.; Sienkowska, M.J.; Monteiro, M.J.; Sahoo, S. Ultrafast synthesis of ultrahigh molar mass polymers by metal-catalyzed living radical polymerization of acrylates, methacrylates, and vinyl chloride mediated by SET at 25 °C. J. Am. Chem. Soc. 2006, 128, 14156–14165. [Google Scholar] [CrossRef]
- Hawker, C.J.; Bosman, A.W.; Harth, E. New polymer synthesis by nitroxide mediated living radical polymerizations. Chem. Rev. 2001, 101, 3661–3688. [Google Scholar] [CrossRef]
- Nicolas, J.; Guillaneuf, Y.; Lefay, C.; Bertin, D.; Gigmes, D.; Charleux, B. Nitroxide-mediated polymerization. Prog. Polym. Sci. 2013, 38, 63–235. [Google Scholar] [CrossRef]
- Chiefari, J.; Chong, Y.K.; Ercole, F.; Krstina, J.; Jeffery, J.; Le, T.P.T.; Mayadunne, R.T.A.; Meijs, G.F.; Moad, C.L.; Moad, G.; et al. Living Free-Radical Polymerization by Reversible Addition-Fragmentation Chain Transfer: The RAFT Process. Macromolecules 1998, 31, 5559–5562. [Google Scholar] [CrossRef]
- Moad, G.; Rizzardo, E.; Thang, S.H. Living Radical Polymerization by the RAFT Process. Aust. J. Chem. 2009, 62, 1402–1472. [Google Scholar] [CrossRef]
- Wang, J.-S.; Matyjaszewski, K.J. Controlled/“Living” Radical Polymerization. Atom Transfer Radical Polymerization in the Presence of Transition-Metal Complexes. Am. Chem. Soc. 1995, 117, 5614–5615. [Google Scholar] [CrossRef]
- Iddon, P.D.; Robinson, K.L.; Armes, S.P. Polymerization of sodium 4-styrenesulfonate via atom transfer radical polymerization in protic media. Polymer 2004, 45, 759–768. [Google Scholar] [CrossRef]
- Teodorescu, M.; Matyjaszewski, K. Atom transfer radical polymerization of (meth)acrylamides. Macromolecules 1999, 32, 4826–4831. [Google Scholar] [CrossRef]
- Teodorescu, M.; Matyjaszewski, K. Controlled polymerization of (meth)acrylamides by atom transfer radical polymerization. Macromol. Rapid Commun. 2000, 21, 190–194. [Google Scholar] [CrossRef]
- Rademacher, J.T.; Baum, M.; Pallack, M.E.; Brittain, W.J.; Simonsick, W.J. Atom transfer radical polymerization of N,N-Dimethylacrylamide. Macromolecules 2000, 33, 284–288. [Google Scholar] [CrossRef]
- Zhang, Q.; Wilson, P.; Li, Z.; McHale, R.; Godfrey, J.; Anastasaki, A.; Waldron, C.; Haddleton, D.M. Aqueous copper-mediated living polymerization: Exploiting rapid disproportionation of CuBr with Me6TREN. J. Am. Chem. Soc. 2013, 135, 7355–7363. [Google Scholar] [CrossRef]
- Alsubaie, F.; Anastasaki, A.; Wilson, P.; Haddleton, D.M. Sequence-controlled multi-block copolymerization of acrylamides via aqueous SET-LRP at 0 °C. Polym. Chem. 2015, 6, 406–417. [Google Scholar] [CrossRef]
- Gody, G.; Maschmeyer, T.; Zetterlund, P.B.; Perrier, S. Rapid and quantitative one-pot synthesis of sequence-controlled polymers by radical polymerization. Nat. Commun. 2013, 4, 2505. [Google Scholar] [CrossRef]
- Gody, G.; Maschmeyer, T.; Zetterlund, P.B.; Perrier, S. Exploitation of the degenerative transfer mechanism in RAFT polymerization for synthesis of polymer of high livingness at full monomer conversion. Macromolecules 2014, 47, 639–649. [Google Scholar] [CrossRef]
- Uemura, Y.; McNulty, J.; Macdonald, P.M. Associative behavior and diffusion coefficients of hydrophobically modified poly(N,N-dimethylacrylamides). Macromolecules 1995, 28, 4150–4158. [Google Scholar] [CrossRef]
- Kondo, S.; Nakashima, N.; Hado, H.; Tsuoa, K. Poly(N,N-dimethylamide-co-styrene)s as a highly efficient catalysts for two-phase reactions. J. Polym. Sci. A Polym. Chem. 1990, 28, 2229–2232. [Google Scholar] [CrossRef]
- Valdebenito, A.; Encinas, M.V. Effect of solvent on the free radical polymerization of N,N-dimethylacrylamide. Polym. Int. 2010, 59, 1246–1251. [Google Scholar] [CrossRef]
- Perrier, S.; Armes, S.P.; Wang, X.S.; Malet, F.; Haddleton, D.M.J. Copper(I)-mediated radical polymerization of methacrylates in aqueous solution. Polym. Sci. Part A Polym. Chem. 2001, 39, 1696. [Google Scholar] [CrossRef]
- Ciampolini, M.; Nardi, N. Five-Coordinated High-Spin Complexes of Bivalent Cobalt, Nickel, and Copper with Tris(2-dimethylaminoethyl)amine. Inorg. Chem. 1966, 5, 41. [Google Scholar] [CrossRef]
- Li, Y.; Pan, S.; Zhang, W.; Du, Z. Novel thermo-sensitive core-shell nanoparticles for targeted paclitaxel delivery. Nanotechnology 2009, 20, 065104. [Google Scholar] [CrossRef]
- Ma, L.; Liu, M.; Liu, H.; Chen, J.; Cui, D. In vitro cytotoxicity and drug release properties of pH- and temperature-sensitive core–shell hydrogel microspheres. Int. J. Pharm. 2010, 385, 86–91. [Google Scholar] [CrossRef]
- Panayiotou, M.; Freitag, R. Influence of the synthesis conditions and ionic additives on the swelling behavior of thermo-responsive polyalkylacrylamide hydrogels. Polymer 2005, 46, 6777–6785. [Google Scholar] [CrossRef]
- Panayiotou, M.; Pöhner, C.; Vandevyver, C.; Wandrey, C.; Hilbrig, F.; Freitag, R. Synthesis and characterisation of thermoresponsive poly(N,N-diethylacrylamide) microgels. React. Funct. Polym. 2007, 76, 807–881. [Google Scholar] [CrossRef]
- Maeda, Y.; Nakamura, T.; Ikeda, I. Change in solvation of poly(N,N-diethylacrylamide) during phase transition in aqueous solutions. Macromolecules 2002, 35, 10172–10177. [Google Scholar] [CrossRef]
- Delaittre, G.; Rieger, J.; Charleux, B.; Living, N.-M. Nitroxide-mediated living/controlled radical polymerization of N, N-diethylacrylamide. Macromolecules 2011, 44, 462–470. [Google Scholar] [CrossRef]
- Liu, H.Y.; Zhu, X.X. Lower critical solution temperatures of N-substituted acrylamide copolymers in aqueous solutions. Polymer 1999, 40, 6985–6990. [Google Scholar] [CrossRef]
- Albarghouthi, M.N.; Buchholz, B.A.; Doherty, E.A.S.; Bogdan, F.M.; Zhou, H.; Barron, A.E. Impact of polymer hydrophobicity on the properties and performance of DNA sequencing matrices for capillary electrophoresis. Electrophoresis 2001, 22, 737–747. [Google Scholar] [CrossRef]
- Bian, F.; Xiang, M.; Yu, Y.; Liu, M. Preparation and characterization of thermo-sensitive micelles composed of PSt-b-P(DEA-co-DMA). e-Polymers 2008, 121, 1–11. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).