A Polymer-Biologic Hybrid Hernia Construct: Review of Data and Early Experiences
Abstract
1. Introduction
1.1. Abdominal Wall Hernia and Treatment Options
1.2. Wound Healing
1.3. Mesh Landscape
1.4. Ideal Mesh
1.5. Hybrid Meshes
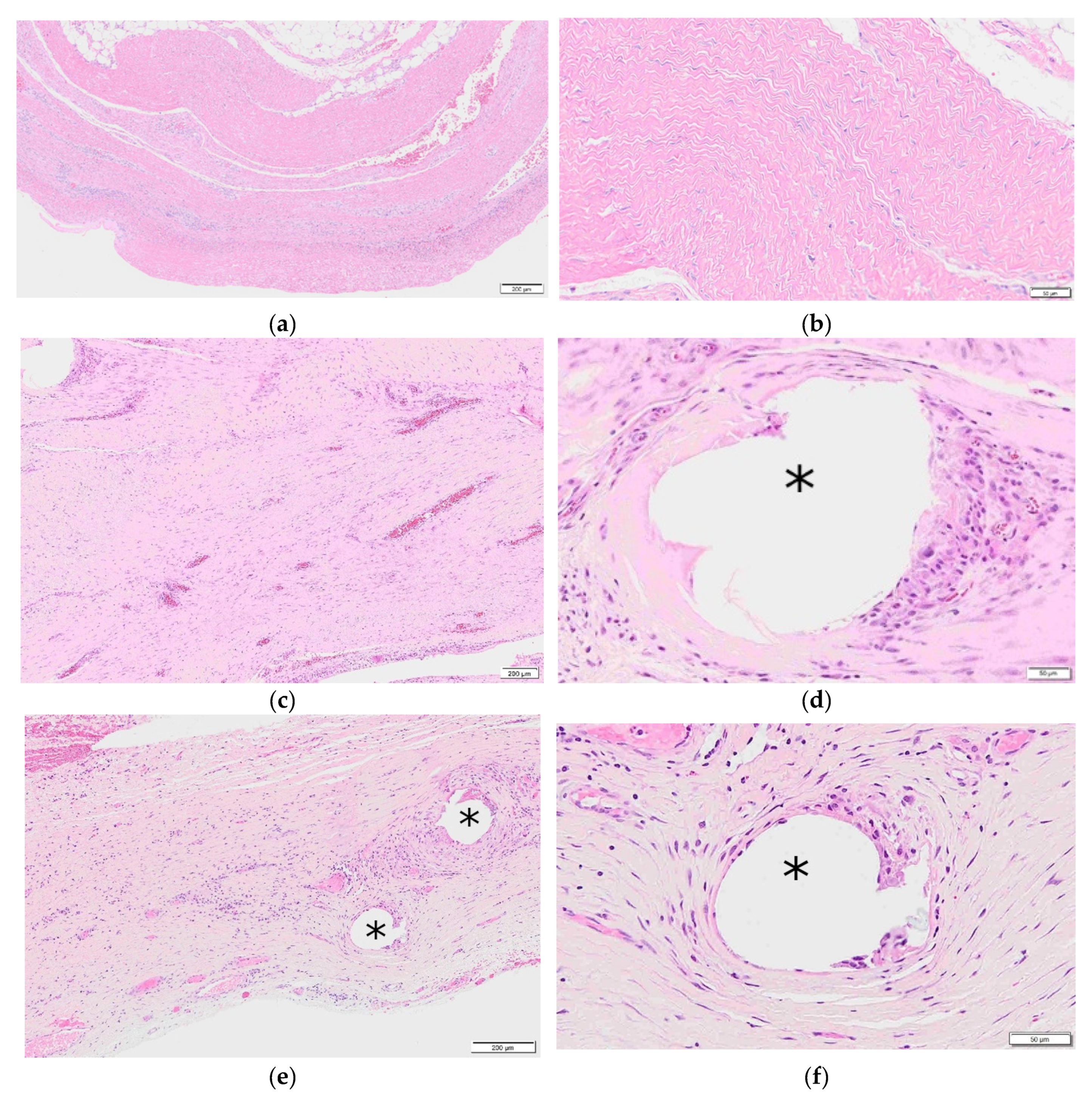
1.6. Reinforced Tissue Matrices
2. Review of Reinforced Tissue Matrices in the Repair of Various Hernia Types
2.1. Ventral Hernia Repair
2.2. Hiatal Hernia Repair
2.3. Inguinal Hernia Repair
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burger, J.W.; Luijendijk, R.W.; Hop, W.C.; Halm, J.A.; Verdaasdonk, E.G.; Jeekel, J. Long-term Follow-up of a Randomized Controlled Trial of Suture Versus Mesh Repair of Incisional Hernia. Ann. Surg. 2004, 240, 578–585. [Google Scholar] [CrossRef]
- Köckerling, F.; Hoffmann, H.; Mayer, F.; Zarras, K.; Reinpold, W.; Fortelny, R.; Weyhe, D.; Lammers, B.; Adolf, D.; Schug-Pass, C. What are the trends in incisional hernia repair? Real-world data over 10 years from the Herniamed registry. Hernia 2021, 25, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Corr, D.T.; Hart, D.A. Biomechanics of Scar Tissue and Uninjured Skin. Adv. Wound Care 2013, 2, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Orenstein, S.B.; Saberski, E.R.; Kreutzer, N.L.; Novitsky, Y.W. Comparative Analysis of Histopathologic Effects of Synthetic Meshes Based on Material, Weight, and Pore Size in Mice. J. Surg. Res. 2012, 176, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Cobb, W.S.; Kercher, K.W.; Heniford, B.T. The Argument for Lightweight Polypropylene Mesh in Hernia Repair. Surg. Innov. 2005, 12, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Klinge, U.; Klosterhalfen, B.; Birkenhauer, V.; Junge, K.; Conze, J.; Schumpelick, V. Impact of Polymer Pore Size on the Interface Scar Formation in a Rat Model. J. Surg. Res. 2002, 103, 208–214. [Google Scholar] [CrossRef]
- Christensen, M.B.; Tresco, P.A. The foreign body response and morphometric changes associated with mesh-style peripheral nerve cuffs. Acta Biomater. 2018, 67, 79–86. [Google Scholar] [CrossRef]
- Hwang, K.; Sim, H.B.; Huan, F.; Kim, D.J. Myofibroblasts and Capsular Tissue Tension in Breast Capsular Contracture. Aesthetic Plast. Surg. 2010, 34, 716–721. [Google Scholar] [CrossRef]
- Schuster, R.; Rockel, J.S.; Kapoor, M.; Hinz, B. The inflammatory speech of fibroblasts. Immunol. Rev. 2021. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef]
- Major, M.R.; Wong, V.W.; Nelson, E.R.; Longaker, M.T.; Gurtner, G.C. The Foreign Body Response. Plast. Reconstr. Surg. 2015, 135, 1489–1498. [Google Scholar] [CrossRef] [PubMed]
- Molina, C.P.; Giglio, R.; Gandhi, R.M.; Sicari, B.M.; Londono, R.; Hussey, G.S.; Bartolacci, J.G.; Luque, L.M.Q.; Cramer, M.C.; Dziki, J.L.; et al. Comparison of the host macrophage response to synthetic and biologic surgical meshes used for ventral hernia repair. J. Immunol. Regen. Med. 2019, 3, 13–25. [Google Scholar] [CrossRef]
- Brown, B.N.; Badylak, S.F. Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Transl. Res. 2014, 163, 268–285. [Google Scholar] [CrossRef] [PubMed]
- Deeken, C.R.; Abdo, M.S.; Frisella, M.M.; Matthews, B.D. Physicomechanical Evaluation of Polypropylene, Polyester, and Polytetrafluoroethylene Meshes for Inguinal Hernia Repair. J. Am. Coll. Surg. 2011, 212, 68–79. [Google Scholar] [CrossRef] [PubMed]
- See, C.W.; Kim, T.; Zhu, D. Hernia Mesh and Hernia Repair: A Review. Eng. Regen. 2020, 1, 19–33. [Google Scholar] [CrossRef]
- White, A.R.; Hirose, F.M.; Sproat, R.W.; Lawrence, R.S.; Nelson, R.J. Histopathologic observations after short-term implantation of two porous elastomers in dogs. Biomaterials 1981, 2, 171–176. [Google Scholar] [CrossRef]
- Engelsman, A.F.; van der Mei, H.C.; Busscher, H.J.; Ploeg, R.J. Morphological aspects of surgical meshes as a risk factor for bacterial colonization. Br. J. Surg. 2008, 95, 1051–1059. [Google Scholar] [CrossRef]
- Brown, C.N.; Finch, J.G. Which mesh for hernia repair? Ann. R. Coll. Surg. Engl. 2010, 92, 272–278. [Google Scholar] [CrossRef]
- Verhorstert, K.W.J.; Guler, Z.; De Boer, L.; Riool, M.; Roovers, J.-P.W.R.; Zaat, S.A.J. In Vitro Bacterial Adhesion and Biofilm Formation on Fully Absorbable Poly-4-hydroxybutyrate and Nonabsorbable Polypropylene Pelvic Floor Implants. ACS Appl. Mater. Interfaces 2020, 12, 53646–53653. [Google Scholar] [CrossRef]
- Olsen, M.A.; Nickel, K.B.; Wallace, A.E.; Mines, D.; Fraser, V.J.; Warren, D.K. Stratification of surgical site infection by operative factors and comparison of infection rates after hernia repair. Infect. Control Hosp. Epidemiol. 2014, 36, 329–335. [Google Scholar] [CrossRef]
- Diamond, M.P.; Burns, E.L.; Accomando, B.; Mian, S.; Holmdahl, L. Seprafilm® adhesion barrier: (1) a review of preclinical, animal, and human investigational studies. Gynecol. Surg. 2012, 9, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Baek, S.; Kang, H.; Lee, D. Biomaterials to Prevent Post-Operative Adhesion. Materials 2020, 13, 3056. [Google Scholar] [CrossRef]
- Roth, J.S.; Anthone, G.J.; Selzer, D.J.; Poulose, B.K.; Pierce, R.A.; Bittner, J.G.; Hope, W.W.; Dunn, R.M.; Martindale, R.G.; Goldblatt, M.I.; et al. Prospective, multicenter study of P4HB (Phasix™) mesh for hernia repair in cohort at risk for complications: 3-Year follow-up. Ann. Med. Surg. 2021, 61, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Overbeck, N.; Nagvajara, G.M.; Ferzoco, S.; May, B.C.H.; Beierschmitt, A.; Qi, S. In-vivo evaluation of a reinforced ovine biologic: A comparative study to available hernia mesh repair materials. Hernia 2020, 24, 1293–1306. [Google Scholar] [CrossRef] [PubMed]
- Garvey, P.B.; Giordano, S.A.; Baumann, D.P.; Liu, J.; Butler, C.E. Long-Term Outcomes after Abdominal Wall Reconstruction with Acellular Dermal Matrix. J. Am. Coll. Surg. 2017, 224, 341–350. [Google Scholar] [CrossRef]
- Hynes, R.O.; Naba, A. Overview of the Matrisome--An Inventory of Extracellular Matrix Constituents and Functions. Cold Spring Harb. Perspect. Biol. 2011, 4, a004903. [Google Scholar] [CrossRef]
- Annor, A.H.; Tang, M.E.; Pui, C.L.; Ebersole, G.C.; Frisella, M.M.; Matthews, B.D.; Deeken, C.R. Effect of enzymatic degradation on the mechanical properties of biological scaffold materials. Surg. Endosc. 2012, 26, 2767–2778. [Google Scholar] [CrossRef]
- Gómez-Gil, V.; Pascual, G.; Bellón, J.M. Biomaterial Implants in Abdominal Wall Hernia Repair: A Review on the Importance of the Peritoneal Interface. Processes 2019, 7, 105. [Google Scholar] [CrossRef]
- Butler, C.E.; Prieto, V.G. Reduction of Adhesions with Composite AlloDerm/Polypropylene Mesh Implants for Abdominal Wall Reconstruction. Plast. Reconstr. Surg. 2004, 114, 464–473. [Google Scholar] [CrossRef]
- Kumar, A.S.; Fitzgerald, J.F. Biologic versus Synthetic Mesh Reinforcement: What are the Pros and Cons? Clin. Colon Rectal Surg. 2014, 27, 140–148. [Google Scholar] [CrossRef]
- Tognetti, L.; Pianigiani, E.; Ierardi, F.; Lorenzini, G.; Casella, D.; Liso, F.G.; De Pascalis, A.; Cinotti, E.; Rubegni, P. The use of human acellular dermal matrices in advanced wound healing and surgical procedures: State of the art. Derm Ther. 2021, 34, e14987. [Google Scholar] [CrossRef]
- Liang, R.; Knight, K.M.; Barone, W.; Powers, R.W.; Nolfi, A.; Palcsey, S.; Abramowitch, S.; Moalli, P.A. Extracellular matrix regenerative graft attenuates the negative impact of polypropylene prolapse mesh on vagina in rhesus macaque. Am. J. Obs. Gynecol. 2017, 216, 153.e1–153.e9. [Google Scholar] [CrossRef]
- Brown, B.N.; Ratner, B.D.; Goodman, S.B.; Amar, S.; Badylak, S.F. Macrophage polarization: An opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials 2012, 33, 3792–3802. [Google Scholar] [CrossRef] [PubMed]
- Londono, R.; Badylak, S.F. Biologic Scaffolds for Regenerative Medicine: Mechanisms of In vivo Remodeling. Ann. Biomed. Eng. 2014, 43, 577–592. [Google Scholar] [CrossRef]
- Kim, H.; Wang, S.Y.; Kwak, G.; Yang, Y.; Kwon, I.C.; Kim, S.H. Exosome-Guided Phenotypic Switch of M1 to M2 Macrophages for Cutaneous Wound Healing. Adv. Sci. 2019, 6, 1900513. [Google Scholar] [CrossRef] [PubMed]
- Deeken, C.R.; Lake, S.P. Mechanical properties of the abdominal wall and biomaterials utilized for hernia repair. J. Mech. Behav. Biomed. Mater. 2017, 74, 411–427. [Google Scholar] [CrossRef]
- Junge, K.; Klinge, U.; Prescher, A.; Giboni, P.; Niewiera, M.; Schumpelick, V. Elasticity of the anterior abdominal wall and impact for reparation of incisional hernias using mesh implants. Hernia 2001, 5, 113–118. [Google Scholar] [CrossRef] [PubMed]
- 2019 AHS Annual Meeting. Hernia 2019, 23, 1–109. [CrossRef]
- Lun, S.; Irvine, S.M.; Johnson, K.D.; Fisher, N.J.; Floden, E.W.; Negron, L.; Dempsey, S.; McLaughlin, R.J.; Vasudevamurthy, M.; Ward, B.R.; et al. A functional extracellular matrix biomaterial derived from ovine forestomach. Biomaterials 2010, 31, 4517–4529. [Google Scholar] [CrossRef]
- Dempsey, S.; Miller, C.H.; Hill, R.C.; Hansen, K.C.; May, B.C.H. Functional Insights from the Proteomic Inventory of Ovine Forestomach Matrix. J. Proteome Res. 2019, 18, 1657–1668. [Google Scholar] [CrossRef]
- Sizeland, K.H.; Wells, H.C.; Kelly, S.J.; Nesdale, K.E.; May, B.C.H.; Dempsey, S.; Miller, C.H.; Kirby, N.; Hawley, A.; Mudie, S.; et al. Collagen Fibril Response to Strain in Scaffolds from Ovine Forestomach for Tissue Engineering. Acs. Biomater. Sci. Eng. 2017, 3, 2550–2558. [Google Scholar] [CrossRef]
- Dempsey, S.G.; Miller, C.H.; Schueler, J.; Veale, R.W.F.; Day, D.J.; May, B.C.H. A novel chemotactic factor derived from the extracellular matrix protein decorin recruits mesenchymal stromal cells in vitro and in vivo. PLoS ONE 2020, 15, e0235784. [Google Scholar] [CrossRef]
- Smith, J.; Parmely, J.D. Ventral Hernia; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Faylona, J.M. Evolution of ventral hernia repair. Asian J. Endosc. Surg. 2017, 10, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Bringman, S.; Conze, J.; Cuccurullo, D.; Deprest, J.; Junge, K.; Klosterhalfen, B.; Parra-Davila, E.; Ramshaw, B.; Schumpelick, V. Hernia repair: The search for ideal meshes. Hernia 2009, 14, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Parker, M.J.; Barrio, M.; House, M.G.; Socas, J.; Reed, R.L.; Nakeeb, A.; Ceppa, E.P. Reinforced BioScaffold Mesh Lowers Recurrent Hernia Rate in High-Risk Ventral Hernia Repair with Surgical Site Occurrences. In Proceedings of the Americas Hernia Society (AHS) Annual Meeting, Las Vegas, NV, USA, 11–14 March 2019. [Google Scholar]
- Kanters, A.E.; Krpata, D.M.; Blatnik, J.A.; Novitsky, Y.M.; Rosen, M.J. Modified Hernia Grading Scale to Stratify Surgical Site Occurrence after Open Ventral Hernia Repairs. J. Am. Coll. Surg. 2012, 215, 787–793. [Google Scholar] [CrossRef]
- Onyekwelu, I.; Yakkanti, R.; Protzer, L.; Pinkston, C.M.; Tucker, C.; Seligson, D. Surgical Wound Classification and Surgical Site Infections in the Orthopaedic Patient. Glob. Res. Rev. 2017, 1, e022. [Google Scholar] [CrossRef] [PubMed]
- Sfara, A.; Dumitrașcu, D.L. The management of hiatal hernia: An update on diagnosis and treatment. Med. Pharm. Rep. 2019, 92, 321–325. [Google Scholar] [CrossRef]
- Schneider, R.; Herrington, J.L.; Granda, A.M. Marlex mesh in repair of a diaphragmatic defect later eroding into the distal esophagus and stomach. Am. Surg. 1979, 45, 337–339. [Google Scholar]
- Hazebroek, E.J.; Leibman, S.; Smith, G.S. Erosion of a Composite PTFE/ePTFE Mesh After Hiatal Hernia Repair. Surg. Laparosc. Endosc. Percutaneous Tech. 2009, 19, 175–177. [Google Scholar] [CrossRef]
- Stadlhuber, R.J.; El Sherif, A.; Mittal, S.K.; Jr, R.J.F.; Brunt, L.M.; Hunter, J.G.; Demeester, T.R.; Swanstrom, L.L.; Smith, C.D.; Filipi, C.J. Mesh complications after prosthetic reinforcement of hiatal closure: A 28-case series. Surg. Endosc. 2008, 23, 1219–1226. [Google Scholar] [CrossRef]
- Sawyer, M.A.J. New Ovine Polymer-Reinforced Bioscaffold in Hiatal Hernia Repair. JSLS J. Soc. Laparoendosc. Surg. 2018, 22. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, B.; Maloley, B.J.; Fitzgibbons, R.J. Inguinal Hernia. Adv. Surg. 2014, 48, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ferzli, G.S.; Edwards, E.D.; Khoury, G.E. Chronic Pain after Inguinal Herniorrhaphy. J. Am. Coll. Surg. 2007, 205, 333–341. [Google Scholar] [CrossRef]
- Ferzoco, S.J. Early experience outcome of a reinforced Bioscaffold in inguinal hernia repair: A case series. Int. J. Surg. Open 2018, 12, 9–11. [Google Scholar] [CrossRef]
- Kokotovic, D.; Bisgaard, T.; Helgstrand, F. Long-term Recurrence and Complications Associated with Elective Incisional Hernia Repair. JAMA 2016, 316, 1575–1582. [Google Scholar] [CrossRef]
- Hawn, M.T.; Gray, S.; Snyder, C.W.; Graham, L.A.; Finan, K.R.; Vick, C.C. Predictors of mesh explantation after incisional hernia repair. Am. J. Surg. 2011, 202, 28–33. [Google Scholar] [CrossRef]
- Klosterhalfen, B.; Lynen, P.; Schumpelick, V.; Junge, K.; Klinge, U.; Rosch, R.; Mertens, P.R.; Kirch, J. Decreased collagen typeI/III ratio in patients with recurring hernia after implantation of alloplastic prostheses. Langenbeck’s Arch. Surg. 2003, 389, 17–22. [Google Scholar] [CrossRef]
- Klinge, U.; Si, Z.; Zheng, H.; Schumpelick, V.; Bhardwaj, R.; Klosterhalfen, B. Abnormal collagen I to III distribution in the skin of patients with incisional hernia. Eur. Surg. Res. 2000, 32, 43–48. [Google Scholar] [CrossRef]
- Itani, K.M.; Rosen, M.; Vargo, D.; Awad, S.S.; DeNoto, G.; Butler, C.E. Prospective study of single-stage repair of contaminated hernias using a biologic porcine tissue matrix: The RICH Study. Surgery 2012, 152, 498–505. [Google Scholar] [CrossRef]
- Rosen, M.J.; Bauer, J.J.; Harmaty, M.; Carbonell, A.M.; Cobb, W.S.; Matthews, B.; Goldblatt, M.I.; Selzer, D.J.; Poulose, B.K.; Hansson, B.M.E.; et al. Multicenter, Prospective, Longitudinal Study of the Recurrence, Surgical Site Infection, and Quality of Life After Contaminated Ventral Hernia Repair Using Biosynthetic Absorbable Mesh. Ann. Surg. 2017, 265, 205–211. [Google Scholar] [CrossRef]

| Study | Parker et al., 2020 | Sawyer 2018 | Ferzoco 2018 |
|---|---|---|---|
| Number of Patients | 50 | 25 | 31 |
| Hernia type | Ventral | Hiatal | Inguinal |
| Hernia severity | 68% modified VHWG grade 3, 70% CDC wound class > I | 56% CDC wound class > I | N/A |
| Months to follow-up | 12 | 14.2 | 12.6 |
| Surgical site occurrence (SSO) | 36% | N/A | 0% at 30 days |
| Recurrence rate | 6% | 0% | 0% |
| Postoperative pain | N/A | 6/7 symptoms resolved between 85.7 and 100% | 0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawyer, M.; Ferzoco, S.; DeNoto, G., III. A Polymer-Biologic Hybrid Hernia Construct: Review of Data and Early Experiences. Polymers 2021, 13, 1928. https://doi.org/10.3390/polym13121928
Sawyer M, Ferzoco S, DeNoto G III. A Polymer-Biologic Hybrid Hernia Construct: Review of Data and Early Experiences. Polymers. 2021; 13(12):1928. https://doi.org/10.3390/polym13121928
Chicago/Turabian StyleSawyer, Michael, Stephen Ferzoco, and George DeNoto, III. 2021. "A Polymer-Biologic Hybrid Hernia Construct: Review of Data and Early Experiences" Polymers 13, no. 12: 1928. https://doi.org/10.3390/polym13121928
APA StyleSawyer, M., Ferzoco, S., & DeNoto, G., III. (2021). A Polymer-Biologic Hybrid Hernia Construct: Review of Data and Early Experiences. Polymers, 13(12), 1928. https://doi.org/10.3390/polym13121928






