Polymer Film Blend of Polyvinyl Alcohol, Trichloroethylene and Cresol Red for Gamma Radiation Dosimetry
Abstract
1. Introduction
2. Materials and Methods
2.1. Polymer Film Preparation
2.2. Polymer Film Irradiation
2.3. Optical Properties Analysis
3. Results and Discussion
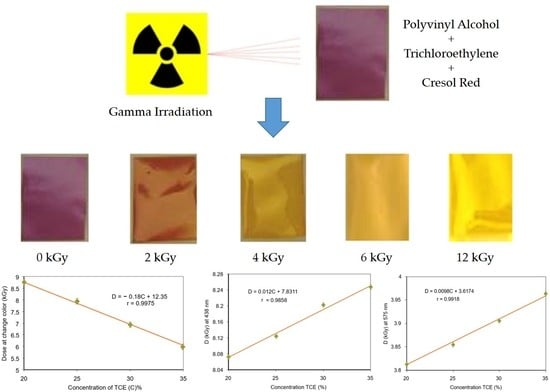
3.1. Discoloration of the Polymer Film before and after Radiation
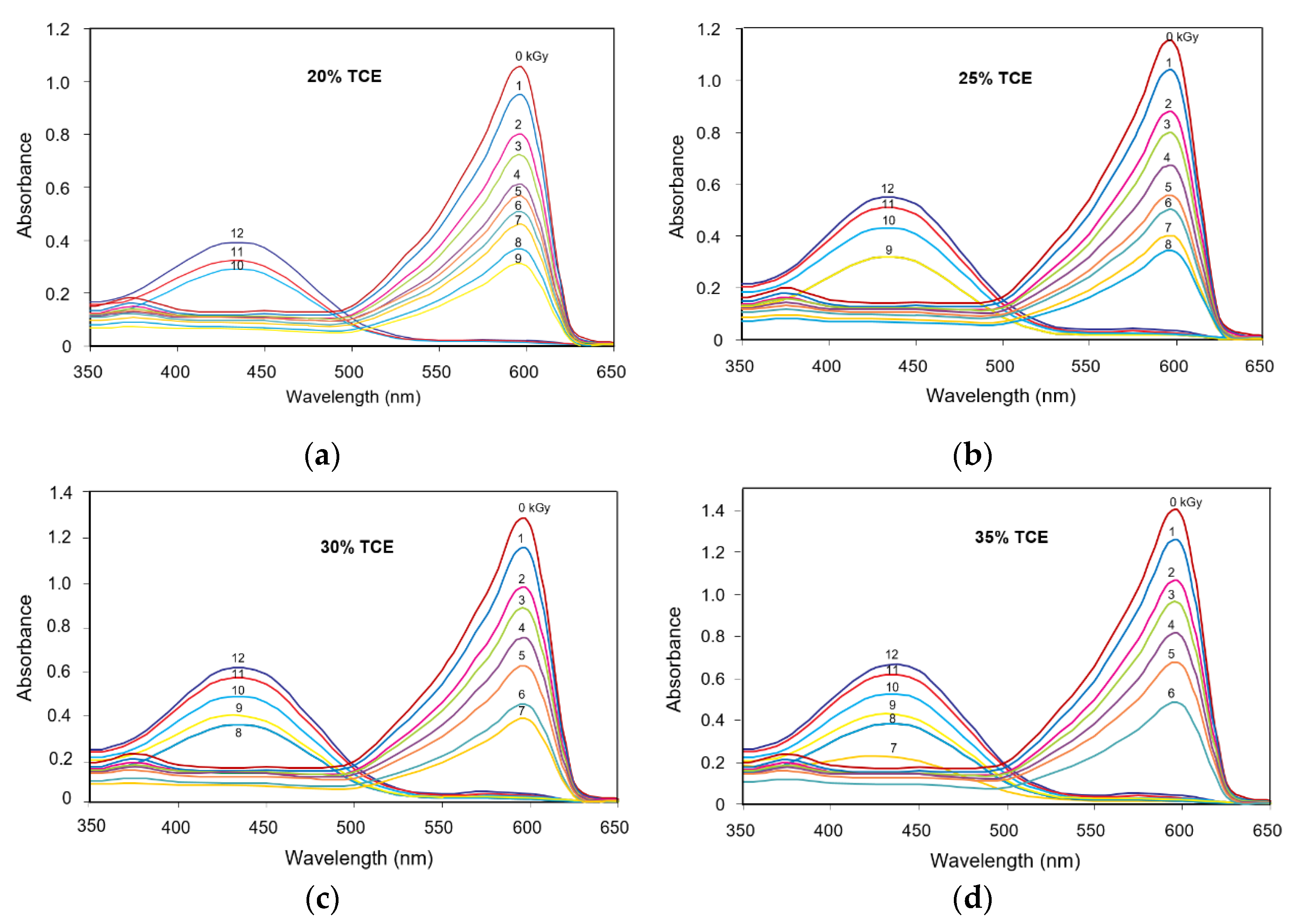
3.2. Absorption Spectra
3.3. Formation of Acid in PVA-TCE Composites
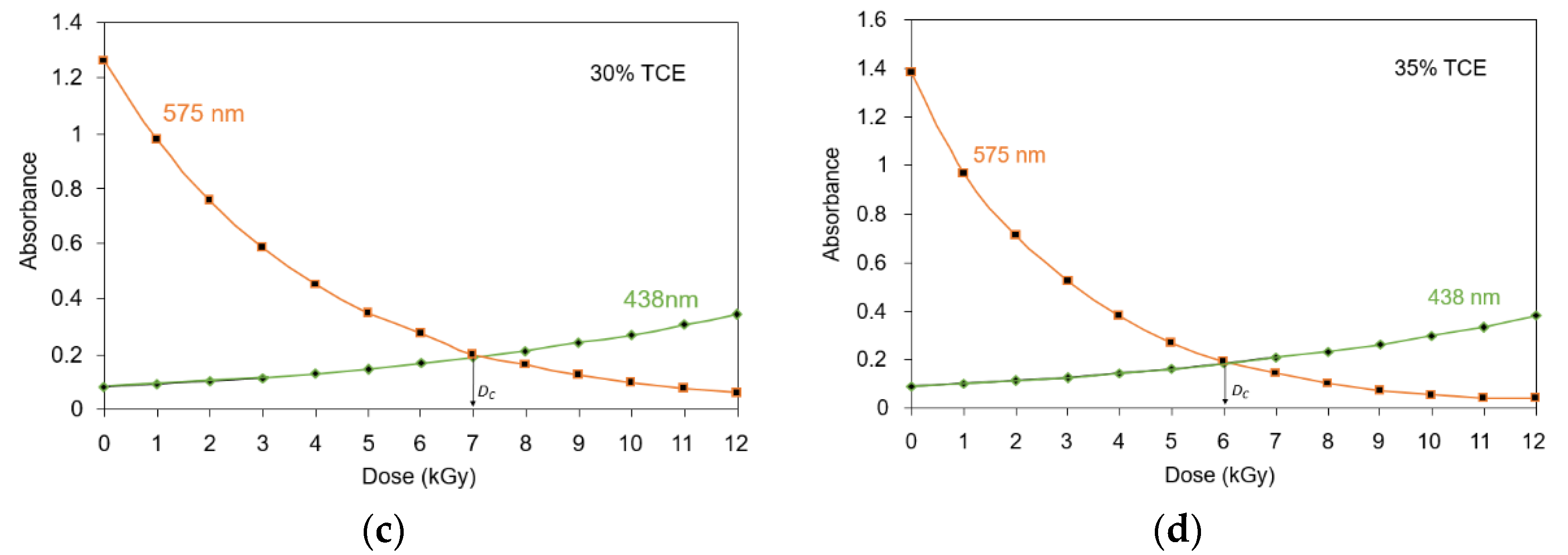
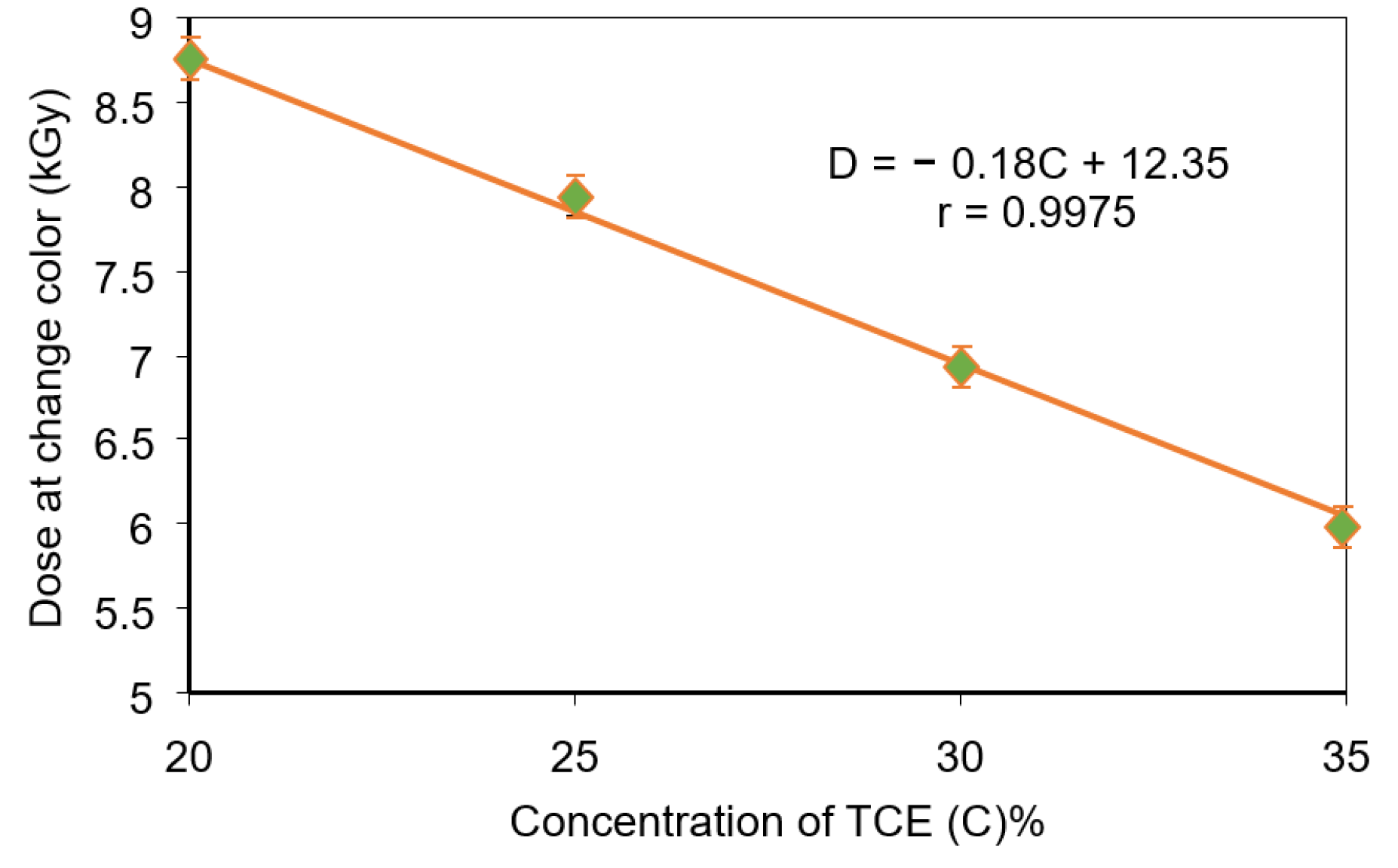
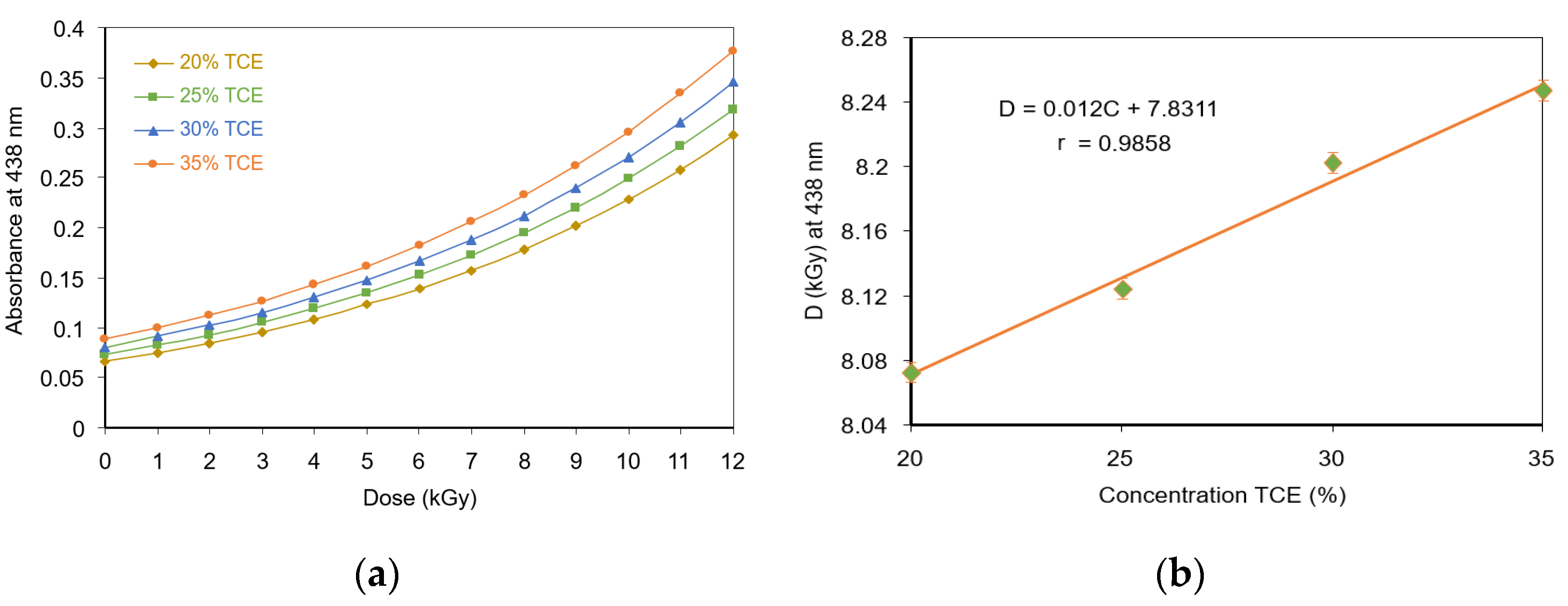
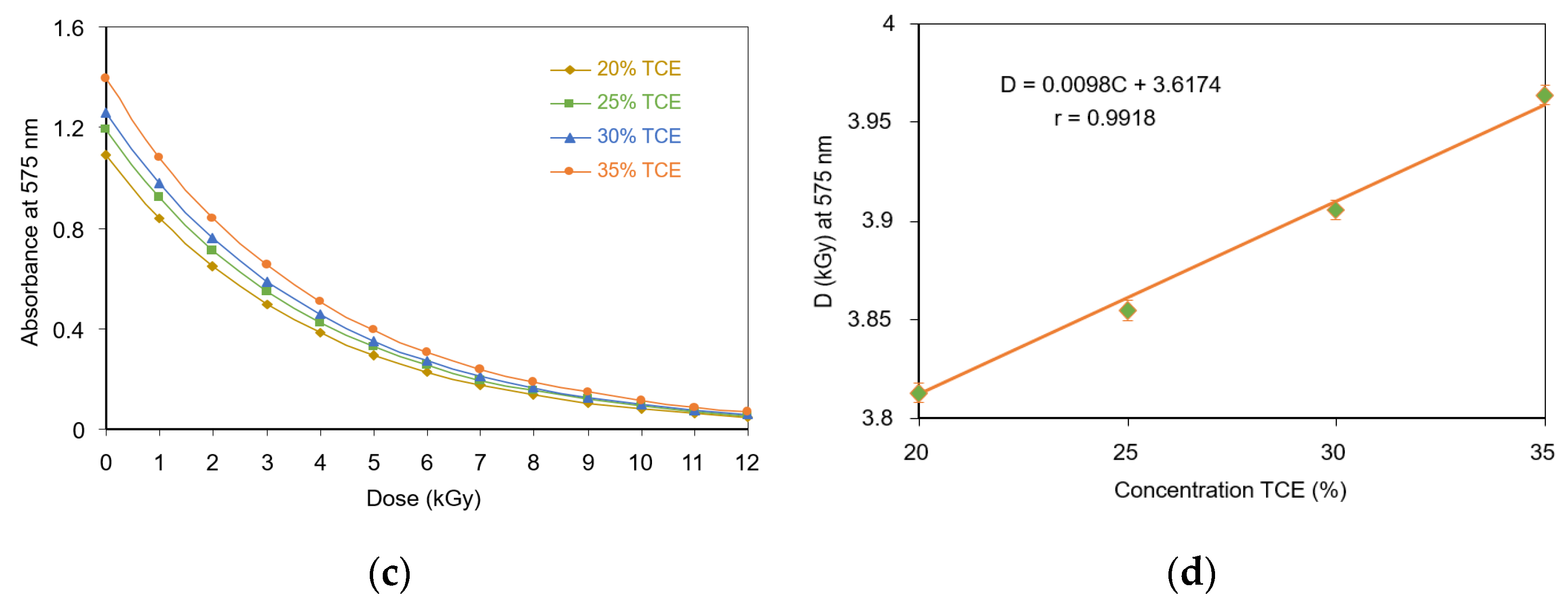
3.4. Critical Dose at Color Change
3.5. Optical Absorption Dose Response
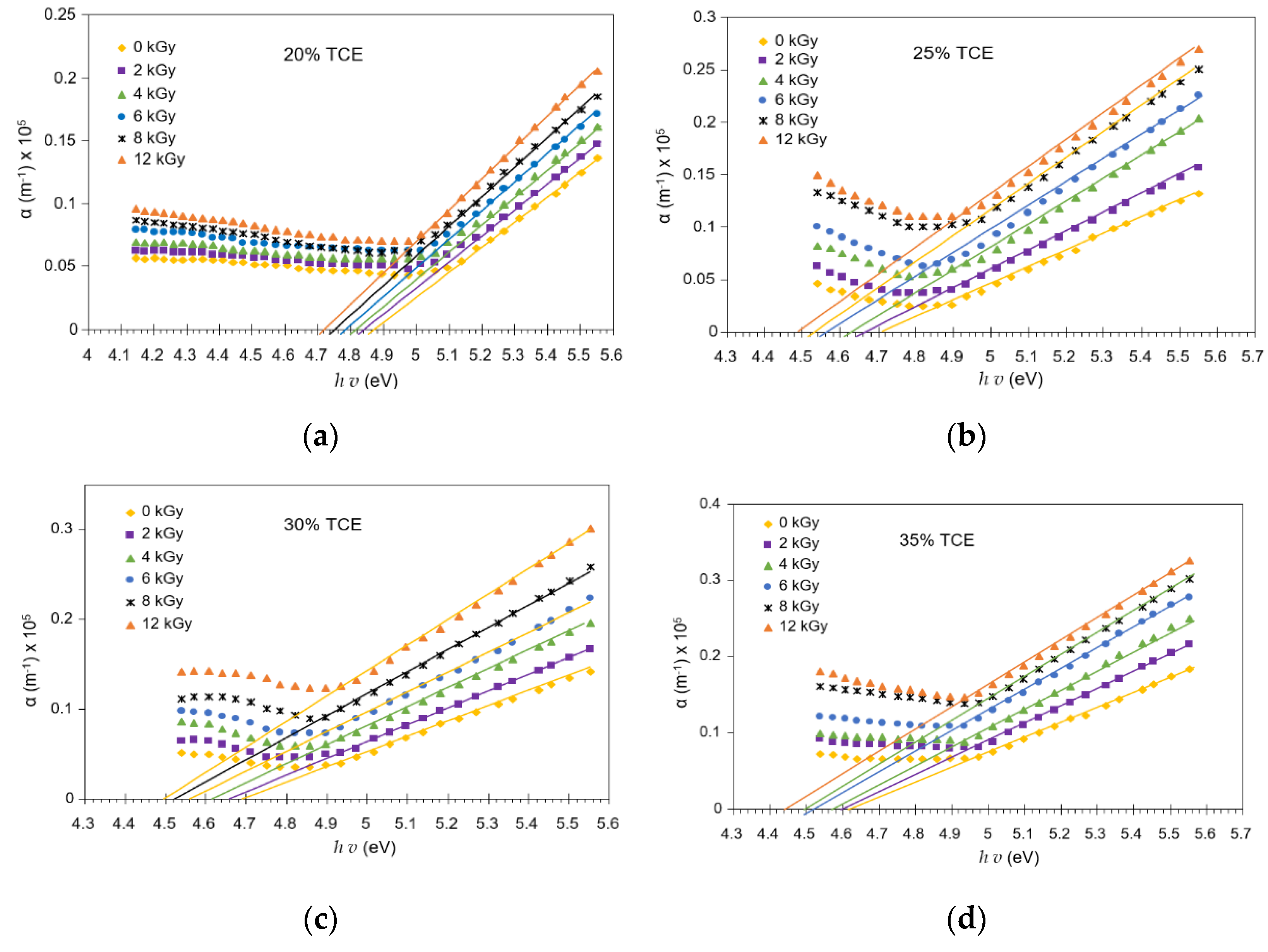
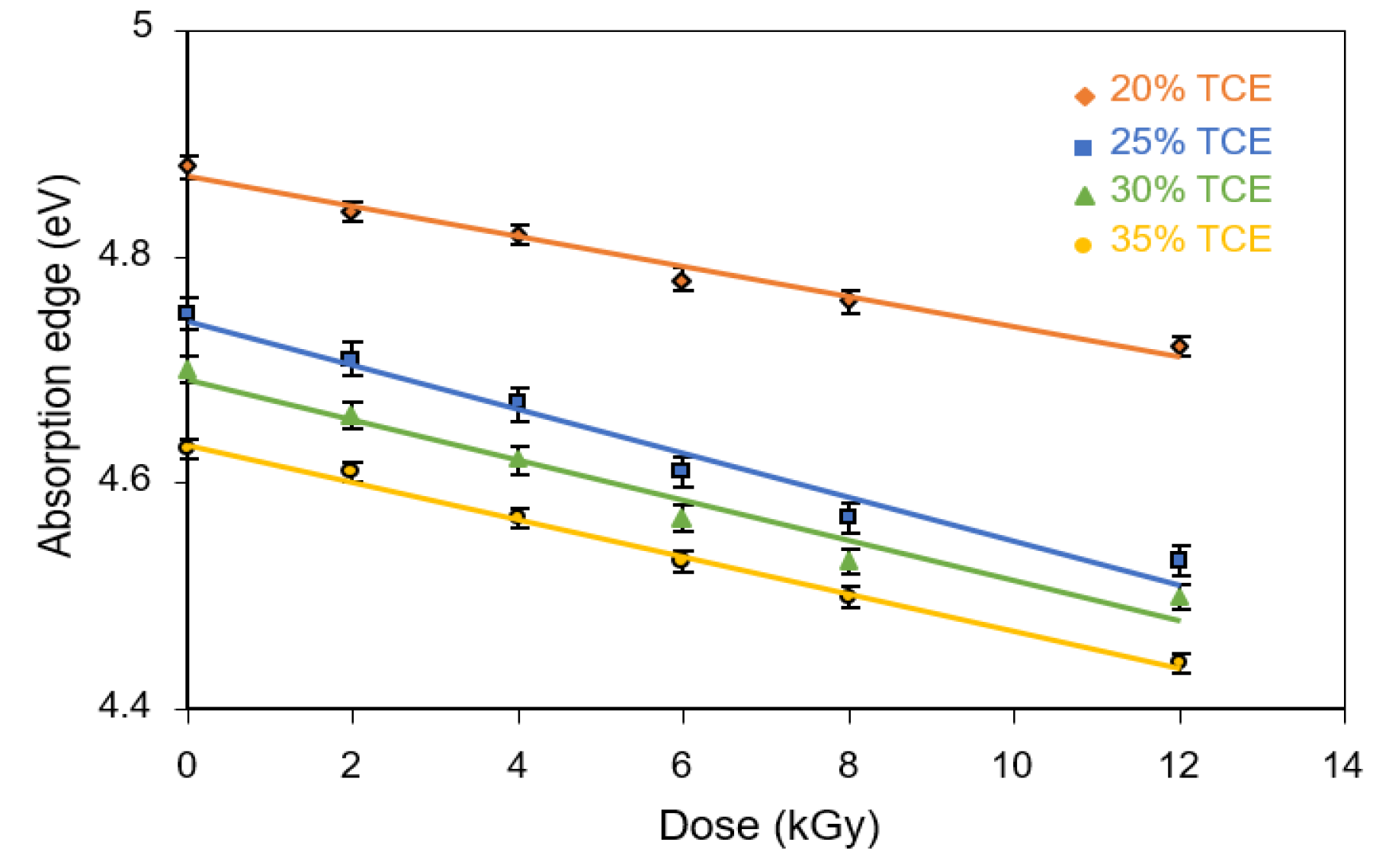
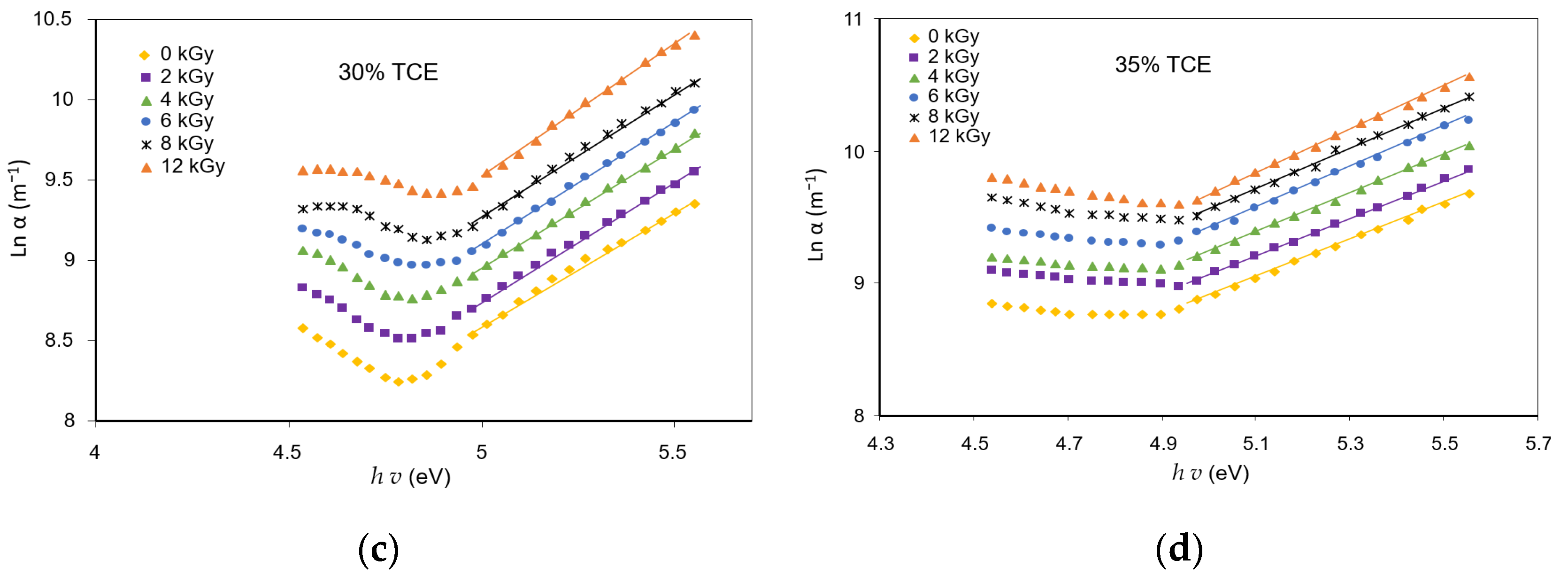
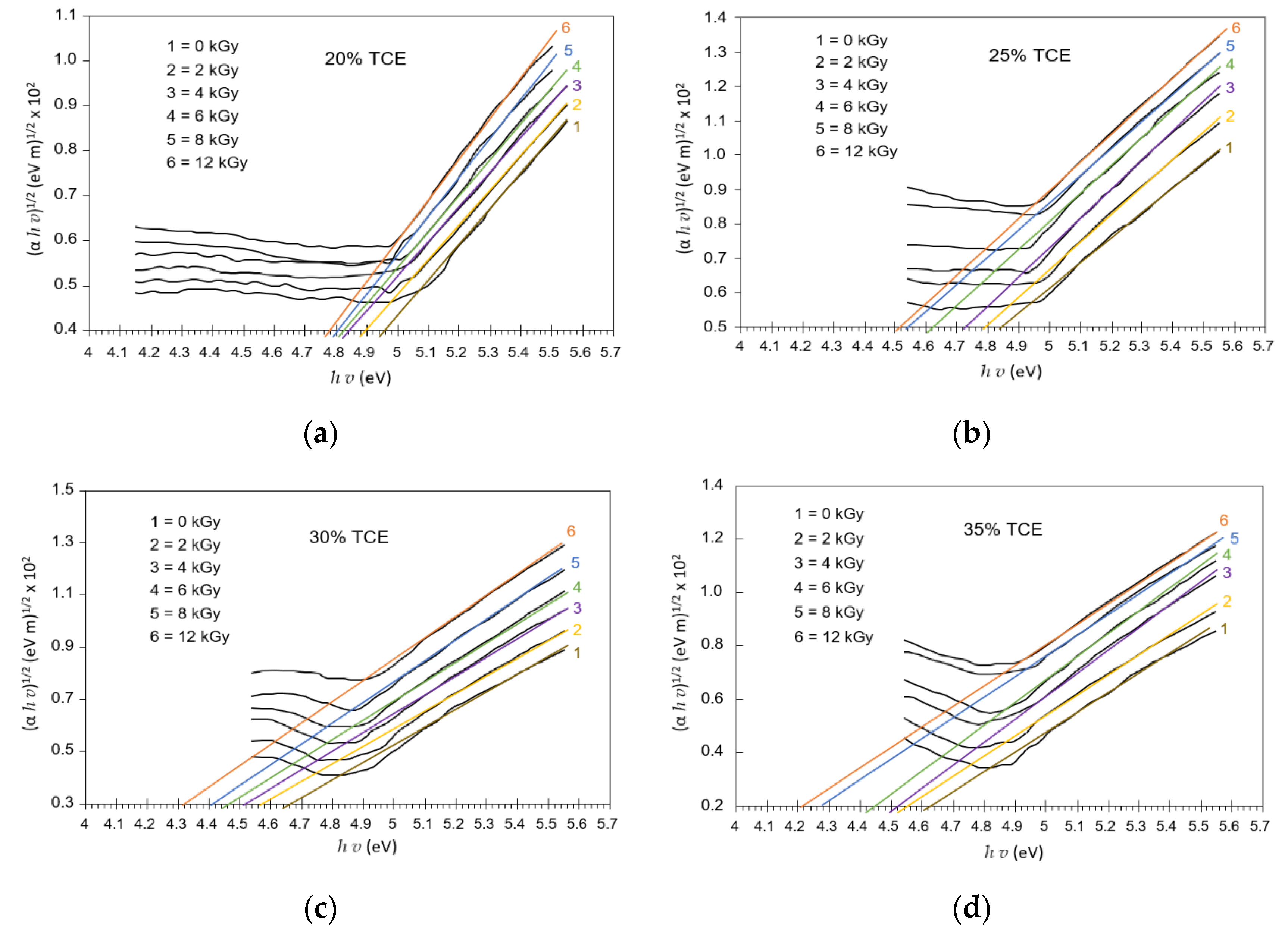
3.6. Absorption Edge
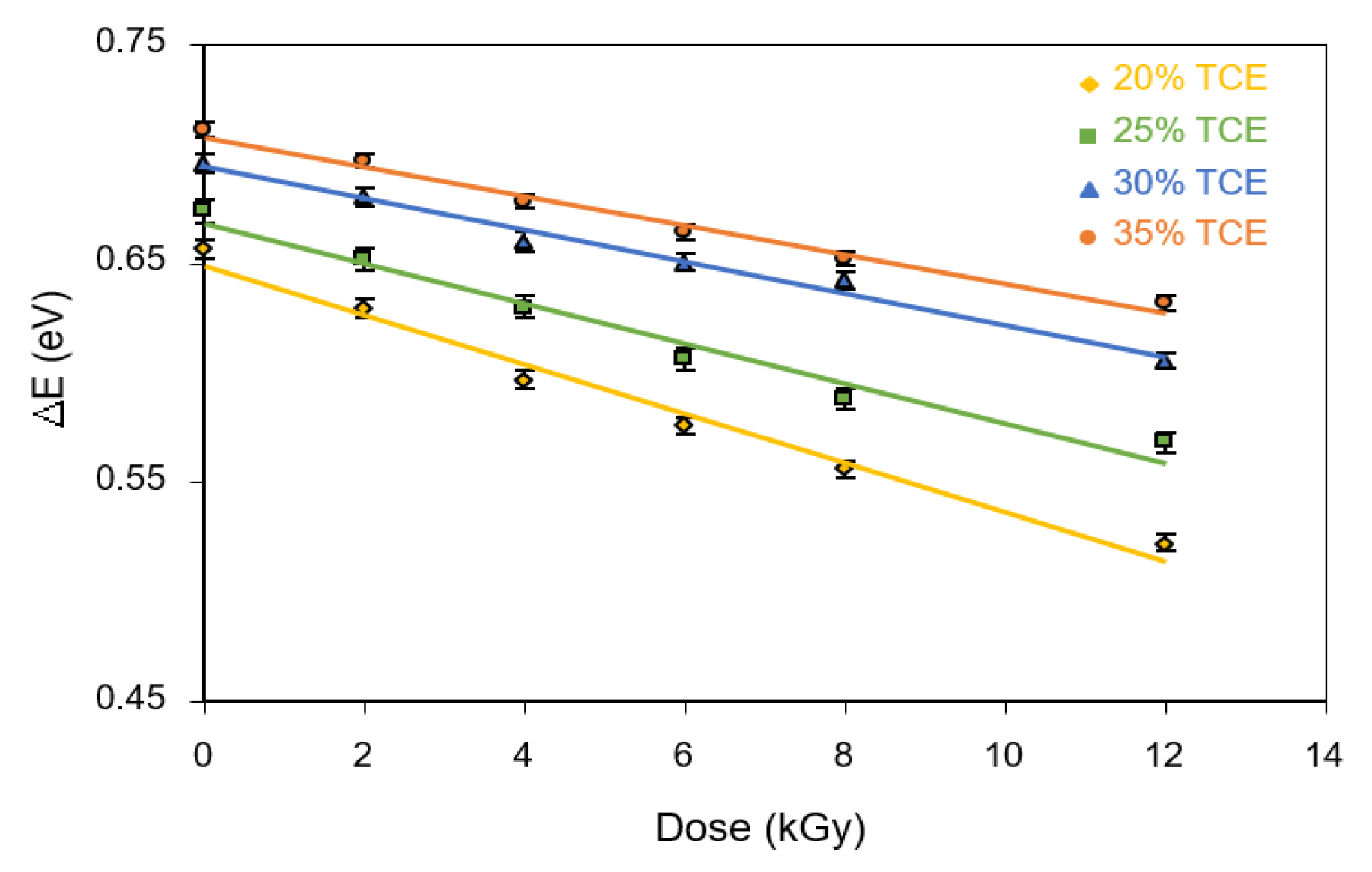
3.7. Activation Energy
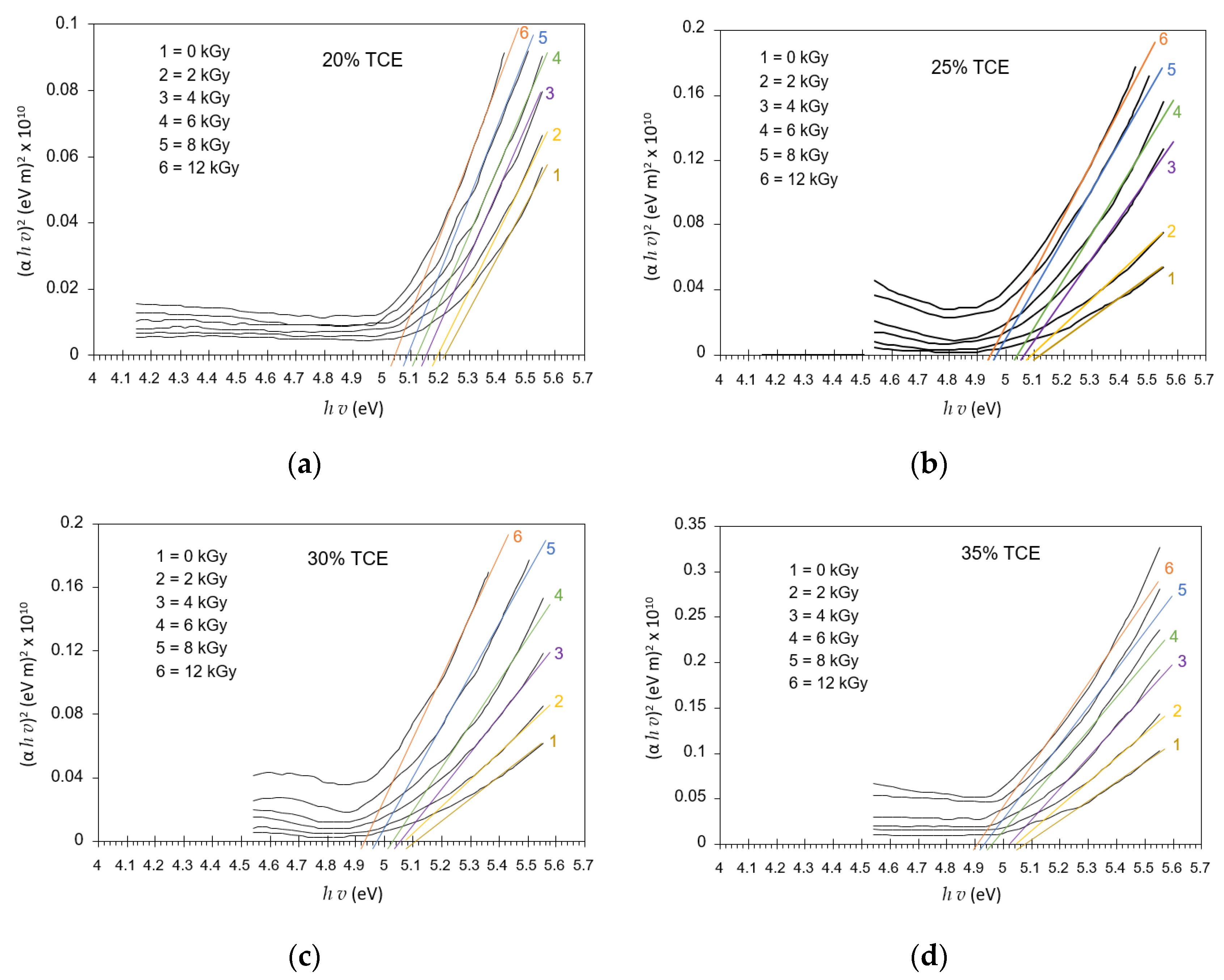
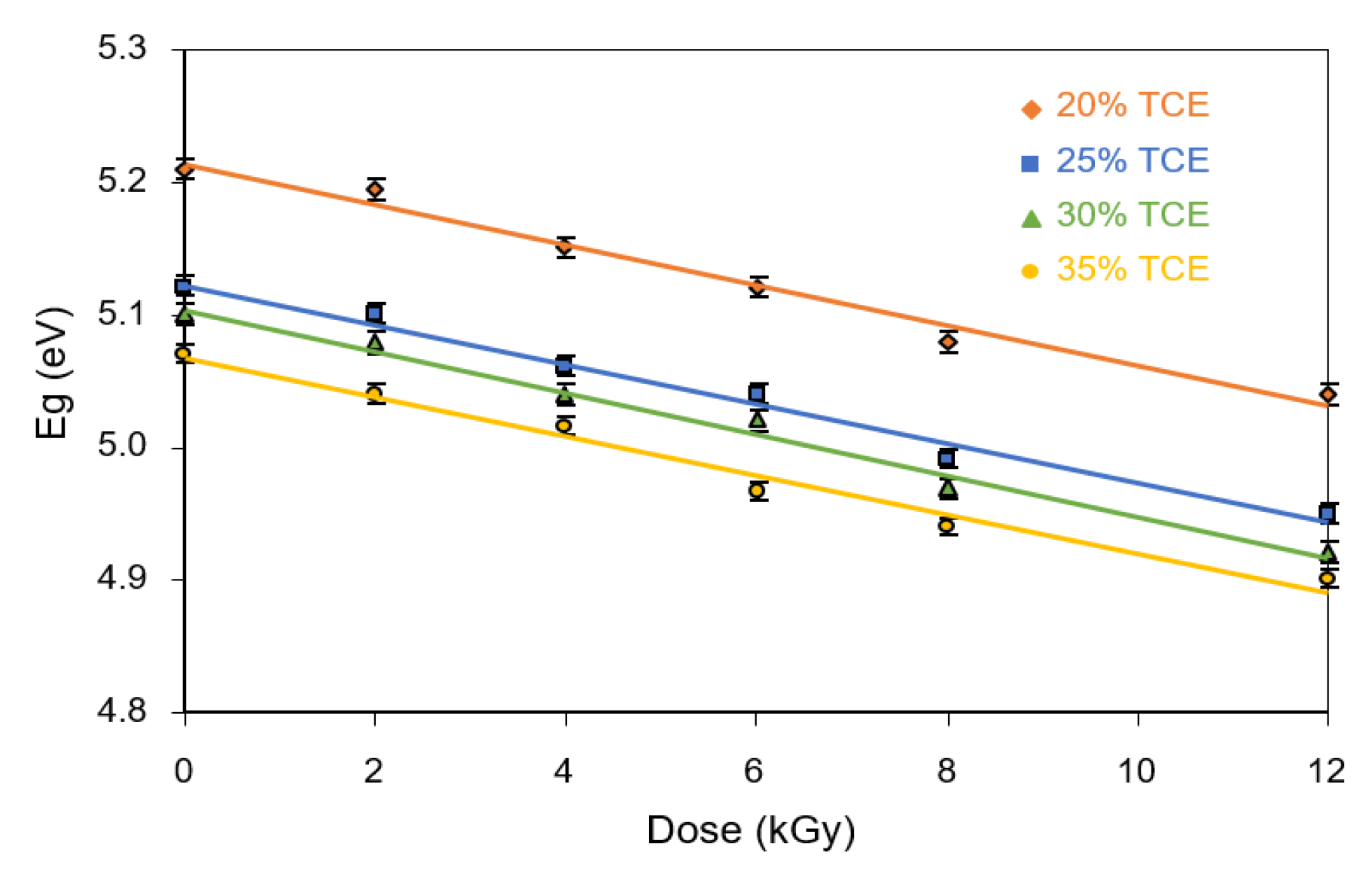
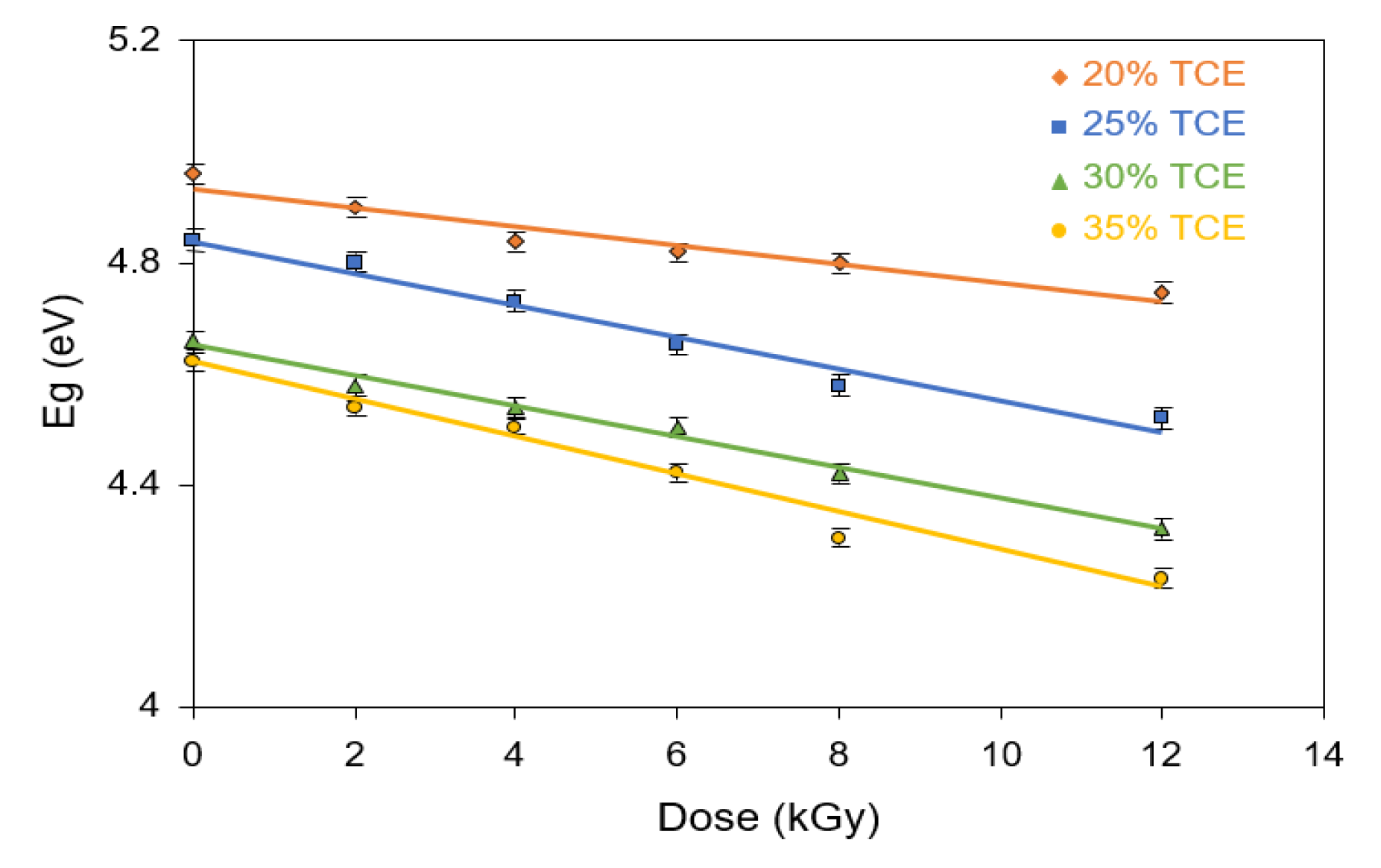
3.8. Band Gap Energy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gafar, S.M.; El-Kelany, M.; El-Ahdal, M. Low-Dose Film Dosimeter Based on Mixture of AY and TBPE Dyed Poly(Vinyl Alcohol). Dyes Pigment. 2017, 140, 1–5. [Google Scholar] [CrossRef]
- Kattan, M.; al Kassiri, H.; Daher, Y. Using Polyvinyl Chloride Dyed with Bromocresol Purple in Radiation Dosimetry. Appl. Radiat. Isot. 2011, 69, 377–380. [Google Scholar] [CrossRef]
- Akhtar, S.; Shahzad, A.; Bashir, S.; Hussain, M.Y.; Akhtar, N. Improved Performance of Radiochromic Films for High-Dose Dosimetry. Radioprotection 2016, 51, 129–133. [Google Scholar] [CrossRef][Green Version]
- Akhtar, S.; Hussain, T.; Shahzad, A.; Qamar-ul-Islam. The Feasibility of Reactive Dye in PVA Films as High Dosimeter. J. Basic Appl. Sci. 2013, 9, 420–423. [Google Scholar] [CrossRef]
- El-Kelany, M.; Gafar, S.M. Preparation of Radiation Monitoring Labels to γ Ray. Optik 2016, 127, 6746–6753. [Google Scholar] [CrossRef]
- Gafar, S.M.; El-Ahdal, M.A. Dosimetric Characteristics of 2,6 Di-Nitro Phenol for High Dose Dosimetry. Dyes Pigment. 2014, 109, 67–71. [Google Scholar] [CrossRef]
- Hassani, H.; Nedaie, H.A.; Zahmatkesh, M.H.; Shirani, K. A Dosimetric Study of Small Photon Fields Using Polymer Gel and Gafchromic EBT Films. Med. Dosim. 2014, 39, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Ali-Omer, M.A.; Ali-Bashir, E.A. Synthesis of Polyvinyl Alcohol and Cuprous Oxide (PVA/Cu2O) Films for Radiation Detection and Personal Dosimeter Based on Optical Properties. J. Radiat. Res. Appl. Sci. 2018, 11, 237–241. [Google Scholar] [CrossRef]
- Aydarous, A.; Badawi, A.; Abdallah, S. The Effects of Electrons and Photons Irradiation on the Optical and Thermophysical Properties of Gafchromic HD-V2 Films. Results Phys. 2016, 6, 952–956. [Google Scholar] [CrossRef]
- Basfar, A.A.; Rabaeh, K.A.; Mousa, A.A. Improved Performance of Nitro-Blue Tetrazolium Polyvinyl Butyral High Dose Film Dosimeters. Radiat. Meas. 2012, 47, 1005–1008. [Google Scholar] [CrossRef]
- Rabaeh, K.A.; Aljammal, S.A.; Eyadeh, M.M.; Abumurad, K.M. Methyl Thymol Blue Solution and Film Dosimeter for High Dose Measurements. Results Phys. 2021, 23, 103980. [Google Scholar] [CrossRef]
- Hosni, F.; Farah, K.; Kaouach, H.; Louati, A.; Chtourou, R.; Hamzaoui, A.H. Effect of Gamma-Irradiation on the Colorimetric Properties of Epoxy-Resin Films: Potential Use in Dosimetric Application. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2013, 311, 1–4. [Google Scholar] [CrossRef]
- Raouafi, A.; Daoudi, M.; Jouini, K.; Charradi, K.; Hamzaoui, A.H.; Blaise, P.; Farah, K.; Hosni, F. Effect of Gamma Irradiation on the Color, Structure and Morphology of Nickel-Doped Polyvinyl Alcohol Films: Alternative Use as Dosimeter or Irradiation Indicator. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2018, 425, 4–10. [Google Scholar] [CrossRef]
- Abdel-Fattah, A.A.; Soliman, Y.S.; Bayomi, A.M.M.; Abdel-Khalek, A.A. Dosimetric Characteristics of a Radiochromic Polyvinyl Butyral Film Containing 2,4-Hexadiyn-1,6-Bis(n-Butyl Urethane). Appl. Radiat. Isot. 2014, 86, 21–27. [Google Scholar] [CrossRef]
- Soliman, Y.S.; Abdel-Fattah, A.A.; Alkhuraiji, T.S. Radiochromic Film Containing Poly(Hexa-2,4-Diynylene Adipate) as a Radiation Dosimeter. Appl. Radiat. Isot. 2018, 141, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Ticoş, D.; Scurtu, A.; Oane, M.; Diplaşu, C.; Giubega, G.; Călina, I.; Ticoş, C.M. Complementary Dosimetry for a 6 MeV Electron Beam. Results Phys. 2019, 14, 102377. [Google Scholar] [CrossRef]
- Galante, A.M.S.; Campos, L.L. Mapping Radiation Fields in Containers for Industrial γ-Irradiation Using Polycarbonate Dosimeters. Appl. Radiat. Isot. 2012, 70, 1264–1266. [Google Scholar] [CrossRef]
- Kattan, M.; Daher, Y. The Use of Polyvinyl Chloride Films Dyed with Methyl Red in Radiation Dosimetry. IJRR 2016, 14, 263–267. [Google Scholar] [CrossRef]
- Ebraheem, S.; El-Kelany, M. Dosimeter Film Based on Ethyl Violet-Bromophenol Blue Dyed Poly(Vinyl Alcohol). OJPChem 2013, 3, 1–5. [Google Scholar] [CrossRef][Green Version]
- Ebraheem, S.; Eid, S.; Kovacs, A. A New Dyed Poly (Vinyl Alcohol) Film for High-Dose Applications. Radiat. Phys. Chem. 2002, 63, 807–811. [Google Scholar] [CrossRef]
- Emi-Reynolds, G.; Kovacs, A.; Fletcher, J.J. Dosimetry Characterization of Tetrazolium Violet-Polyvinylalcohol Films. Radiat. Phys. Chem. 2007, 76, 1519–1522. [Google Scholar] [CrossRef]
- Lavalle, M.; Corda, U.; Fuochi, P.G.; Caminati, S.; Venturi, M.; Kovács, A.; Baranyai, M.; Sáfrány, A.; Miller, A. Radiochromic Film Containing Methyl Viologen for Radiation Dosimetry. Radiat. Phys. Chem. 2007, 76, 1502–1506. [Google Scholar] [CrossRef]
- Ang, S.L.; Sivashankari, R.; Shaharuddin, B.; Chuah, J.-A.; Tsuge, T.; Abe, H.; Sudesh, K. Potential Applications of Polyhydroxyalkanoates as a Biomaterial for the Aging Population. Polym. Degrad. Stab. 2020, 181, 109371. [Google Scholar] [CrossRef]
- Wong, C.Y.; Wong, W.Y.; Loh, K.S.; Daud, W.R.W.; Lim, K.L.; Khalid, M.; Walvekar, R. Development of Poly(Vinyl Alcohol)-Based Polymers as Proton Exchange Membranes and Challenges in Fuel Cell Application: A Review. Polym. Rev. 2020, 60, 171–202. [Google Scholar] [CrossRef]
- Chaturvedi, A.; Bajpai, A.K.; Bajpai, J.; Sharma, A. Antimicrobial Poly(Vinyl Alcohol) Cryogel–Copper Nanocomposites for Possible Applications in Biomedical Fields. Des. Monomers Polym. 2015, 18, 385–400. [Google Scholar] [CrossRef][Green Version]
- Abdel-Fattah, A.A.; El-Kelany, M.; Abdel-Rehim, F. Development of a Radiation-Sensitive Indicator. Radiat. Phys. Chem. 1996, 48, 497–503. [Google Scholar] [CrossRef]
- Abdel-Fattah, A.A.; El-Kelany, M.; Abdel-Rehim, F.; El Miligy, A.A. UV-Sensitive Indicators Based on Bromophenol Blue and Chloral Hydrate Dyed Poly(Vinyl Butyral). J. Photochem. Photobiol. A Chem. 1997, 110, 291–297. [Google Scholar] [CrossRef]
- Nayef, U.M.; Khudhair, I.M. Study of Porous Silicon Humidity Sensor Vapors by Photoluminescence Quenching for Organic Solvents. Optik 2017, 135, 169–173. [Google Scholar] [CrossRef]
- Susilawati, U.; Doyan, A. Dose Response and Optical Properties of Dyed Poly Vinyl Alcohol-Trichloroacetic Acid Polymeric Blends Irradiated with Gamma-Rays. Am. J. Appl. Sci. 2009, 6, 2071–2077. [Google Scholar] [CrossRef]
- Dhara, B.; Sappati, S.; Singh, S.K.; Kurungot, S.; Ghosh, P.; Ballav, N. Coordination Polymers of Fe(III) and Al(III) Ions with TCA Ligand: Distinctive Fluorescence, CO2 Uptake, Redox-Activity and Oxygen Evolution Reaction. Dalton Trans. 2016, 45, 6901–6908. [Google Scholar] [CrossRef]
- Susilawati, S.; Prayogi, S.; Arif, M.F.; Ismail, N.M.; Bilad, M.R.; Asy’ari, M. Optical Properties and Conductivity of PVA–H3PO4 (Polyvinyl Alcohol–Phosphoric Acid) Film Blend Irradiated by γ-Rays. Polymers 2021, 13, 1065. [Google Scholar] [CrossRef]
- Skuja, L.; Kajihara, K.; Ikuta, Y.; Hirano, M.; Hosono, H. Urbach Absorption Edge of Silica: Reduction of Glassy Disorder by Fluorine Doping. J. Non-Cryst. Solids 2004, 345–346, 328–331. [Google Scholar] [CrossRef]
- Mott, N.F.; Davis, E.A. Electronic Processes in Non-Crystalline Materials, 2nd ed.; International Series of Monographs on Physics; Clarendon Press: Oxford, UK, 2012; ISBN 978-0-19-964533-6. [Google Scholar]
- Aldweri, F.M.; Rabaeh, K.A.; Al-Ahmad, K.N. Novel Radiochromic Dosimeters Based on Calcein Dye for High Dose Applications. Radiat. Phys. Chem. 2017, 139, 1–4. [Google Scholar] [CrossRef]
- Rabaeh, K.A.; Basfar, A.A. A Polystyrene Film Dosimeter Containing Dithizone Dye for High Dose Applications of Gamma-Ray Source. Radiat. Phys. Chem. 2020, 170, 108646. [Google Scholar] [CrossRef]
- Aldweri, F.M.; Abuzayed, M.H.; Al-Ajaleen, M.S.; Rabaeh, K.A. Characterization of Thymol Blue Radiochromic Dosimeters for High Dose Applications. Results Phys. 2018, 8, 1001–1005. [Google Scholar] [CrossRef]
- Saion, E.; Susilawati; Doyan, A.; Zainal Abidin, S.; Azmi, Z.; Zulkfli, A.; Zaki, A.R.M.; Dahlan, K.Z.H.; Karni, T. Changes in the Optical Band Gap and Absorption Edge of Gamma-Irradiated Polymer Blends. J. Appl. Sci. 2005, 5, 1825–1829. [Google Scholar] [CrossRef]
- Abdel-Fattah, A.A.; Abdel-Hamid, H.M.; Radwan, R.M. Changes in the Optical Energy Gap and ESR Spectra of Proton-Irradiated Unplasticized PVC Copolymer and Its Possible Use in Radiation Dosimetry. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2002, 196, 279–285. [Google Scholar] [CrossRef]
- Ali, H.E.; Abdel-Aziz, M.M.; Algarni, H.; Yahia, I.S. The Structure Analysis and Optical Performance of PVA Films Doped with Fe3+-Metal for UV- Limiter, and Optoelectronics. Mater. Res. Express 2019, 6, 085334. [Google Scholar] [CrossRef]
- Salah, M.; Gad, M.; Elkattan, M.; Sabry, Y.M. Effect of Gamma-Irradiation and Doping on the Absorption Edge and the Optical Bandgap of Silver-Doped PVA Films. Opt. Commun. 2020, 473, 125933. [Google Scholar] [CrossRef]
- Otero, T.F.; Martinez, J.G. Activation Energy for Polypyrrole Oxidation: Film Thickness Influence. J. Solid State Electrochem. 2011, 15, 1169–1178. [Google Scholar] [CrossRef]
- Singh, S.; Neerja. The Effect of Gamma-Irradiation on the Activation Energy of Bulk and Track Etching in CR-39 Plastic Track Detector. Radiat. Meas. 2007, 42, 1507–1509. [Google Scholar] [CrossRef]
- Isac, J. Optical Band Gap Analysis of Nano-Crystalline Ceramic PbSrCaCuO. JAP 2014, 5, 816–822. [Google Scholar] [CrossRef]
- Aziz, S.B.; Brza, M.A.; Nofal, M.M.; Abdulwahid, R.T.; Hussen, S.A.; Hussein, A.M.; Karim, W.O. A Comprehensive Review on Optical Properties of Polymer Electrolytes and Composites. Materials 2020, 13, 3675. [Google Scholar] [CrossRef]
- Costa, J.C.S.; Taveira, R.J.S.; Lima, C.F.R.A.C.; Mendes, A.; Santos, L.M.N.B.F. Optical Band Gaps of Organic Semiconductor Materials. Opt. Mater. 2016, 58, 51–60. [Google Scholar] [CrossRef]
- Escobedo-Morales, A.; Ruiz-López, I.I.; Ruiz-Peralta, M.d.L.; Tepech-Carrillo, L.; Sánchez-Cantú, M.; Moreno-Orea, J.E. Automated Method for the Determination of the Band Gap Energy of Pure and Mixed Powder Samples Using Diffuse Reflectance Spectroscopy. Heliyon 2019, 5, e01505. [Google Scholar] [CrossRef] [PubMed]
- Meftah, A.; Gharibshahi, E.; Soltani, N.; Yunus, W.; Saion, E. Structural, Optical and Electrical Properties of PVA/PANI/Nickel Nanocomposites Synthesized by Gamma Radiolytic Method. Polymers 2014, 6, 2435–2450. [Google Scholar] [CrossRef]
- Arshak, K.; Korostynska, O. Gamma Radiation Dosimetry Using Tellurium Dioxide Thin Film Structures. Sensors 2002, 2, 347–355. [Google Scholar] [CrossRef]
- Talebi, M.; Zahedifar, M.; Sadeghi, E. UVC Dosimetry Properties of Mn and Ce Doped KCl Thermoluminescent Phosphor Produced by Co-Precipitation Method. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2019, 458, 97–104. [Google Scholar] [CrossRef]
- Horowitz, Y.S. Theory of Thermoluminescence Gamma Dose Response: The Unified Interaction Model. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2001, 184, 68–84. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doyan, A.; Susilawati, S.; Prayogi, S.; Bilad, M.R.; Arif, M.F.; Ismail, N.M. Polymer Film Blend of Polyvinyl Alcohol, Trichloroethylene and Cresol Red for Gamma Radiation Dosimetry. Polymers 2021, 13, 1866. https://doi.org/10.3390/polym13111866
Doyan A, Susilawati S, Prayogi S, Bilad MR, Arif MF, Ismail NM. Polymer Film Blend of Polyvinyl Alcohol, Trichloroethylene and Cresol Red for Gamma Radiation Dosimetry. Polymers. 2021; 13(11):1866. https://doi.org/10.3390/polym13111866
Chicago/Turabian StyleDoyan, Aris, Susilawati Susilawati, Saiful Prayogi, Muhammad Roil Bilad, Muhamad Fatikul Arif, and Noor Maizura Ismail. 2021. "Polymer Film Blend of Polyvinyl Alcohol, Trichloroethylene and Cresol Red for Gamma Radiation Dosimetry" Polymers 13, no. 11: 1866. https://doi.org/10.3390/polym13111866
APA StyleDoyan, A., Susilawati, S., Prayogi, S., Bilad, M. R., Arif, M. F., & Ismail, N. M. (2021). Polymer Film Blend of Polyvinyl Alcohol, Trichloroethylene and Cresol Red for Gamma Radiation Dosimetry. Polymers, 13(11), 1866. https://doi.org/10.3390/polym13111866