Abstract
Herein we report new multiblock chalcone conjugate phthalimide and naphthalimide functionalized copolymers with a topologically novel architecture synthesis using nucleophilic substitution and polycondensation methodology. The structures of the synthesized novolacs were elucidated on the basis of their spectroscopic analysis including FTIR, 1H NMR, and 13C NMR spectroscopy. Further, the number-average and weight-average molecular weights of the novolac polymers were determined by gel permeation chromatography (GPC). We examined the solubility of the synthesized polymers in various organic solvents including CHCl3, CH3CN, THF, H2O, CH3OH, DMSO, and DMF and found they are insoluble in both methanol and water. The novolac polymers were evaluated for their photophysical properties and microbial activities. The investigation of the antimicrobial activities of these polymers reveals significant antimicrobial activity against the pathogens E. coli, S. aureus, C. albicans, and A. niger.
1. Introduction
Polymeric fluorescent compounds are an attractive research area for researchers in both academia and industries including textiles, food, cosmetics, and biomedicines. Among the various available fluorescent materials in the literature, polymeric compounds containing phthalimides and 1,8-naphthalimide analogs exhibit a wide range of applications [1,2,3]. The structural feature of –CO–N(R)–CO– and the imide ring functionality in phthalimides are responsible for the bioactivity and pharmaceutical properties of the analogs. Interestingly, due to their hydrophobic and neutral nature, phthalimides can cross biological membranes in vivo [4]. Moreover, they exhibit antioxidant and anti-inflammatory properties [5,6], have antimicrobial potential [7], and possess anthelmintic activity [8]. In 2012, Basaric et al. found that phthalimides can produce tumor necrosis factor (TNFα) to suppress tumor cell proliferation [9]. Furthermore, phthalimide polymers are widely used in biomedical and biological fields with a variety of properties including antimalarial, anti-influenza, anticancer, anti-inflammatory, and antifungal activities, apoptotic-inducing properties, and in applications such as protein sensors, n-type organic semiconductors, and electroluminescence [10,11,12,13,14].
Similarly, naphthalimide and its derivatives are a unique class of fused naphthalene compounds in which a chalcone is fused to a naphthalimide moiety. Naphthalimide derivatives possess a broad range of biological activities such as antiviral [15], antioxidant [16], antitumor [17], and antimicrobial activities [18]. In 2017, Al-Azzawi and Faiq reported a variety of new phenolic resins bearing 1,8-naphthalimide pendent groups for various applications [19]. They also act as blue-light-emitting materials because of their highly efficient fluorescence and photoluminescence, thermal and oxidative stability, and emission of polarized blue light [20]. Hence, during recent decades, remarkable advances have been made in the production of naphthalimide polymeric derivatives. For instance, allyloxy- and alkoxy-substituted 1,8-naphthalimide copolymeric materials emit bright blue fluorescence and can be used as brightening agents for polymers [21]. In 2020, Hudson et al. [22] reported that 1,8-naphthalimide-based deep-red acrylic polymers could be applied as ratiometric temperature sensors. Very recently, a ratiometric fluorescent nanoprobe based on naphthalimidefunctionalized carbon dots was developed for HeLa cellline studies [23]. Motivated by their notable fluorescent and biological characteristics, we were interested in investigating phthalamide- and naphthalimide- based fluorescent novolac analogs. In this work, we propose the synthesis and characterization of a new type of highly structured chalcone conjugated fluorescent resins bearing naphthalimide and phthalimide pendants on the novolac backbone. Further, the synthesized fluorescence novolac resins exhibited in vitro inhibitory activity against highly threatening pathogens.
2. Materials and Methods
All required chemicals were purchased from Sigma (Bangalore, Karnataka, India), Merck (Mumbai, Maharashtra, India), and Alfa Aesar (Melbourne, Australia). 1H and 13C NMR spectra were recorded on a Bruker 400 MHz instrument (SAIF-IITM, Chennai, India). UV–Vis absorption spectra were recorded on a Perkin Elmer LAMBDA 950 spectrophotometer (SAIF-IITM, Chennai, India), and fluorescence measurements were performed on a Spectra Max Fluorolog-3 instrument (SAIF-IITM, Chennai, India) at room temperature. Silica gel (60–230 mesh) (LOBA Chemie, India) was employed for routine column chromatography separations. Thin layer chromatography (TLC) was performed on precoated (0.25 mm) silica gel F 254 plates (E. Merck, Germany); naphthalimide derivatives were detected using a 254 nm UV lamp. Melting points were recorded on MEL-TEMP Electrothermal melting point apparatus (Electrothermal, Germany) and were uncorrected.
2.1. General Procedure for the Synthesis of Isoindoline-1,3-Diones 3 and 7
Isoindoline-1,3-diones 3 and 7 were prepared following a literature procedure [24]. Briefly, 4-aminoacetophenone was reacted with the appropriate acid anhydride in glacial acetic acid (50 mL). The reaction mixture was heated to boiling temperature (118 °C) for 16 h with constant stirring and monitored by TLC to determine the completion of the reaction. Then, the reaction mixture was allowed to cool to room temperature, and ice-cold water was added. The resulting solid was filtered off and dried, and the crude product was purified by recrystallization from a hot EtOH:CHCl3 (1:1) mixture.
2.2. General Procedure for the Condensation Reaction to Synthesize 4a–c and 8a–d
An equimolar mixture of the appropriate aromatic aldehyde and isoindoline-1,3-dione 3 or 7 was stirred with 10–20 mL of ethanol, and 10 mL of a 40% aqueous KOH solution was added at room temperature. The reaction mixture was stirred overnight, and the completion of the reaction was determined by TLC. The reaction mixture was poured into ice-cold water and acidified with dilute HCl to precipitate the crude product, which was then recrystallized with hot ethanol.
2.3. General Procedure for the Synthesis of Compounds 5a–c and 9a–d
An equimolar mixture of chalcone 4a and formaldehyde was placed in a 250 mL round bottom flask fitted with a mechanical stirrer and a condenser to allow melting at 60 °C with constant stirring under N2 atmosphere. Then, 5 mL of TEA was added dropwise, and the reaction mixture was allowed to reflux for a further 6–7 h under N2 atmosphere. After TLC indicated consumption of the reactants, the reaction mixture was allowed to cool down. Then, the excess of TEA was removed by washing with diethylether (3 × 15 mL), and the solid mass was poured into hot ethanol and dried overnight under vacuum in a hot oven at 50 °C.
2.4. Determination of Molecular Weight
The most significant parameter in polymer characterization is the determination of molecular weight. In the current study, we found the molecular weights of the novalac polymers using gas permutation chromatography (GPC), a reliable and systematic technique. The number-average molecular weights (Mn) and the weight-average molecular weights (Mw) of the polymer samples were determined using a Waters 510 HPLC pump (Waters, Hayward, NJ, USA) equipped with a Waters 410 Differential Refractometer instrument (Waters, Hayward, NJ, USA) at an operating wavelength (λ) of 930 nm. Gel permeation chromatography (GPC) consisted of three sets of Waters Styragel columns, viz., HR1, HR2, and HR3, having a size of 7.8 mm in diameter and 300 mm in length. Initially, the instrument calibration techniques were done according to the standard method with poly(styrene) standard and the experiment was carried out at 40 ± 1 °C using HPLC grade acetonitrile (CH3CN) as the mobile phase at a flow rate of 1.0 mL/min.
2.5. Spectrophotometric Studies
2.5.1. FourierTransform Infrared Spectroscopy
The Fouriertransform infrared spectroscopy (FTIR, Shimadzu, Japan) spectra were recorded in a Shimadzu FTIR 8400 spectrophotometer instrument using a KBr disc back as a reference. Spectra were acquired between 4000 and 400 cm−1, accumulating up to 45 spectra with a resolution of 2 cm−1.
2.5.2. Absorption and Emission Spectral Studies
The UV–Vis double beam UVD-3500 Labomed Inc. (Los Angeles, CA, USA) instrument was used to record absorption spectra at room temperature, and the emission spectra were recorded in a PerkinElmer Luminescence PE 45 instrument. Spectroscopic grade acetonitrile was used as a solvent to record the emission spectra, and the concentration of 1 × 10−6 M synthesized novolac solutions was prepared by sonication for 10–15 min as it has poor solubility. The slit width was 5 nm at input and output pathways and the path length of the cuvette was 10 mm.
2.6. Antimicrobial Studies
Antimicrobial activities of the synthesized phthalimide and naphthalimide novolacs were used to screen primary biological studies. All the synthesized compounds 5a–c and 9a–d were subjected to antimicrobial activity against common pathogens such as Gram-negative bacteria (Escherichia coli MTCC 25922), Gram-positive bacteria (Staphylococcus aureus MTCC 25923), pathogenic yeast (Candida albicans MTCC 282), and a fungus (Aspergillus niger MTCC 227), which are usually found in vegetables and fruits. The agar diffusion method was used for the determination of antimicrobial activities against the above-mentioned microorganisms by standard methodology [25]. All the cultures were maintained in the standard laboratory conditions. Wells were made with a cork borer in the solid agar and loaded with 50, 75, and 100 mg/mL of the novolacs dissolved in DMSO with tetracycline and carbendazim as control for E. coli, S. aureus, C. albicans, and A. niger, respectively. Petri dishes were incubated at 35 °C for 24 h and the resulting inhibition zone diameter was measured in millimeters.
3. Results and Discussion
3.1. Synthesis
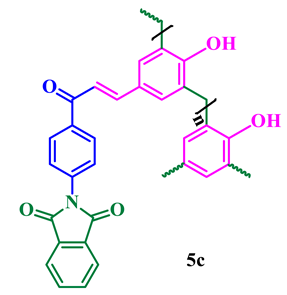
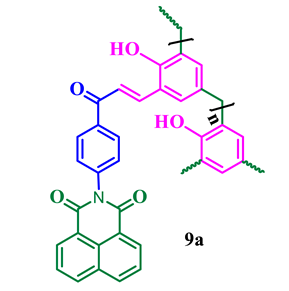
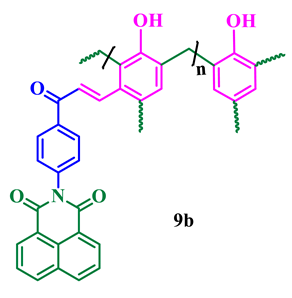
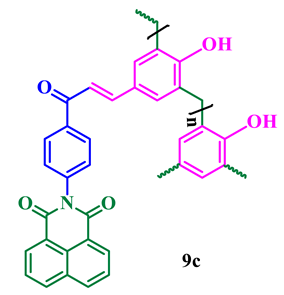
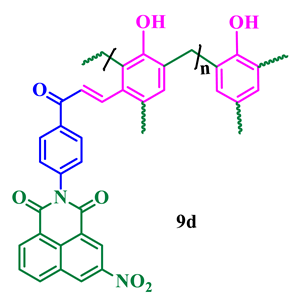
The synthesis of phthalimide analogs 5a–c and 1,8-naphthalimide analogs 9a–d was performed as depicted in Scheme 1 and Scheme 2, starting from key materials 1 and 2 and 1 and 6, respectively. Initially, 2-(4-acetylphenyl)isoindoline-1,3-dione (3) and 2-(4-acetylphenyl)-5-substituted-1H-benzo[de]isoquinoline-1,3(2H)-dione (7) were synthesized according to a literature procedure [24] by the reaction of the corresponding aromatic anhydride with 4-aminoacetophenone in acetic acid under reflux. In the second step, the required chalcone was obtained by aldol condensation and subsequent dehydration of 1,3-diones 3 and 7 with various substituted aromatic aldehydes in ethanolic solution in KOH medium. The obtained crude products were dissolved with hot ethanol and recrystallized to produce chalcones 4a–c and 8a–d. These chalcones underwent polymerization by the reaction with formaldehyde in a ratio of 1:8 in the presence of triethylamine (TEA) in ethanolic solution at 65 °C under N2 atmosphere, producing phenolic-formaldehyde resins 5a–c and 9a–d in 50–56% yields.
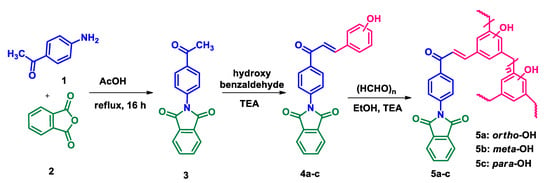
Scheme 1.
Synthesis of N-substituted phthalimide phenol analogs 5a–c.
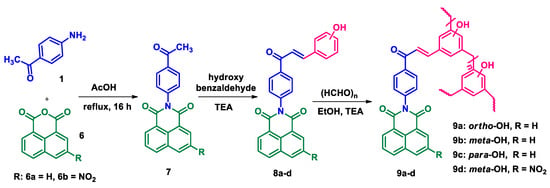
Scheme 2.
Synthesis of N-substituted naphthalimide phenol analogs 9a–d.
The structures of the newly synthesized compounds were confirmed by spectroscopic analysis. Thus, the IR spectra of 5a–c and 9a–d show two strong absorption peaks between 1777 and 1660 cm−1, which revealed the presence of two carbonyl groups. The absorption peak at 1402 cm−1 (imide), together with the obvious bands around 2920 cm−1, are attributed to the amide group, which further supports the proposed structures. A representative FTIR spectrum of phthalimide pendent novolac 5a is shown in Figure 1. The remarkable signatures around 1790 cm−1 and 1769 cm−1 denote the asymmetrical and symmetrical stretching frequencies of imide carbonyl groups, respectively. The intense band of chalcone carbonyl carbon symmetrical stretching absorption appeared at 1727 cm−1. An absorption band that appeared around 1306 cm−1 corresponds to C–N stretching and the sharp absorption bands that appeared around 1012 and 771 cm−1 are due to imide ring deformation. The aromatic C–H stretching frequency has been observed at 3071 cm−1 and the absorption at 3460 cm−1 represents the presence of a phenolic OH group in the novolac polymer.
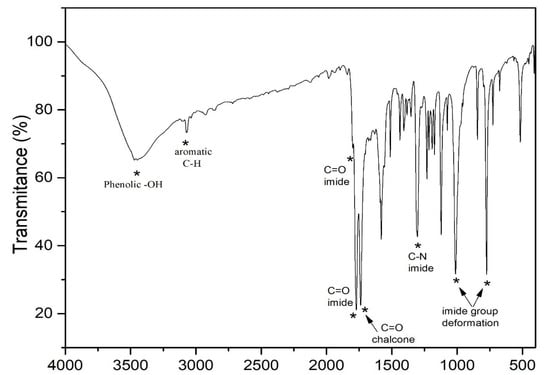
Figure 1.
FTIR spectrum of phthalimide pendent novolac 5a.
All the newly synthesized compounds structures were determined based on the standard literature procedure [26,27]. As a representative example, the 1H NMR spectrum of compound 5a shows a characteristic singlet at δ 5.46 ppm, which is due to the presence of phenolic OH and a distorted doublet at δ 3.19 ppm corresponding to the newly generated methylene group between the phenolic rings. The aromatic protons are observed between δ 7.19 and 8.22 ppm (Figure 2). The 13C NMR of compound 5a shows a characteristic peak at δ 29.71 ppm that can be assigned to methylene carbons. The downfield peaks at 158.30 and 186.20 ppm confirm the presence of 1,3-diketonyl and α,β-unsaturated carbonyl carbons in compound 5a, respectively. The structures of the synthesized compounds 5a–c and 9a–d are presented in Table 1, and complete NMR data can be found in the supplementary materials (S1).
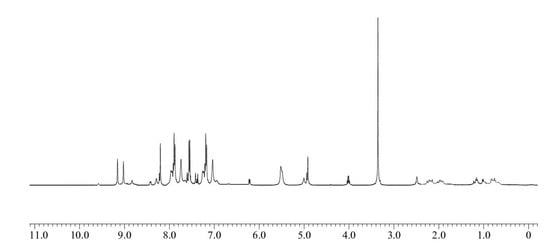
Figure 2.
1H NMR spectrum of phthalimide pendent novolac 5a.

Table 1.
Phthalimide- and naphthalimide-substituted phenol-formaldehyde polymeric resins 5a–c and 9a–d.
3.2. Average Molecular Weight Analysis
The average molecular weights (Mn and Mw) and polydispersity index (Ð = Mw/Mn) of the synthesized polymers were calculated and the results are presented in Table 2. The chromatogram of novolac 5a is presented in Figure 3.

Table 2.
Molecular weight Mn, Mw, and Ð of the synthesis novolac polymers 5a–c and 9a–d. (Results are expressed as rounded to the third significant number).
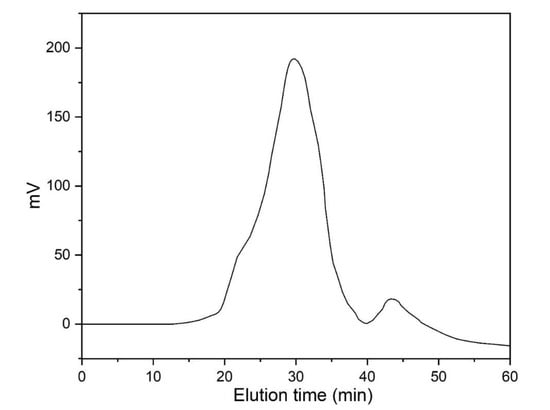
Figure 3.
Molecular weight distribution chromatogram of novolac 5a from GPC.
The average molecular weight of phthalimide novolacs5a–c (Mn = 15,169–16,734, Mw = 46,512–52,110, and Ð = 2.82–3.43) was found to be lower than 1,8-naphthalimide novolacs 9a–d (Mn = 12,056–18,599, Mw = 50,218–60,327, and Ð = 2.70–4.41). The obtained high Ð values (Ð > 3.00) of the synthesized polymers are due to fragmentation and re-combination of the monomers. Further, we attribute the high Ð values of the polymers to their branched structures. In addition, all other polymers have Ð values > 2.00 (Ð = 2.70, 2.82, and 2.91), which indicates the presence of a hyper-branching chain in the synthesized polymers. The naphthalimide analog 9d showed the lowest Ð value (Mn = 9514, Mw = 18,326, and Ð = 1.92) compared to the other analogs due to the substituted electron-withdrawing nitro group in the naphthalimide ring.
3.3. Absorbance Spectra
The UV–visible absorption spectra of naphthalimide analogs 9a–d recorded in acetonitrile are shown in Figure 4a. It is obvious that the photochemical properties of the 1,8-naphthalimide core are influenced by the substituents present in the aromatic ring [28]. All the synthesized naphthalimide derivatives were UV active, with maximum absorption wavelengths (absλmax) in the region 336–343 nm, and the acetonitrile solutions were colorless to the naked eye. The observed blue shift of the absorbance of 1,8-naphthalimide is due to the presence of the chalcone. In general, the n–π* transition of chalcone compounds is stronger than their π–π* transition owing to the presence of an extended π–conjugation system in the structure. Xiao et al. [29] reported the synthesis and characterization of 4-(2-methoxyethoxy)-N-butyl-1,8-naphthalimide (MEBN), and they found that MEBN had a absλmax value at 355 nm, whereas that of 4-methoxy-N-butyl-1,8-naphthalimide was 350 nm, which was attributed to the presence of the more strongly electron-donating methoxy-ethoxy group compared to the methoxy group. The fluorescent molecules based on 1,8-naphthalimide thio and amino derivatives exhibit maximum excitation wavelengths ranging between 390 and 398 nm, which agrees well with literature reports [30].
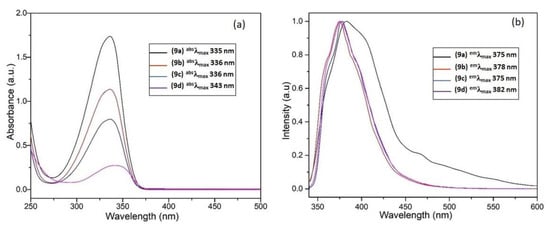
Figure 4.
(a) UV–visible spectra of 9a–d in acetonitrile solution; (b) normalized fluorescence spectra of 9a–d at a concentration of 1 × 10−6 M in acetonitrile solution.
3.4. Fluorescence Emission
The excited state behavior of naphthalimide analogs 9a–d was evaluated by recording their fluorescent emission spectra in acetonitrile solutions (emλmax = 375–382 nm). As shown in Figure 4b, all the naphthalimide analogs showed a similar range of emission, which is slightly red-shifted to emλmax = 382 nm in the case of compound 9d. The spectrum of this compound also exhibits a shoulder at emλmax = 411 nm, which is due to the presence of the electron-withdrawing NO2 group in the 5-position of the naphthalimide ring, reducing the π–electron conjugation between the chalcone spacer and the naphthalimide aromatic ring. Table 3 summarizes the photophysical properties of naphthalimides 9a–d. In addition, Figure 5 displays the fluorescence emissions of these compounds upon exposure to UV light.

Table 3.
Photophysical properties of naphthalimide analogs 9a–d in acetonitrile at a concentration of 1 × 10−6 M.

Figure 5.
Blue light emission of 9a–d under 365 nm UV irradiation.
As reported by Xiao et al., the fluorescence emission of MEBN was sensitive to solvent polarity, and the corresponding bands were gradually shifted to lower energies with increasing solvent polarity [29]. The effect of the polarity of the medium on the photoluminescent emission maximum was more pronounced than on the absorption maximum. The long wavelength of fluorescence emission observed in more polar solvents is due to an intramolecular charge-transfer transition. In this context, Saito et al. [30] designed three fluorescent probes integrated in a molecular system, in which the introduction of amines as receptors enabled the photo-induced electron transfer process.
3.5. Antimicrobial Activity
3.5.1. In Vitro Antibacterial Activities
The agar well diffusion method was used to determine the antibacterial activities of the synthesized imide compounds 5a–c and 9a–d. The bacterial strains used in these studies were S. aureus MTCC 25923 and E. coli MTCC 25922, and tetracycline was used as a positive control. Compound 9b was effective in controlling both pathogens with a large zone of inhibition at a concentration of 50, 75, and 100μg/mL. However, interestingly, the zone of inhibition was almost the same as that of the positive control at the higher concentration (100 μg/mL). Moreover, 5c, 9c, and 5b showed antibacterial effects for both strains, with zones of inhibitions of 10–23 mm, 9–18 mm, and 8–18 mm, respectively (Table 2).
3.5.2. In Vitro Antifungal Activity
The antifungal activity of compounds 5a–c and 9a–d was evaluated against two fungal strains, viz., A. niger MTCC 227 and C. albicans MTCC 282, at various concentrations (μg/mL) using the disk diffusion technique and the inhibition data are presented in Table 4. Compound 9b could effectively control both fungal pathogens with zone of inhibition values of 11–28 mm at concentrations of 50–100 μg/mL. The polymer 5c showed a moderate zone of inhibition between 11 and 26 mm, and 5b and 9c exhibited efficient inhibition ranges compared with carbendazim as a positive control. Figure 6 displays a representative graph summarizing these results and the zone of inhibition of high-efficacy polymer 9b is presented in Figure 7.

Table 4.
(a) Zone of inhibition of compounds 5a–c and 9a–d against bacterial pathogens. (b) Zone of inhibition of compounds 5a–c and 9a–d against fungal pathogens.
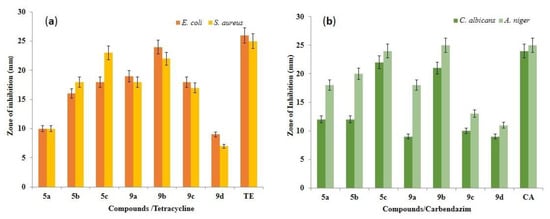
Figure 6.
Antimicrobial efficiency of compounds 5a–c and 9a–d. (a) Zone of inhibition of tested bacteria, i.e., E. coli MTCC 25922 and S. aureus MTCC 25923. (b) Zone of inhibition of tested fungi, i.e., C. albicans MTCC 282 and A. niger MTCC 227. Results are expressed as mean ± SD for n = 3. Bars with the same letter are not significantly different at p > 0.05.
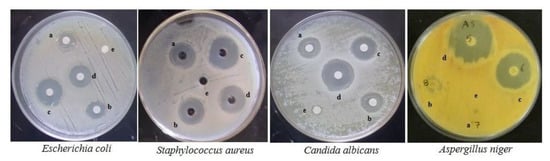
Figure 7.
Antimicrobial activity of compound 9b against common bacterial pathogens. a: 50 µL, b: 75 µL, c: 100 µL, d: positive control, e: negative control.
4. Conclusions
The synthesis of new multiblock chalcone conjugate phthalimide and naphthalimide functionalized copolymers with a topologically novel architecture synthesis using nucleophilic substitution and polycondensation methodology have been successfully explored. We have synthesized phthalimide- and naphthalimide-containing novolac analogs conjugated with a chalcone spacer via sequential nucleophilic substitution and condensation reaction in a triethylamine base. Spectroscopic analysis including FTIR, 1H NMR, and 13C NMR spectroscopy confirms the structures of the synthesized novolacs. The synthesized multi–block novolacs are photoactive; notably, 1,8-naphthalimide pendant organic functional group novolacs showed interesting optical activity compared to the other phthalimide pendent functionalized novolacs. All the 1,8-naphthalimide derivatives showed UV absorption and emission.
Employing gel permeation chromatography (GPC), the number- and weight-average molecular weights of the novolac polymers were determined. On examining the solubility of synthesized polymers in various organic solvents including CHCl3, CH3CN, THF, H2O, CH3OH, DMSO, DMF, etc., these novolac polymers were insoluble in methanol and water. Photophysical properties and microbial activities of the synthesized novolac polymers were evaluated. The investigation of the antimicrobial activities of these polymers reveals significant antimicrobial activity against the pathogens E. coli, S. aureus, C. albicans, and A. niger. The in vitro antibacterial activities against E. coli and S. aureus and the in vitro antifungal activities against C. albicans and A. niger were evaluated for the novolacs using the MIC method. The antimicrobial efficacy of all the synthesized novolacs showed interesting results for all the tested pathogens. The MIC values of all synthesized compounds are found to be effective compared to the standard drugs tetracycline and carbendazim. Particularly, compounds 9b and 5c showed their effective inhibition of both Gram-positive and Gram-negative pathogens. The present study strongly supports that the synthesized organic multi–block functionalized novolacs are optically active materials for materials science applications and also act as suitable material for a wide range of antimicrobial applications in health-associated sectors.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/polym13111859/s1, S1.
Author Contributions
Conceptualization, P.D. and G.P.; methodology, G.P.; validation, P.A.V., C.U. and M.R.; formal analysis, C.U.; investigation, P.D.; data curation, G.P.; writing original draft preparation, P.D.; writing—review and editing, G.P. and P.A.V.; supervision, C.U. and M.R.; funding acquisition, G.P.; All authors have read and agreed to the published version of the manuscript.
Funding
The authors express their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group no. RG-1440-111.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors express their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group no. RG-1440-111. The authors thank the Deanship of Scientific Research and RSSU at King Saud University for their technical support. We would like to express their gratitude and thanks to Masilmani Jaganmohan, Faculty of Chemistry, Indian Institute Madras, India, for providing the NMR data.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, L.; Fujii, M.; Yamaji, M.; Okamoto, H. Fluorescence behaviour of 2-, 3- and 4-amino-1,8-naphthalimides: Effects of the substitution positions of the amino functionality on the photophysical properties. Photochem. Photobiol. Sci. 2018, 17, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Orita, R.; Franckeviclus, M.; Vysniauskas, A.; Gulbinas, V.; Sugiyama, H.; Uekusa, H.; KanosueKishige, R.; Ando, S. Enhanced fluorescence of phthalimide compounds induced by the incorporation of electron-donating alicyclic amino groups. Phys. Chem. Chem. Phys. 2018, 20, 16033–16044. [Google Scholar] [CrossRef] [PubMed]
- Yoon, U.C. and Mariano, P.S. The Synthetic Potential of Phthalimide SET Photochemistry. Acc. Chem. Res. 2001, 34, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Bansode, T.N.; Shelke, J.V.; Dongre, V.G. Synthesis and antimicrobial activity of some new N-acyl substituted phenothiazines. Eur. J. Med. Chem. 2009, 44, 5094–5098. [Google Scholar] [CrossRef]
- Kamal, A.; Bolla, N.R.; Srikanth, P.S.; Srivastava, A.K. Naphthalimide derivatives with therapeutic characteristics: A patent review. Expert Opin. Ther. Pat. 2013, 23, 299–317. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Rao, G.K.; Pai, S.; Ranjan, A. Design, Synthesis, Antibacterial and invitro Antioxidant activity of substituted 2HBenzopyran-2-one derivatives. Int. J. Chem. Tech. Res. 2011, 3, 555–559. [Google Scholar]
- Santos, J.L.; Yamasaki, P.R.; Chin, C.M.; Takashi, C.H.; Pavan, F.R.; Leite, C.Q. Synthesis and in vitro anti Mycobacterium tuberculosis activity of a series of phthalimide derivatives. Bioorganic Med. Chem. 2009, 17, 3795–3799. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Kumar, K.R.; Kumar, P.P. Synthesis and Antimicrobial Activity of some New α N-Phthilimido Amino Acids Analogues. Int. J. Chem. Tech. Res. 2010, 2, 895–898. [Google Scholar]
- Horvat, M.; Uzelac, L.; Marjanovic, M.; Cindro, N.; Frankovic, O.; Mlinaric-Majerski, K.; Kralj, M.; Basaric, N. Evaluation of antiproliferative effect of N-(alkyladamantyl)phthalimides in vitro. Chem. Biol. Drug. Des. 2012, 79, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Rajendran, V.; Pant, A.; Ghosh, P.C.; Singh, N.; Latha, N.; Rathi, B. Design, synthesis and biological evaluation of functionalized phthalimides: A new class of antimalarials and inhibitors of falcipain-2, a major hemoglobinase of malaria parasite. Bioorganic Med. Chem. 2015, 23, 1817–1827. [Google Scholar] [CrossRef]
- Iwai, Y.; Takahashi, H.; Hatakeyama, D.; Motoshima, D.; Ishikawa, M.; Sugita, K.; Kuzuhara, T. Anti-influenza activity of phenethylphenylphthalimide analogs derived from thalidomide. Bioorganic Medic. Chem. 2010, 18, 5379–5390. [Google Scholar] [CrossRef]
- Vivekanand, P.A.; Wang, M.L.; Hsieh, Y.M. Sonolytic and Silent Polymerization of Methacrlyic Acid ButylEster Catalyzed by a New Onium Salt with bis-Active Sites in a Biphasic System—A Comparative Investigation. Molecules 2013, 18, 2419–2437. [Google Scholar] [CrossRef] [PubMed]
- Coêlho, L.C.D.; Cardoso, M.V.; Moreira, O.d.D.R.M.; Gomes, P.A.T.; Cavalcanti, M.d.S.M.T.; Oliveira, A.R.; Leite, A.C.L. Novel phthalimide derivatives with TNF-α and IL-1β expression inhibitory and apoptotic inducing properties. Med. Chem. Commun. 2016, 5, 758–765. [Google Scholar] [CrossRef]
- Pan, L.; Li, X.; Gong, C.; Jin, H.; Qin, B. Synthesis of N-substituted phthalimides and their antifungal activity against Alternaria solani and Botrytis cinerea. Micro Pathog. 2016, 95, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Chanh, T.C.; Lewis, D.E.; Judy, M.M.; Sogandares-Bernal, F.; Michalek, G.R.; Utecht, R.E.; Matthews, J.L. Inhibition of retrovirus-induced syncytium formation by photoproducts of a brominated 1,8-naphthalimide compound. Antivir. Res. 1994, 25, 133–146. [Google Scholar] [CrossRef]
- Ghosh, P.; Singha Roy, S.; Basu, A.; Bhattacharjee, A.; Bhattacharya, S. Sensitization of cis-platin therapy by a naphthalimide based organoselenium compound through modulation of antioxidant enzymes and p53 mediated apoptosis. Free Radic. Res. 2015, 49, 453–471. [Google Scholar] [CrossRef]
- Chen, Z.; Liang, X.; Zhang, H.; Xie, H.; Liu, J.; Xu, Y.; Qian, X. A New Class of Naphthalimide-Based Antitumor Agents That Inhibit Topoisomerase II and Induce Lysosomal Membrane Permeabilization and Apoptosis. J. Med. Chem. 2010, 53, 2589–2600. [Google Scholar] [CrossRef]
- Staneva, D.; Vasileva-Tonkova, E.; Grabchev, I. A New Bioactive Complex between Zn(II) and a Fluorescent Symmetrical Benzanthrone Tripod for an Antibacterial Textile. J. Photochem. Photobiol. A Chem. 2019, 375, 24–29. [Google Scholar] [CrossRef]
- Al-Azzawi, A.M.; Faiq, E. Synthesis and Modification of New Phenolic Resins Bearing Pendant 1,8-Naphthalimides. Int. J. Sci. Res. 2017, 6, 1498–1504. [Google Scholar]
- Zhang, X.; Zhang, J.; Lu, H.; Wu, J.; Li, G.; Li, C.; Bo, Z. A 1,8-naphthalimide based small molecular acceptor for polymer solar cells with high open circuit voltage. J. Mater. Chem. C 2015, 3, 6979–6985. [Google Scholar] [CrossRef]
- Grabchev, I.; Petkov, C.; Bojinov, V. 1,8-Naphthalimides as Blue Emitting Fluorophores for Polymer Materials. Macromol. Mater. Eng. 2002, 287, 904–908. [Google Scholar] [CrossRef]
- Christopherson, C.J.; Mayder, D.M.; Poisson, J.; Paisley, N.R.; Tonge, C.M.; Hudson, Z.M. 1,8-Naphthalimide-Based Polymers Exhibiting Deep-Red Thermally Activated Delayed Fluorescence and Their Application in Ratiometric Temperature Sensing. ACS Appl. Mater. Interfaces 2020, 12, 20000–20011. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jia, Y.; Zou, G.-Y.; Yu, Y.-L.; Wang, J.-H. A ratiometric fluorescent nanoprobe based on naphthalimide derivative-functionalized carbon dots for imaging lysosomal formaldehyde in HeLa cells. Nanoscale 2019, 11, 6377–6383. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, A.A.-M.; El-Azab, A.S.; El-Enin, M.A.A.; Almehizia, A.A.; Supuran, C.T.; Nocentini. A. Synthesis of novel isoindoline-1,3-dione-based oximes and benzenesulfonamide hydrazones as selective inhibitors of the tumor-associated carbonic anhydrase IX. Bioorganic Chem. 2018, 80, 706–713. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Cao, Y.; Gao, M.; Chen, C.; Fan, A.; Zhang, J.; Kong, D.; Wang, Z.; Peer, D.; Zhao, Y. Triggered-release polymeric conjugate micelles for on-demand intracellular drug delivery. Nanotechnology 2015, 26, 115101. [Google Scholar] [CrossRef] [PubMed]
- Al-Azzawi, A.M.; Yaseen, H. Synthesis and curing of new phenolic resins containing pendant tetrachlorophthalimides. Iraqi J. Sci. 2016, 57, 1345–1356. [Google Scholar]
- Bojinov, V.B.; Panova, I.P.; Simeonov, D.B.; Georgiev, N.I. Synthesis and sensor activity of photostable blue emitting 1,8-naphthalimides containing s-triazine UV absorber and HALS fragments. J. Photochem. Photobiol. A Chem. 2010, 210, 89–99. [Google Scholar] [CrossRef]
- Xiao, H.; Chen, M.; Shi, G.; Wang, L.; Yin, H.; Mei, C. A novel fluorescent molecule based on 1,8-naphthalimide: Synthesis, spectral properties, and application in cell imaging. Res. Chem. Intermed. 2010, 36, 1021–1026. [Google Scholar] [CrossRef]
- Saito, G.; Velluto, D.; Resmini, M.R. Synthesis of 1,8-naphthalimide-based probes with fluorescent switch triggered by flufenamic acid. Soc. Open. Sci. 2018, 5, 172137. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).