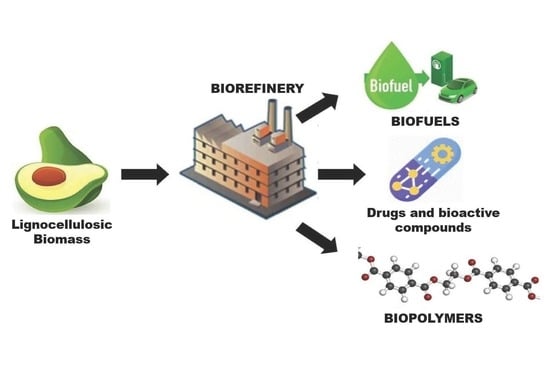
Persea Americana Agro-Industrial Waste Biorefinery for Sustainable High-Value-Added Products
Abstract
1. Introduction
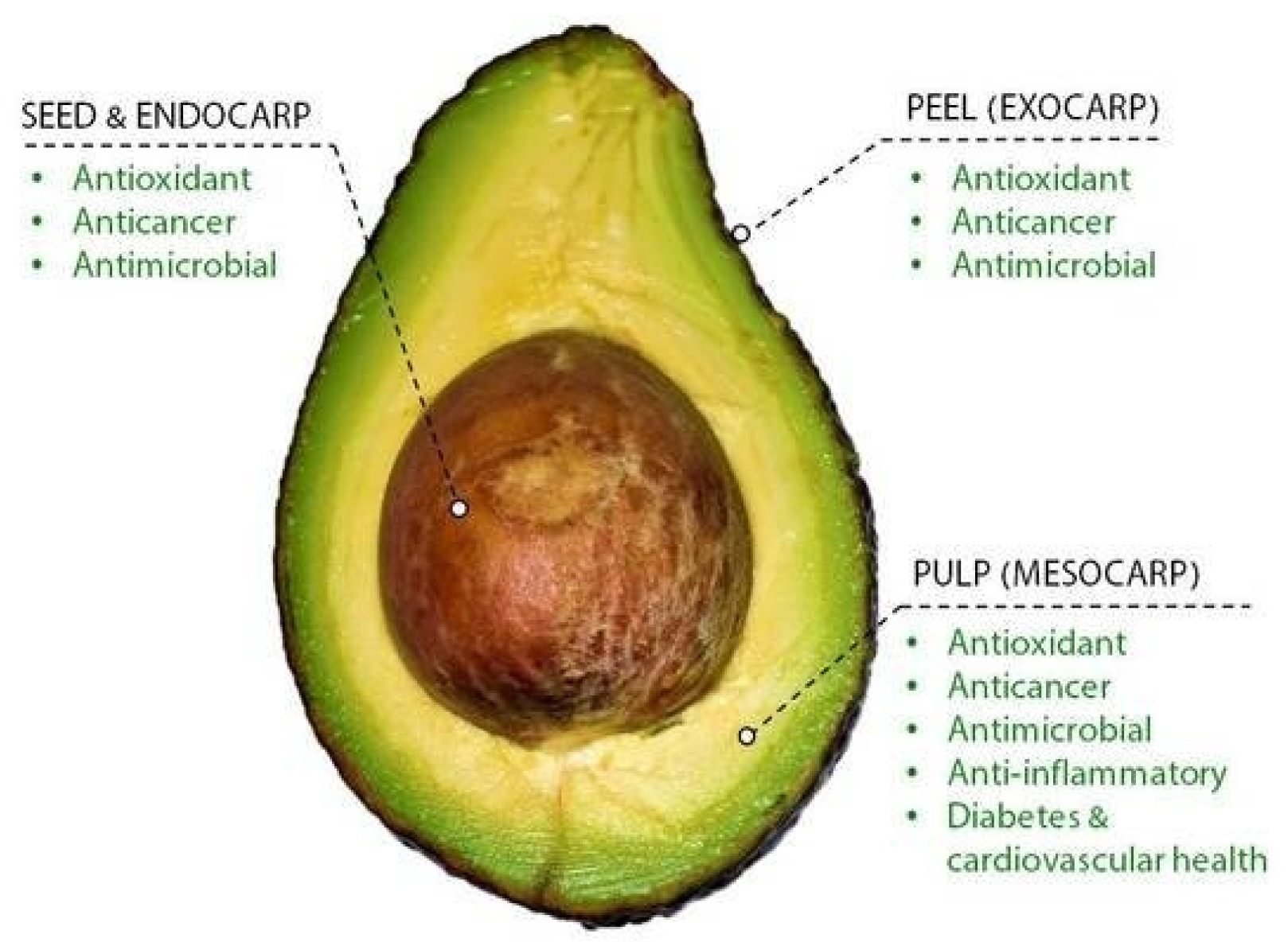
2. Applications of Avocado Agro-Industrial Wastes
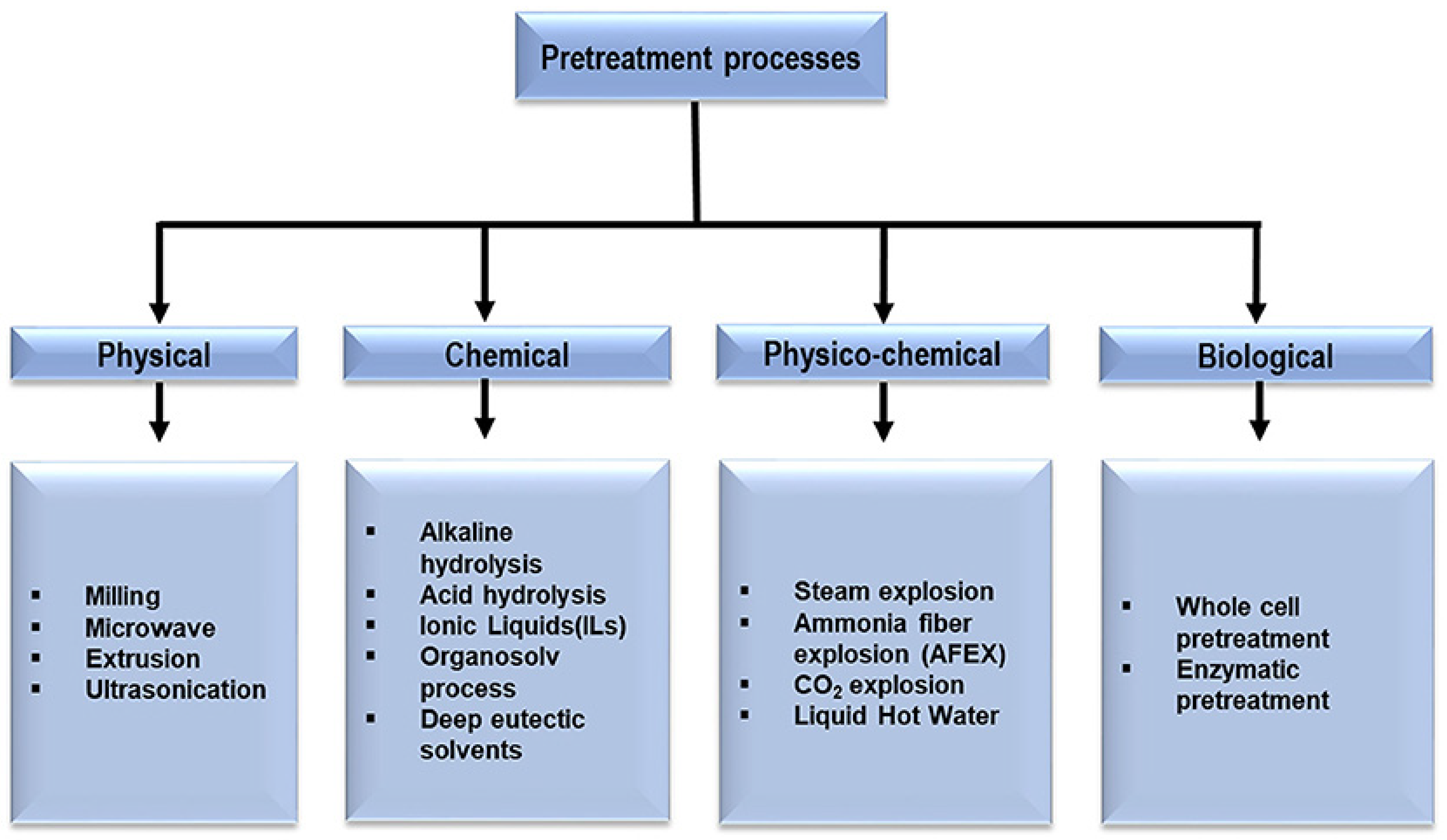
2.1. Waste and Pretreatments
2.2. Potential Biorefinery
3. High-Value Products Obtained from Avocado Wastes
3.1. Biofuels and Bioenergy
3.2. Drugs and Bioactive Compounds
3.3. Biopolymers
3.3.1. PolylacticAcid
3.3.2. Polyhydroxybutyrate (PHB)
3.3.3. Starch Applicability
3.3.4. Nanocellulose Fibers (NCFs)
4. Nanotechnology
5. Challenges and Future Perspectives
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- De Buck, V.; Polanska, M.; Van Impe, J. Modeling Biowaste Biorefineries: A Review. Front. Sustain. Food Syst. 2020, 4. [Google Scholar] [CrossRef]
- Morais, A.R.; Bogel-Lukasik, R. Green chemistry and the biorefinery concept. Sustain. Chem. Process. 2013, 1, 18. [Google Scholar] [CrossRef]
- Dávila, J.A.; Rosenberg, M.; Castro, E.; Cardona, C.A. A model biorefinery for avocado (Persea americanamill.) processing. Bioresour. Technol. 2017, 243, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, H.A. Foreword. High Pressure Processing for the Valorization of Biomass; Royal Society of Chemistry (RSC): London, UK, 2017; pp. P007–P008. [Google Scholar]
- Cherubini, F. The biorefinery concept: Using biomass instead of oil for producing energy and chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Pauliuk, S.; Arvesen, A.; Stadler, K.; Hertwich, E.G. Industrial ecology in integrated assessment models. Nat. Clim. Chang. 2017, 7, 13–20. [Google Scholar] [CrossRef]
- Redondo-Gómez, C.; Quesada, M.R.; Astúa, S.V.; Zamora, J.P.M.; Lopretti, M.; Vega-Baudrit, J.R. Biorefinery of Biomass of Agro-Industrial Banana Waste to Obtain High-Value Biopolymers. Molecules 2020, 25, 3829. [Google Scholar] [CrossRef]
- Alfaro, C.C.; Arias, J.M. Enzymatic conversion of treated oil palm empty fruit bunches fiber into fermentable sugars: Optimization of solid and protein loadings and surfactant effects. Biomass Convers. Biorefinery 2020. [Google Scholar] [CrossRef]
- Moreno, A.J.P.; Aguilera-Ureña, M.-J.; Manzano-Agugliaro, F. Fuel properties of avocado stone. Fuel 2016, 186, 358–364. [Google Scholar] [CrossRef]
- Hurtado-Fernández, E.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. Avocado fruit—Persea americana. In Exotic Fruits; Elsevier: Amsterdam, The Netherlands, 2018; pp. 37–48. [Google Scholar]
- Li, Y.; Bundeesomchok, K.; Rakotomanomana, N.; Fabiano-Tixier, A.-S.; Bott, R.; Wang, Y.; Chemat, F. Towards a Zero-Waste Biorefinery Using Edible Oils as Solvents for the Green Extraction of Volatile and Non-Volatile Bioactive Compounds from Rosemary. Antioxidants 2019, 8, 140. [Google Scholar] [CrossRef] [PubMed]
- Zafar, T.; Sidhu, J.S. Avocado Production, Processing, and Nutrition. In Handbook of Vegetables and Vegetable Processing, 2nd ed.; John Wiley & Sons: Chichester, UK, 2018; pp. 509–534. [Google Scholar]
- Trujillo-Mayol, I.; Badillo-Muñoz, G.; Céspedes-Acuña, C.; Alarcón-Enos, J. The Relationship between Fruit Size and Phenolic and Enzymatic Composition of Avocado Byproducts (Persea americana Mill.): The Importance for Biorefinery Applications. Horticulturae 2020, 6, 91. [Google Scholar] [CrossRef]
- Villacís-Chiriboga, J.; Elst, K.; Van Camp, J.; Vera, E.; Ruales, J. Valorization of byproducts from tropical fruits: Extraction methodologies, applications, environmental, and economic assessment: A review (Part 1: General overview of the byproducts, traditional biorefinery practices, and possible applications). Compr. Rev. Food Sci. Food Saf. 2020, 19, 405–447. [Google Scholar] [CrossRef] [PubMed]
- Bayón, B.; Berti, I.R.; Gagneten, A.M.; Castro, G.R. Biopolymers from Wastes to High-Value Products in Biomedicine. In Applications of Paleoenvironmental Techniques in Estuarine Studies; Springer: Singapore, 2018; pp. 1–44. [Google Scholar]
- Páramos, P.R.; Granjo, J.F.; Corazza, M.L.; Matos, H.A. Extraction of high value products from avocado waste biomass. J. Supercrit. Fluids 2020, 165, 104988. [Google Scholar] [CrossRef]
- Colombo, R.; Papetti, A. Avocado (Persea americana Mill.) by-products and their impact: From bioactive compounds to biomass energy and sorbent material for removing contaminants. A review. Int. J. Food Sci. Technol. 2019, 54, 943–951. [Google Scholar] [CrossRef]
- Duarte, P.F.; Chaves, M.A.; Borges, C.D.; Mendonça, C.R.B. Avocado: Characteristics, health benefits and uses. Ciênc. Rural 2016, 46, 747–754. [Google Scholar] [CrossRef]
- Jimenez, P.; Garcia, P.; Quitral, V.; Vasquez, K.; Parra-Ruiz, C.; Reyes-Farias, M.; Garcia-Diaz, D.F.; Robert, P.; Encina, C.; Soto-Covasich, J. Pulp, Leaf, Peel and Seed of Avocado Fruit: A Review of Bioactive Compounds and Healthy Benefits. Food Rev. Int. 2020, 1–37. [Google Scholar] [CrossRef]
- Ghodake, H.; Goswami, T.; Chakraverty, A. Moisture sorption isotherms, heat of sorption and vaporization of withered leaves, black and green tea. J. Food Eng. 2007, 78, 827–835. [Google Scholar] [CrossRef]
- Bhuyan, D.J.; Alsherbiny, M.A.; Perera, S.; Low, M.; Basu, A.; Devi, O.A.; Barooah, M.S.; Li, C.G.; Papoutsis, K. The Odyssey of Bioactive Compounds in Avocado (Persea americana) and their Health Benefits. Antioxidants 2019, 8, 426. [Google Scholar] [CrossRef]
- Zhang, Z.; Huber, D.J.; Rao, J. Antioxidant systems of ripening avocado (Persea americana Mill.) fruit following treatment at the preclimacteric stage with aqueous 1-methylcyclopropene. Postharvest Biol. Technol. 2013, 76, 58–64. [Google Scholar] [CrossRef]
- Araújo, R.G.; Rodriguez-Jasso, R.M.; Ruiz, H.A.; Pintado, M.M.E.; Aguilar, C.N. Avocado by-products: Nutritional and functional properties. Trends Food Sci. Technol. 2018, 80, 51–60. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef]
- Akhtar, N.; Gupta, K.; Goyal, D.; Goyal, A. Recent advances in pretreatment technologies for efficient hydrolysis of lignocellulosic biomass. Environ. Prog. Sustain. Energy 2016, 35, 489–511. [Google Scholar] [CrossRef]
- Sun, Y.-G.; Ma, Y.-L.; Wang, L.-Q.; Wang, F.-Z.; Wu, Q.-Q.; Pan, G.-Y. Physicochemical properties of corn stalk after treatment using steam explosion coupled with acid or alkali. Carbohydr. Polym. 2015, 117, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Loow, Y.; Wu, T.Y.; Tan, K.A.; Lim, Y.S.; Siow, L.F.; Jahim, J.M.; Mohammad, A.W.; Teoh, W.H. Recent Advances in the Application of Inorganic Salt Pretreatment for Transforming Lignocellulosic Biomass into Reducing Sugars. J. Agric. Food Chem. 2015, 63, 8349–8363. [Google Scholar] [CrossRef] [PubMed]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent Trends in the Pretreatment of Lignocellulosic Biomass for Value-Added Products. Front. Energy Res. 2018, 6, 1–19. [Google Scholar] [CrossRef]
- Kumari, D.; Singh, R. Pretreatment of lignocellulosic wastes for biofuel production: A critical review. Renew. Sustain. Energy Rev. 2018, 90, 877–891. [Google Scholar] [CrossRef]
- Chen, H.; Liu, J.; Chang, X.; Chen, D.; Xue, Y.; Liu, P.; Lin, H.; Han, S. A review on the pretreatment of lignocellulose for high-value chemicals. Fuel Process. Technol. 2017, 160, 196–206. [Google Scholar] [CrossRef]
- Mirmohamadsadeghi, S.; Chen, Z.; Wan, C. Reducing biomass recalcitrance via mild sodium carbonate pretreatment. Bioresour. Technol. 2016, 209, 386–390. [Google Scholar] [CrossRef]
- Dahadha, S.; Amin, Z.; Lakeh, A.A.B.; Elbeshbishy, E. Evaluation of Different Pretreatment Processes of Lignocellulosic Biomass for Enhanced Biomethane Production. Energy Fuels 2017, 31. [Google Scholar] [CrossRef]
- Seidl, P.R.; Goulart, A.K. Pretreatment processes for lignocellulosic biomass conversion to biofuels and bioproducts. Curr. Opin. Green Sustain. Chem. 2016, 2, 48–53. [Google Scholar] [CrossRef]
- Rajendran, K.; Drielak, E.; Varma, V.S.; Muthusamy, S.; Kumar, G. Updates on the pretreatment of lignocellulosic feedstocks for bioenergy production—A review. Biomass Convers. Biorefinery 2018, 8, 471–483. [Google Scholar] [CrossRef]
- Alvira, P.; Tomás-Pejó, E.; Ballesteros, M.; Negro, M. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef] [PubMed]
- Shirkavand, E.; Baroutian, S.; Gapes, D.J.; Young, B.R. Combination of fungal and physicochemical processes for lignocellulosic biomass pretreatment—A review. Renew. Sustain. Energy Rev. 2016, 54, 217–234. [Google Scholar] [CrossRef]
- Bai, X.; Wang, G.; Yu, Y.; Wang, D.; Wang, Z. Changes in the physicochemical structure and pyrolysis characteristics of wheat straw after rod-milling pretreatment. Bioresour. Technol. 2018, 250, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 1–19. [Google Scholar] [CrossRef]
- Wahid, R.; Hjorth, M.; Kristensen, S.; Møller, H.B. Extrusion as Pretreatment for Boosting Methane Production: Effect of Screw Configurations. Energy Fuels 2015, 29, 4030–4037. [Google Scholar] [CrossRef]
- Duque, A.; Manzanares, P.; Ballesteros, M. Extrusion as a pretreatment for lignocellulosic biomass: Fundamentals and applications. Renew. Energy 2017, 114, 1427–1441. [Google Scholar] [CrossRef]
- Aguilar-Reynosa, A.; Romaní, A.; Rodríguez-Jasso, R.M.; Aguilar, C.N.; Garrote, G.; Ruiz, H.A. Microwave heating processing as alternative of pretreatment in second-generation biorefinery: An overview. Energy Convers. Manag. 2017, 136, 50–65. [Google Scholar] [CrossRef]
- Li, H.; Qu, Y.; Yang, Y.; Chang, S.; Xu, J. Microwave irradiation—A green and efficient way to pretreat biomass. Bioresour. Technol. 2016, 199, 34–41. [Google Scholar] [CrossRef]
- Duan, D.; Ruan, R.; Wang, Y.; Liu, Y.; Dai, L.; Zhao, Y.; Zhou, Y.; Wu, Q. Microwave-assisted acid pretreatment of alkali lignin: Effect on characteristics and pyrolysis behavior. Bioresour. Technol. 2018, 251, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, R.; Jaiswal, A.K. A comprehensive review on pre-treatment strategy for lignocellulosic food industry waste: Challenges and opportunities. Bioresour. Technol. 2016, 199, 92–102. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Zhang, M.; Xiu, H.; He, H. Ultrasonic enhance acid hydrolysis selectivity of cellulose with HCl–FeCl3 as catalyst. Carbohydr. Polym. 2015, 117, 917–922. [Google Scholar] [CrossRef]
- Trujillo-Mayol, I.; Céspedes-Acuña, C.; Silva, F.L.; Alarcón-Enos, J. Improvement of the polyphenol extraction from avocado peel by assisted ultrasound and microwaves. J. Food Process. Eng. 2019, 42, 13197. [Google Scholar] [CrossRef]
- Araújo, R.G.; Rodriguez-Jasso, R.M.; Ruiz, H.A.; Govea-Salas, M.; Pintado, M.E.; Aguilar, C.N. Process optimization of microwave-assisted extraction of bioactive molecules from avocado seeds. Ind. Crop Prod. 2020, 154, 112623. [Google Scholar] [CrossRef]
- Lei, H.; Cybulska, I.; Julson, J. Hydrothermal Pretreatment of Lignocellulosic Biomass and Kinetics. J. Sustain. Bioenergy Syst. 2013, 03, 250–259. [Google Scholar] [CrossRef][Green Version]
- Stanton, J.; Xue, Y.; Pandher, P.; Malek, L.; Brown, T.; Hu, X.; la Cruz, D.S.-D. Impact of ionic liquid type on the structure, morphology and properties of silk-cellulose biocomposite materials. Int. J. Biol. Macromol. 2018, 108, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Smuga-Kogut, M.; Zgórska, K.; Kogut, T.; Kukiełka, K.; Wojdalski, J.; Kupczyk, A.; Dróżdż, B.; Wielewska, I. The use of ionic liquid pretreatment of rye straw for bioethanol production. Fuel 2017, 191, 266–274. [Google Scholar] [CrossRef]
- Brandt-Talbot, A.; Gschwend, F.J.V.; Fennell, P.S.; Lammens, T.M.; Tan, B.; Weale, J.; Hallett, J.P. An economically viable ionic liquid for the fractionation of lignocellulosic biomass. Green Chem. 2017, 19, 3078–3102. [Google Scholar] [CrossRef]
- Koo, B.-W.; Min, B.-C.; Gwak, K.-S.; Lee, S.-M.; Choi, J.-W.; Yeo, H.; Choi, I.-G. Structural changes in lignin during organosolv pretreatment of Liriodendron tulipifera and the effect on enzymatic hydrolysis. Biomass Bioenergy 2012, 42, 24–32. [Google Scholar] [CrossRef]
- Ahmad, E.; Pant, K.K. Chapter 14—Lignin Conversion: A Key to the Concept of Lignocellulosic Biomass-Based Integrated Biorefinery. In Waste Biorefinery; Bhaskar, T., Pandey, A., Mohan, S.V., Lee, D.-J., Khanal, S.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 409–444. [Google Scholar]
- Borand, M.N.; Karaosmanoğlu, F. Effects of organosolvpretreatment conditions for lignocellulosic biomass in biorefinery applications: A review. J. Renew. Sustain. Energy 2018, 10, 033104. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, S.-J.; Zhong, C.; Li, B.-Z.; Yuan, Y.-J. Alkali-Based Pretreatment-Facilitated Lignin Valorization: A Review. Ind. Eng. Chem. Res. 2020, 59, 16923–16938. [Google Scholar] [CrossRef]
- Chen, Y.; Stevens, M.A.; Zhu, Y.; Holmes, J.; Xu, H. Understanding of Alkaline Pretreatment Parameters for Corn Stover Enzymatic Saccharification. Biotechnol. Biofuels 2013, 6, 8. [Google Scholar] [CrossRef]
- Xu, H.; Li, B.; Mu, X. Review of Alkali-Based Pretreatment to Enhance Enzymatic Saccharification for Lignocellulosic Biomass Conversion. Ind. Eng. Chem. Res. 2016, 55, 8691–8705. [Google Scholar] [CrossRef]
- Oriez, V.; Peydecastaing, J.; Pontalier, P.-Y. Lignocellulosic Biomass Mild Alkaline Fractionation and Resulting Extract Purification Processes: Conditions, Yields, and Purities. Clean Technol. 2020, 2, 91–115. [Google Scholar] [CrossRef]
- Zhong, L.; Wang, C.; Xu, M.; Ji, X.; Yang, G.; Chen, J.; Janaswamy, S.; Lyu, G. Alkali-Catalyzed Organosolv Pretreatment of Lignocellulose Enhances Enzymatic Hydrolysis and Results in Highly Antioxidative Lignin. Energy Fuels 2021, 35, 5039–5048. [Google Scholar] [CrossRef]
- Solarte-Toro, J.C.; Romero-García, J.M.; Martínez-Patiño, J.C.; Ruiz-Ramos, E.; Castro-Galiano, E.; Cardona-Alzate, C.A. Acid pretreatment of lignocellulosic biomass for energy vectors production: A review focused on operational conditions and techno-economic assessment for bioethanol production. Renew. Sustain. Energy Rev. 2019, 107, 587–601. [Google Scholar] [CrossRef]
- Mafe, O.A.; Davies, S.M.; Hancock, J.; Du, C. Development of an estimation model for the evaluation of the energy requirement of dilute acid pretreatments of biomass. Biomass Bioenergy 2015, 72, 28–38. [Google Scholar] [CrossRef]
- Binod, P.; Pandey, A. Chapter 1—Introduction. In Pretreatment of Biomass; Pandey, A., Negi, S., Binod, P., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 3–6. [Google Scholar]
- Keskin, T.; Abubackar, H.N.; Arslan, K.; Azbar, N. Chapter 12—Biohydrogen Production from Solid Wastes. In Biohydrogen; Pandey, A., Mohan, S.V., Chang, J.-S., Hallenbeck, P.C., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 321–346. [Google Scholar]
- Brodeur, G.; Yau, E.; Badal, K.; Collier, J.; Ramachandran, K.B.; Ramakrishnan, S. Chemical and Physicochemical Pretreatment of Lignocellulosic Biomass: A Review. Enzym. Res. 2011, 2011, 1–17. [Google Scholar] [CrossRef]
- Yu, G.; Yano, S.; Inoue, H.; Inoue, S.; Endo, T.; Sawayama, S. Pretreatment of Rice Straw by a Hot-Compressed Water Process for Enzymatic Hydrolysis. Appl. Biochem. Biotechnol. 2010, 160, 539–551. [Google Scholar] [CrossRef]
- Ingram, T.; Rogalinski, T.; Bockemühl, V.; Antranikian, G.; Brunner, G. Semi-continuous liquid hot water pretreatment of rye straw. J. Supercrit. Fluids 2009, 48, 238–246. [Google Scholar] [CrossRef]
- Chen, H.-Z.; Liu, Z.-H. Steam explosion and its combinatorial pretreatment refining technology of plant biomass to bio-based products. Biotechnol. J. 2015, 10, 866–885. [Google Scholar] [CrossRef]
- Rabemanolontsoa, H.; Saka, S. Various pretreatments of lignocellulosics. Bioresour. Technol. 2016, 199, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, H.; Teymouri, F.; Gilbert, T.I.; Dale, B.E. Pretreatment of Switchgrass by Ammonia Fiber Explosion (AFEX). Appl.Biochem. Biotechnol. 2005, 124, 1133–1142. [Google Scholar] [CrossRef]
- Wan, C.; Li, Y. Solid-State Biological Pretreatment of Lignocellulosic Biomass. In Green Biomass Pretreatment for Biofuels Production; Springer: Berlin/Heidelberg, Germany, 2013; pp. 67–86. [Google Scholar]
- Yepes-Betancur, D.P.; Márquez-Cardozo, C.J.; Cadena-Chamorro, E.M.; Martinez-Saldarriaga, J.; Torres-León, C.; Ascacio-Valdes, A.; Aguilar, C.N. Solid-state fermentation-assisted extraction of bioactive compounds from hass avocado seeds. Food Bioprod. Process. 2021, 126, 155–163. [Google Scholar] [CrossRef]
- Anwar, Z.; Gulfraz, M.; Irshad, M. Agro-industrial lignocellulosic biomass a key to unlock the future bioenergy: A brief review. J. Radiat. Res. Appl. Sci. 2014, 7, 163–173. [Google Scholar] [CrossRef]
- Dhamodharan, K.; Ahlawat, S.; Kaushal, M.; Rajendran, K. 25—Economics and cost analysis of waste biorefineries. In Refining Biomass Residues for Sustainable Energy and Bioproducts; Kumar, R.P., Gnansounou, E., Raman, J.K., Baskar, G., Eds.; Academic Press: London, UK, 2020; pp. 545–565. [Google Scholar]
- Rodríguez, S.P.; Alzate, C.A.C. Chapter 12—Small-scale biorefineries based on plantain and avocado residues. In Waste Biore-finery; Bhaskar, T., Varjani, S., Pandey, A., Rene, E.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 349–374. [Google Scholar]
- Poveda-Giraldo, J.A.; Piedrahita-Rodríguez, S.; Alzate, C.A.C. Life Cycle Analysis of Biotechnological Processes Based on the Composition of the Raw Material: Eucalyptus, Avocado, and Plantain Cases in a Biorefinery System. Chem. Eng. Trans. 2021, 83, 397–402. [Google Scholar] [CrossRef]
- Melo, P.S.; Arrivetti, L.D.O.R.; De Alencar, S.M.; Skibsted, L.H. Antioxidative and prooxidative effects in food lipids and synergism with α-tocopherol of açaí seed extracts and grape rachis extracts. Food Chem. 2016, 213, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Rezende, Y.R.R.S.; Nogueira, J.P.; Narain, N. Comparison and optimization of conventional and ultrasound assisted extraction for bioactive compounds and antioxidant activity from agro-industrial acerola (Malpighia emarginata DC) residue. LWT 2017, 85, 158–169. [Google Scholar] [CrossRef]
- Fu, X.; Cheng, S.; Liao, Y.; Huang, B.; Du, B.; Zeng, W.; Jiang, Y.; Duan, X.; Yang, Z. Comparative analysis of pigments in red and yellow banana fruit. Food Chem. 2018, 239, 1009–1018. [Google Scholar] [CrossRef]
- Lima, R.D.S.; Ferreira, S.R.S.; Vitali, L.; Block, J.M. May the superfruit red guava and its processing waste be a potential ingredient in functional foods? Food Res. Int. 2019, 115, 451–459. [Google Scholar] [CrossRef]
- Mugwagwa, L.; Chimphango, A. Box-Behnken design based multi-objective optimisation of sequential extraction of pectin and anthocyanins from mango peels. Carbohydr. Polym. 2019, 219, 29–38. [Google Scholar] [CrossRef]
- García-Vargas, M.C.; Contreras, M.D.M.; Castro, E. Avocado-Derived Biomass as a Source of Bioenergy and Bioproducts. Appl. Sci. 2020, 10, 8195. [Google Scholar] [CrossRef]
- Hennessey-Ramos, L.; Murillo-Arango, W.; Guayabo, G.T. Evaluation of a colorant and oil extracted from avocado waste as functional components of a liquid soap formulation. Rev. Fac. Nac. Agron. Medellin 2019, 72, 8855–8862. [Google Scholar] [CrossRef]
- Salazar-López, N.J.; Domínguez-Avila, J.A.; Yahia, E.M.; Belmonte-Herrera, B.H.; Wall-Medrano, A.; Montalvo-González, E.; González-Aguilar, G. Avocado fruit and by-products as potential sources of bioactive compounds. Food Res. Int. 2020, 138, 109774. [Google Scholar] [CrossRef]
- Del Castillo-Llamosas, A.; del Río, P.G.; Pérez-Pérez, A.; Yáñez, R.; Garrote, G.; Gullón, B. Recent advances to recover value-added compounds from avocado by-products following a biorefinery approach. Curr. Opin. Green Sustain. Chem. 2021, 28, 100433. [Google Scholar] [CrossRef]
- Chen, H. Future perspectives for lignocellulose biorefinery engineering. In Lignocellulose Biorefinery Engineering; Woodhead Publishing: Cambridge, MA, USA, 2015; pp. 247–251. [Google Scholar]
- Weschenfelder, C.; dos Santos, J.L.; de Souza, P.A.L.; de Campos, V.P.; Marcadenti, A. Avocado and Cardiovascular Health. Open J. Endocr. Metab. Dis. 2015, 5, 77–83. [Google Scholar] [CrossRef]
- Figueroa, J.G.; Borrás-Linares, I.; Lozano-Sánchez, J.; Quirantes-Piné, R.; Segura-Carretero, A. Optimization of drying process and pressurized liquid extraction for recovery of bioactive compounds from avocado peel by-product. Electrophoresis 2018, 39, 1908–1916. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.O.; Dorantes, L.; Galíndez, A.J.; Guzmán, R.I. Effect of Different Extraction Methods on Fatty Acids, Volatile Compounds, and Physical and Chemical Properties of Avocado (Perseaamericana Mill.) Oil. J. Agric. Food Chem. 2003, 51, 2216–2221. [Google Scholar] [CrossRef] [PubMed]
- Permal, R.; Chang, W.L.; Seale, B.; Hamid, N.; Kam, R. Converting industrial organic waste from the cold-pressed avocado oil production line into a potential food preservative. Food Chem. 2020, 306, 125635. [Google Scholar] [CrossRef] [PubMed]
- El-Ahmady, N.; Deraz, S.; Khalil, A. Bioethanol Production from Lignocellulosic Feedstocks Based on Enzymatic Hydrolysis: Current Status and Recent Developments. Biotechnology 2013, 13, 1–21. [Google Scholar] [CrossRef]
- Baidhe, E.; Kiggundu, N.; Banadda, N. The Bioprocessing Quick Wins from Avocado Fruit in Uganda. Adv. Biosci. Biotechnol. 2020, 11, 405–419. [Google Scholar] [CrossRef]
- Di Bitonto, L.; Reynel-Ávila, H.E.; Castillo, D.I.M.; Bonilla-Petriciolet, A.; Pastore, C. Residual Mexican biomasses for bioenergy and fine chemical production: Correlation between composition and specific applications. Biomass Convers. Biorefinery 2021, 11, 619–631. [Google Scholar] [CrossRef]
- Karagoz, P.; Bill, R.M.; Ozkan, M. Lignocellulosic ethanol production: Evaluation of new approaches, cell immobilization and reactor configurations. Renew. Energy 2019, 143, 741–752. [Google Scholar] [CrossRef]
- Ginting, M.H.S.; Misran, E.; Maulina, S. Potential of durian, avocado and jackfruit seed as raw material of bioethanol: A review. IOP Conf. Ser. Mater. Sci. Eng. 2020, 801, 012045. [Google Scholar] [CrossRef]
- Woldu, A.R.; Ashagrie, Y.N.; Tsigie, Y.A. Bioethanol Production from Avocado Seed Wastes Using Saccharomyces Cerevisiae. Am. J. Environ. Energy Power Res. 2015, 3, 1–9. [Google Scholar]
- Pratywi, C.D.; Marantika, S.; Dwijananti, P. Masturi Characterization of starch degradation during simple heating for bioethanol production from the avocado seed. IOP Conf. Ser. Mater. Sci. Eng. 2018, 432, 012042. [Google Scholar] [CrossRef]
- Faik, A. “Plant Cell Wall Structure-Pretreatment” the Critical Relationship in Biomass Conversion to Fermentable Sugars. In Green Biomass Pretreatment for Biofuels Production; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–30. [Google Scholar]
- Silvello, M.A.D.C.; Martínez, J.; Goldbeck, R. Alternative technology for intensification of fermentable sugars released from enzymatic hydrolysis of sugarcane bagasse. Biomass Convers. Biorefinery 2020, 1–7. [Google Scholar] [CrossRef]
- Gu, T. Pretreatment of Lignocellulosic Biomass Using Supercritical Carbon Dioxide as a Green Solvent. In Green Biomass Pretreatment for Biofuels Production; Springer: Berlin/Heidelberg, Germany, 2013; pp. 107–125. [Google Scholar]
- Dagde, K. Extraction of vegetable oil from avocado seeds for production of biodiesel. J. Appl. Sci. Environ. Manag. 2019, 23, 215. [Google Scholar] [CrossRef]
- Melgar, B.; Dias, M.I.; Ciric, A.; Sokovic, M.; Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Barros, L.; Ferreira, I.C. Bioactive characterization of Persea americana Mill. by-products: A rich source of inherent antioxidants. Ind. Crop Prod. 2018, 111, 212–218. [Google Scholar] [CrossRef]
- Alkhalaf, M.I.; Alansari, W.S.; Ibrahim, E.A.; Elhalwagy, M.E. Anti-oxidant, anti-inflammatory and anti-cancer activities of avocado (Persea americana) fruit and seed extract. J. King Saud Univ. Sci. 2019, 31, 1358–1362. [Google Scholar] [CrossRef]
- Cires, M.J.; Navarrete, P.; Pastene, E.; Carrasco-Pozo, C.; Valenzuela, R.; Medina, D.; Andriamihaja, M.; Beaumont, M.; Blachier, F.; Gotteland, M. Protective Effect of an Avocado Peel Polyphenolic Extract Rich in Proanthocyanidins on the Alterations of Colonic Homeostasis Induced by a High-Protein Diet. J. Agric. Food Chem. 2019, 67, 11616–11626. [Google Scholar] [CrossRef]
- Torres, E.; García, A.; Aranda, M.; Saéz, V.; Zúñiga, F.; Alarcón, J.; Avello, M.; Pastene, E. One-step purification of two semi-synthetic epicatechin adducts prepared from avocado peels procyanidins by centrifugal partition chromatography and evaluation of their anti-inflammatory effects on adenocarcinoma gastric cells infected with Helicobacter pylori. J. Chil. Chem. Soc. 2018, 63, 4222–4228. [Google Scholar] [CrossRef]
- Athaydes, B.R.; Alves, G.M.; de Assis, A.L.E.M.; Gomes, J.V.D.; Rodrigues, R.P.; Campagnaro, B.P.; Nogueira, B.V.; Silveira, D.; Kuster, R.M.; Pereira, T.M.C.; et al. Avocado seeds (Persea americana Mill.) prevents indomethacin-induced gastric ulcer in mice. Food Res. Int. 2019, 119, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, J.; Zhang, F.; Dou, X.; Ma, A.; Zhang, X.; Shao, H.; Zhao, S.; Ling, P.; Liu, F.; et al. Lower range of molecular weight of xanthan gum inhibits apoptosis of chondrocytes through MAPK signaling pathways. Int. J. Biol. Macromol. 2019, 130, 79–87. [Google Scholar] [CrossRef]
- Dabas, D.; Ziegler, G.R.; Lambert, J.D. Anti-Inflammatory Properties of a Colored Avocado Seed Extract. Adv. Food Technol. Nutr. Sci. Open J. 2019, 5, 8–12. [Google Scholar] [CrossRef]
- Willyard, C. The drug-resistant bacteria that pose the greatest health threats. Nat. Cell Biol. 2017, 543, 15. [Google Scholar] [CrossRef] [PubMed]
- Amado, D.A.V.; Helmann, G.A.; Detoni, A.M.; De Carvalho, S.L.C.; De Aguiar, C.M.; Martin, C.A.; Tiuman, T.S.; Cottica, S.M. Antioxidant and antibacterial activity and preliminary toxicity analysis of four varieties of avocado (Persea americana Mill.). Braz. J. Food Technol. 2019, 22. [Google Scholar] [CrossRef]
- Villarreal-Lara, R.; Rodríguez-Sánchez, D.G.; De La Garza, R.I.D.; García-Cruz, M.I.; Castillo, A.; Pacheco, A.; Hernández-Brenes, C. Purified avocado seed acetogenins: Antimicrobial spectrum and complete inhibition of Listeria monocytogenes in a refrigerated food matrix. CyTAJ. Food 2019, 17, 228–239. [Google Scholar] [CrossRef]
- Salinas-Salazar, C.; Hernández-Brenes, C.; Rodríguez-Sánchez, D.G.; Castillo, E.C.; Navarro-Silva, J.M.; Pacheco, A. Inhibitory Activity of Avocado Seed Fatty Acid Derivatives (Acetogenins) against Listeria Monocytogenes. J. Food Sci. 2016, 82, 134–144. [Google Scholar] [CrossRef]
- Gonçalves, B.; Moeenfard, M.; Rocha, F.; Alves, A.; Estevinho, B.N.; Santos, L. Microencapsulation of a Natural Antioxidant from Coffee—Chlorogenic Acid (3-Caffeoylquinic Acid). Food Bioprocess Technol. 2017, 10, 1521–1530. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Zhang, X.; Zu, Y.; Yang, Y.; Liu, W.; Xu, Z.; Gao, H.; Sun, X.; Jiang, X.; et al. Current Advances in Naturally Occurring Caffeoylquinic Acids: Structure, Bioactivity, and Synthesis. J. Agric. Food Chem. 2020, 68, 10489–10516. [Google Scholar] [CrossRef]
- You, Z.; Wang, Y. A Versatile Synthetic Platform for a Wide Range of Functionalized Biomaterials. Adv. Funct. Mater. 2012, 22, 2812–2820. [Google Scholar] [CrossRef]
- Gullón, P.; Astray, G.; Gullón, B.; Tomasevic, I.; Lorenzo, J.M. Pomegranate Peel as Suitable Source of High-Added Value Bioactives: Tailored Functionalized Meat Products. Molecules 2020, 25, 2859. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Abdel-Rahman, M.A.; Sonomoto, K. Biorefinery-Based Lactic Acid Fermentation: Microbial Production of Pure Monomer Product. In Synthesis, Structure and Properties of Poly(lactic acid); Di Lorenzo, M.L., Androsch, R., Eds.; Springer: Cham, Switzerland, 2018; pp. 27–66. [Google Scholar]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Yeo, J.C.C.; Muiruri, J.K.; Thitsartarn, W.; Li, Z.; He, C. Recent advances in the development of biodegradable PHB-based toughening materials: Approaches, advantages and applications. Mater. Sci. Eng. C 2018, 92, 1092–1116. [Google Scholar] [CrossRef] [PubMed]
- Naranjo, J.M.; Cardona, C.A.; Higuita, J.C. Use of residual banana for polyhydroxybutyrate (PHB) production: Case of study in an integrated biorefinery. Waste Manag. 2014, 34, 2634–2640. [Google Scholar] [CrossRef] [PubMed]
- Getachew, A.; Woldesenbet, F. Production of biodegradable plastic by polyhydroxybutyrate (PHB) accumulating bacteria using low cost agricultural waste material. BMC Res. Notes 2016, 9, 1–9. [Google Scholar] [CrossRef]
- Manavitehrani, I.; Fathi, A.; Badr, H.; Daly, S.; Shirazi, A.N.; Dehghani, F. Biomedical Applications of Biodegradable Polyesters. Polymers 2016, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Krucińska, I.; Żywicka, B.; Komisarczyk, A.; Szymonowicz, M.; Kowalska, S.; Zaczyńska, E.; Struszczyk, M.; Czarny, A.; Jadczyk, P.; Umińska-Wasiluk, B.; et al. Biological Properties of Low-Toxicity PLGA and PLGA/PHB Fibrous Nanocomposite Implants for Osseous Tissue Regeneration. Part I: Evaluation of Potential Biotoxicity. Molecules 2017, 22, 2092. [Google Scholar] [CrossRef]
- Kai, D.; Zhang, K.; Liow, S.S.; Loh, X.J. New Dual Functional PHB-Grafted Lignin Copolymer: Synthesis, Mechanical Properties, and Biocompatibility Studies. ACS Appl. Bio Mater. 2018, 2, 127–134. [Google Scholar] [CrossRef]
- Santos, D.M.d.; Ascheri, D.P.R.; Bukzem, A.d.; Morais, C.C.; Carvalho, C.W.P.; Ascheri, J.L.R. Physicochemical properties of starch from avocado seed (Persea americana Mill). Dos Santos 2016, 34. [Google Scholar] [CrossRef]
- Chel-Guerrero, L.; Barbosa-Martín, E.; Martínez-Antonio, A.; González-Mondragón, E.; Betancur-Ancona, D. Some physicochemical and rheological properties of starch isolated from avocado seeds. Int. J. Biol. Macromol. 2016, 86, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, T.; Gibril, M.; Sithole, B.; Ramjugernath, D.; Chavan, R.; Chunilall, V.; Gounden, N. Valorisation of avocado seeds: Extraction and characterisation of starch for textile applications. Clean Technol. Environ. Policy 2018, 20, 2135–2154. [Google Scholar] [CrossRef]
- Tesfaye, T.; Ayele, M.; Ferede, E.; Gibril, M.; Kong, F.; Sithole, B. A techno-economic feasibility of a process for extraction of starch from waste avocado seeds. Clean Technol. Environ. Policy 2020, 1, 1–15. [Google Scholar] [CrossRef]
- Ubwa, S.T.; Abah, J.; Asemave, K.; Shambe, T. Studies on the Gelatinization Temperature of Some Cereal Starches. Int. J. Chem. 2012, 4. [Google Scholar] [CrossRef]
- Ginting, M.; Hasibuan, R.; Lubis, M.; Alanjani, F.; Winoto, F.; Siregar, R. Utilization of Avocado Seeds as Bioplastic Films Filler Chitosan and Ethylene Glycol Plasticizer. Asian J. Chem. 2018, 30, 1569–1573. [Google Scholar] [CrossRef]
- Lubis, M.; Harahap, M.B.; Ginting, H.S.; Sartika, M.; Azmi, H. Production of bioplastic from avocado seed starch reinforced with microcrystalline cellulose from sugar palm fibers. Chemistry 2018, 13, 13. [Google Scholar]
- Araújo, R.G.; Rodríguez-Jasso, R.M.; Ruiz, H.A.; Govea-Salas, M.; Rosas-Flores, W.; Aguilar-González, M.A.; Pintado, M.E.; Lopez-Badillo, C.; Luevanos, C.; Aguilar, C.N. Hydrothermal–Microwave Processing for Starch Extraction from Mexican Avocado Seeds: Operational Conditions and Characterization. Processes 2020, 8, 759. [Google Scholar] [CrossRef]
- George, J. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54. [Google Scholar] [CrossRef]
- Gómez, H.C.; Serpa, A.; Velásquez-Cock, J.; Gañán, P.; Castro, C.; Vélez, L.; Zuluaga, R. Vegetable Nanocellulose in Food Science: A Review. Food Hydrocoll. 2016, 57, 178–186. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef]
- RajeshKumar, S.; Rinitha, G. Nanostructural characterization of antimicrobial and antioxidant copper nanoparticles synthesized using novel Persea americana seeds. OpenNano 2018, 3, 18–27. [Google Scholar] [CrossRef]
- Azeredo, H.M.; Rosa, M.F.; Mattoso, L.H.C. Nanocellulose in bio-based food packaging applications. Ind. Crop Prod. 2017, 97, 664–671. [Google Scholar] [CrossRef]
- Rajwade, J.M.; Chikte, R.G.; Paknikar, K.M. Nanomaterials: New weapons in a crusade against phytopathogens. Appl. Microbiol. Biotechnol. 2020, 104, 1437–1461. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Avena-Bustillos, R.J.; Chiou, B.-S.; Li, Y.; Ma, Y.; Williams, T.G.; Wood, D.F.; McHugh, T.H.; Zhong, F. Controlled-release of tea polyphenol from gelatin films incorporated with different ratios of free/nanoencapsulated tea polyphenols into fatty food simulants. Food Hydrocoll. 2017, 62, 212–221. [Google Scholar] [CrossRef]
- Adebayo, A.E.; Oke, A.M.; Lateef, A.; Oyatokun, A.A.; Abisoye, O.D.; Adiji, I.P.; Fagbenro, D.O.; Amusan, T.V.; Badmus, J.A.; Asafa, T.B.; et al. Biosynthesis of silver, gold and silver–gold alloy nanoparticles using Persea americana fruit peel aqueous extract for their biomedical properties. Nanotechnol. Environ. Eng. 2019, 4, 13. [Google Scholar] [CrossRef]
- Lara-Flores, A.A.; Araújo, R.G.; Rodríguez-Jasso, R.M.; Aguedo, M.; Aguilar, C.N.; Trajano, H.L.; Ruiz, H.A. Bioeconomy and Biorefinery: Valorization of Hemicellulose from Lignocellulosic Biomass and Potential Use of Avocado Residues as a Promising Resource of Bioproducts. In Waste to Wealth; Singhania, R.R., Agarwal, R.A., Kumar, R.P., Sukumaran, R.K., Eds.; Springer: Singapore, 2018; pp. 141–170. [Google Scholar]
- Antunes, F.A.F.; Gaikwad, S.; Ingle, A.P.; Pandit, R.; dos Santos, J.C.; Rai, M.; da Silva, S.S. Bioenergy and Biofuels: Nano-technological Solutions for Sustainable Production. In Nanotechnology for Bioenergy and Biofuel Production; Rai, M., da Silva, S.S., Eds.; Springer: Cham, Switzerland, 2017; pp. 3–18. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mora-Sandí, A.; Ramírez-González, A.; Castillo-Henríquez, L.; Lopretti-Correa, M.; Vega-Baudrit, J.R. Persea Americana Agro-Industrial Waste Biorefinery for Sustainable High-Value-Added Products. Polymers 2021, 13, 1727. https://doi.org/10.3390/polym13111727
Mora-Sandí A, Ramírez-González A, Castillo-Henríquez L, Lopretti-Correa M, Vega-Baudrit JR. Persea Americana Agro-Industrial Waste Biorefinery for Sustainable High-Value-Added Products. Polymers. 2021; 13(11):1727. https://doi.org/10.3390/polym13111727
Chicago/Turabian StyleMora-Sandí, Anthony, Abigail Ramírez-González, Luis Castillo-Henríquez, Mary Lopretti-Correa, and José Roberto Vega-Baudrit. 2021. "Persea Americana Agro-Industrial Waste Biorefinery for Sustainable High-Value-Added Products" Polymers 13, no. 11: 1727. https://doi.org/10.3390/polym13111727
APA StyleMora-Sandí, A., Ramírez-González, A., Castillo-Henríquez, L., Lopretti-Correa, M., & Vega-Baudrit, J. R. (2021). Persea Americana Agro-Industrial Waste Biorefinery for Sustainable High-Value-Added Products. Polymers, 13(11), 1727. https://doi.org/10.3390/polym13111727