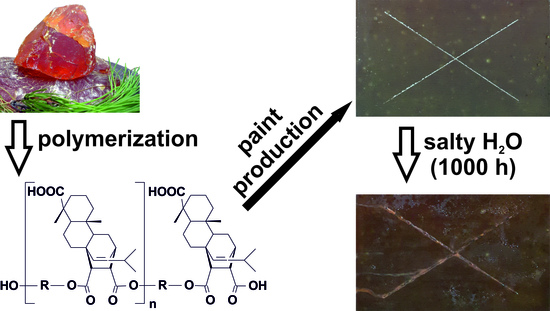
Composition and Properties of Protective Coatings Made of Biologically-Derived Polyester Reactive Binder
Abstract
1. Introduction
- the flexibility of chains in molecules enabling non-brittle curing of coatings;
- and softening temperatures in a range of ca. 100–140 °C, which should be lower than curing temperatures of powder coatings (usually 140–220 °C).
2. Materials and Methods
2.1. Materials
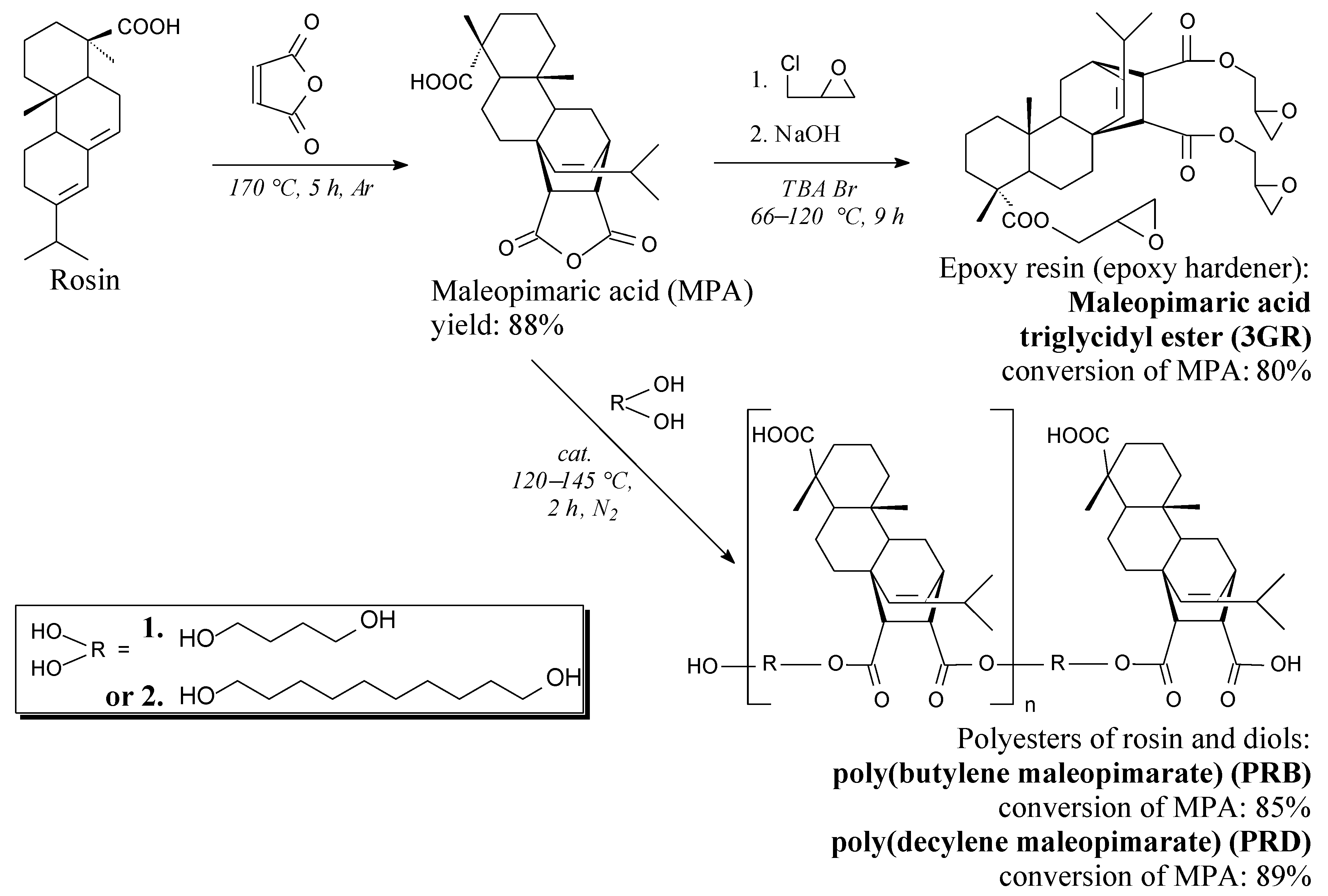
2.2. Synthesis of Maleopimaric Acid and its Triglycidyl Ester
2.3. Synthesis of Rosin-Biodiol Polyesters
2.4. Preparation of Natural Origin Anti-Corrosive Filler
2.5. Preparation of Natural-Resources-Rich Powder Coatings
2.6. Evaluation Methods of the Components, Coating Compositions, and Coatings
3. Results and Discussion
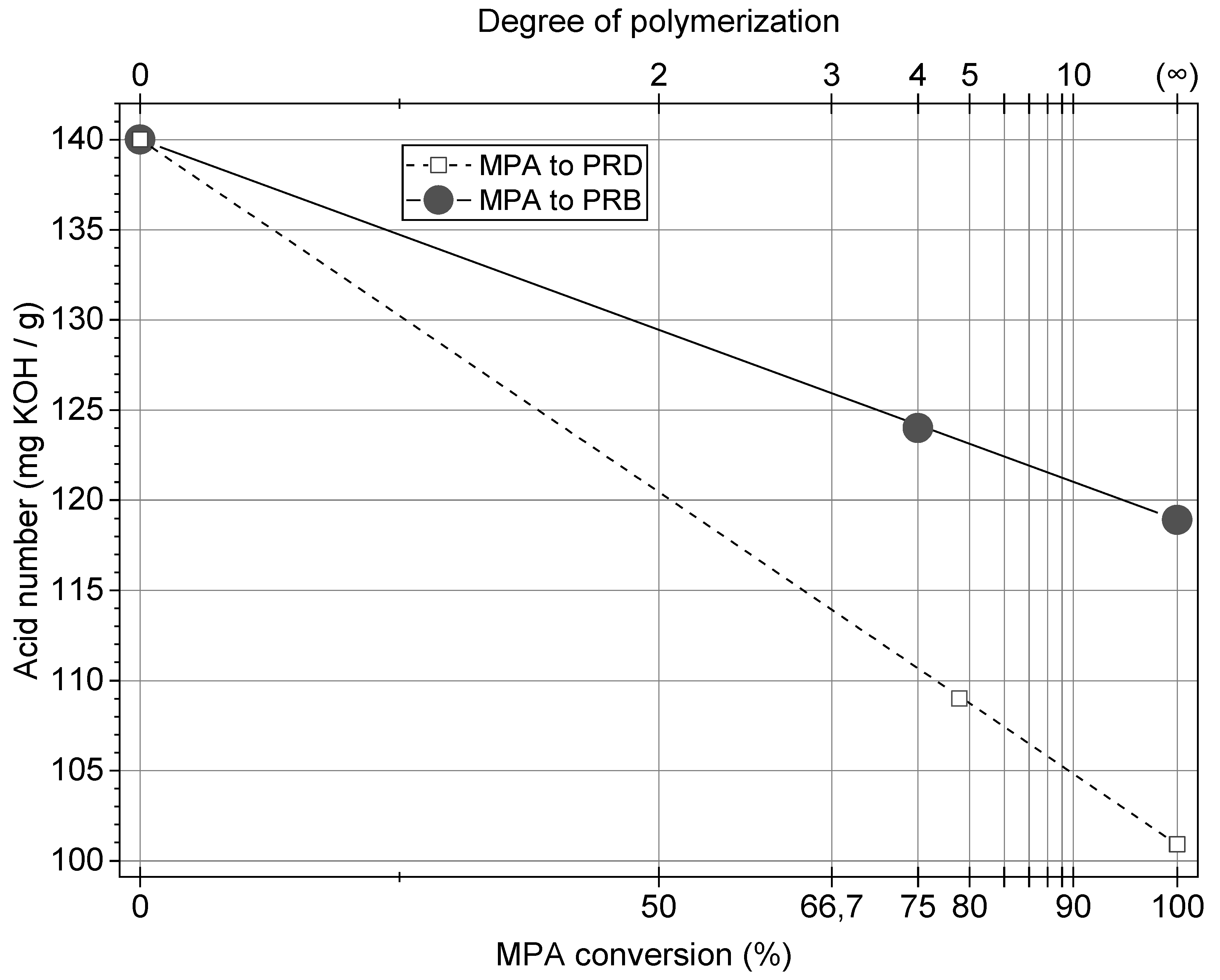
3.1. Properties of Rosin-Derived Polyester Resins
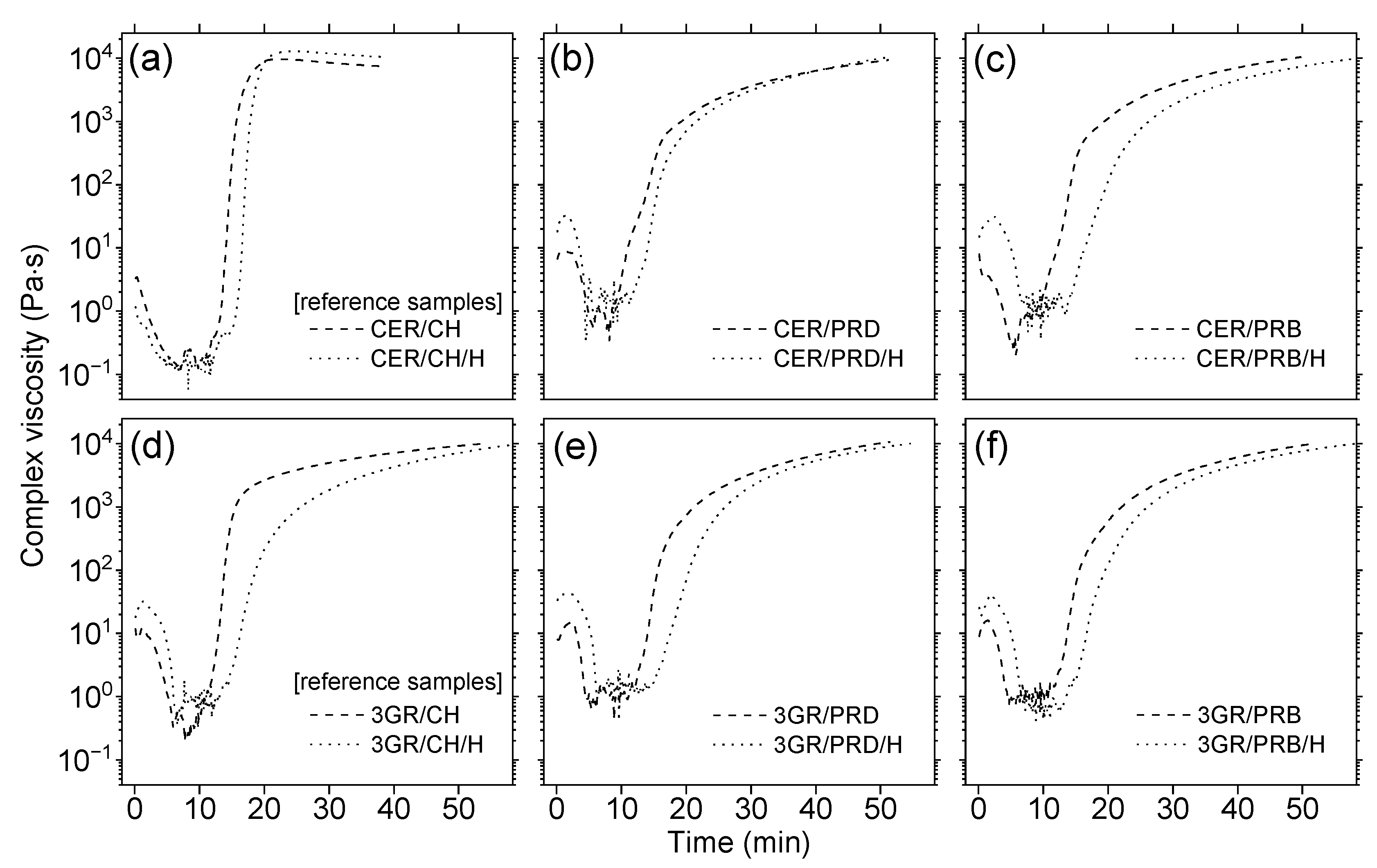
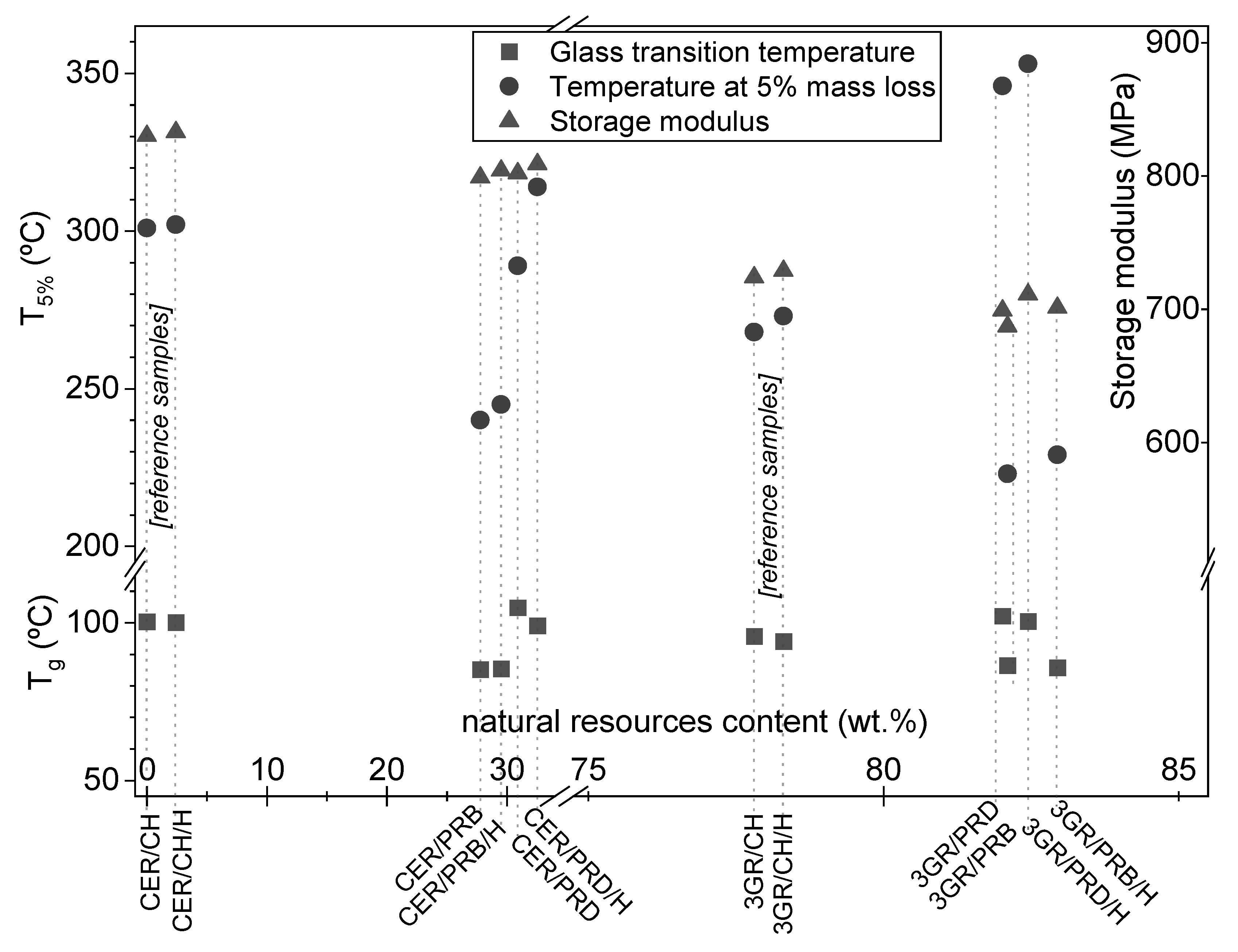
3.2. Thermal Properties of Coating Materials
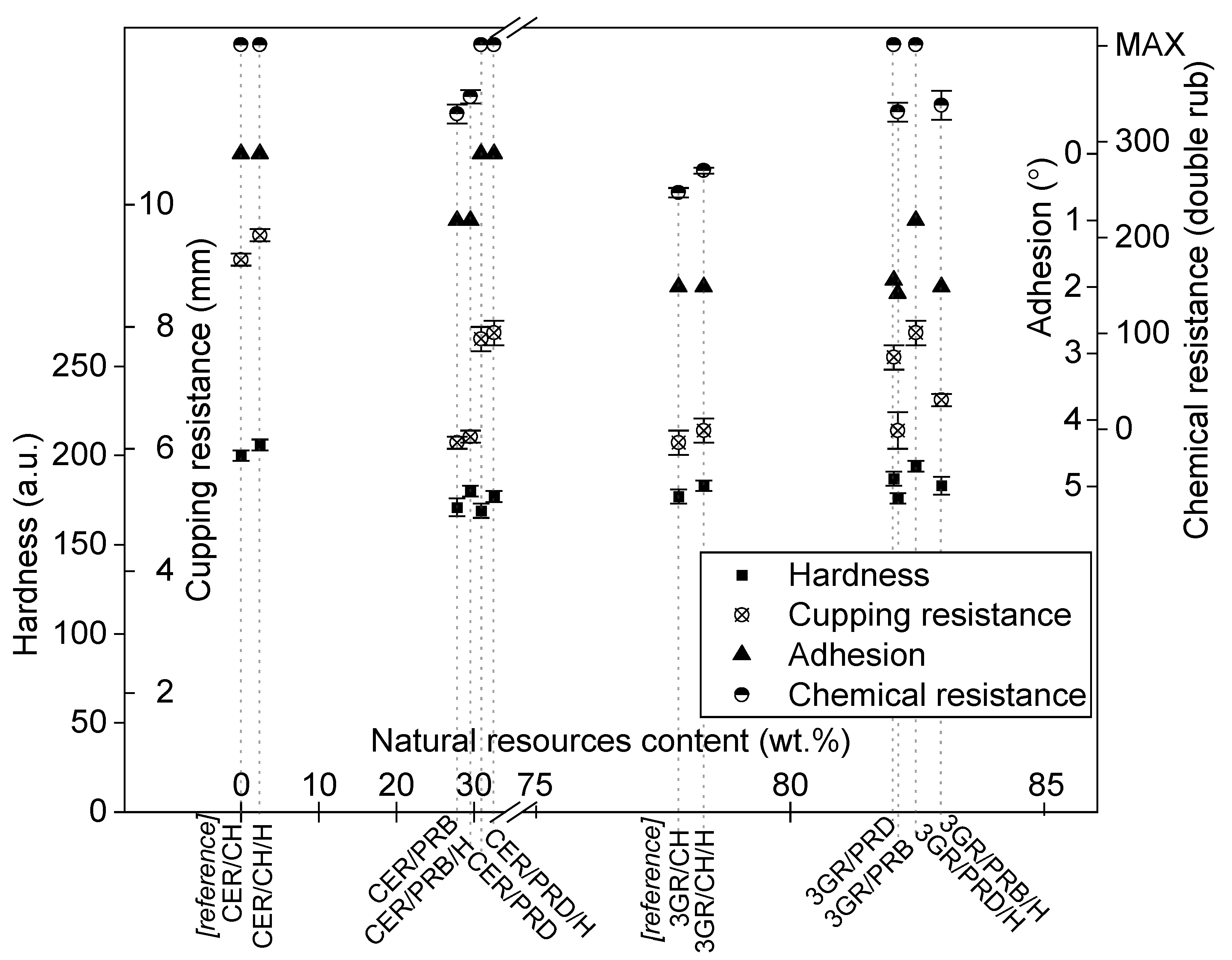
3.3. Mechanical Properties of Coatings
3.4. Functional Properties of Coatings
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Geyer, R. Chapter 2—Production, use, and fate of synthetic polymers. In Plastic Waste and Recycling; Letcher, T.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 13–32. ISBN 978-0-12-817880-5. [Google Scholar]
- Ritchie, H.; Roser, M. CO2 and Greenhouse Gas Emissions. 2020. Available online: https://ourworldindata.org/co2-and-other-greenhouse-gas-emissions?fbclid=IwAR1-222h-a-w2N6erxdE8kSEBVGCY0XZnxaFhhG8R4KjvPaEwlrXA0sMkxk (accessed on 15 April 2021).
- Spyrou, E. Powder Coatings Chemistry and Technology: 3rd Revised Edition; Vincentz Network: Hannover, Germany, 2014; ISBN 978-3-7486-0236-1. [Google Scholar]
- Powder Coatings Market Size, Share|Industry Report. 2021. Available online: https://www.grandviewresearch.com/industry-analysis/powder-coatings-market-analysis (accessed on 1 February 2021).
- Kumar, S.; Krishnan, S.; Mohanty, S.; Nayak, S.K. Synthesis and Characterization of Petroleum and Biobased Epoxy Resins: A Review. Polym. Int. 2018, 67, 815–839. [Google Scholar] [CrossRef]
- Ma, S.; Li, T.; Liu, X.; Zhu, J. Research Progress on Bio-Based Thermosetting Resins. Polym. Int. 2016, 65, 164–173. [Google Scholar] [CrossRef]
- Kumar, S.; Samal, S.K.; Mohanty, S.; Nayak, S.K. Recent Development of Biobased Epoxy Resins: A Review. Polym. Plast. Technol. 2018, 57, 133–155. [Google Scholar] [CrossRef]
- Ding, C.; Matharu, A.S. Recent Developments on Biobased Curing Agents: A Review of Their Preparation and Use. ACS Sustain. Chem. Eng. 2014, 2, 2217–2236. [Google Scholar] [CrossRef]
- Baroncini, E.A.; Yadav, S.K.; Palmese, G.R.; Stanzione, J.F. Recent Advances in Bio-Based Epoxy Resins and Bio-Based Epoxy Curing Agents. J. Appl. Polym. Sci. 2016, 133, 44103. [Google Scholar] [CrossRef]
- Kugler, S.; Ossowicz, P.; Malarczyk-Matusiak, K.; Wierzbicka, E. Advances in Rosin-Based Chemicals: The Latest Recipes, Applications and Future Trends. Molecules 2019, 24, 1651. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.J.; Xu, R.S.; Zhao, Z.D.; Zhang, P.H.; Wang, M.X. Recent Progress on Derivation and Chemical Modification of Rosin Acids. Adv. Mater. Res. 2013, 785–786, 1111–1116. [Google Scholar] [CrossRef]
- Parthiban, A. Monomers and polymers derived from renewable or partially renewable resources. In Synthesis and Applications of Copolymers; Wiley-Blackwell: Hoboken, NJ, USA, 2014; pp. 101–124. ISBN 978-1-118-86016-8. [Google Scholar]
- Gandini, A.; Lacerda, T.M. From Monomers to Polymers from Renewable Resources: Recent Advances. Prog. Polym. Sci. 2015, 48, 1–39. [Google Scholar] [CrossRef]
- Zia, K.M.; Noreen, A.; Zuber, M.; Tabasum, S.; Mujahid, M. Recent Developments and Future Prospects on Bio-Based Polyesters Derived from Renewable Resources: A Review. Int. J. Biol. Macromol. 2016, 82, 1028–1040. [Google Scholar] [CrossRef]
- Wilbon, P.A.; Chu, F.; Tang, C. Progress in Renewable Polymers from Natural Terpenes, Terpenoids, and Rosin. Macromol. Rapid Commun. 2013, 34, 8–37. [Google Scholar] [CrossRef]
- Liu, X.; Xin, W.; Zhang, J. Rosin-Based Acid Anhydrides as Alternatives to Petrochemical Curing Agents. Green Chem. 2009, 11, 1018–1025. [Google Scholar] [CrossRef]
- Liu, X.; Xin, W.; Zhang, J. Rosin-Derived Imide-Diacids as Epoxy Curing Agents for Enhanced Performance. Bioresource Technol. 2010, 101, 2520–2524. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, X.; Liu, B.; Zhang, J.; Xian, M. Synthesis of Rosin-Based Flexible Anhydride-Type Curing Agents and Properties of the Cured Epoxy. Polym. Int. 2009, 58, 1435–1441. [Google Scholar] [CrossRef]
- Mustata, F.R.; Tudorachi, N. Epoxy Resins Cross-Linked with Rosin Adduct Derivatives. Cross-Linking and Thermal Behaviors. Ind. Eng. Chem. Res. 2010, 49, 12414–12422. [Google Scholar] [CrossRef]
- Liu, X.Q.; Huang, W.; Jiang, Y.H.; Zhu, J.; Zhang, C.Z. Preparation of a Bio-Based Epoxy with Comparable Properties to Those of Petroleum-Based Counterparts. Express Polym. Lett. 2012, 6, 293–298. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J. High-Performance Biobased Epoxy Derived from Rosin. Polym. Int. 2010, 59, 607–609. [Google Scholar] [CrossRef]
- Jaswal, S.; Gaur, B. Structure-Property Correlation Study of Bio-Based Multifunctional Vinyl Ester Resin in Presence of Methacrylated Lignin Model Compounds. Polym. Sci. Ser. B 2015, 57, 417–433. [Google Scholar] [CrossRef]
- Li, T.; Liu, X.; Jiang, Y.; Ma, S.; Zhu, J. Bio-Based Shape Memory Epoxy Resin Synthesized from Rosin Acid. Iran. Polym. J. 2016, 25, 957–965. [Google Scholar] [CrossRef]
- Deng, L.; Shen, M.; Yu, J.; Wu, K.; Ha, C. Preparation, Characterization, and Flame Retardancy of Novel Rosin-Based Siloxane Epoxy Resins. Ind. Eng. Chem. Res. 2012, 51, 8178–8184. [Google Scholar] [CrossRef]
- Li, C.; Liu, X.; Zhu, J.; Zhang, C.; Guo, J. Synthesis, Characterization of a Rosin-Based Epoxy Monomer and Its Comparison with a Petroleum-Based Counterpart. J. Macromol. Sci. A 2013, 50, 321–329. [Google Scholar] [CrossRef]
- El-Ghazawy, R.A.; El-Saeed, A.M.; Al-Shafey, H.I.; Abdul-Raheim, A.-R.M.; El-Sockary, M.A. Rosin Based Epoxy Coating: Synthesis, Identification and Characterization. Eur. Polym. J. 2015, 69, 403–415. [Google Scholar] [CrossRef]
- Brocas, A.-L.; Llevot, A.; Mantzaridis, C.; Cendejas, G.; Auvergne, R.; Caillol, S.; Carlotti, S.; Cramail, H. Epoxidized Rosin Acids as Co-Precursors for Epoxy Resins. Des. Monomers Polym. 2014, 17, 301–310. [Google Scholar] [CrossRef]
- Mantzaridis, C.; Brocas, A.-L.; Llevot, A.; Cendejas, G.; Auvergne, R.; Caillol, S.; Carlotti, S.; Cramail, H. Rosin Acid Oligomers as Precursors of DGEBA-Free Epoxy Resins. Green Chem. 2013, 15, 3091–3098. [Google Scholar] [CrossRef]
- Wierzbicka, E.; Kugler, S.; Ossowicz, P.; Malarczyk-Matusiak, K. Modifier of Epoxy Coating Compositions Properties. Preparation and Characterization. Mater. Eng. 2020, 5. [Google Scholar] [CrossRef]
- Fenouillot, F.; Rousseau, A.; Colomines, G.; Saint-Loup, R.; Pascault, J.-P. Polymers from Renewable 1,4:3,6-Dianhydrohexitols (Isosorbide, Isomannide and Isoidide): A Review. Prog. Polym. Sci. 2010, 35, 578–622. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Patel, M.K. Plastics Derived from Biological Sources: Present and Future: A Technical and Environmental Review. Chem. Rev. 2012, 112, 2082–2099. [Google Scholar] [CrossRef] [PubMed]
- Albertsson, A.-C.; Varma, I.K. Recent Developments in Ring Opening Polymerization of Lactones for Biomedical Applications. Biomacromolecules 2003, 4, 1466–1486. [Google Scholar] [CrossRef] [PubMed]
- Lavilla, C.; de Ilarduya, A.M.; Alla, A.; García-Martín, M.G.; Galbis, J.A.; Muñoz-Guerra, S. Bio-Based Aromatic Polyesters from a Novel Bicyclic Diol Derived from d-Mannitol. Macromolecules 2012, 45, 8257–8266. [Google Scholar] [CrossRef]
- Noordover, B.A.J.; Duchateau, R.; van Benthem, R.A.T.M.; Ming, W.; Koning, C.E. Enhancing the Functionality of Biobased Polyester Coating Resins through Modification with Citric Acid. Biomacromolecules 2007, 8, 3860–3870. [Google Scholar] [CrossRef]
- de Miranda, M.I.G.; Tomedi, C.; Bica, C.I.D.; Samios, D. A d.s.c. Kinetic Study on the Effect of Filler Concentration on Crosslinking of Diglycidylether of Bisphenol-A with 4,4′-Diaminodiphenylmethane. Polymer 1997, 38, 1017–1020. [Google Scholar] [CrossRef]
- ISO 1520:2006. Paints and Varnishes—Cupping Test; ISO: Geneva, Switzerland, 2006; Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/04/09/40923.html (accessed on 27 April 2021).
- ISO 2409:2020. Paints and varnishes—Cross-cut test; ISO: Geneva, Switzerland, 2020; Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/07/60/76041.html (accessed on 27 April 2021).
- ISO 9227:2017. Corrosion Tests in Artificial Atmospheres—Salt Spray Tests; ISO: Geneva, Switzerland, 2017; Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/06/35/63543.html (accessed on 27 April 2021).
- PN-EN 13523-11:2020-01. Metale Powlekane Metodą Ciągłą–Metody Badań–Część 11: Odporność na Rozpuszczalniki (Próba Pocierania); Polish Committee for Standardization: Warsaw, Poland, 2020; Available online: https://sklep.pkn.pl/pn-en-13523-11-2020-01e.html (accessed on 27 April 2021).
- ISO 2813:2014. Paints and Varnishes—Determination of Gloss Value at 20°, 60° and 85°; ISO: Geneva, Switzerland, 2014; Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/05/68/56807.html (accessed on 27 April 2021).

| Sample Symbol | Used Bio-Diol | Catalyst | Reaction Temperature (°C) | Softening Temperature (°C) | Acid Number (mg KOH/g) |
|---|---|---|---|---|---|
| PRB | butanediol | dibutyltin oxide | 140 ± 5 | 100 | 190 |
| PRD | decanediol | 3-ethanolamine | 125 ± 5 | 120 | 170 |
| Sample Symbol | Weight Ratio of Components in Coating Composition (wt. Part) | |||||
|---|---|---|---|---|---|---|
| Commercial Epoxy Resin (CER) | Rosin-Based Epoxy Resin (3GR) | Commercial Hardener (CH) | BD/Rosin Polyester (PRB) | DD/Rosin Polyester (PRD) | Modified Halloysite (H) | |
| CER/CH a | 97.8 | - | 2.2 | - | - | - |
| CER/CH/H a | 97.8 | - | 2.2 | - | - | 2.5 |
| CER/PRB | 65.7 | - | - | 34.3 | - | - |
| CER/PRB/H | 65.7 | - | - | 34.3 | - | 2.5 |
| CER/PRD | 61.9 | - | - | - | 38.1 | - |
| CER/PRD/H | 61.9 | - | - | - | 38.1 | 2.5 |
| 3GR/CH a | - | 93.7 | 6.3 | - | - | - |
| 3GR/CH/H a | - | 93.7 | 6.3 | - | - | 2.5 |
| 3GR/PRB | - | 38.8 | - | 61.2 | - | - |
| 3GR/PRB/H | - | 38.8 | - | 61.2 | - | 2.5 |
| 3GR/PRD | - | 35 | - | - | 65 | - |
| 3GR/PRD/H | - | 35 | - | - | 65 | 2.5 |
| Sample Symbol | Gloss (G.U.) | Haze (a.u.) | Color (RAL) |
|---|---|---|---|
| CER/CH | 70 ± 3 | 7 ± 1 | 7034 (yellow grey) |
| CER/CH/H | 62 ± 4 | 10 ± 1 | 8000 (green brown) |
| CER/PRB | 70 ± 4 | 6 ± 1 | 7008 (khaki grey) |
| CER/PRB/H | 65 ± 2 | 8 ± 1 | 8008 (olive brown) |
| CER/PRD | 63 ± 3 | 9 ± 1 | 1020 (olive yellow) |
| CER/PRD/H | 50 ± 3 | 15 ± 1 | 7006 (beige grey) |
| 3GR/CH | 61 ± 2 | 9 ± 1 | 1036 (pearl gold) |
| 3GR/CH/H | 55 ± 3 | 14 ± 1 | 7002 (olive grey) |
| 3GR/PRB | 68 ± 1 | 10 ± 1 | 8024 (beige brown) |
| 3GR/PRB/H | 60 ± 5 | 16 ± 1 | 7013 (brown grey) |
| 3GR/PRD | 52 ± 3 | 14 ± 1 | 8025 (pale brown) |
| 3GR/PRD/H | 51 ± 2 | 16 ± 1 | 7006 (beige grey) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kugler, S.; Wierzbicka, E.; Ossowicz-Rupniewska, P.; Łopiński, J. Composition and Properties of Protective Coatings Made of Biologically-Derived Polyester Reactive Binder. Polymers 2021, 13, 1700. https://doi.org/10.3390/polym13111700
Kugler S, Wierzbicka E, Ossowicz-Rupniewska P, Łopiński J. Composition and Properties of Protective Coatings Made of Biologically-Derived Polyester Reactive Binder. Polymers. 2021; 13(11):1700. https://doi.org/10.3390/polym13111700
Chicago/Turabian StyleKugler, Szymon, Ewa Wierzbicka, Paula Ossowicz-Rupniewska, and Jakub Łopiński. 2021. "Composition and Properties of Protective Coatings Made of Biologically-Derived Polyester Reactive Binder" Polymers 13, no. 11: 1700. https://doi.org/10.3390/polym13111700
APA StyleKugler, S., Wierzbicka, E., Ossowicz-Rupniewska, P., & Łopiński, J. (2021). Composition and Properties of Protective Coatings Made of Biologically-Derived Polyester Reactive Binder. Polymers, 13(11), 1700. https://doi.org/10.3390/polym13111700