Anion Exchange Membrane Based on Sulfonated Poly (Styrene-Ethylene-Butylene-Styrene) Copolymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
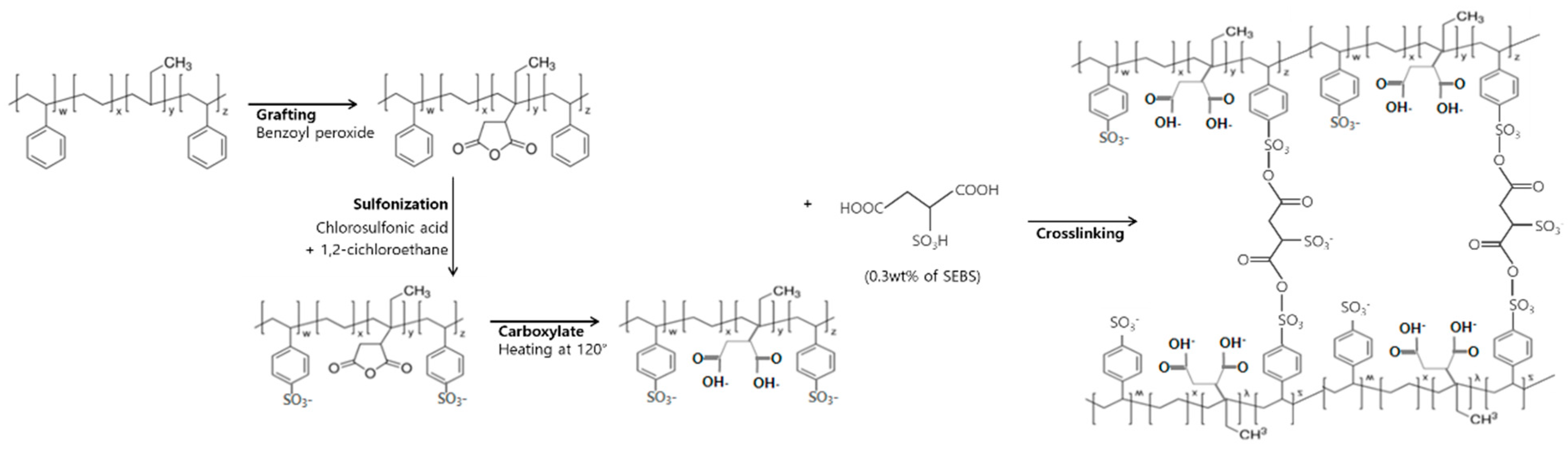
2.2. Synthesis of S-SEBS and S-SEBS-g-MA Membrane
2.2.1. Sulfonated SEBS (S-SEBS)
2.2.2. Synthesis MA Grafted S-SEBS (S-SEBS-g-MA)
2.3. Characterizations
2.3.1. Fourier Transform Infrared (FT-IR) Spectroscopy
2.3.2. Gel Permeation Chromatography (GPC)
2.3.3. Topographical Analysis
2.3.4. Ionic Conductivity
2.3.5. Ion Exchange Capacity (IEC)
2.3.6. Sulfonation Degree (SD)
2.3.7. Water Uptake
2.3.8. Mechanical Properties
3. Results and Discussion
3.1. Vibrational and GPC Analysis
3.2. Topographical Analysis
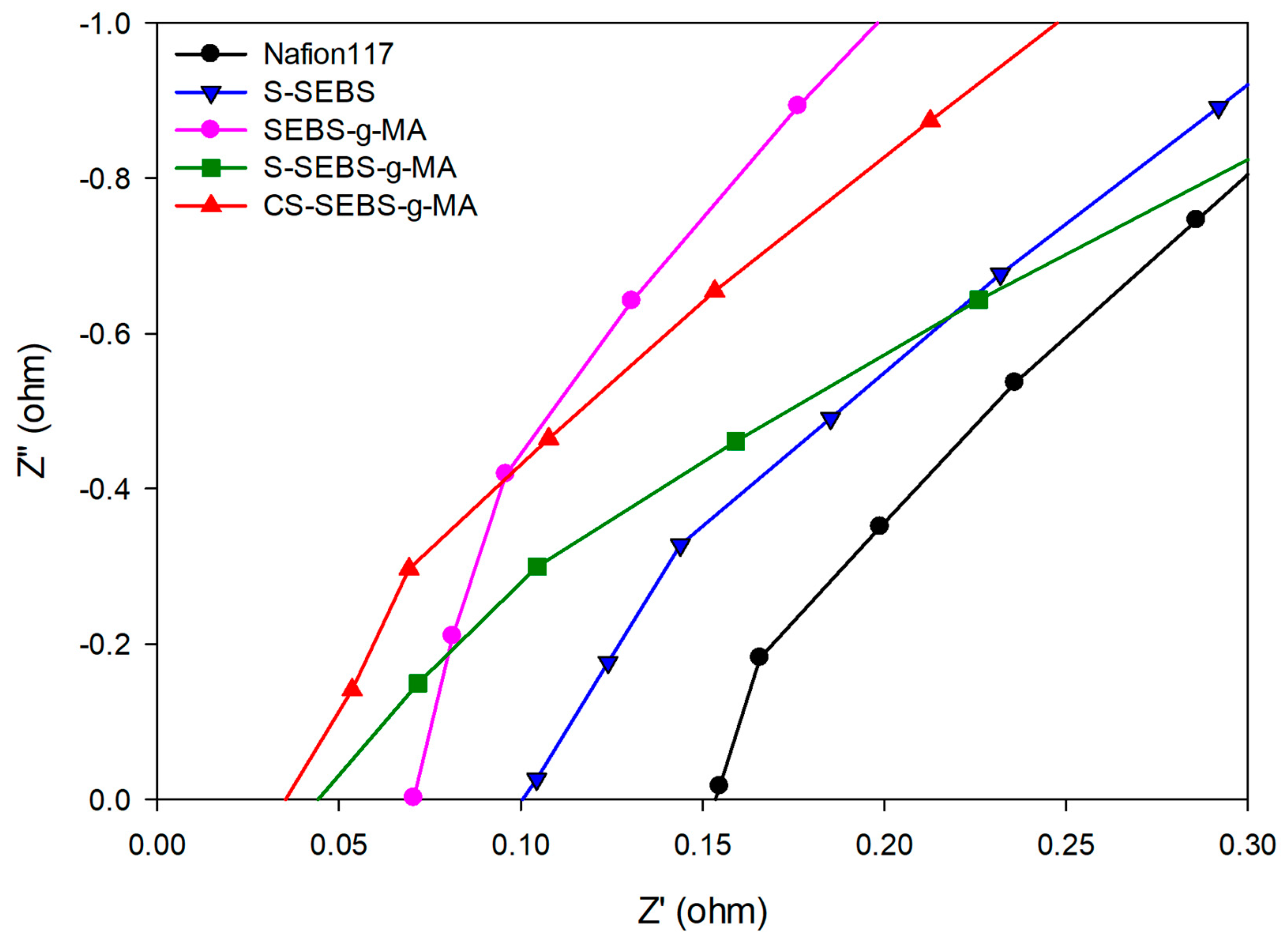
3.3. Ionic Conductivity and Ion Exchange Capacity (IEC)
3.4. Water Uptake
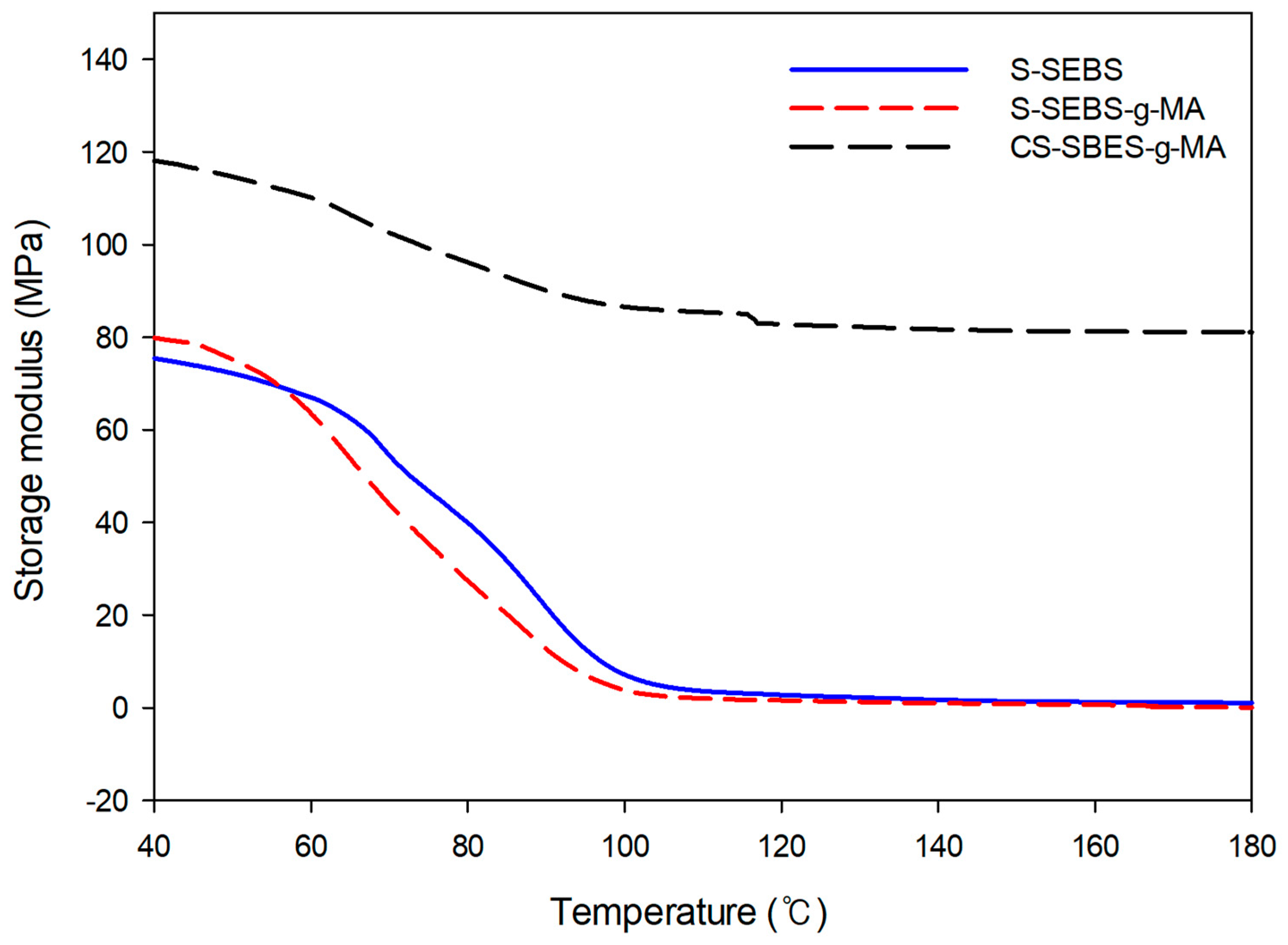
3.5. Dynamic Mechanical Analysis (DMA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, M.; Liu, Y.; Xiao, B.; Yang, S.; Wang, Z.; Han, H. An analytical model for the transverse permeability of gas diffusion layer with electrical double layer effects in proton exchange membrane fuel cells. Int. J. Hydrog. Energy 2018, 43, 17880–17888. [Google Scholar] [CrossRef]
- Xiao, B.; Wang, W.; Zhang, X.; Long, G.; Chen, H.; Cai, H.; Deng, L. A novel fractal model for relative permeability of gas diffusion layer in proton exchange membrane fuel cell with capillary pressure effect. Fractals 2019, 27, 1950012. [Google Scholar] [CrossRef]
- Parasuramana, A.; Lima, T.M.; Menictas, C.; Skyllas-Kazacos, M. Review of material research and development for vanadium redox flow battery applications. Electrochim. Acta 2013, 101, 27–40. [Google Scholar] [CrossRef]
- Sum, E.; Skylaa-kazacos, M. A study of the V(II)/V(III) redox couple for redox cell applications. J. Power Sources 1985, 15, 179–190. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Mai, Z.; Zhang, H.; Vankelecom, I. Ion exchange membranes for vanadium redox flow battery (VRB) applications. Energy Environ. Sci. 2011, 4, 1147–1160. [Google Scholar] [CrossRef]
- Oei, D.G. Permeation of vanadium cation through anionic and cationic membranes. J. Appl. Electrochem. 1985, 15, 231–235. [Google Scholar] [CrossRef]
- Jia, C.; Liu, J.; Yan, C. A significantly improved membrane for vanadium redox flow battery. J. Power Sources 2010, 13, 4380–4383. [Google Scholar] [CrossRef]
- Yandrasits, M.A.; Lindell, M.J.; Hamroc, S.J. New directions in perfluoroalkyl sulfonic acid–based proton-exchange membranes. Curr. Opin. Electrochem. 2019, 18, 90–98. [Google Scholar] [CrossRef]
- Arora, P.; Zhang, Z. Battery Separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef]
- Bae, B.; Miyatake, K.; Watanabe, M. Watanabe, Synthesis and properties of sulfonated block copolymers having fluorenyl groups for Fuel-Cell applications. ACS Appl. Mater. Interfaces 2009, 1, 1279–1286. [Google Scholar] [CrossRef]
- Li, N.N.; Fane, A.G.; Ho, W.S.W.; Matsuura, T. Advanced Membrane Technology and Application; Wiley: Weinheim, Germany, 2011; pp. 841–847. [Google Scholar]
- Leibler, L. Theory of microphase separation in block copolymers. Macromolecules 1980, 13, 1602–1617. [Google Scholar] [CrossRef]
- Zeng, Q.H.; Liu, Q.L.; Broadwell, I.; Zhu, A.M.; Xiong, Y.; Tu, X.P. Anion exchange membranes based on quaternized polystyrene-block-poly(ethylene-ran-butylene)-block-polystyrene for direct methanol alkaline fuel cells. J. Membr. Sci. 2010, 349, 237–243. [Google Scholar] [CrossRef]
- Vinodh, R.; Ilakkiya, A.; Elamathi, S.; Sangeetha, D. A novel anion exchange membrane from polystyrene (ethylene butylene) polystyrene: Synthesis and characterization. Mater. Sci. Eng. B 2010, 167, 43–50. [Google Scholar] [CrossRef]
- Castaneda, S.; Ribadeneira, R. Theoretical description of the structural characteristics of the quaternized SEBS Anion-Exchange Membrane Using DFT. J. Phys. Chem. C 2015, 119, 28235–28246. [Google Scholar] [CrossRef]
- Elabd, Y.A.; Napadensky, E.; Sloan, J.M.; Crawford, D.M.; Walker, C.W. Triblock copolymer ionomer membranes: Part1. Methanol and proton transport. J. Membr. Sci. 2003, 217, 227–242. [Google Scholar] [CrossRef]
- Edmondson, C.A.; Fontanella, J.J.; Chung, S.H.; Greenbaum, S.G.; Wnek, G.E. Complex impedance studies of S-SEBS block polymer proton-conducting membrane. Electrochim. Acta 2001, 46, 1623–1628. [Google Scholar] [CrossRef]
- Wintersgill, M.C.; Fontanella, J.J. Complex impedance measurements on Nafion. Electrochim. Acta 1998, 43, 1533–1538. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications, 2nd ed.; Wiley: Weinheim, Germany, 2004; pp. 400–421. [Google Scholar]
- Schreiber, M.; Harrer, M.; Whitehead, A.; Bucsich, H.; Dragschitz, M.; Seifert, E.; Tymciw, P. Practical and commercial issues in the design and manufacture of vanadium flow batteries. J. Power Sources 2012, 206, 483–489. [Google Scholar] [CrossRef]
- Mokrini, A.; Acosta, J. Studies of sulfonated block copolymer and its blends. Polymer 2001, 42, 9–15. [Google Scholar] [CrossRef]
- Weiss, R.A.; Sen, A.; Willis, C.L.; Pottick, L.A. Block copolymer ionomers: 1. Synthesis and physical properties of sulphonated poly(styrene-ethylene/butylene-styrene). Polymer 1991, 32, 1867–1874. [Google Scholar] [CrossRef]
- Wootthikanokkhan, J.; Changsuwan, P. Dehydrofluorination of PVDF and Proton Conductivity of the modified PVDF/sulfonated SEBS blend membranes. J. Met. 2008, 18, 57–62. [Google Scholar]
- Won, J.; Choi, S.W.; Kang, Y.S. Structural characterization and surface modification of sulfonated polystyrene–(ethylene–butylene)–styrene triblock proton exchange membranes. J. Membr. Sci. 2003, 214, 245–257. [Google Scholar] [CrossRef]
- Wang, D.; Nakajima, K.; Fujinami, S.; Shibasaki, Y.; Wang, J.Q.; Nishi, T. Characterization of morphology and mechanical properties of block copolymers using atomic force microscopy: Effects of processing conditions. Polymer 2012, 53, 1960–1965. [Google Scholar] [CrossRef]
- Shi, Y.; Zhao, Z.; Liu, W.; Zhang, C. Physically self-cross-linked SEBS anion exchange membranes. Energy Fuels 2020, 34, 16746–16755. [Google Scholar] [CrossRef]
- Laurer, J.H.; Bukovnik, R.; Spontak, R.J. Morphological characteristics of SEBS thermoplastic elastomer gels. Macromolecules 1996, 29, 5760–5762. [Google Scholar] [CrossRef]
- Han, X.; Hu, J.; Liu, H.; Hu, Y. SEBS aggregate patterning at a surface studied by atomic force microscopy. Langmuir 2006, 22, 3428–3433. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Steckle, W.P.; Weiss, R.A. Ionic aggregation in a block copolymer ionomer. Macromolecules 1993, 26, 5876–5884. [Google Scholar] [CrossRef]
- Barrera, G.M.; Lopez, H.; Castano, V.M.; Rodriuez, R. Studies on the rubber phase stability in gamma irradiated polystyrene-SBR blends by using FT-IR and Raman spectroscopy. Radiat. Phys. Chem. 2004, 69, 155–162. [Google Scholar] [CrossRef]
- Kerres, J.A. Development of ionomer membranes for fuel cells. J. Membr. Sci. 2001, 185, 3–27. [Google Scholar] [CrossRef]





| Samples | SEBS | S-SEBS | SEBS-g-MA | S-SEEB-g-MA | CS-SEBS-g-MA |
|---|---|---|---|---|---|
| Mw (g/mol) | 108,589 | 108,493 | 110,179 | 109,986 | 114,241 |
| Samples Name | Resistance (Ω/cm2) | Sulfonation Degree (%) | Ionic Conductivity (S/cm) | IEC (meq) |
|---|---|---|---|---|
| Nafion 117 | 0.145 ± 0.002 | 38.8 ± 0.4 | 0.06 ± 0.001 | 0.9 ± 0.002 |
| S-SEBS | 0.085 ± 0.0001 | 45.6 ± 0.1 | 0.1 ± 0.004 | 2.1 ± 0.004 |
| SEBS-g-MA | 0.06 ± 0.008 | 65.2 ± 0.1 | 0.12 ± 0.004 | 2.8 ± 0.004 |
| S-SEBS-g-MA | 0.047 ± 0.003 | 83.1 ± 0.2 | 0.18 ± 0.004 | 3.36 ± 0.06 |
| CS-SEBS-g-MA | 0.037 ± 0.002 | 102.6 ± 0.2 | 0.25 ± 0.004 | 3.9 ± 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.-S.; Hong, C.-K. Anion Exchange Membrane Based on Sulfonated Poly (Styrene-Ethylene-Butylene-Styrene) Copolymers. Polymers 2021, 13, 1669. https://doi.org/10.3390/polym13101669
Park H-S, Hong C-K. Anion Exchange Membrane Based on Sulfonated Poly (Styrene-Ethylene-Butylene-Styrene) Copolymers. Polymers. 2021; 13(10):1669. https://doi.org/10.3390/polym13101669
Chicago/Turabian StylePark, Hye-Seon, and Chang-Kook Hong. 2021. "Anion Exchange Membrane Based on Sulfonated Poly (Styrene-Ethylene-Butylene-Styrene) Copolymers" Polymers 13, no. 10: 1669. https://doi.org/10.3390/polym13101669
APA StylePark, H.-S., & Hong, C.-K. (2021). Anion Exchange Membrane Based on Sulfonated Poly (Styrene-Ethylene-Butylene-Styrene) Copolymers. Polymers, 13(10), 1669. https://doi.org/10.3390/polym13101669





