Classification and Production of Polymeric Foams among the Systems for Wound Treatment
Abstract
1. Introduction
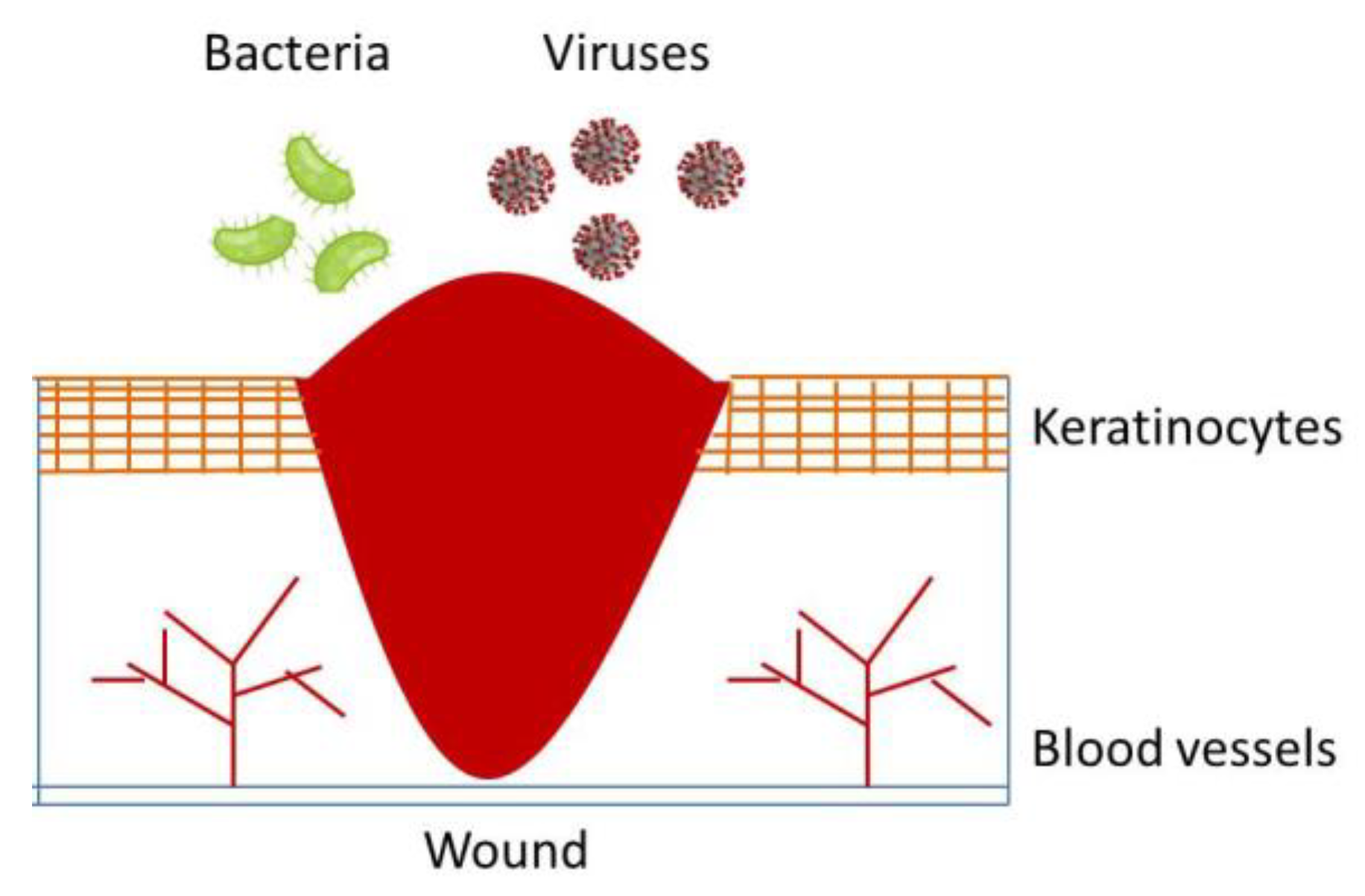
2. Classification of Wound Healing Processes
3. Biological Wound Healing Process
4. Local and System Factors Affecting Wound Healing
5. History of Wound Treatment
6. Types of Wound Treatment in Modern Times
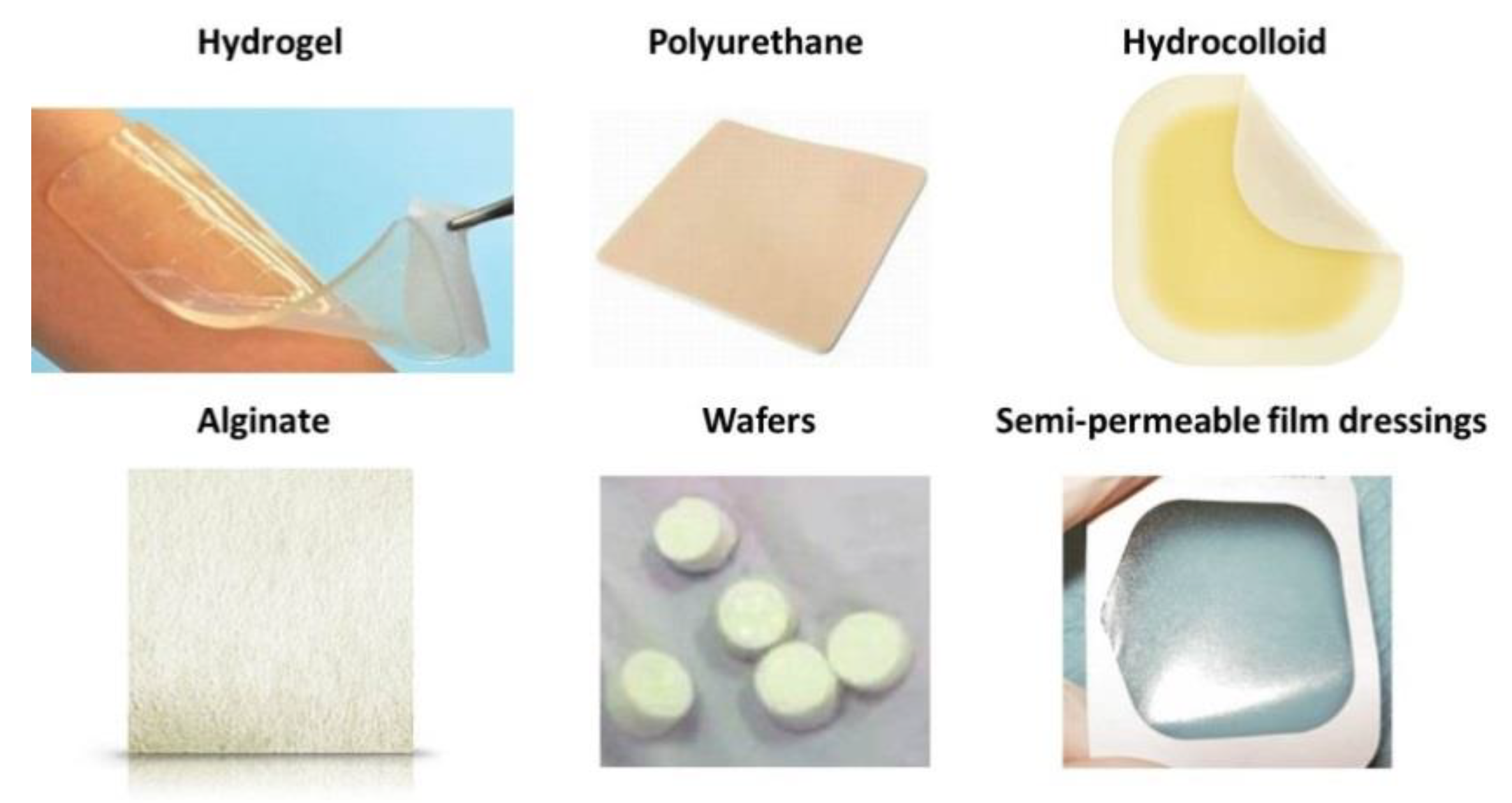
7. Overview of Synthetic Polymeric Dressing Methods
8. A Focus on Foams
- Passive products are non-occlusive used just to cover the wound.
- Interactive dressings, are semi- occlusive or occlusive. Interaction is mainly related to the barrier action against penetration of the bacteria in the wound environment. Semipermeable foam dressings are either hydrophobic or hydrophilic, with adhesive borders for proper positioning.
- Bioactive wound dressings, which are known for their biocompatibility, biodegradability and nontoxic nature, are the latest type and play important, active roles in the healing process, often containing collagen, hyaluronic acid, chitosan, alginate and elastin to this aim. In order to enhance the wound healing process, additives such as growth factors and/or antimicrobials may be incorporated with the base polymer [10].
9. Conventional Methods for Foams Production
10. Supercritical Methods for Foams Production
11. Drug Loaded into Foams
12. In Vitro Tests: Definition and Description
13. In Vitro Tests: Examples and Case Studies
14. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mohil, R.S. Classification of wounds. In Principles and Practice of Wound Care; Sarabahi, S., Tiwari, T.K., Eds.; JP Medical: London, UK, 2012; pp. 42–52. [Google Scholar]
- Luna, A.; Solano, C.; Gomez, M.; Bañon, R. Incised wound margins caused by steel blades. Scanning Electron Microscopy to determine wound direction. Forensic Sci. Int. 1989, 43, 21–26. [Google Scholar] [CrossRef]
- Dai, T.; Tanaka, M.; Huang, Y.Y.; Hamblin, M.R. Chitosan preparations for wounds and burns: Antimicrobial and wound-healing effects. Expert Rev. Anti-Infect. Ther. 2011, 9, 857–879. [Google Scholar] [CrossRef] [PubMed]
- Fackler, M.L. Gunshot wound review. Ann. Emerg. Med. 1996, 28, 194–203. [Google Scholar] [CrossRef]
- Ahmed, M.; Alam, S.N.; Khan, O.; Manzar, S. Postoperative wound infection: A surgeon’s dilemma. Pak. J. Surg. 2007, 23, 41–47. [Google Scholar]
- Daunton, C.; Kothari, S.; Smith, L.; Steele, D. A history of materials and practices for wound management. Wound Practice & Research. J. Aust. Wound Manag. Assoc. 2012, 20, 174–186. [Google Scholar]
- Van Rijswijk, L. Ingredient-based wound dressing classification: A paradigm that is passé and in need of replacement. J. Wound Care 2006, 15, 11–14. [Google Scholar] [CrossRef] [PubMed]
- Khandelwal, P.; Das, A.; Sen, C.K.; Srinivas, S.P.; Roy, S.; Khanna, S. A surfactant polymer wound dressing protects human keratinocytes from inducible necroptosis. Sci. Rep. 2021, 11, 4357. [Google Scholar] [CrossRef]
- Blessing, A.A. Efficacy of Polymer-Based Wound Dressings in Chronic Wounds. In Modeling and Control of Drug Delivery Systems; Azar, A.T., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 79–110. [Google Scholar]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Lazarus, G.S.; Cooper, D.M.; Knighton, D.R.; Margolis, D.J.; Percoraro, R.E.; Rodeheaver, G.; Robson, M.C. Definitions and guidelines for assessment of wounds and evaluation of healing. Wound Repair Regen. 1994, 2, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Percival, N.J. Classification of wounds and their management. Surgery 2002, 2, 114–117. [Google Scholar] [CrossRef]
- Tiwari, V.K. Burn wound: How it differs from other wounds? Indian J. Plast. Surg. 2012, 45, 364–373. [Google Scholar] [CrossRef]
- Jiang, S.C.; Ma, N.; Li, H.J.; Zhang, X.X. Effects of thermal properties and geometrical dimensions on skin burn injuries. Burns 2002, 28, 713–717. [Google Scholar] [CrossRef]
- Bolton, L.; Van Rijswijk, L. Wound dressings: Meeting clinical and biological needs. Dermatol. Nurs. 1991, 3, 146–161. [Google Scholar] [PubMed]
- Harding, K.G.; Morris, H.L.; Patel, G.K. Healing chronic wounds. BMJ 2002, 324, 160. [Google Scholar] [CrossRef] [PubMed]
- Laitiff, A.A.; Teoh, S.L.; Das, S. Wound healing in diabetes mellitus: Traditional treatment modalities. Clin. Ter. 2010, 161, 359–364. [Google Scholar] [PubMed]
- Baltzis, D.; Eleftheriadou, I.; Veves, A. Pathogenesis and treatment of impaired wound healing in diabetes mellitus: New insights. Adv. Ther. 2014, 31, 817–836. [Google Scholar] [CrossRef]
- Hamed, S.; Bennett, C.L.; Demiot, C.; Ullmann, Y.; Teot, L.; Desmoulière, A. Erythropoietin, a novel repurposed drug: An innovative treatment for wound healing in patients with diabetes mellitus. Wound Repair Regen. 2014, 22, 23–33. [Google Scholar] [CrossRef]
- Greenhalgh, D.G. Wound healing and diabetes mellitus. Clin. Plast. Surg. 2003, 30, 37–45. [Google Scholar] [CrossRef]
- Bansal, C.; Scott, R.; Stewart, D.; Cockerell, C.J. Decubitus ulcers: A review of the literature. Int. J. Dermatol. 2005, 44, 805–810. [Google Scholar] [CrossRef]
- Parish, L.C.; Witkowski, J.A. Controversies about the decubitus ulcer. Dermatol. Clin. 2004, 22, 87–91. [Google Scholar] [CrossRef]
- Andersen, K.E.; Kvorning, S.A. Medical aspects of the decubitus ulcer. Int. J. Dermatol. 1982, 21, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Liptak, J.M. An overview of the topical management of wounds. Aust. Vet. J. 1997, 75, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, T.; Newton, H. Wound dressings: Principles and practice. Surgery 2011, 29, 491–495. [Google Scholar] [CrossRef]
- Mostow, E.N. Diagnosis and classification of chronic wounds. Clin. Dermatol. 1994, 12, 3–9. [Google Scholar] [CrossRef]
- Singhal, A.; Reis, E.D.; Kerstein, M.D. Options for nonsurgical debridement of necrotic wounds. Adv. Skin Wound Care 2001, 14, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S. Assessment and management of wound exudate. J. Wound Care 1997, 6, 327–330. [Google Scholar]
- Robson, M.C.; Edstrom, L.E.; Krizek, T.J.; Groskin, M.G. The efficacy of systemic antibiotics in the treatment of granulating wounds. J. Surg. Res. 1974, 16, 299–306. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Strodtbeck, F. Physiology of wound healing. Newborn Infant Nurs. Rev. 2001, 1, 43–52. [Google Scholar] [CrossRef]
- Russell, L. Understanding physiology of wound healing and how dressings help. Br. J. Nurs. 2000, 9, 10–21. [Google Scholar] [CrossRef]
- Diegelmann, R.F.; Evans, M.C. Wound healing: An overview of acute, fibrotic and delayed healing. Front. Biosci. 2004, 9, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, G. Stages of wound healing and their clinical relevance. Vet. Clin. N. Am. Small Anim. Pract. 2006, 36, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Schultz, G.S. Molecular Regulation of Wound Healing. Acute and Chronic Wounds: Nursing Management, 2nd ed.; Mosby: St. Loius, MO, USA, 1999; pp. 413–429. [Google Scholar]
- Rothe, M.; Falanga, V. Growth factors: Their biology and promise in dermatologic diseases and tissue repair. Arch. Dermatol. 1989, 125, 1390–1398. [Google Scholar] [CrossRef]
- Shakespeare, P. Burn wound healing and skin substitutes. Burns 2001, 27, 517–522. [Google Scholar] [CrossRef]
- Cooper, D.M. Wound healing: New understandings. Nurse Pract. Forum 1999, 10, 74–86. [Google Scholar] [PubMed]
- Hess, C.T. Checklist for factors affecting wound healing. Adv. Skin Wound Care 2011, 24, 192. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.A.; DiPietro, L.A. Factors affecting wound healing. J. Dent. Res. 2020, 89, 219–229. [Google Scholar] [CrossRef]
- Khalil, H.; Cullen, M.; Chambers, H.; Carroll, M.; Walker, J. Elements affecting wound healing time: An evidence based analysis. Wound Repair Regen. 2015, 23, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Eneroth, M. Factors affecting wound healing after major amputation for vascular disease: A review. Prosthet. Orthot. Int. 1999, 23, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Bishop, A. Role of oxygen in wound healing. J. Wound Care 2008, 17, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Mantey, I.; Foster, A.V.M.; Spencer, S.; Edmonds, M.E. Why do foot ulcers recur in diabetic patients? Diabet. Med. 1999, 16, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Boutoille, D.; Féraille, A.; Maulaz, D.; Krempf, M. Quality of life with diabetes-associated foot complications: Comparison between lower-limb amputation and chronic foot ulceration. Foot Ankle Int. 2008, 29, 1074–1078. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed. Pharmacother. 2019, 112, 108615. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Hamm, R.L. Factors that impair wound healing. J. Am. Coll. Clin. Wound Spec. 2012, 4, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.K.; Triplett, R.G. The relationship of cigarette smoking to impaired intraoral wound healing: A review of evidence and implications for patient care. J. Oral Maxillofac. Surg. 1992, 50, 237–239. [Google Scholar] [CrossRef]
- Forrest, R.D. Early history of wound treatment. J. R. Soc. Med. 1982, 75, 198–205. [Google Scholar]
- Nickell, L.G. Antimicrobial activity of vascular plants. Econ. Bot. 1959, 13, 281–318. [Google Scholar] [CrossRef]
- Teall, E.K. Medicine and doctoring in ancient mesopotamia. Grand Val. J. Hist. 2014, 3, 2. [Google Scholar]
- Akkol, E.K.; Koca, U.; Pesin, I.; Yilmazer, D. Evaluation of the wound healing potential of Achillea biebersteinii Afan.(Asteraceae) by in vivo excision and incision models. Evid. Based Complement. Altern. Med. 2011, 2011, 474026. [Google Scholar] [CrossRef]
- Aldini, N.N.; Fini, M.; Giardino, R. From Hippocrates to tissue engineering: Surgical strategies in wound treatment. World J. Surg. 2008, 32, 2114–2121. [Google Scholar] [CrossRef] [PubMed]
- Bull, J.P. The historical development of clinical therapeutic trials. J. Chronic Dis. 1959, 10, 218–248. [Google Scholar] [CrossRef]
- Brocke, T.; Barr, J. The History of Wound Healing. Surg. Clin. 2020, 100, 787–806. [Google Scholar] [CrossRef]
- Ferraris, Z.A.; Ferraris, V.A. The women of Salerno: Contribution to the origins of surgery from medieval Italy. Ann. Thorac. Surg. 1997, 64, 1855–1857. [Google Scholar] [CrossRef]
- Castiglioni, A. The School of Salerno. Bull. Inst. Hist. Med. 1938, 6, 883–898. [Google Scholar]
- De Divitiis, E.; Cappabianca, P.; De Divitiis, O. The “schola medica salernitana”: The forerunner of the modern university medical schools. Neurosurgery 2004, 55, 722–745. [Google Scholar] [CrossRef] [PubMed]
- Manring, M.M.; Hawk, A.; Calhoun, J.H.; Andersen, R.C. Treatment of war wounds: A historical review. Clin. Orthop. Relat. Res. 2009, 467, 2168–2191. [Google Scholar] [CrossRef]
- Tsoucalas, G.; Karamanou, M.; Laios, K.; Markatos, K.; Androutsos, G. Oncologic conceptions in the work of the surgeon Guy de Chauliac (c. 1300–1368). Off. J. Balk. Union Oncol. 2019, 24, 410–414. [Google Scholar]
- Mir, M.; Ali, M.N.; Barakullah, A.; Gulzar, A.; Arshad, M.; Fatima, S.; Asad, M. Synthetic polymeric biomaterials for wound healing: A review. Prog. Biomater. 2008, 7, 1–21. [Google Scholar] [CrossRef]
- Thomas, S. Wound Management and Dressings; Pharmaceutical Press: London, UK, 1990. [Google Scholar]
- Helfman, T.; Ovington, L.; Falanga, V. Occlusive dressings and wound healing. Clin. Dermatol. 1994, 12, 121–127. [Google Scholar] [CrossRef]
- Zahedi, P.; Rezaeian, I.; Ranaei-Siadat, S.O.; Jafari, S.H.; Supaphol, P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym. Adv. Technol. 2010, 21, 77–95. [Google Scholar] [CrossRef]
- Jayakumar, R.; Prabaharan, M.; Kumar, P.S.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Choi, E.Y.; Oh, J.S.; Lee, H.C.; Park, S.S.; Cho, C.S. Possibility of wound dressing using poly(l-leucine)/poly(ethylene glycol)/poly(l-leucine) triblock copolymer. Biomaterials 2000, 21, 131–141. [Google Scholar] [CrossRef]
- Lee, Y.M.; Kim, S.S.; Park, M.H.; Song, K.W.; Sung, Y.K.; Kang, I.K. β-Chitin-based wound dressing containing silver sulfurdiazine. J. Mater. Sci. Mater. Med. 2000, 11, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, E.; Jordan, X.; Clauss, M.; Borens, O.; Mäder, M.; Trampuz, A. High bacterial load in negative pressure wound therapy (NPWT) foams used in the treatment of chronic wounds. Wound Repair Regen. 2013, 21, 677–681. [Google Scholar] [CrossRef]
- Du, L.; Tong, L.; Jin, Y.; Jia, J.; Liu, Y.; Su, C.; Yu, S.; Li, X. A multifunctional in situ-forming hydrogel for wound healing. Wound Repair Regen. 2012, 20, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.; Harding, K.G.; Moore, K. Alginates from wound dressings activate human macrophages to secrete tumour necrosis factor-α. Biomaterials 2000, 21, 1797–1802. [Google Scholar] [CrossRef]
- Finnie, A. Hydrocolloids in wound management: Pros and cons. Br. J. Community Nurs. 2002, 7, 338–345. [Google Scholar] [CrossRef]
- Lee, S.M.; Park, I.K.; Kim, Y.S.; Kim, H.J.; Moon, H.; Mueller, S.; Jeong, Y.I. Physical, morphological, and wound healing properties of a polyurethane foam-film dressing. Biomater. Res. 2016, 20, 15. [Google Scholar] [CrossRef]
- Lai, H.L.; Abu’Khalil, A.; Craig, D.Q. The preparation and characterisation of drug-loaded alginate and chitosan sponges. Int. J. Pharm. 2003, 251, 175–181. [Google Scholar] [CrossRef]
- Varaprasad, K.; Jayaramudu, T.; Kanikireddy, V.; Toro, C.; Sadiku, E.R. Alginate-based composite materials for wound dressing application: A mini review. Carbohydr. Polym. 2020, 236, 116025. [Google Scholar] [CrossRef] [PubMed]
- Derakhshandeh, H.; Kashaf, S.S.; Aghabaglou, F.; Ghanavati, I.O.; Tamayol, A. Smart bandages: The future of wound care. Trends Biotechnol. 2018, 36, 1259–1274. [Google Scholar] [CrossRef] [PubMed]
- Khil, M.S.; Cha, D.I.; Kim, H.Y.; Kim, I.S.; Bhattarai, N. Electrospun nanofibrous polyurethane membrane as wound dressing. J. Biomed. Mater. Res. Part B Appl. Biomater. 2003, 67, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S. Hydrocolloid dressings in the management of acute wounds: A review of the literature. Int. Wound J. 2008, 5, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Tønnesen, H.H.; Karlsen, J. Alginate in drug delivery systems. Drug Dev. Ind. Pharm. 2002, 28, 621–630. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Yusof, N.L.B.M.; Wee, A.; Lim, L.Y.; Khor, E. Flexible chitin films as potential wound-dressing materials: Wound model studies. J. Biomed. Mater. Res. Part A 2003, 66, 224–232. [Google Scholar] [CrossRef]
- Kumar, P.S.; Abhilash, S.; Manzoor, K.; Nair, S.V.; Tamura, H.; Jayakumar, R. Preparation and characterization of novel β-chitin/nanosilver composite scaffolds for wound dressing applications. Carbohydr. Polym. 2010, 80, 761–767. [Google Scholar] [CrossRef]
- Pawar, H.V.; Boateng, J.S.; Ayensu, I.; Tetteh, J. Multifunctional medicated lyophilised wafer dressing for effective chronic wound healing. J. Pharm. Sci. 2014, 103, 1720–1733. [Google Scholar] [CrossRef]
- Saratale, R.G.; Cho, S.K.; Saratale, G.D.; Kadam, A.A.; Ghodake, G.S.; Kumar, M.; Kim, D.S.; Mulla, S.I.; Shin, H.S. A comprehensive overview and recent advances on polyhydroxyalkanoates (PHA) production using various organic waste streams. Bioresour. Technol. 2021, 325, 124685. [Google Scholar] [CrossRef]
- Wang, K.; Chen, F.; Li, Z.; Fu, Q. Control of the hierarchical structure of polymer articles via “structuring” processing. Prog. Polym. Sci. 2014, 39, 891–920. [Google Scholar] [CrossRef]
- Bianchera, A.; Catanzano, O.; Boateng, J.; Elviri, L. The place of biomaterials in wound healing. Ther. Dress. Wound Heal. Appl. 2020, 15, 337–366. [Google Scholar]
- Ghomi, E.R.; Khalili, S.; Khorasani, S.N.; Neisiany, R.E.; Ramakrishna, S. Wound dressings: Current advances and future directions. J. Appl. Polym. Sci. 2019, 136, 47738. [Google Scholar] [CrossRef]
- Del Bakhshayesh, A.R.; Annabi, N.; Khalilov, R.; Akbarzadeh, A.; Samiei, M.; Alizadeh, E.; Alizadeh-Ghodsi, M.; Davaran, S.; Montaseri, A. Recent advances on biomedical applications of scaffolds in wound healing and dermal tissue engineering. Artif. Cells Nanomed. Biotechnol. 2018, 46, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Granick, M.S.; Tomaselli, N.L. Wound dressings and comparative effectiveness data. Adv. Wound Care 2014, 3, 511–529. [Google Scholar] [CrossRef]
- Liu, X.; Niu, Y.; Chen, K.C.; Chen, S. Rapid hemostatic and mild polyurethane-urea foam wound dressing for promoting wound healing. Mater. Sci. Eng. C 2017, 71, 289–297. [Google Scholar] [CrossRef]
- Song, E.H.; Jeong, S.H.; Park, J.U.; Kim, S.; Kim, H.E.; Song, J. Polyurethane-silica hybrid foams from a one-step foaming reaction, coupled with a sol-gel process, for enhanced wound healing. Mater. Sci. Eng. C 2017, 79, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Arcangeli, G.; Cupelli, V.; Giuliano, G. Effects of silica on human lung fibroblast in culture. Sci. Total Environ. 2001, 270, 135–139. [Google Scholar] [CrossRef]
- Kaur, N.; Garg, T.; Goyal, A.K.; Rath, G. Formulation, optimization and evaluation of curcumin-β-cyclodextrin-loaded sponge for effective drug delivery in thermal burns chemotherapy. Drug Deliv. 2016, 23, 2245–2254. [Google Scholar] [CrossRef] [PubMed]
- Sathiyaseelan, A.; Shajahan, A.; Kalaichelvan, P.T.; Kaviyarasan, V. Fungal chitosan based nanocomposites sponges—An alternative medicine for wound dressing. Int. J. Biol. Macromol. 2017, 104, 1905–1915. [Google Scholar] [CrossRef]
- Anbazhagan, S.; Thangavelu, K.P. Application of tetracycline hydrochloride loaded-fungal chitosan and Aloe vera extract based composite sponges for wound dressing. J. Adv. Res. 2018, 14, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Ito, A.; Taki, K.; Ohshima, M. A new technique for foaming submicron size poly(methyl methacrylate) particles. J. Appl. Polym. Sci. 2007, 106, 2825–2830. [Google Scholar] [CrossRef]
- Hou, W.H.; Lloyd, T.B. A new technique for preparing monodisperse polymer particles. J. Appl. Polym. Sci. 1992, 45, 1783–1788. [Google Scholar] [CrossRef]
- Zhou, W.; Zhu, Y.; Grundish, N.; Xin, S.; Wang, S.; You, Y.; Wu, N.; Gao, J.; Cui, Z.; Li, Y.; et al. Polymer lithium-garnet interphase for an all-solid-state rechargeable battery. Nano Energy 2018, 53, 926–931. [Google Scholar] [CrossRef]
- Ma, Z.; Gao, C.; Gong, Y.; Shen, J. Paraffin spheres as porogen to fabricate poly(l-lactic acid) scaffolds with improved cytocompatibility for cartilage tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2003, 67, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Xanthos, M.; Dey, S.K.; Zhang, Q.; Quintans, J. Parameters affecting extrusion foaming of PET by gas injection. J. Cell. Plast. 2000, 36, 102–111. [Google Scholar] [CrossRef]
- Jacobs, L.J.; Kemmere, M.F.; Keurentjes, J.T. Sustainable polymer foaming using high pressure carbon dioxide: A review on fundamentals, processes and applications. Green Chem. 2008, 10, 731–738. [Google Scholar] [CrossRef]
- Reverchon, E.; Cardea, S. Production of controlled polymeric foams by supercritical CO2. J. Supercrit. Fluids 2007, 40, 144–152. [Google Scholar] [CrossRef]
- Chauvet, M.; Sauceau, M.; Fages, J. Extrusion assisted by supercritical CO2: A review on its application to biopolymers. J. Supercrit. Fluids 2017, 120, 408–420. [Google Scholar] [CrossRef]
- Trucillo, P. Drug Carriers: Classification, Administration, Release Profiles, and Industrial Approach. Processes 2021, 9, 470. [Google Scholar] [CrossRef]
- Bužarovska, A.; Dinescu, S.; Lazar, A.D.; Serban, M.; Pircalabioru, G.G.; Costache, M.; Gualandi, C.; Avérous, L. Nanocomposite foams based on flexible biobased thermoplastic polyurethane and ZnO nanoparticles as potential wound dressing materials. Mater. Sci. Eng. C 2019, 104, 109893. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cheng, J.; Ao, Q. Preparation of Alginate-Based Biomaterials and Their Applications in Biomedicine. Marine Drugs. 2021, 19, 264. [Google Scholar] [CrossRef]
- Bueno, C.Z.; Moraes, Â.M. Influence of the incorporation of the antimicrobial agent polyhexamethylene biguanide on the properties of dense and porous chitosan-alginate membranes. Mater. Sci. Eng. C 2018, 93, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Andersen, T.; Melvik, J.E.; Gåserød, O.; Alsberg, E.; Christensen, B.E. Ionically gelled alginate foams: Physical properties controlled by type, amount and source of gelling ions. Carbohydr. Polym. 2014, 99, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Bueno, C.Z.; Dias, A.M.A.; de Sousa, H.J.C.; Braga, M.E.M.; Moraes, A.M. Control of the properties of porous chitosan-alginate membranes through the addition of different proportions of Pluronic F68. Mater. Sci. Eng. C 2014, 44, 117–125. [Google Scholar] [CrossRef]
- Barbetta, A.; Carrino, A.; Costantini, M.; Dentini, M. Polysaccharide based scaffolds obtained by freezing the external phase of gas-in-liquid foams. Soft Matter 2010, 6, 5213–5224. [Google Scholar] [CrossRef]
- Calcagnile, P.; Fragouli, D.; Mele, E.; Ruffilli, R.; Athanassiou, A. Polymeric foams with functional nanocomposite cells. RSC Adv. 2014, 4, 19177–19182. [Google Scholar] [CrossRef]
- Oh, S.T.; Kim, S.H.; Jeong, H.Y.; Lee, J.M.; Cho, J.W.; Park, J.S. The mechanical properties of polyurethane foam wound dressing hybridized with alginate hydrogel and jute fiber. Fibers Polym. 2013, 14, 173–181. [Google Scholar] [CrossRef]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug delivery systems and materials for wound healing applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.-C.; Park, A.Y.; Guang, J.-L. In vitro scratch assay: A conveniente and inexpensive method for analysis cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Faulkner, C.L.; Nelson-Overton, L.K.; Wiley, J.A.; Quinn, M.T. Macrophage immunomodulatory activity of polysaccharides isolated from Juniperus scopolorum. Int. Immunopharmacol. 2005, 5, 1783–1799. [Google Scholar] [CrossRef] [PubMed]
- Raman, S.P.; Keil, C.; Dieringer, P.; Hübner, C.; Bueno, A.; Gurikov, P.; Nissen, J.; Holtkamp, M.; Karst, U.; Haase, H. Alginate aerogels carrying calcium, zinc and silver cations for wound care: Fabrication and metal detection. J. Supercrit. Fluids 2019, 153, 104545. [Google Scholar] [CrossRef]
- Pyun, D.G.; Yoon, H.S.; Chung, H.Y.; Choi, H.J.; Thambi, T.; Kim, B.S.; Lee, D.S. Evaluation of AgHAP-containing polyurethane foam dressing or wound healing: Synthesis, characterizatoin, in vitro and in vivo studies. J. Mater. Chem. B 2015, 3, 7752–7763. [Google Scholar] [CrossRef]
- Baldino, L.; González-Garcinuño, A.; Tabernero, A.; Cardea, S.; Martín del Valle, E.M.; Reverchon, E. Production of fungistatic porous structures of cellulose acetate loaded with quercetin, using supercritical CO2. J. Supercrit. Fluids 2020, 160, 105129. [Google Scholar]
- Zsikó, S.; Csányi, E.; Kovács, A.; Budai-Szűcs, M.; Gácsi, A.; Berkó, S. Methods to evaluate skin penetration in vitro. Sci. Pharm. 2019, 87, 19. [Google Scholar] [CrossRef]
- Moser, K.; Kriwet, K.; Naik, A.; Kalia, Y.N.; Guy, R.H. Passive skin penetration enhancement and its quantification in vitro. Eur. J. Pharm. Biopharm. 2001, 52, 103–112. [Google Scholar] [CrossRef]
- Wilhelm, K.-P.; Wilhelm, D.; Bielfeldt, S. Models of wound healing: An emphasis on clinical studies. Skin Res. Technol. 2017, 23, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Kukowska, M.; Pikuta, M.; Kukowska-Kaszuba, M.; Schumacher, A.; Dzierbicka, K.; Tzronkowski, P. Synthetic lipopeptides as potential topical therapeutics in wound and skin care: In vitro studies of permeation and skin cells behavior. RSC Adv. 2016, 6, 115120–115131. [Google Scholar] [CrossRef]
- Milovanovic, S.; Stamenic, M.; Markovic, D.; Ivanovic, J.; Zizovic, I. Supercritical impregnation of cellulose acetate with thymol. J. Supercrit. Fluids 2015, 97, 107–115. [Google Scholar] [CrossRef]
- Franco, P.; Belvedere, R.; Pessolano, E.; Liparoti, S.; Pantani, R.; Petrella, A.; De Marco, I. PCL/Mesoglycan devices obtained by supercritical foaming and impregnation. Pharmaceutics 2019, 11, 631. [Google Scholar] [CrossRef]
- Lee, J.W.; Song, K.Y. Evaluation of polyurethane foam dressing impregnated with 3% povidone-iodine (Betafoam) in a rat wound model. Ann. Surg. Treat. Res. 2018, 94, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Joodaki, H.; Panzer, M.B. Skin mechanical properties and modeling: A review. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2018, 232, 323–343. [Google Scholar] [CrossRef]
- Zaman, H.U.; Islam, J.M.M.; Khan, M.A.; Khan, R.A. Physico-mechanical properties of wound dressing material and its biomedical application. J. Mech. Behav. Biomed. Mater. 2011, 4, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Xiong, Z.; Chen, S.; Tu, G. The use of models in “target” theory to evaluate the survival curves of human ovarian carcinoma cell lines exposur to Adriamycin combined with ultrasounds. Ultrason. Sonochem. 2005, 12, 345–348. [Google Scholar] [CrossRef]
- Peppas, N.; Narasimhan, B. Mathematical models in drug delivery: How modeling has shaped the way we design new drug delivery systems. J. Control Release 2014, 190, 75–81. [Google Scholar] [CrossRef] [PubMed]
- González-Garcinuño, A.; Masa, R.; Hernández, M.; Domínguez, A.; Tabernero, A.; del Valle, E.M. Levan-capped silver nanoparticles for bactericidal formulations: Release and activity modelling. Int. J. Mol. Sci. 2019, 20, 1502. [Google Scholar] [CrossRef] [PubMed]



| Physical | Chemical | Mechanical | Biological |
|---|---|---|---|
| Moisture control | Adhesive | Mechanical protection | Protection from microorganism |
| Gas permeability | Wettability | stiffness | Necrosis prevention |
| Exudate transport/absorption | --- | strength | biocompatibility |
| Biodegradability | --- | ---- | Nontoxic |
| --- | --- | --- | Pain relief |
| Brand Name | Production Country | Use | Base Polymer | Additive |
|---|---|---|---|---|
| Flexzan | Dow Hickam Inc., Sugar Land, TX, USA | Chronic wounds | Polyurethane | --- |
| Biopatch | Johnson & Johnson, Malvern, PA, USA | General, with broad-spectrum antimicrobial and antifungal activity | Hydrophilic polyurethane | Chlorhexidine Gluconate |
| Biatain | Coloplast, Humlebæk, Denmark | Mohs surgery and wounds | Hydrophilic polyurethane | --- |
| Cultinova | Cultinova, München, Germany | Laser resurfacing | Polyurethane | Polyacrylate superabsorbent |
| Lyofoam | Convatec, Bridgewater, NJ, USA | From moderate to highly exuding wounds | Polyurethane | --- |
| Allevyn | Smith & Nephew, Watford, UK | Chronic or acute exuding wounds | Hydrophilic polyurethane | --- |
| Unilene | Unilene S.A.C., Lima, Peru | Burn and minor injuries | Hydrophilic polyurethane | --- |
| Tielle | 3M, St. Paul, MN, USA | Ulcers, post-surgical or traumatic | Silicone and polyurethane foam | --- |
| CuraSpon | CuraMedical B.V., Assendelft, The Nederlands | Hemostasis | Gelatine | --- |
| Kendall | H&R Healthcare Ltd., North Ferriby, UK | General, Bactericidal | Polyurethane | Polyhexamethylene biguanide |
| Hydrasorb | Hartmann Group, Heidenheim an der Brenz, Germany | General | Polyurethane | --- |
| Polymer in the Foam | Drug/Molecule Loaded in the Foam | Field of Application and Effect | Reference |
|---|---|---|---|
| PU | ZnO | Reducing bacterial infection | [105] |
| Alginate | Azidophyneyl | Drug delivery and wound healing | [106] |
| Chitosan/alginate | Polyhexamethylene biguanide | Wound dressing | [107] |
| Alginate | Calcium, Strontium | Drug delivery and tissue engineering | [108] |
| Alginate | Chitosan, Pluronic F68 | biomedical | [109] |
| Alginate | Hyaluronic acid, chitosan | Tissue engineering | [110] |
| Alginate | gold nanoparticles, Poly(dimethylsioxane) | biomedical | [111] |
| Polyurethane/alginate | Jute fiber | Wound dressing | [112] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trucillo, P.; Di Maio, E. Classification and Production of Polymeric Foams among the Systems for Wound Treatment. Polymers 2021, 13, 1608. https://doi.org/10.3390/polym13101608
Trucillo P, Di Maio E. Classification and Production of Polymeric Foams among the Systems for Wound Treatment. Polymers. 2021; 13(10):1608. https://doi.org/10.3390/polym13101608
Chicago/Turabian StyleTrucillo, Paolo, and Ernesto Di Maio. 2021. "Classification and Production of Polymeric Foams among the Systems for Wound Treatment" Polymers 13, no. 10: 1608. https://doi.org/10.3390/polym13101608
APA StyleTrucillo, P., & Di Maio, E. (2021). Classification and Production of Polymeric Foams among the Systems for Wound Treatment. Polymers, 13(10), 1608. https://doi.org/10.3390/polym13101608








