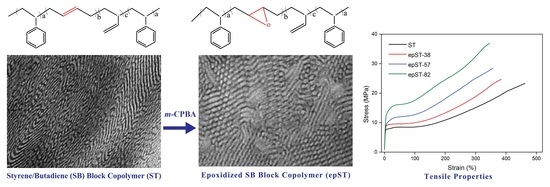
Influence of Controlled Epoxidation of an Asymmetric Styrene/Butadiene Star Block Copolymer on Structural and Mechanical Properties
Abstract
1. Introduction
2. Experimental
2.1. Materials
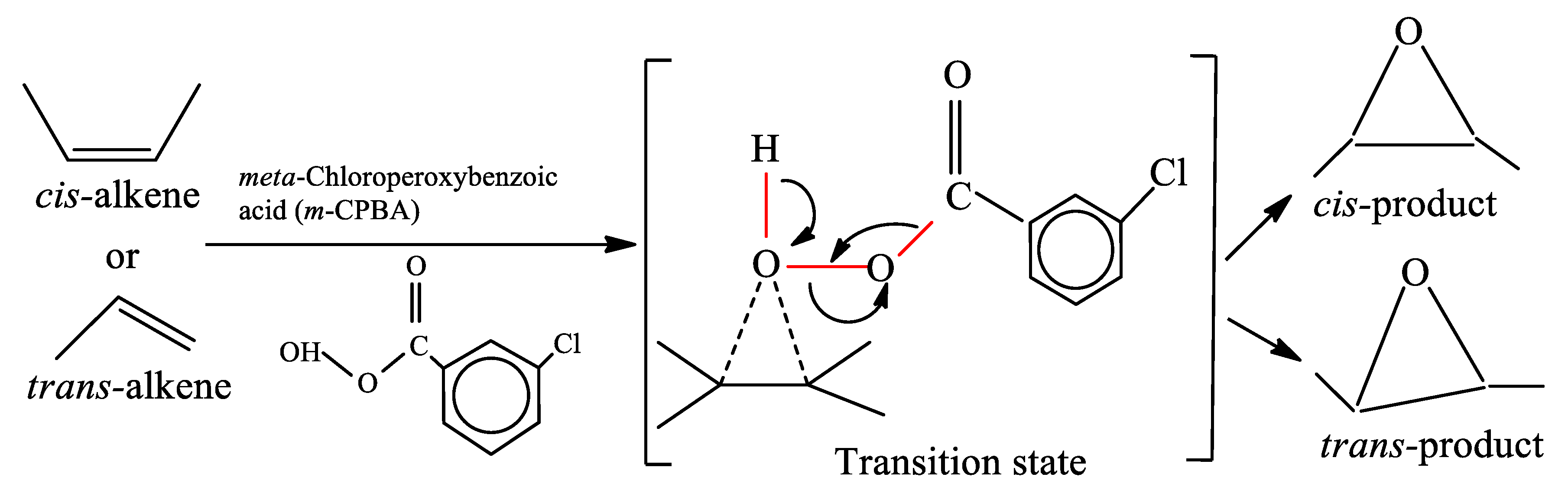
2.2. Epoxidation of BCP with m-CPBA
2.3. Sample Preparation
2.4. Molecular Characterization
2.5. Morphological Characterization
2.6. Dynamic Mechanical Analysis (DMA)
2.7. Mechanical Characterization
3. Results and Discussion
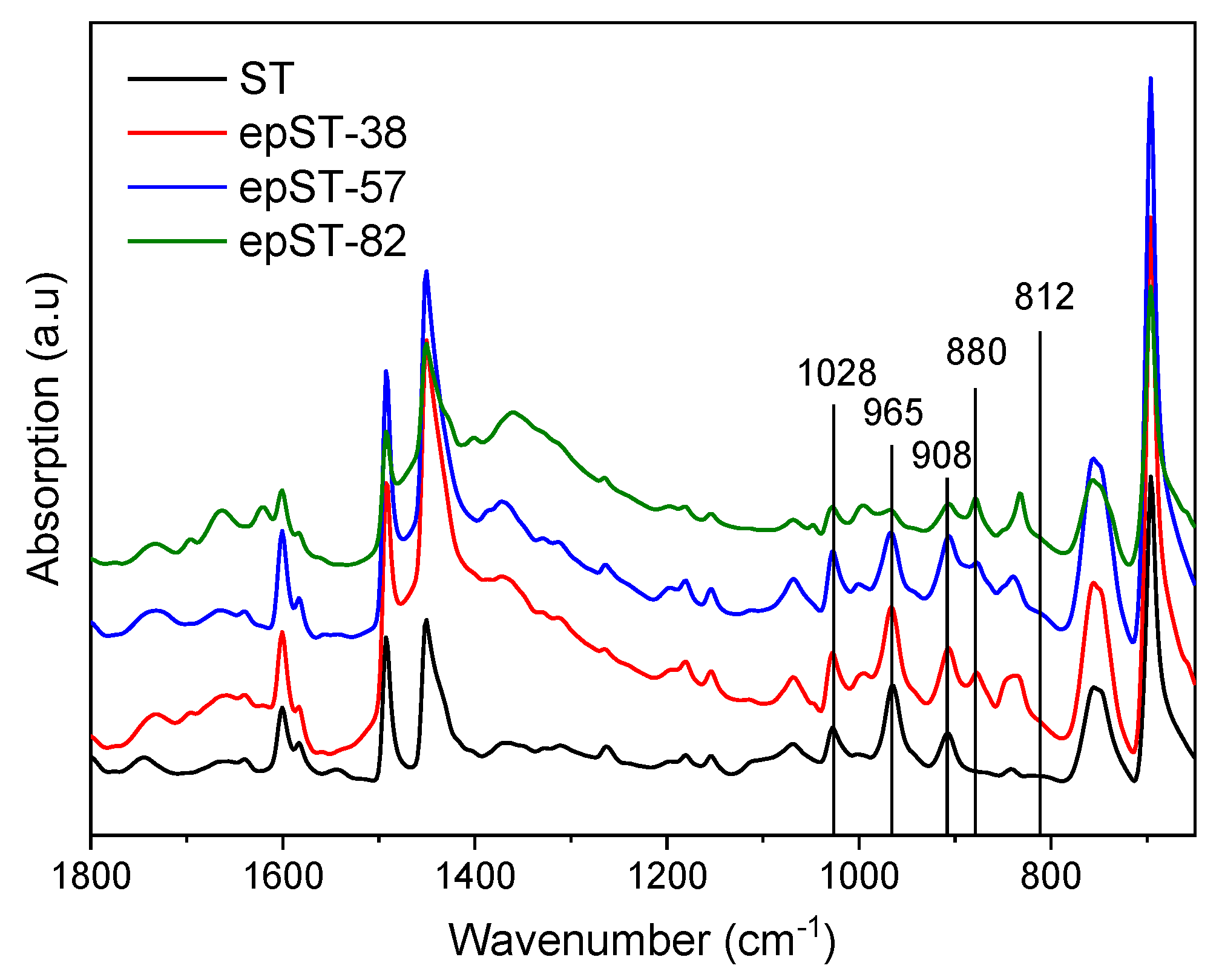
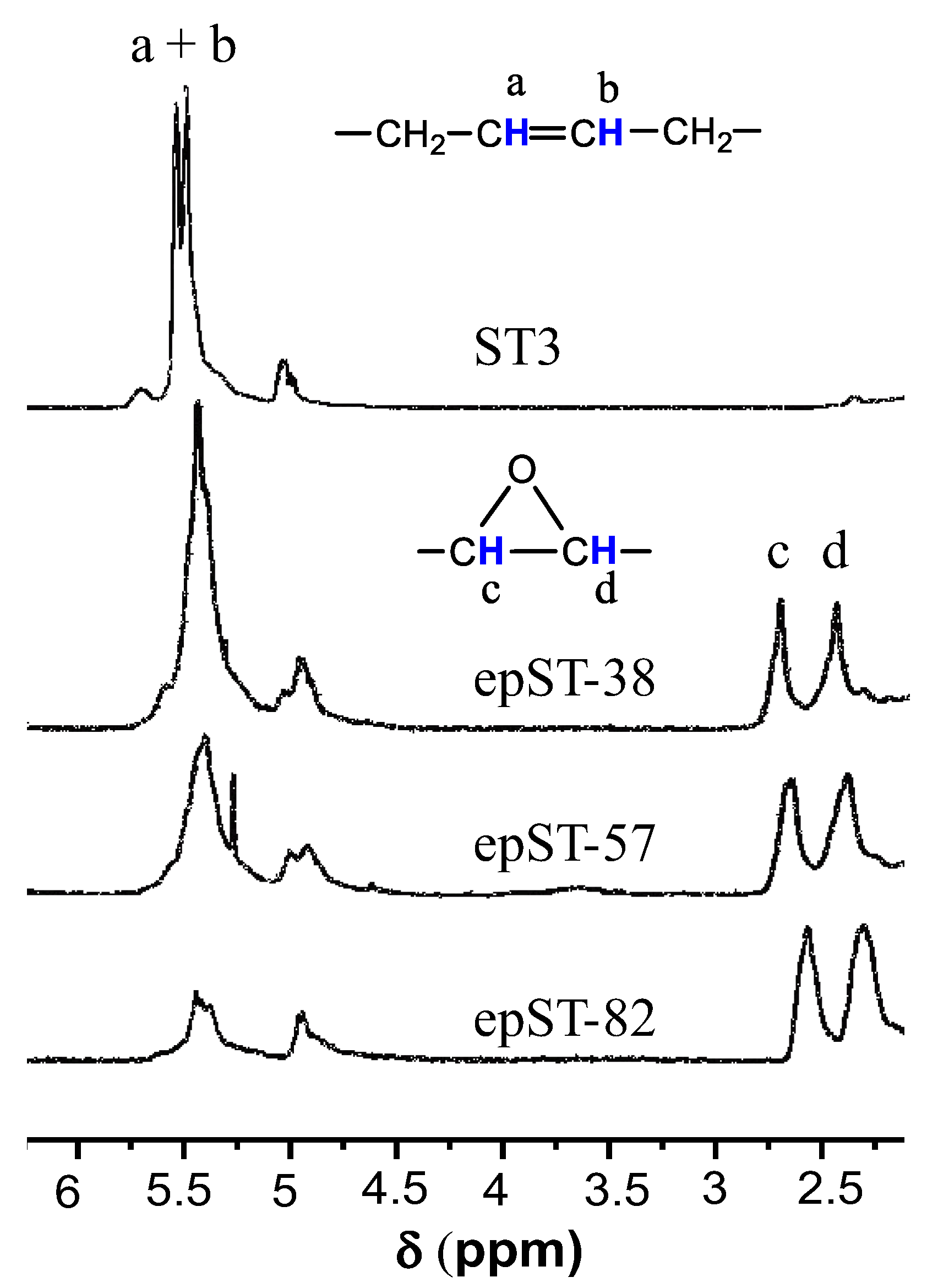
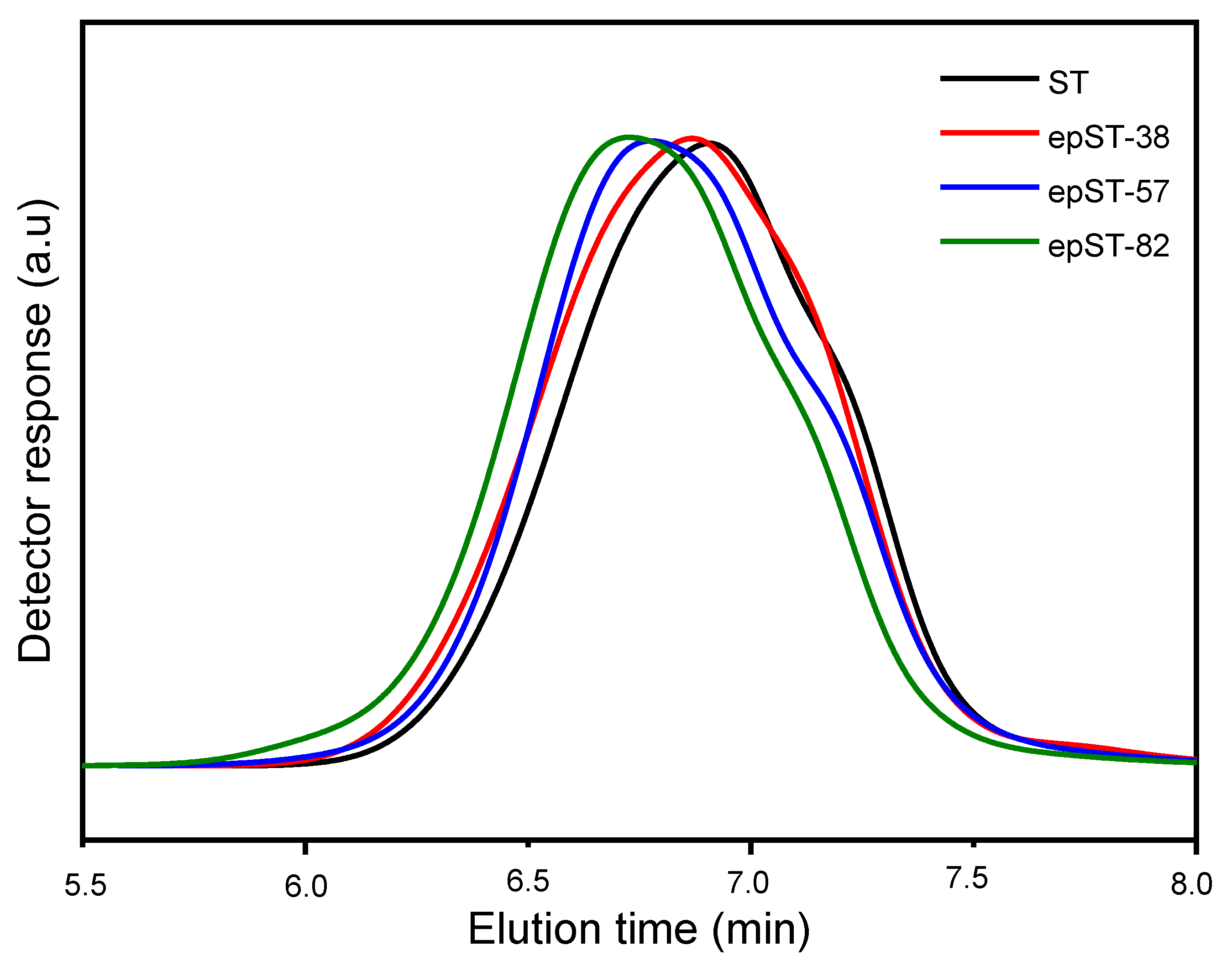
3.1. Validation of Epoxidation
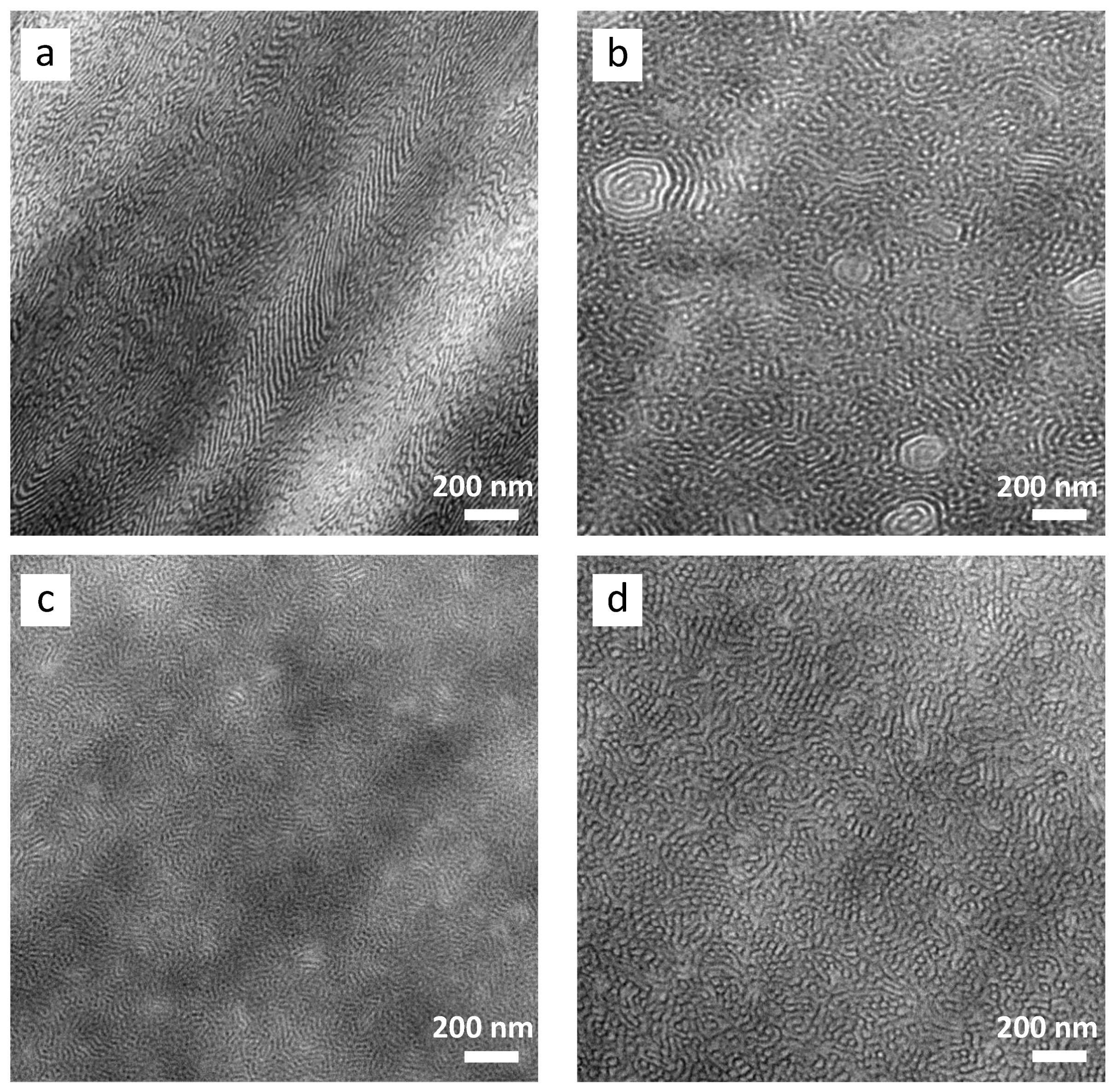
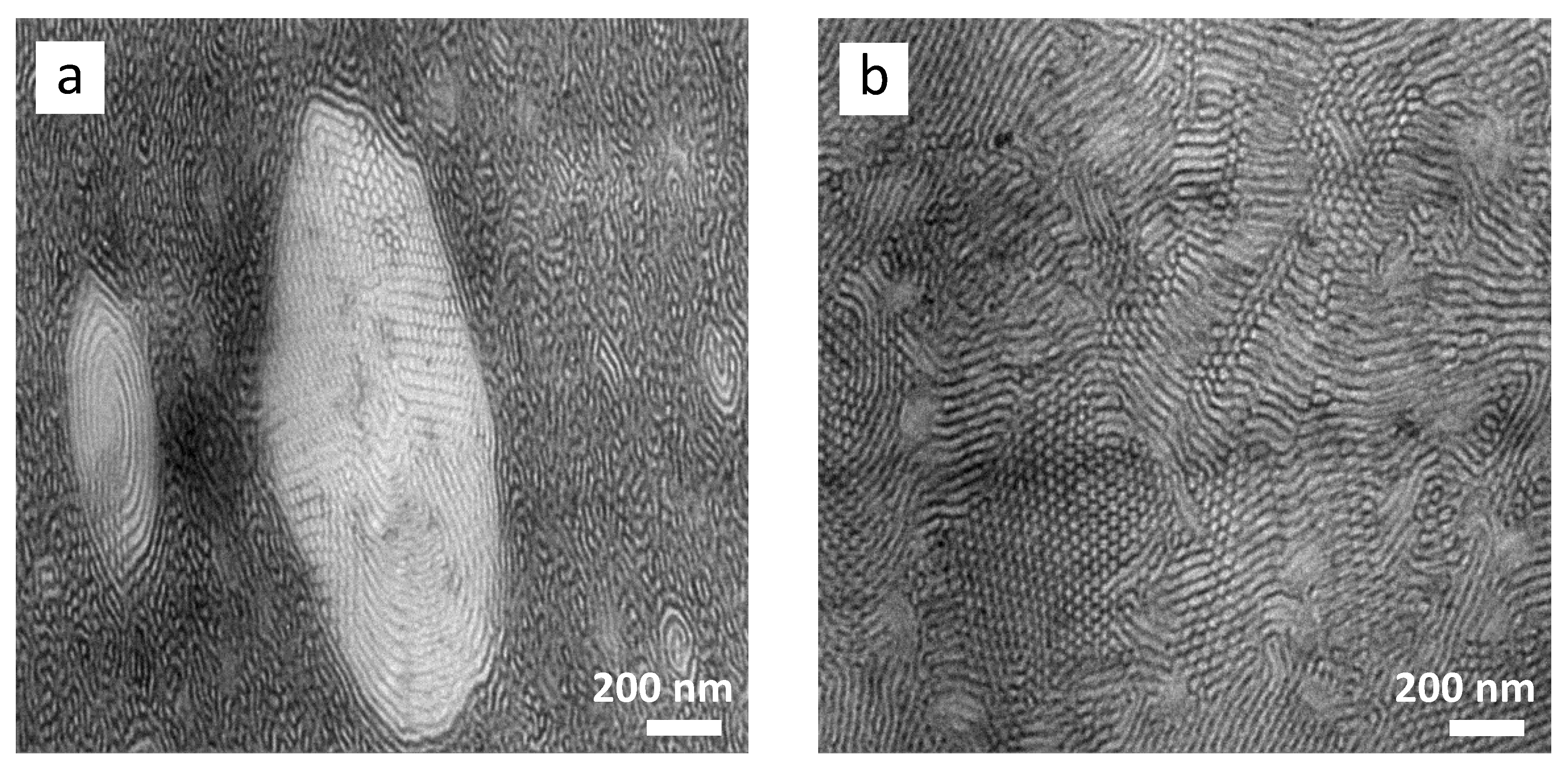
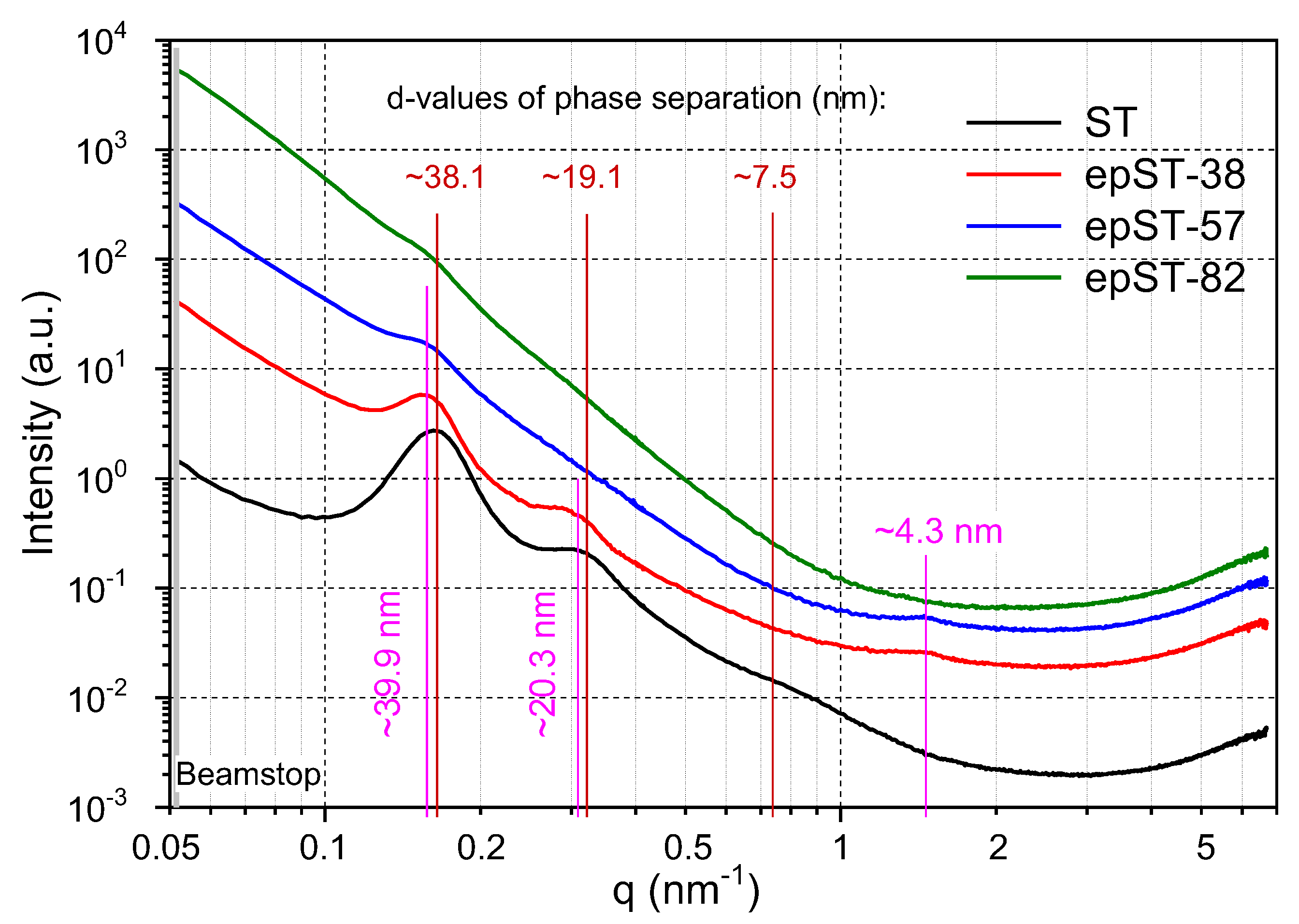
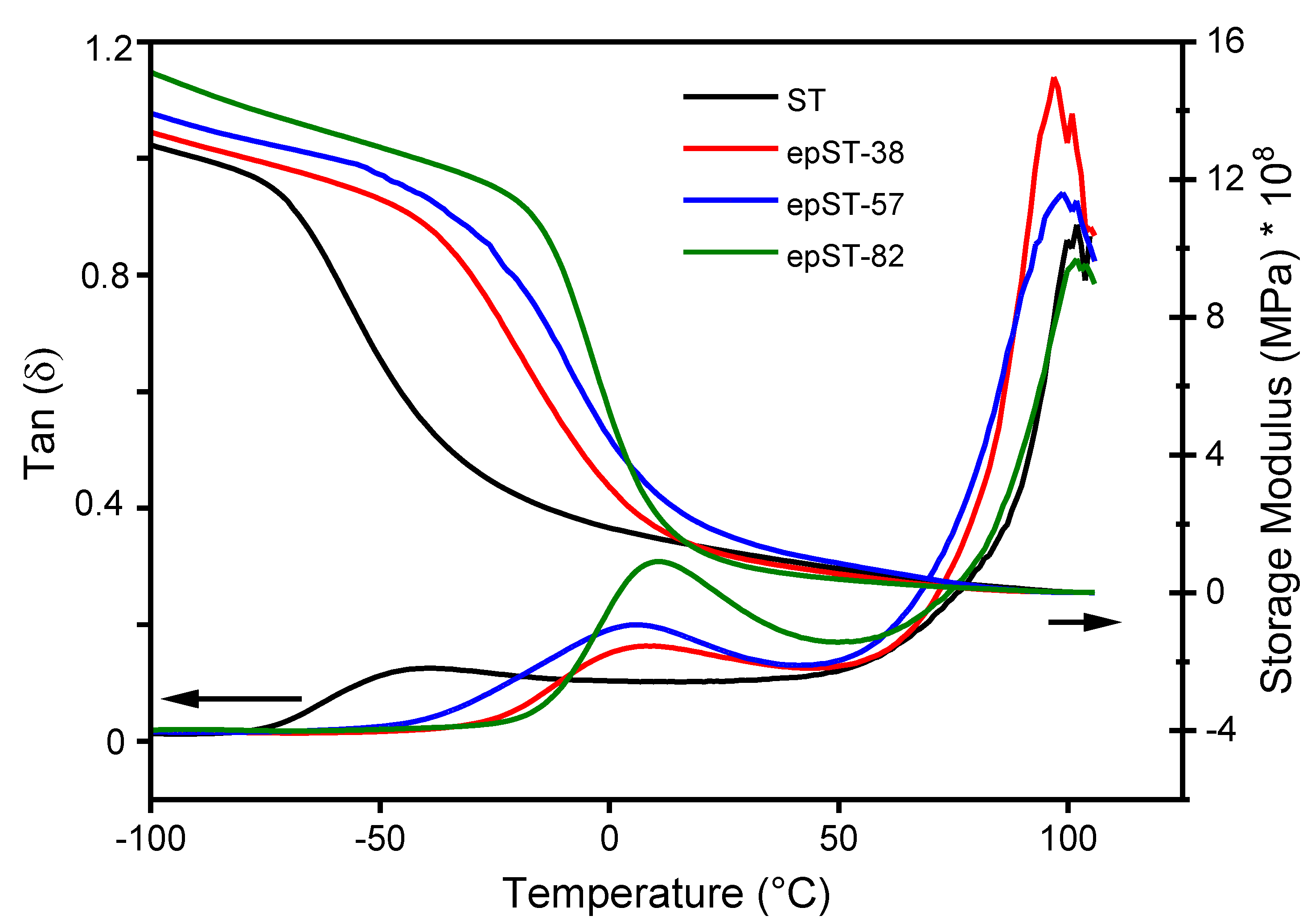
3.2. Morphology and Phase Behavior
3.3. Mechanical Behavior
4. Conclusions
- The experimental condition for epoxidation of BCP under precisely controlled conditions was optimized for the first time.
- Varying degrees of epoxidation (38 mol%, 58 mol%, and 82 mol%) were attained and validated by FTIR, 1H-NMR and GPC analyses.
- A transition in nanostructured morphology from a lamellae-like structure (in the BCP) to a cylindrical structure (for the sample with the highest epoxidation grade) could be observed via TEM, accompanied by a significant shift in soft phase Tg values towards higher temperatures.
- The predominantly elastomeric property of the neat BCP was largely maintained while stiffness and tensile strength were enhanced by epoxidation.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Niessner, N. Practical Guide to Structures, Properties and Applications of Styrenic Polymers; Smithers Rapra Technology, Shrewsbury: Shropshire, UK, 2013. [Google Scholar]
- Legge, N.R.; Holden, G.; Schroeder, H.E. Thermoplastic Elastomers: A Comprehensive Review; Hanser Publishers: Munich, Germany, 1987. [Google Scholar]
- Bates, F.S.; Fredrickson, G.H. Block Copolymer Thermodynamics: Theory and Experiment. Annu. Rev. Phys. Chem. 1990, 41, 525–557. [Google Scholar] [CrossRef] [PubMed]
- Hamley, I.W. The Physics of Block Copolymers; Oxford University Press: Oxford, UK; New York, NY, USA; Tokyo, Japan, 1998. [Google Scholar]
- Abetz, V.; Hadjichristidis, N.; Iatrou, H.; Pitsikalis, M.; Simon, P.F.W. Block Copolymers I; Advances in Polymer Science 189; Springer: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Feng, H.; Lu, X.; Zhang, W.; Kang, N.-G.; Mays, J. Block Copolymers: Synthesis, Self-Assembly, and Applications. Polymers 2017, 9, 494. [Google Scholar] [CrossRef] [PubMed]
- Horechyy, A.; Nandan, B.; Stamm, M. Functional Nanostructured Materials via Self-Assembly of Block Copolymers. In World Scientific Reference of Hybrid Materials: Volume 1. Block Copolymers; Wang, Y., Ed.; World Scientific: Singapore, 2019; pp. 1–44. [Google Scholar] [CrossRef]
- Holden, G. Understanding Thermoplastic Elastomers; Hanser Publishers: Munich, Germany, 2000. [Google Scholar]
- Wang, W.; Lu, W.; Goodwin, A.; Wang, H.; Yin, P.; Kang, N.-G.; Hong, K.; Mays, J. Recent Advances in Thermoplastic Elastomers from Living Polymerizations: Macromolecular Architectures and Supramolecular Chemistry. Prog. Polym. Sci. 2019, 95, 31. [Google Scholar] [CrossRef]
- Michler, G.H.; Adhikari, R.; Lebek, W.; Goerlitz, S.; Weidisch, R.; Knoll, K. Morphology and micromechanical deformation behavior of styrene/butadiene-block copolymers. I. Toughening mechanisms in asymmetric star block copolymers. J. Appl. Polym. Sci. 2002, 85, 683–700. [Google Scholar] [CrossRef]
- Adhikari, R.; Huy, T.A.; Buschnakowski, M.; Michler, G.H.; Knoll, K. Asymmetric PS-block-(PS-co-PB)-block-PS block copolymers: Morphology formation and deformation behaviour. New J. Phys. 2004, 6, 28. [Google Scholar] [CrossRef][Green Version]
- Satapathy, B.K.; Staudinger, U.; Thunga, M.; Lach, R.; Weidisch, R. Influence of phase miscibility on the crack propagation kinetics of nanostructured binary S-(S/B)-S triblock copolymer blends. Macromol. Rapid Commun. 2006, 27, 1814–1820. [Google Scholar] [CrossRef]
- Staudinger, U.; Satapathy, B.K.; Thunga, M.; Weidisch, R.; Janke, A.; Knoll, K. Enhancement of mechanical properties of triblock copolymers by random copolymer middle blocks. Eur. Polym. J. 2007, 43, 2750–2758. [Google Scholar] [CrossRef]
- Staudinger, U.; Satapathy, B.K.; Weidisch, R. Influence of block composition on crack toughness behaviour of styrene-b-(styrene-random-butadiene)-b-styrene triblock copolymers. Eur. Polym. J. 2008, 44, 1822–1833. [Google Scholar] [CrossRef]
- Ganss, M.; Satapathy, B.K.; Thunga, M.; Weidisch, R.; Knoll, K. Molecular-Weight-Controlled Brittle-to-Semiductile-to-Ductile Transition in S-(S/B)-S Triblock Copolymers. Macromol. Mater. Eng. 2010, 295, 178–188. [Google Scholar] [CrossRef]
- Hadjichristidis, N.; Pispas, S.; Floudas, G. Block Copolymers: Synthetic Strategies, Physical Properties, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Ruzette, A.-V.; Leibler, L. Block copolymers in tomorrow’s plastics. Nat. Mater. 2005, 4, 19–31. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, F.; Shi, J.; Li, Z.; Liang, X. Experimental Study on Nano-Parameters of Styrene-Butadiene-Styrene Block Copolymer Modified Bitumen Based on Atomic Force Microscopy. Polymers 2019, 11, 989. [Google Scholar] [CrossRef] [PubMed]
- Airey, G. Styrene butadiene styrene polymer modification of road bitumen. J. Mater. Sci. 2004, 39, 951–959. [Google Scholar] [CrossRef]
- Serrano, E.; Martin, M.D.; Tercjak, A.; Pomposo, J.A.; Mecerreyes, D.; Mondragon, I. Nanostructured Thermosetting Systems from Epoxidized Styrene Butadiene Block Copolymers. Macromol. Rapid Commun. 2005, 26, 982–985. [Google Scholar] [CrossRef]
- Serrano, E.; Tercjak, A.; Kortaberria, G.; Pomposo, J.A.; Mecerreyes, D.; Zafeiropoulos, N.E.; Stamm, M.; Mondragon, I. Nanostructured Thermosetting Systems by Modification with Epoxidized Styrene−Butadiene Star Block Copolymers. Effect of Epoxidation Degree. Macromolecules 2006, 39, 2254–2261. [Google Scholar] [CrossRef]
- Ocando, C.; Tercjak, A.; Serrano, E.; Ramos, J.A.; Corona-Galván, S.; Parellada, M.D.; Fernández-Berridi, M.J.; Mondragon, I. Micro- and macrophase separation of thermosetting systems modified with epoxidized styrene-block-butadiene- block-styrene linear triblock copolymers and their influence on final mechanical properties. Polym. Int. 2008, 57, 1333–1342. [Google Scholar] [CrossRef]
- Pandit, R.; Giri, J.; Michler, G.H.; Lach, R.; Grellmann, W.; Youssef, B.; Saiter, J.M.; Adhikari, R. Effect of Epoxidation of Diene Component of SBS Block Copolymer on Morphology and Mechanical Properties. Macromol. Symp. 2012, 315, 152–159. [Google Scholar] [CrossRef]
- Garate, H.; Mondragon, I.; Goyanes, S.; D’Accorso, N.B. Controlled epoxidation of poly(styrene-b-isoprene-b-styrene) block copolymer for the development of nanostructured epoxy thermosets. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 4505–4513. [Google Scholar] [CrossRef]
- Udipi, K. Epoxidation of styrene–butadiene block polymers. I. J. Appl. Polym. Sci. 1979, 23, 3301–3309. [Google Scholar] [CrossRef]
- Khatiwada, S.; Yadav, A.; Lebek, W.; Saiter, J.; Adhikari, R. Synthesis and design of laboratory device from proton conducting block copolymer membrane. Bibechana 2012, 9. [Google Scholar] [CrossRef]
- Zuchowska, D. Polybutadiene modified by epoxidation. 1. Effect of polybutadiene microstructure on the reactivity of double bonds. Polymer 1980, 21, 514–520. [Google Scholar] [CrossRef]
- Antonietti, M.; Förster, S.; Hartmann, J.; Oestreich, S. Novel Amphiphilic Block Copolymers by Polymer Reactions and Their Use for Solubilization of Metal Salts and Metal Colloids. Macromolecules 1996, 29, 3800–3806. [Google Scholar] [CrossRef]
- Margaritis, A.G.; Kallitsis, J.K.; Kalfoglou, N.K. Compatibility of poly(vinyl chloride) with epoxidized copolymers of butadiene-styrene. Polymer 1989, 30, 2253–2257. [Google Scholar] [CrossRef]
- Aguiar, M.; de Menezes, S.C.; Akcelrud, L. Configurational double bond selectivity in the epoxidation of hydroxy-terminated polybutadiene with m-chloroperbenzoic acid. Macromol. Chem. Phys. 1994, 195, 3937–3948. [Google Scholar] [CrossRef]
- Saffer, A.; Johnson, B.L. Measurement of Internal Double Bonds in Polymers by Per-benzoic Acid Addition. Ind. Eng. Chem. 1948, 40, 538–541. [Google Scholar] [CrossRef]
- Serrano, E.; Larrañaga, M.; Remiro, P.M.; Mondragon, I.; Carrasco, P.M.; Pomposo, J.A.; Mecerreyes, D. Synthesis and Characterization of Epoxidized Styrene-Butadiene Block Copolymers as Templates for Nanostructured Thermosets. Macromol. Chem. Phys. 2004, 205, 987–996. [Google Scholar] [CrossRef]
- Pandit, R.; Michler, G.H.; Lach, R.; Grellmann, W.; Saiter, J.M.; Berkessel, A.; Adhikari, R. Epoxidation of Styrene/Butadiene Star Block Copolymer by Different Methods and Characterization of the Blends with Epoxy Resin. Macromol. Symp. 2014, 341, 67–74. [Google Scholar] [CrossRef]
- Khatiwada, S.P.; Sarath Chandran, C.; Lach, R.; Liebscher, M.; Marc Saiter, J.; Thomas, S.; Heinrich, G.; Adhikari, R. Morphology and Mechanical Properties of Star Block Copolymer Modified Epoxy Resin Blends. Mater. Today Proc. 2017, 4, 5734–5742. [Google Scholar] [CrossRef]
- Knoll, K.; Niessner, N. Styrolux(+) and Styroflex(+)—From transparent high impact polystyrene to new thermoplastic elastomers—Syntheses, applications and blends with other styrene based polymers. Macromol. Symp. 1998, 132, 231–243. [Google Scholar] [CrossRef]
- Saalwächter, K.; Thomann, Y.; Hasenhindl, A.; Schneider, H. Direct Observation of Interphase Composition in Block Copolymers. Macromolecules 2008, 41, 9187–9191. [Google Scholar] [CrossRef]
- Khatiwada, S.P.; Gohs, U.; Lach, R.; Heinrich, G.; Adhikari, R. A New Way of Toughening of Thermoset by Dual-Cured Thermoplastic/Thermosetting Blend. Materials 2019, 12, 548. [Google Scholar] [CrossRef]
- Munteanu, S.; Vasile, C. Spectral and Thermal Characterization of Styrene-Butadiene Copolymers with different Architectures. J. Optoelectron. Adv. Mater. 2005, 7, 3135–3148. [Google Scholar]
- Nikje, M.; Mozaffari, Z. Selective epoxidation of hydroxyl terminated polybutadiene using in situ generated dimethyl dioxirane in the presence Of MoO3. Polymers 2007, 52, 820–826. [Google Scholar] [CrossRef]
- Nikje, M.M.A.; Hajifatheali, H. Performance of dimethyl dioxirane/nano-TiO2 on epoxidation of polybutadiene and hydroxyl terminated polybutadiene. J. Elastomers Plast. 2013, 45, 457–469. [Google Scholar] [CrossRef]
- Holden, G.; Hansen, D.R. Styrenic Thermoplastic Elastomers. In Thermoplastic Elastomers, 3rd ed.; Holden, G., Kricheldorf, H.R., Quirk, R.P., Eds.; Hanser Publishers: Munich, Germany, 2004. [Google Scholar]
- Matsen, M.W.; Schick, M. Microphase Separation in Starblock Copolymer Melts. Macromolecules 1994, 27, 6761–6767. [Google Scholar] [CrossRef]
- Fox, T.G. Influence of Diluent and of Copolymer Composition on the Glass Temperature of a Polymer System. Bull. Am. Phys. Soc. 1956, 1, 123. [Google Scholar]
- Staudinger, U.; Satapathy, B.K. Influence of Carbon Nanotubes on the Morphology and Properties of Styrene-Butadiene Based Block Copolymers. Macromol. Symp. 2017, 373, 1700030. [Google Scholar] [CrossRef]

| Sample Code | Targeted DOE (mol%) | Calculated DOE (mol%) |
|---|---|---|
| ST3 | 0 | 0 |
| epST3-38 | 50 | 38 |
| epST3-57 | 80 | 57 |
| epST3-82 | 100 | 82 |
| Sample Code | Mn (g/mol) | Mw (g/mol) | Ð = Mw/Mn |
|---|---|---|---|
| ST | 82,000 | 172,500 | 2.1 |
| epST-38 | 84,500 | 188,800 | 2.2 |
| epST-57 | 88,300 | 201,200 | 2.3 |
| epST-82 | 95,400 | 223,900 | 2.4 |
| Sample | ET (MPa) | εB (%) | σB (MPa) |
|---|---|---|---|
| ST3 | 182 | 464 | 23.5 |
| epST3-38 | 187 | 386 | 24.7 |
| epST3-57 | 211 | 360 | 28.5 |
| epST3-82 | 221 | 345 | 36.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khatiwada, S.P.; Staudinger, U.; Jehnichen, D.; Heinrich, G.; Adhikari, R. Influence of Controlled Epoxidation of an Asymmetric Styrene/Butadiene Star Block Copolymer on Structural and Mechanical Properties. Polymers 2021, 13, 96. https://doi.org/10.3390/polym13010096
Khatiwada SP, Staudinger U, Jehnichen D, Heinrich G, Adhikari R. Influence of Controlled Epoxidation of an Asymmetric Styrene/Butadiene Star Block Copolymer on Structural and Mechanical Properties. Polymers. 2021; 13(1):96. https://doi.org/10.3390/polym13010096
Chicago/Turabian StyleKhatiwada, Shankar P., Ulrike Staudinger, Dieter Jehnichen, Gert Heinrich, and Rameshwar Adhikari. 2021. "Influence of Controlled Epoxidation of an Asymmetric Styrene/Butadiene Star Block Copolymer on Structural and Mechanical Properties" Polymers 13, no. 1: 96. https://doi.org/10.3390/polym13010096
APA StyleKhatiwada, S. P., Staudinger, U., Jehnichen, D., Heinrich, G., & Adhikari, R. (2021). Influence of Controlled Epoxidation of an Asymmetric Styrene/Butadiene Star Block Copolymer on Structural and Mechanical Properties. Polymers, 13(1), 96. https://doi.org/10.3390/polym13010096