Impact of Ergothioneine, Hercynine, and Histidine on Oxidative Degradation of Hyaluronan and Wound Healing
Abstract
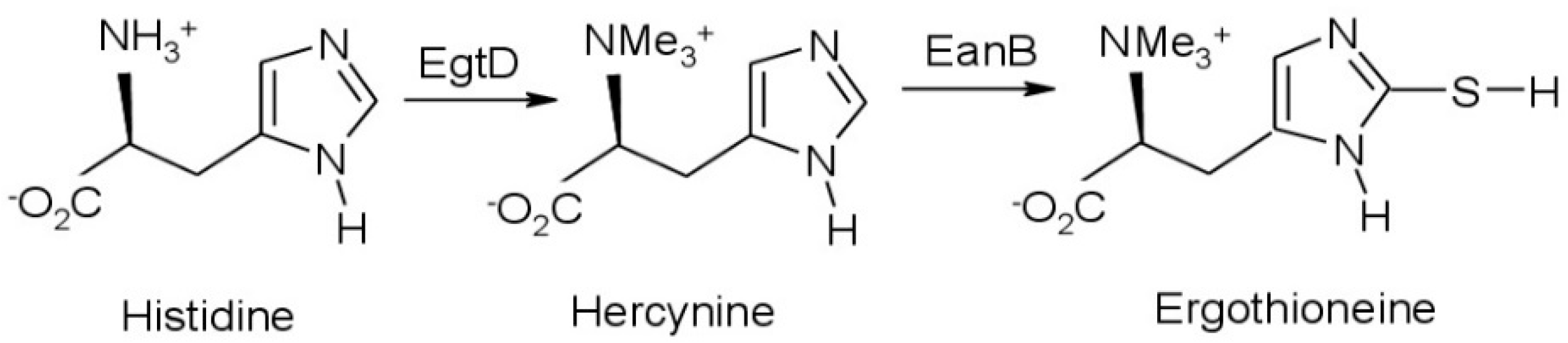
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Stock and Working Solutions
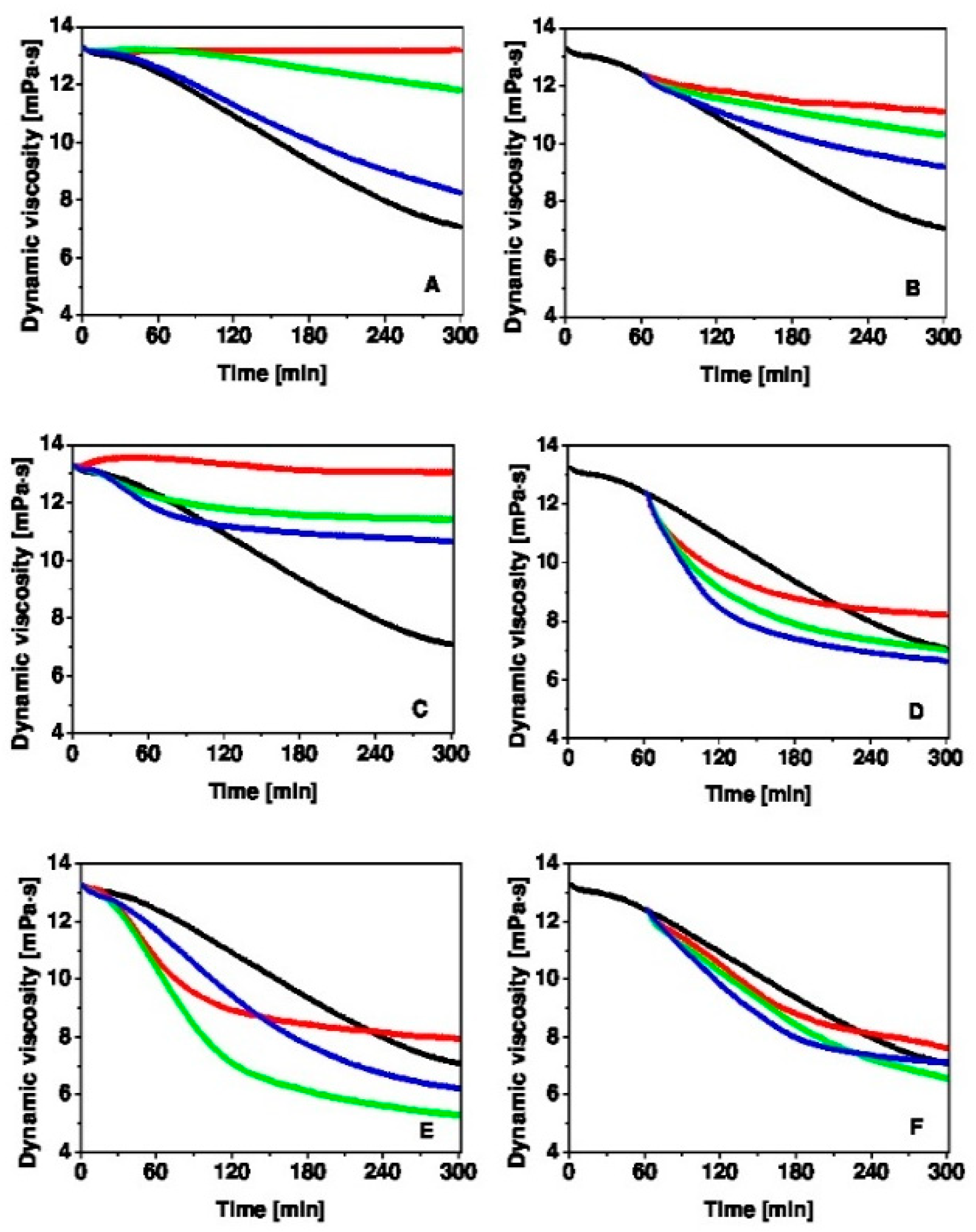
2.3. Hyaluronan Degradation
2.4. Rotational Viscometry
2.5. Preparation of Composite Membranes
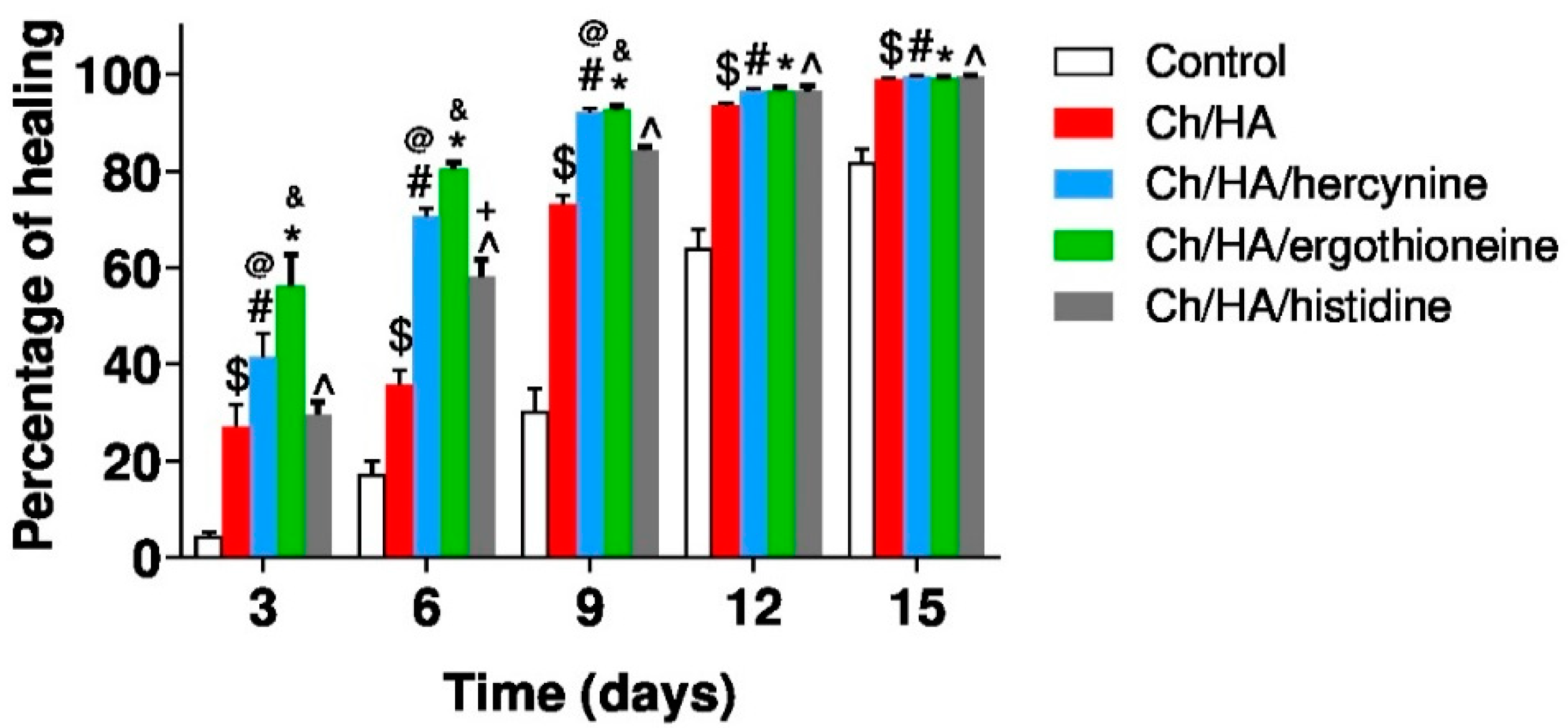
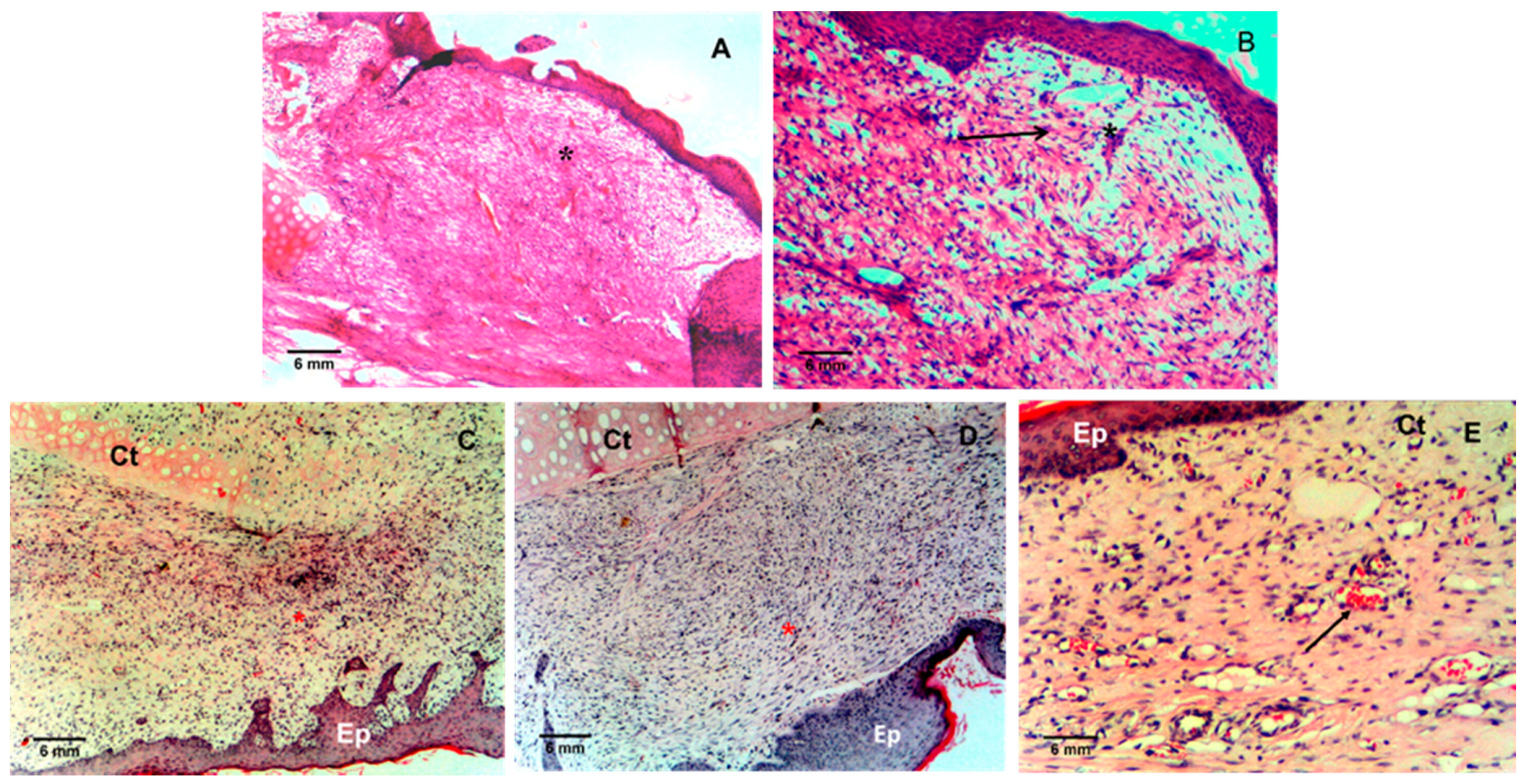
2.6. Skin Wound Healing in Ischemic Rabbits
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stern, R.; Maibach, H.I. Hyaluronan in skin: Aspects of aging and its pharmacologic modulation. Clin. Dermatol. 2008, 26, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Ågren, U.M.; Tammi, R.H.; Tammi, M. Reactive oxygen species contribute to epidermal hyaluronan catabolism in human skin organ culture. Free Radic. Biol. Med. 1997, 23, 996–1001. [Google Scholar] [CrossRef]
- Wu, W.; Jiang, H.; Guo, X.; Wang, Y.; Ying, S.; Feng, L.; Li, T.; Xia, H.; Zhang, Y.; Chen, R.; et al. The pro-tective role of hyaluronic acid in Cr(VI)-induced oxidative damage in corneal epithelial cell. J. Ophthalmol. 2017, 2017, 3678586. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.C.; Lall, R.; Srivastava, A.; Sinha, A. Hyaluronic Acid: Molecular mechanisms and therapeutic trajectory. Front. Vet. Sci. 2019, 6, 192. [Google Scholar] [CrossRef] [PubMed]
- Stankovska, M.; Soltes, L.; Vikartovska, A.; Mendichi, R.; Lath, D.; Molnarova, M.; Gemeiner, P. Study of hyaluronan degradation by means of rotational viscometry. Chem. Pap. 2004, 58, 348–352. [Google Scholar]
- Valachová, K.; Volpi, N.; Stern, R.; Soltes, L. Hyaluronan in medical practice. Curr. Med. Chem. 2016, 23, 3607–3617. [Google Scholar] [CrossRef]
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Frenkel, J.S. The role of hyaluronan in wound healing. Int. Wound J. 2012, 11, 159–163. [Google Scholar] [CrossRef]
- Weindl, G.; Schaller, M.; Schäfer-Korting, M.; Korting, H. Hyaluronic acid in the treatment and prevention of skin diseases: Molecular biological, pharmaceutical and clinical aspects. Skin Pharmacol. Physiol. 2004, 17, 207–213. [Google Scholar] [CrossRef]
- Elieh-Ali-Komi, D.; Hamblin, M.R. Chitin and chitosan: Production and application of versatile biomedical nano-materials. Int. J. Adv. Res. 2016, 4, 411–427. [Google Scholar]
- Chatelet, C.; Damour, O.; Domard, A. Influence of the degree of acetylation on some biological properties of chitosan films. Biomaterials 2001, 22, 261–268. [Google Scholar] [CrossRef]
- Foster, J.L.; Ho, S.; Hook, J.; Basuki, M.; Marçal, H. Chitosan as a biomaterial: Influence of degree of deacetylation on its physiochemical, material and biological Properties. PLoS ONE 2015, 10, e0135153. [Google Scholar] [CrossRef] [PubMed]
- Radwan-Pragłowska, J.; Piątkowski, M.; Deineka, V.; Janus, Ł.; Korniienko, V.; Husak, Y.; Holubnycha, V.; Liubchak, I.; Zhurba, V.; Sierakowska, A.; et al. Chitosan-based bioactive hemostatic agents with antibacterial properties-synthesis and characterization. Molecules 2019, 24, 2629. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Tanaka, M.; Huang, Y.-Y.; Hamblin, M.R. Chitosan preparations for wounds and burns: Antimicrobial and wound-healing effects. Expert Rev. Anti Infect. Ther. 2011, 9, 857–879. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.; Durack, E.; Collins, M.N.; Hudson, S. Tuning the strength and swelling of an injectable polysaccharide hydrogel and the subsequent release of a broad spectrum bacteriocin, nisin A. J. Mater. Chem. B 2020, 8, 4029–4038. [Google Scholar] [CrossRef]
- Valachová, K.; Mach, M.; Dubovický, M.; Šoltés, L. The importance of ergothioneine synthesis in ancient time by organisms living in oxygen free atmosphere. Med. Hypotheses 2019, 123, 72–73. [Google Scholar] [CrossRef]
- Bazela, K.; Solyga-Zurek, A.; Debowska, R.; Rogiewicz, K.; Bartnik, E.; Eris, I. l-Ergothioneine protects skin cells against UV-induced damage—A Preliminary study. Cosmetics 2014, 1, 51–60. [Google Scholar] [CrossRef]
- Hseu, Y.-C.; Lo, H.-W.; Korivi, M.; Tsai, Y.-C.; Tang, M.-J.; Yang, H.-L. Dermato-protective properties of ergothioneine through induction of Nrf2/ARE-mediated antioxidant genes in UVA-irradiated human keratinocytes. Free Radic. Biol. Med. 2015, 86, 102–117. [Google Scholar] [CrossRef]
- Paul, B.D.; Snyder, S.H. The unusual amino acid l-ergothioneine is a physiologic cytoprotectant. Cell Death Differ. 2010, 17, 1134–1140. [Google Scholar] [CrossRef]
- Gründemann, D. The ergothioneine transporter controls and indicates ergothioneine activity—A review. Prev. Med. 2012, 54, S71–S74. [Google Scholar] [CrossRef]
- Seebeck, F.P. Thiohistidine biosynthesis. Chimia 2013, 67, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Li, R.W.S.; Yang, C.; Sit, A.S.M.; Kwan, Y.W.; Lee, S.M.Y.; Hoi, M.P.M.; Chan, S.-W.; Hausman, M.; Vanhoutte, P.M.; Leung, G.P.-H. Uptake and protective effects of ergothioneine in human endothelial cells. J. Pharmacol. Exp. Ther. 2014, 350, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Cheah, I.K.; Drum, C.L. Ergothioneine, an adaptive antioxidant for the protection of injured tissues? A hypothesis. Biochem. Biophys. Res. Commun. 2016, 470, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Cheah, I.K.; Tang, R.M.Y. Ergothioneine–A diet-derived antioxidant with therapeutic potential. FEBS Lett. 2018, 592, 3357–3366. [Google Scholar] [CrossRef] [PubMed]
- Forster, R.; Spezia, F.; Papineau, D.; Sabadie, C.; Erdelmeier, I.; Moutet, M.; Yadan, J.-C. Reproductive safety evaluation of l-ergothioneine. Food Chem. Toxicol. 2015, 80, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Cheah, I.; Halliwell, B. Could ergothioneine aid in the treatment of coronavirus patients? Antioxidants 2020, 9, 595. [Google Scholar] [CrossRef]
- Sotgia, S.; Zinellu, A.; Forteschi, M.; Paliogiannis, P.; Pinna, G.A.; Mangoni, A.A.; Carru, C. Hercynine content in widely consumed commercial beverages. LWT Food Sci. Technol. 2018, 98, 465–469. [Google Scholar] [CrossRef]
- Ząbek-Adamska, A.; Drożdż, R.; Naskalski, J.W. Dynamics of reactive oxygen species generation in the presence of copper(II)-histidine complex and cysteine. Acta Biochim. Pol. 2013, 60, 565–571. [Google Scholar] [CrossRef]
- Moro, J.; Tomé, D.; Schmidely, P.; Demersay, T.-C.; Azzout-Marniche, D. Histidine: A systematic review on metabolism and physiological effects in human and different animal species. Nutrients 2020, 12, 1414. [Google Scholar] [CrossRef]
- Orofino, D.P. Topical Transdermal Method for Delivering Nutrients through the Skin for Expedited Wound Healing and Skin Rejuvenation. U.S. Patent 9,549,960 B1, 24 January 2017. [Google Scholar]
- Tan, S.P.; Brown, S.B.; Griffiths, C.E.; Weller, R.B.; Gibbs, N.K. Feeding filaggrin: Effects of l-histidine supplementation in atopic dermatitis. Clin. Cosmet. Investig. Dermatol. 2017, 10, 403–411. [Google Scholar] [CrossRef]
- Rothkopf, M. Results of histidine (His) supplementation in a case of severe nummular eczema (NE). Curr. Dev. Nutr. 2020, 4, 1141. [Google Scholar] [CrossRef]
- Tamer, M.T.; Valachova, K.; Hassan, M.A.; Omer, A.M.; El-Shafeey, M.; Mohy Eldin, M.S.; Soltes, L. Chitosan/hyaluronan/edaravone membranes for anti-inflammatory wound dressing: In vitro and in vivo evaluation studies. Mater. Sci. Eng. C 2018, 90, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Tamer, T.M.; Collins, M.N.; Valachová, K.; Hassan, M.A.; Omer, A.M.; Eldin, M.S.M.; Švík, K.; Jurčík, R.; Ondruška, Ľ.; Biró, C.; et al. MitoQ loaded chitosan-hyaluronan composite membranes for wound healing. Materials 2018, 11, 569. [Google Scholar] [CrossRef] [PubMed]
- Valachová, K.; Svik, K.; Biro, C.; Soltes, L. Skin wound healing with composite biomembranes loaded by tiopronin or captopril. J. Biotechnol. 2020, 310, 49–53. [Google Scholar] [CrossRef]
- Tamer, M.T.; Hassan, M.A.; Valachová, K.; Omer, A.M.; El-Shafeey, M.E.; Eldin, M.S.M.; Šoltés, L. Enhancement of wound healing by chitosan/hyaluronan polyelectrolyte membrane loaded with glutathione: in vitro and in vivo evaluations. J. Biotechnol. 2020, 310, 103–113. [Google Scholar] [CrossRef]
- Valachová, K.; Vargová, A.; Rapta, P.; Hrabárová, E.; Drafi, F.; Bauerová, K.; Juránek, I.; Šoltés, L. Aurothiomalate as preventive and chain-breaking antioxidant in radical degradation of high-molar-mass hyaluronan. Chem. Biodivers. 2011, 8, 1274–1283. [Google Scholar] [CrossRef]
- Baňasová, M.; Valachová, K.; Rychlý, J.; Janigová, I.; Csomorová, K.; Mendichi, R.; Mislovičová, D.; Juránek, I.; Šoltés, L. Effect of bucillamine on free-radical-mediated degradation of high-molar-mass hyaluronan induced in vitro by ascorbic acid and Cu(II) ions. Polymers 2014, 6, 2625–2644. [Google Scholar] [CrossRef]
- DiPietro, L.A.; Burns, A.L. Wound Healing Methods and Protocols; Humana Press: New York, NY, USA, 2003. [Google Scholar]
- Soltes, L.; Valachova, K.; Mach, M.; Juranek, I. Composite Membranes Containing a Smart-Released Cytoprotectant Targeting the Inflamed Tissue and Use Thereof. European Patent EP20020280.2, 20 June 2017. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valachova, K.; Svik, K.; Biro, C.; Collins, M.N.; Jurcik, R.; Ondruska, L.; Soltes, L. Impact of Ergothioneine, Hercynine, and Histidine on Oxidative Degradation of Hyaluronan and Wound Healing. Polymers 2021, 13, 95. https://doi.org/10.3390/polym13010095
Valachova K, Svik K, Biro C, Collins MN, Jurcik R, Ondruska L, Soltes L. Impact of Ergothioneine, Hercynine, and Histidine on Oxidative Degradation of Hyaluronan and Wound Healing. Polymers. 2021; 13(1):95. https://doi.org/10.3390/polym13010095
Chicago/Turabian StyleValachova, Katarina, Karol Svik, Csaba Biro, Maurice N. Collins, Rastislav Jurcik, Lubomir Ondruska, and Ladislav Soltes. 2021. "Impact of Ergothioneine, Hercynine, and Histidine on Oxidative Degradation of Hyaluronan and Wound Healing" Polymers 13, no. 1: 95. https://doi.org/10.3390/polym13010095
APA StyleValachova, K., Svik, K., Biro, C., Collins, M. N., Jurcik, R., Ondruska, L., & Soltes, L. (2021). Impact of Ergothioneine, Hercynine, and Histidine on Oxidative Degradation of Hyaluronan and Wound Healing. Polymers, 13(1), 95. https://doi.org/10.3390/polym13010095






