Microfiltration Membranes Modified with Composition of Titanium Oxide and Silver Oxide by Magnetron Sputtering
Abstract
1. Introduction
2. Materials and Methods
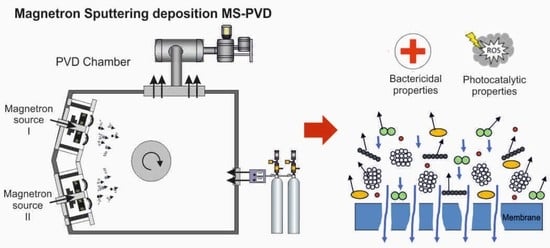
2.1. Coatings Deposition
2.2. Structure and Elemental Composition Characterization
2.3. Bactericidal Properties
2.4. Photocatalytic Properties
2.5. Filtration Properties
3. Results and Discussion
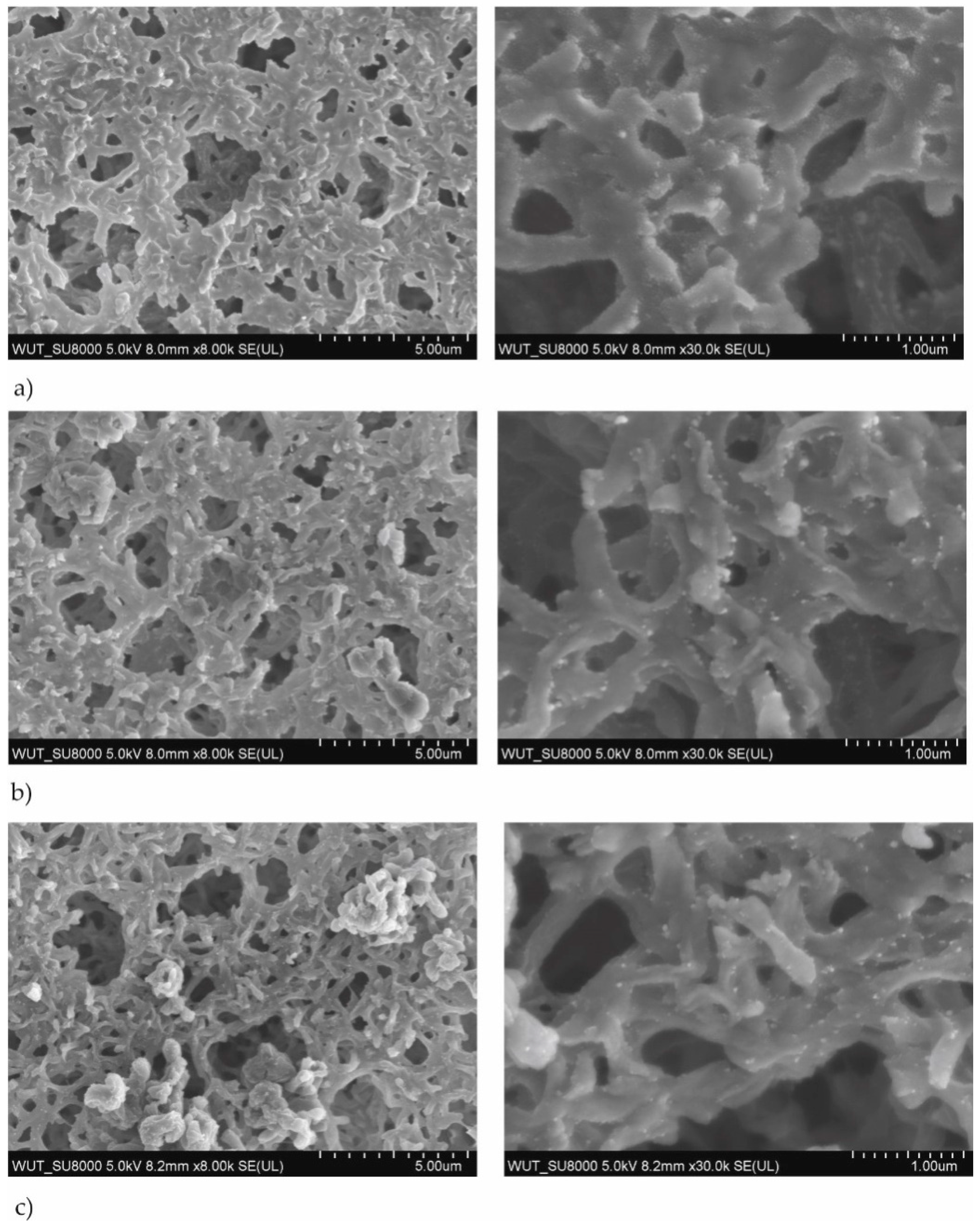
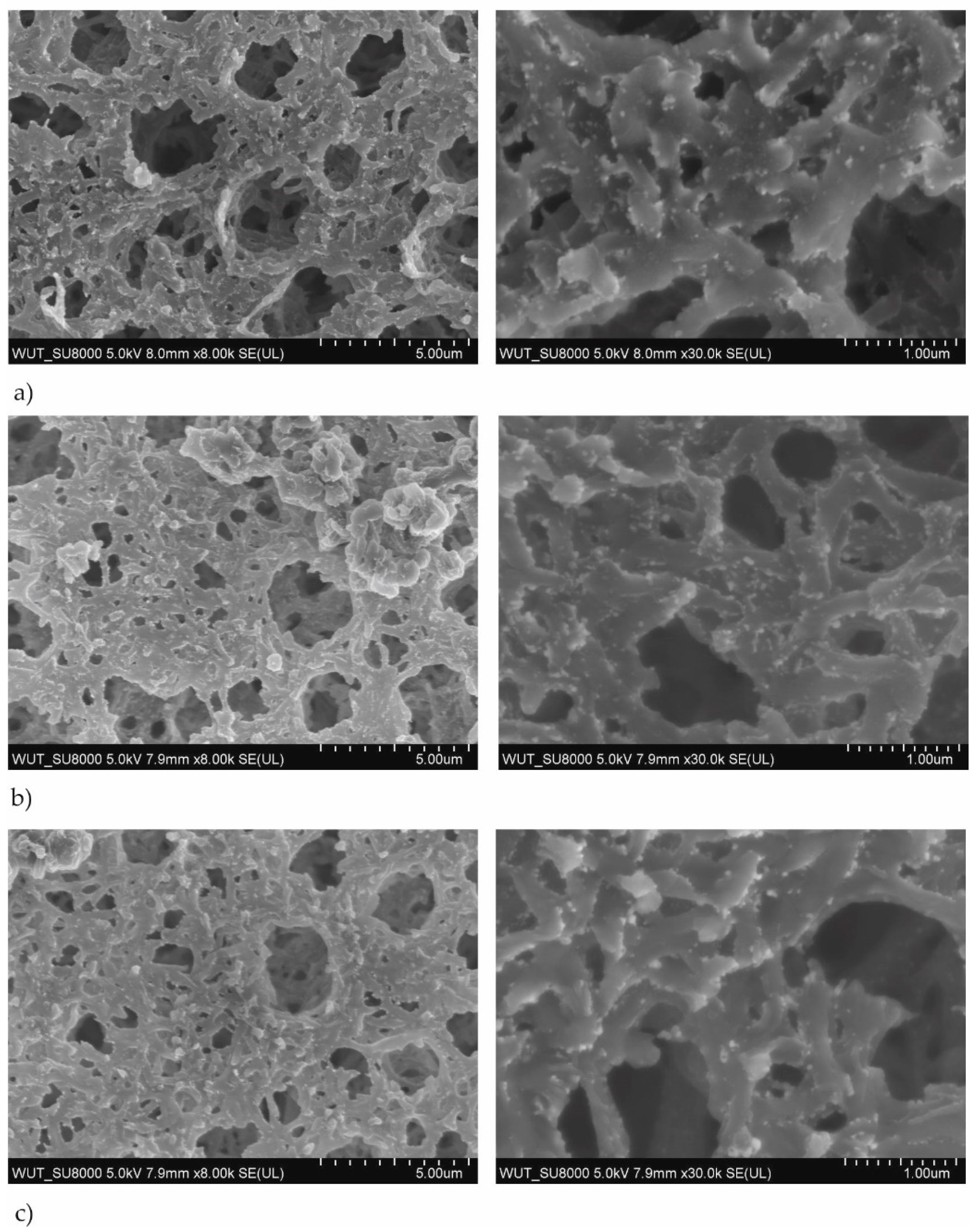
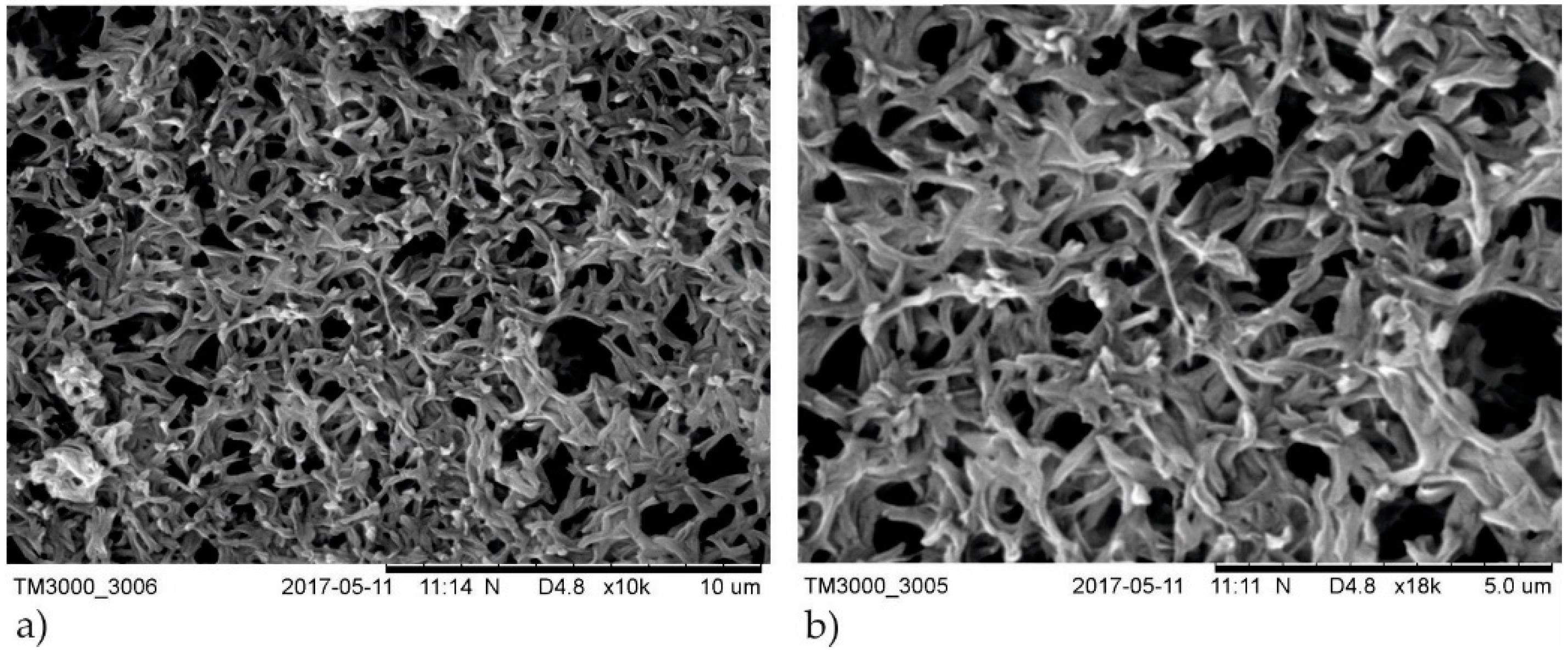
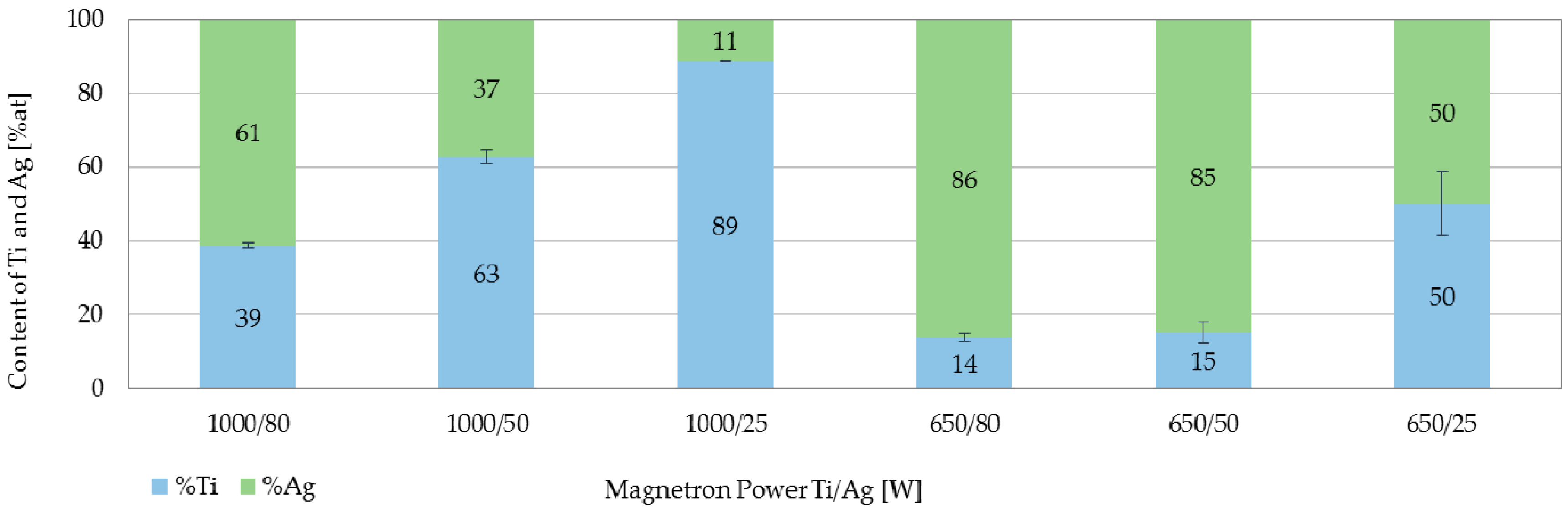
3.1. Structure and Elemental Composition Characterization
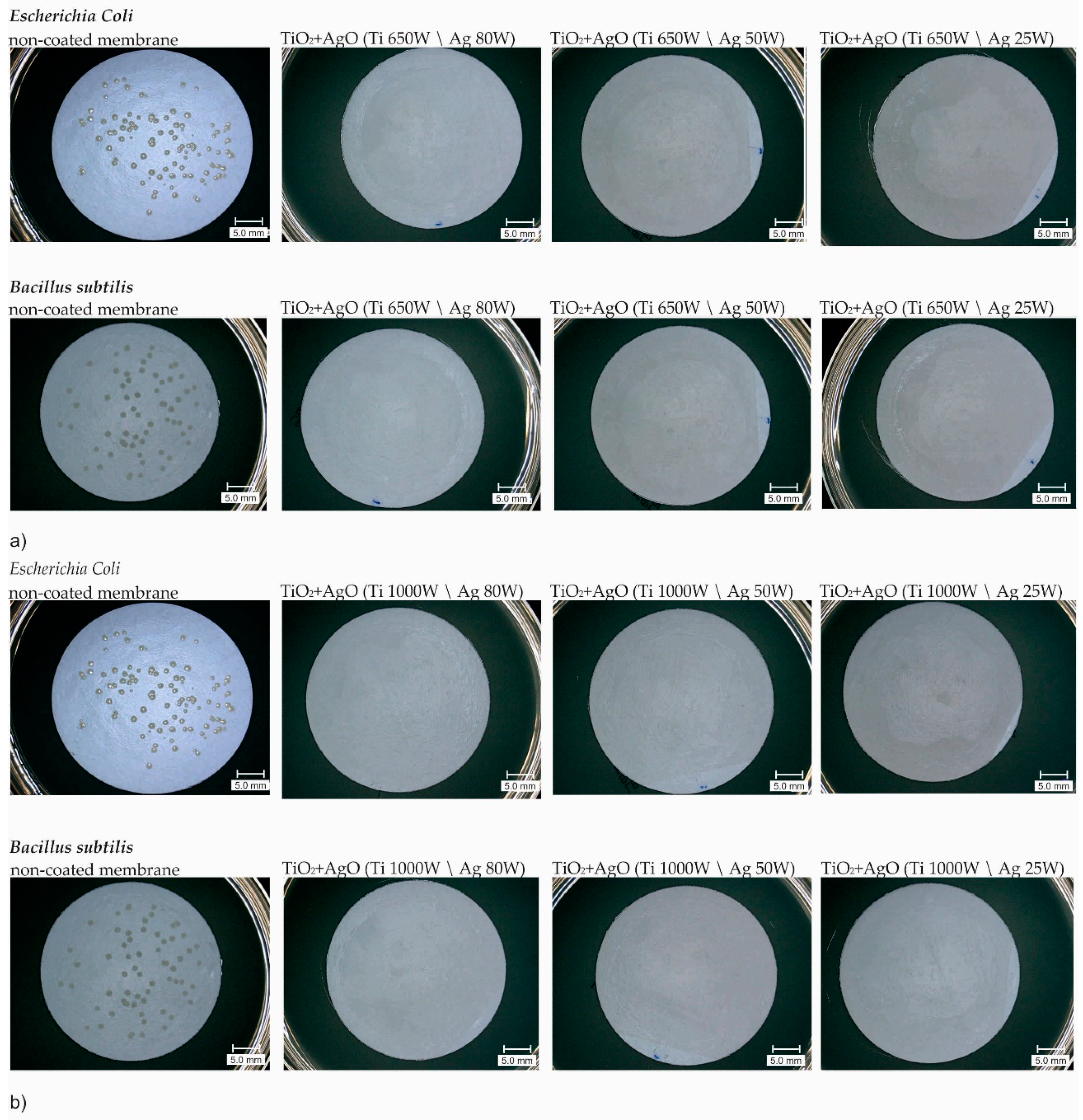
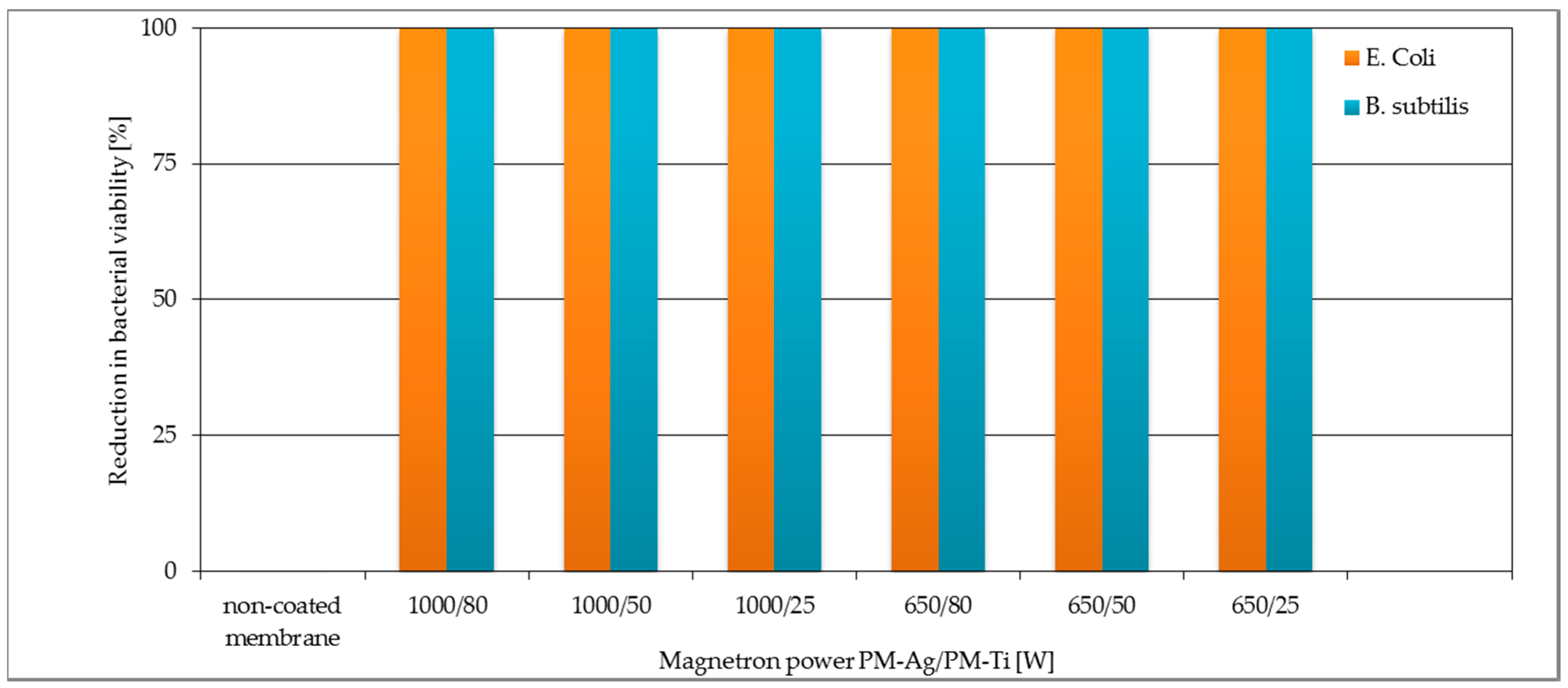
3.2. Antibacterial Properties
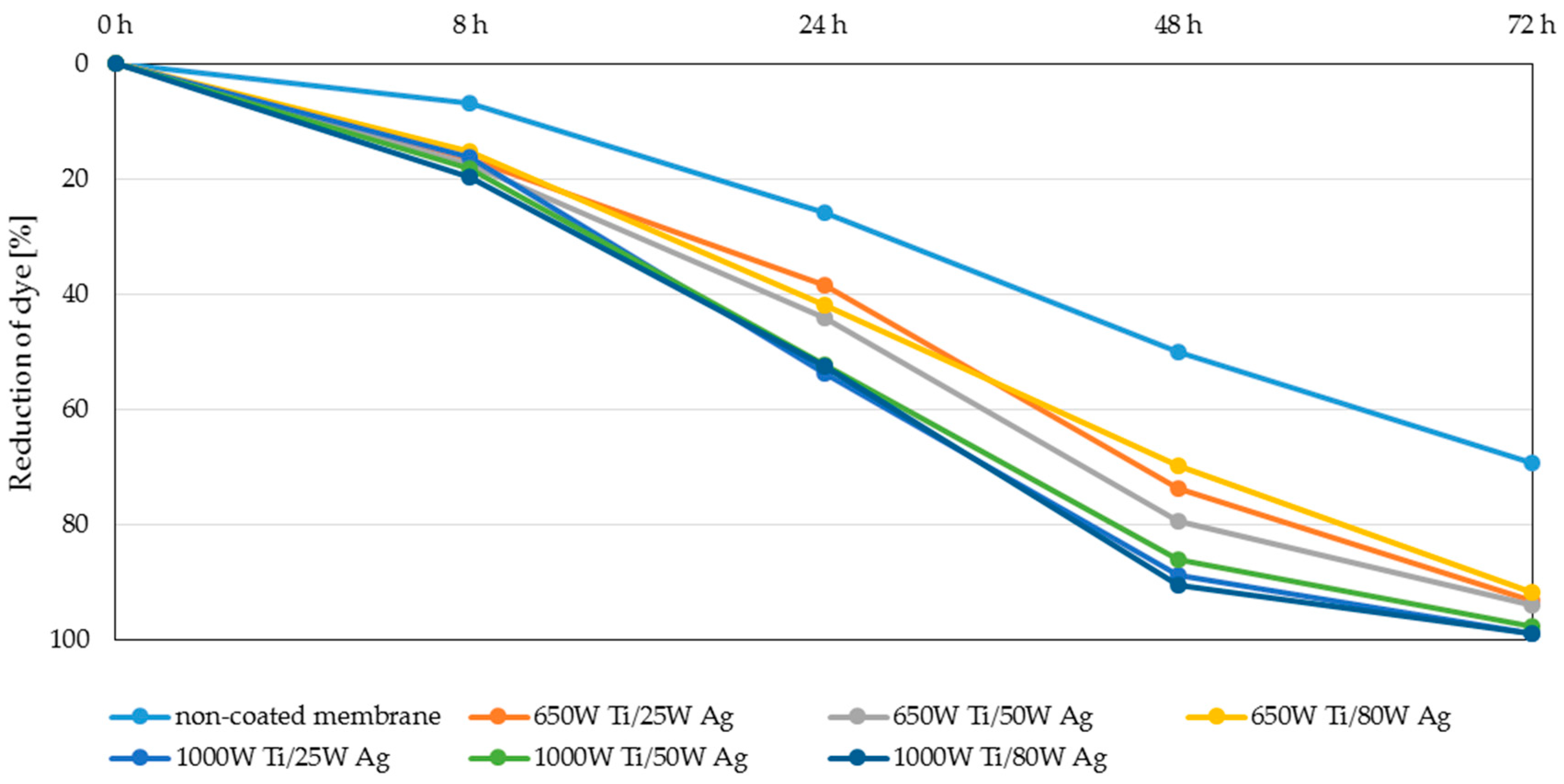
3.3. Photocatalytic Properties
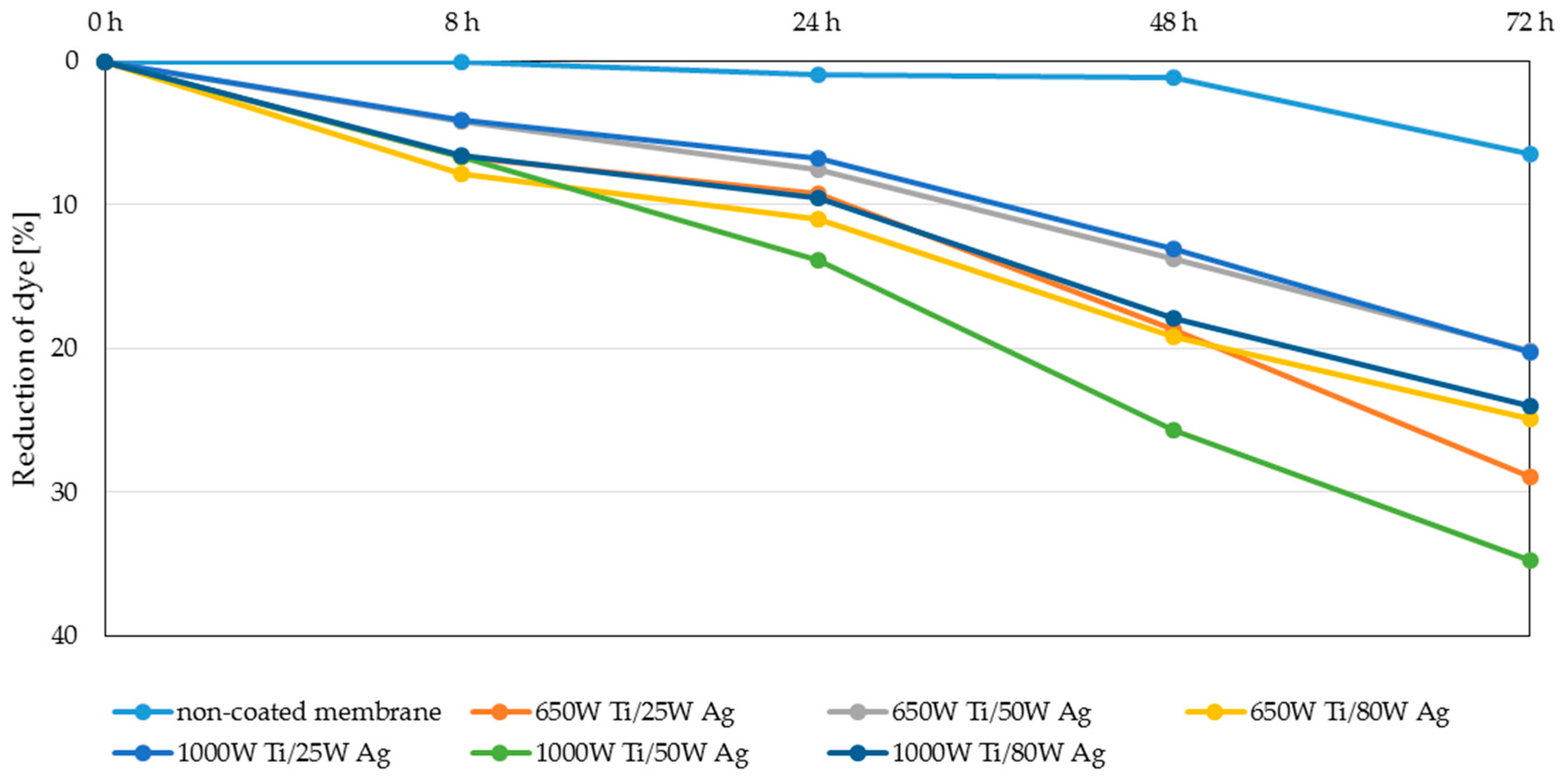
3.4. Filtration Properties and Stability of the Coatings
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kołtuniewicz, A.B.; Drioli, E. Membranes in Clean Technologies. Theory and Practice, Vol. 1–2; WILEY-VCH Verlag GmbH & Co: Weinheim, Germany, 2008. [Google Scholar]
- Ursino, C.; Castro-Muñoz, R.; Drioli, E.; Gzara, L.; Albeirutty, M.H.; Figoli, A. Progress of Nanocomposite Membranes for Water Treatment. Membranes 2018, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Roddick, F.A.; Fan, L. Biofouling of Water Treatment Membranes: A Review of the Underlying Causes, Monitoring Techniques and Control Measures. Membranes 2012, 2, 804–840. [Google Scholar] [CrossRef] [PubMed]
- Subramani, A.; Hoek, E.M.V. Direct observation of initial microbial deposition onto reverse osmosis and nanofiltration membranes. J. Membr. Sci. 2008, 319, 111–125. [Google Scholar] [CrossRef]
- Boussu, K.; Belpaire, A.; Volodin, A.; van Haesendonck, C.; van der Meeren, P.; Vandecasteele, C.; van der Bruggen, B. Influence of membrane and colloid characteristics on fouling of nanofiltration membranes. J. Membr. Sci. 2007, 289, 220–230. [Google Scholar] [CrossRef]
- Najjar, A.; Sabri, S.; Al-Gaashani, R.; Atieh, M.A.; Kochkodan, V. Antibiofouling Performance by Polyethersulfone Membranes Cast with Oxidized Multiwalled Carbon Nanotubes and Arabic Gum. Membranes 2019, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Tilahun, A.; Haddis, S.; Hadush, A.T.T. Review on Biofilm and Microbial Adhesion. Int. J. Microbiol. Res. 2016, 3, 63–73. [Google Scholar] [CrossRef]
- Garrett, T.R.; Bhakoo, M.; Zhang, Z. Bacterial adhesion and biofilms on surfaces. Progr. Nat. Sci. 2008, 18, 1049–1056. [Google Scholar] [CrossRef]
- Vanysacker, L.; Denis, C.; Declerck, P.; Piasecka, A.; Vankelecom, I.F.J. Microbial Adhesion and Biofilm Formation on Microfiltration Membranes: A Detailed Characterization Using Model Organisms with Increasing Complexity. BioMed Res. Int. 2013, 2013, 470867. [Google Scholar] [CrossRef]
- Kacprzyńska-Gołacka, J.; Kowalik-Klimczak, A.; Skowroński, J.; Rajewska, P.; Wieciński, P.; Smolik, J. Możliwości wykorzystania plazmowych technik inżynierii powierzchni do modyfikacji membran polimerowych. Polimery 2018, 5, 353–361. [Google Scholar]
- Kacprzyńska-Gołacka, J.; Kowalik-Klimczak, A.; Woskowicz, E.; Wieciński, P.; Łożyńska, M.; Sowa, S.; Barszcz, W.; Kaźmierczak, B. Microfiltration Membranes Modified with Silver Oxide by Plasma Treatment. Membranes 2020, 10, 133. [Google Scholar] [CrossRef]
- Yadav, H.M.; Kim, J.; Pawar, S.H. Developments in photocatalytic antibacterial activity of nano TiO2: A review. Korean J. Chem. Eng. 2016, 33, 1989–1998. [Google Scholar] [CrossRef]
- Vatanpoura, V.; Madaenia, S.S.; Khataeeb, A.R.; Salehia, E.; Zinadinia, S.; Monfareda, H.A. TiO2 embedded mixed matrix PES nanocomposite membranes: Influence of different sizes and types of nanoparticles on antifouling and performance. Desalination 2012, 292, 19–29. [Google Scholar] [CrossRef]
- Wafi, A.; Szabó-Bárdos, E.; Horváth, O.; Pósfai, M.; Makó, É.; Juzsakova, T.; Fónagy, O. The Photocatalytic and Antibacterial Performance of Nitrogen-Doped TiO2: Surface-Structure Dependence and Silver-Deposition Effect. Nanomaterials 2020, 10, 2261. [Google Scholar] [CrossRef] [PubMed]
- Kacprzyńska-Gołacka, J.; Osuch-Słomka, E.; Wieciński, P.; Garbacz, H.; Skowroński, J.; Kowalik-Klimczak, A.; Smolik, J.; Mazurkiewicz, A. The impact of working gas atmosphere on phase composition and microstructure of TiO2 coating prepared by reactive magnetron sputtering. In Proceedings of the 26th International Conference on Metallurgy and Materials, Brno, Czech Republic, 24–26 May 2017. [Google Scholar]
- Kacprzyńska-Gołacka, J.; Sowa, S.; Kowalik-Klimczak, A.; Rajewska, P.; Stanisławek, E.; Wieciński, P. The Conception and Design of Polymeric Membranes Modification Technology Using Surface Engineering Techniques. In Proceedings of the 9th International Conference Nanomaterials: Applications & Properties (NAP), Odesa, Ukraine, 15–20 September 2019; pp. 01TFC31-1–01TFC31-3. [Google Scholar] [CrossRef]
- Alotaibi, A.M.; Williamson, B.A.D.; Sathasivam, S.; Kafizas, A.; Alqahtani, M.; Sotelo-Vazquez, C.; Buckeridge, J.; Wu, J.; Nair, S.P.; Scanlon, D.O.; et al. Enhanced Photocatalytic and Antibacterial Ability of Cu-Doped Anatase TiO2 Thin Films: Theory and Experiment. ACS Appl. Mater. Interfaces 2020, 12, 15348–15361. [Google Scholar] [CrossRef]
- He, X.; Zhang, G.; Wang, X.; Hang, R.; Huang, X.; Qin, L.; Tang, B.; Zhang, X. Biocompatibility, corrosion resistance and antibacterial activity of TiO2/CuO coating on titanium. Ceram. Int. 2017, 43, 16185–16195. [Google Scholar] [CrossRef]
- Sethi, D.; Sakthivel, R. ZnO/TiO2 composites for photocatalytic inactivation of Escherichia coli. J. Photochem. Photobiol. B Biol. 2017, 168, 17–123. [Google Scholar] [CrossRef]
- Wysocka, I.; Kowalska, E.; Trzciński, K.; Łapiński, M.; Nowaczyk, G.; Zielińska-Jurek, A. UV–Vis-induced degradation of phenol over magnetic photocatalysts modified with Pt, Pd, Cu and Au nanoparticles. Nanomaterials 2018, 8, 28. [Google Scholar] [CrossRef]
- Harikishore, M.; Sandhyarani, M.; Venkateswarlu, K.; Nellaippan, T.A.; Rameshbabu, N. Effect of Ag Doping on Antibacterial and Photocatalytic Activity of Nanocrystalline TiO2. Procedia Mater. Sci. 2014, 6, 557–566. [Google Scholar] [CrossRef]
- Al Suliman, N.; Awada, C.; Alshoaibi, A.; Shaalan, N.M. Simple Preparation of Ceramic-Like Materials Based on 1D-Agx(x=0, 5, 10, 20, 40 mM)/TiO2 Nanostructures and Their Photocatalysis Performance. Crystals 2020, 10, 1024. [Google Scholar] [CrossRef]
- Komaraiah, D.; Radha, E.; Sivakumar, J.; Ramana Reddy, M.V.; Sayanna, R. Photoluminescence and photocatalytic activity of spin coated Ag+ doped anatase TiO2 thin films. Opt. Mater. 2020, 108, 110401. [Google Scholar] [CrossRef]
- Spilarewicz-Stanek, K.; Jakimińska, A.; Kisielewska, A.; Batory, D.; Piwoński, I. Understanding the Role of Silver Nanostructures and Graphene Oxide Applied as Surface Modification of TiO2 in Photocatalytic Transformations of Rhodamine B under UV and Vis Irradiation. Materials 2020, 13, 4653. [Google Scholar] [CrossRef] [PubMed]
- Thukkaram, M.; Cools, P.; Nikiforov, A.; Rigole, P.; Coenye, T.; Van Der Voort, P.; Du Laing, G.; Vercruysse, C.; Declercq, H.; Morent, R.; et al. Antibacterial activity of a porous silver doped TiO2 coating on titanium substrates synthesized by plasma electrolytic oxidation. Appl. Surf. Sci. 2020, 500, 144–235. [Google Scholar] [CrossRef]
- Navabpour, P.; Ostovarpour, S.; Hampshire, J.; Kelly, P.; Verran, J.; Cooke, K. The effect of process parameters on the structure, photocatalytic and self-cleaning properties of TiO2 and Ag-TiO2 coatings deposited using reactive magnetron sputtering. Thin Solid Film 2014, 571, 75–83. [Google Scholar] [CrossRef]
- Le Ouay, B.; Stellaci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Sri Devi, P.; Vijayalakshmi, K.A. Analysis of antibacterial activity and cytotoxicity of silver oxide doped hydroxyapatite exposed to DC glow discharge plasma. Mater. Today Proc. 2020, 24, 3604–3608. [Google Scholar] [CrossRef]
- Duffy, L.L.; Osmond-McLeod, M.J.; Judy, J.; King, T. Investigation into the antibacterial activity of silver, zinc oxide and copper oxide nanoparticles against poultry-relevant isolates of Salmonella and Campylobacter. Food Control 2018, 92, 293–300. [Google Scholar] [CrossRef]
- Slavin, S.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Niu, J.; Chen, Y. Mechanism of Photogenerated Reactive Oxygen Species and Correlation with the Antibacterial Properties of Engineered Metal-Oxide Nanoparticles. ACS Nano 2012, 6, 5164–5173. [Google Scholar] [CrossRef]
- Albiter, E.; Valenzuela, M.A.; Alfaro, S.; Valverde-Aguilar, G.; Martinez-Pallares, F.M. Photocatalytic deposition of Ag nanoparticles on TiO2: Metal precursor effect on the structural and photoactivity properties. J. Saudi Chem. Soc. 2015, 19, 563–573. [Google Scholar] [CrossRef]
- Mogal, S.I.; Gandhi, V.G.; Mishra, M.; Tripathi, S.; Shripathi, T.; Joshi, P.A.; Shah, D.O. Single-Step Synthesis of Silver-Doped Titanium Dioxide: Influence of Silver on Structural, Textural, and Photocatalytic Properties. Ind. Eng. Chem. Res. 2014, 53, 5749–5758. [Google Scholar] [CrossRef]
- Din, M.I.; Khalid, R.; Hussain, Z. Minireview: Silver-Doped Titanium Dioxide and Silver-Doped Zinc Oxide Photocatalysts. Anal. Lett. 2018, 51, 892–907. [Google Scholar] [CrossRef]
- Glez, V.R.; Hernandez-Gordillo, L. Silver-Based Photocatalysts: A Special Class. In American Jewish Year Book; Springer: Cham, Switzerland, 2018; Chapter 8; pp. 221–239. [Google Scholar]
- Kodom, T.; Rusen, E.; Calinescu, I.; Mocanu, A.; Somoghi, R.; Dinescu, A.; Diacon, A.; Boscornea, C. Silver Nanoparticles Influence on Photocatalytic Activity of Hybrid Materials Based on TiO2 P25. J. Nanomater. 2015, 2015, 210734. [Google Scholar] [CrossRef]
- Seery, M.K.; George, R.; Floris, P.; Pillai, S.C. Silver doped titanium dioxide nanomaterials for enhanced visible light photocatalysis. J. Photochem. Photobiol. A Chem. 2007, 189, 258–263. [Google Scholar] [CrossRef]
- Xu, J.; Xiao, X.; Ren, F.; Wu, W.; Dai, Z.; Cai, G.; Zhang, S.; Zhou, J.; Mei, F.; Jaing, C. Enhanced photocatalysis by coupling of anatase TiO2 film to triangular Ag nanoparticle island. Nanoscale Res. Lett. 2012, 7, 239–245. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kacprzyńska-Gołacka, J.; Łożyńska, M.; Barszcz, W.; Sowa, S.; Wieciński, P.; Woskowicz, E. Microfiltration Membranes Modified with Composition of Titanium Oxide and Silver Oxide by Magnetron Sputtering. Polymers 2021, 13, 141. https://doi.org/10.3390/polym13010141
Kacprzyńska-Gołacka J, Łożyńska M, Barszcz W, Sowa S, Wieciński P, Woskowicz E. Microfiltration Membranes Modified with Composition of Titanium Oxide and Silver Oxide by Magnetron Sputtering. Polymers. 2021; 13(1):141. https://doi.org/10.3390/polym13010141
Chicago/Turabian StyleKacprzyńska-Gołacka, Joanna, Monika Łożyńska, Wioletta Barszcz, Sylwia Sowa, Piotr Wieciński, and Ewa Woskowicz. 2021. "Microfiltration Membranes Modified with Composition of Titanium Oxide and Silver Oxide by Magnetron Sputtering" Polymers 13, no. 1: 141. https://doi.org/10.3390/polym13010141
APA StyleKacprzyńska-Gołacka, J., Łożyńska, M., Barszcz, W., Sowa, S., Wieciński, P., & Woskowicz, E. (2021). Microfiltration Membranes Modified with Composition of Titanium Oxide and Silver Oxide by Magnetron Sputtering. Polymers, 13(1), 141. https://doi.org/10.3390/polym13010141