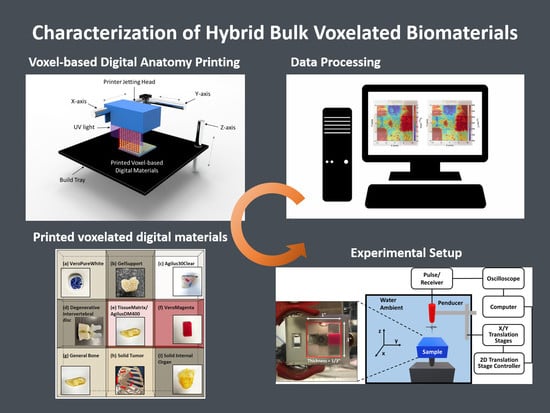
Manufacturing and Characterization of Hybrid Bulk Voxelated Biomaterials Printed by Digital Anatomy 3D Printing
Abstract
1. Introduction
2. Materials and Methods
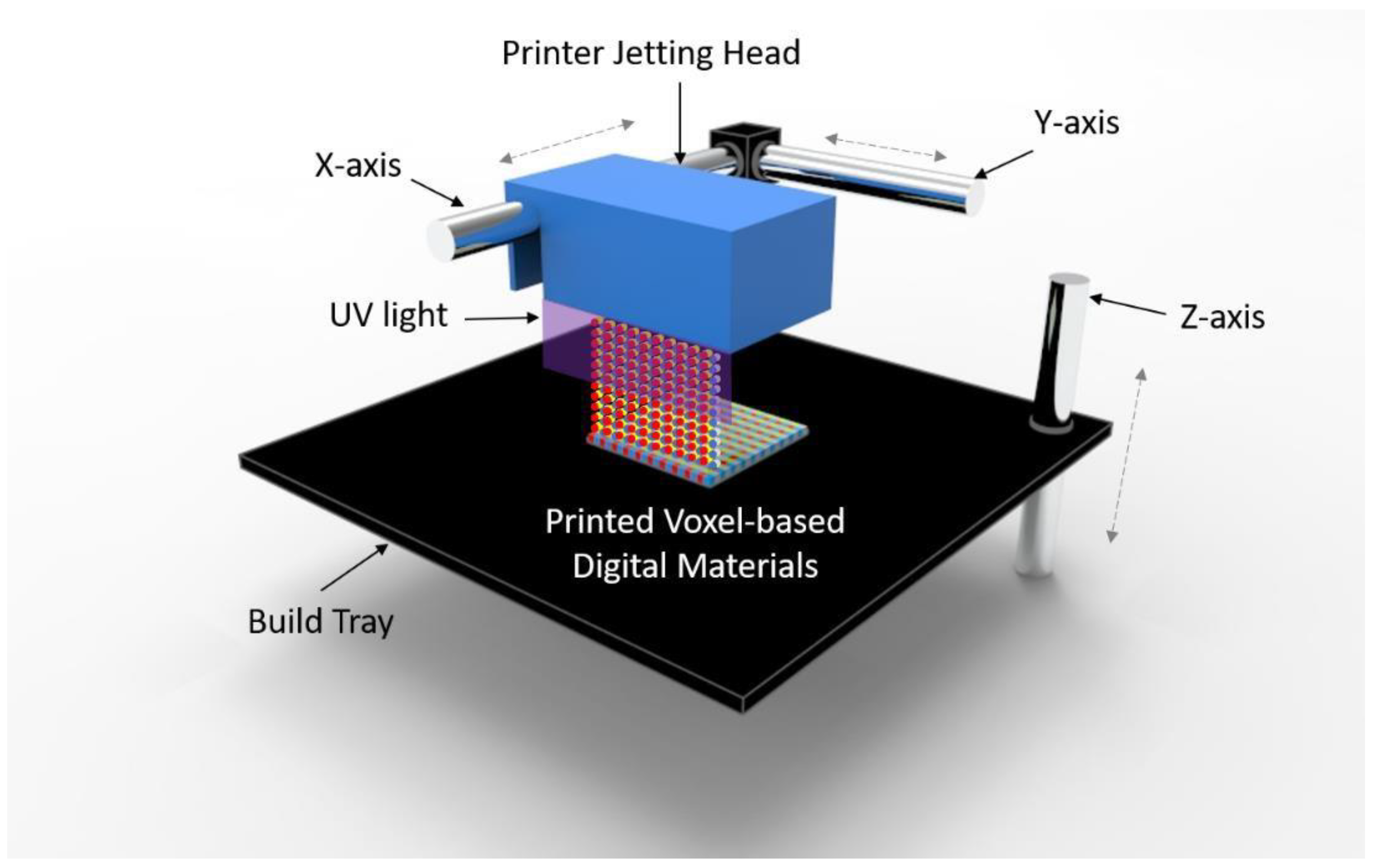
2.1. A Voxel-Based Digital Materials (Gel-, Tissue-, and Bone-Like Materials)
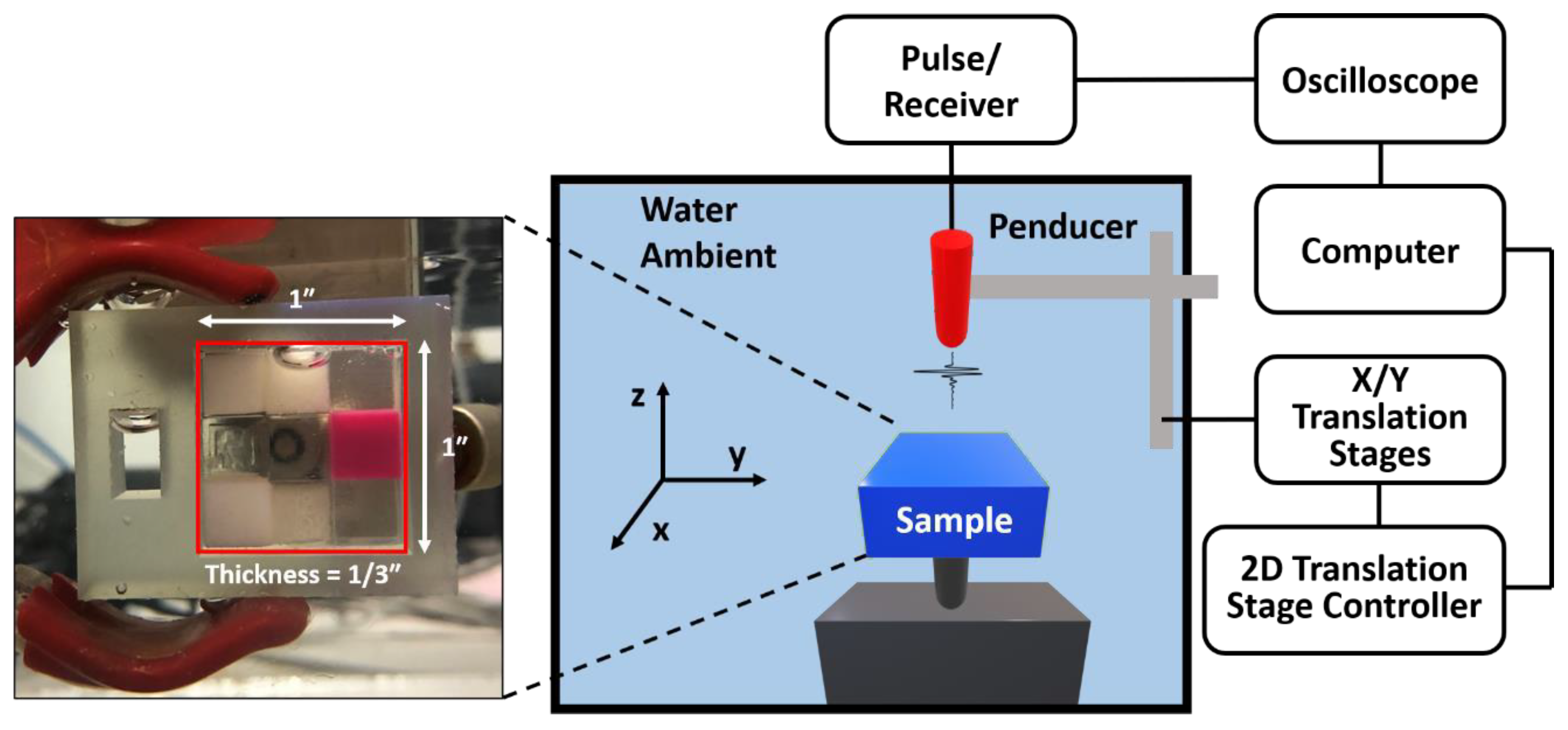
2.2. Experiment Setup
3. Theoretical Framework
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hosny, A.; Keating, S.J.; Dilley, J.D.; Ripley, B.; Kelil, T.; Pieper, S.; Kolb, D.; Bader, C.; Pobloth, A.M.; Griffin, M.; et al. From Improved Diagnostics to Presurgical Planning: High-Resolution Functionally Graded Multimaterial 3D Printing of Biomedical Tomographic Data Sets. 3D Print. Addtive Manuf. 2018, 5, 103–113. [Google Scholar] [CrossRef]
- Marro, A.; Bandukwala, T.; Mak, W. Three-dimensional printing and medical imaging: A review of the methods and applications. Curr. Probl. Diagn. Radiol. 2016, 45, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Mavroidis, C.; Ranky, R.G.; Sivak, M.L.; Patritti, B.L.; DiPisa, J.; Caddle, A.; Gilhooly, K.; Govoni, L.; Sivak, S.; Lancia, M.; et al. Patient specific ankle-foot orthoses using rapid prototyping. J. Neuroeng. Rehabil. 2011, 8, 1. [Google Scholar] [CrossRef]
- Alhnan, M.A.; Okwuosa, T.C.; Sadia, M.; Wan, K.W.; Ahmed, W.; Arafat, B. Emergenceof3D printed dosage forms: Opportunities and challenges. Pharm. Res. 2016, 33, 1817–1832. [Google Scholar] [CrossRef]
- McGurk, M.; Amis, A.A.; Potamianos, P.; Goodger, N.M. Rapid prototyping techniques for anatomical modelling in medicine. Ann. R. Coll. Surg. Engl. 1997, 79, 169. [Google Scholar]
- Cui, J.; Chen, L.; Guan, X.; Ye, L.; Wang, H.; Liu, L. Surgical planning, three-dimensional model surgery and preshaped implants in treatment of bilateral craniomaxillofacial post-traumatic deformities. J. Oral Maxillofac. Surg. 2014, 72, 1138.e1–1138.e14. [Google Scholar] [CrossRef]
- Jacobs, S.; Grunert, R.; Mohr, F.W.; Falk, V. 3D-Imaging of cardiac structures using 3D heart models for planning in heart surgery: A preliminary study. Interact. Cardiovasc. Thorac. Surg. 2008, 7, 6–9. [Google Scholar] [CrossRef]
- Hosny, A.; Shen, T.; Kuo, A.S.; Long, D.; Andrawes, M.N.; Dilley, J.D. Unlocking vendor-specific tags: Three-dimensional printing of echocardiographic data sets. J. Thorac. Cardiovasc. Surg. 2018, 155, 143–145. [Google Scholar] [CrossRef]
- Kiarashi, N.; Nolte, A.C.; Sturgeon, G.M.; Segars, W.P.; Ghate, S.V.; Nolte, L.W.; Samei, E.; Lo, J.Y. Development of realistic physical breast phantoms matched to virtual breast phantoms based on human subject data. Med. Phys. 2015, 42, 4116–4126. [Google Scholar] [CrossRef]
- Severseike, L.; Lee, V.; Bakken, C.; Brandon, T.; Bhatia, V. Polyjet 3D printing of tissue-mimicking materials: How well can 3D printed synthetic myocardium replicate mechanical properties of organic myocardium? BioRxiv 2019, 825794. [Google Scholar] [CrossRef]
- Doubrovski, E.L.; Tsai, E.Y.; Dikovsky, D.; Geraedts, J.M.; Herr, H.; Oxman, N. Voxel-based fabrication through material property mapping: A design method for bitmap printing. Comput. Aided Des. 2015, 60, 3–13. [Google Scholar] [CrossRef]
- Erickson, A. Create the Future with New Grabcad Voxel Print. Available online: https://www.cati.com/blog/2017/12/create-the-future-with-new-grabcad-voxel-print/ (accessed on 10 October 2020).
- Wood, Z.; Lynn, L.; Nguyen, J.T.; Black, M.A.; Patel, M.; Barak, M.M. Are we crying Wolff? 3D printed replicas of trabecular bone structure demonstrate higher stiffness and strength during off-axis loading. Bone 2019, 127, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Vulkicevic, M.; Puperi, D.S.; Grande-Allen, K.J.; Little, S.H. 3D Printed Modeling of the Mitral Valve for Catheter-Based Structural Interventions. Ann. Biomed. Eng. 2017, 45, 508–519. [Google Scholar] [CrossRef]
- Masood, S.H.; Mau, K.; Song, W.Q. Tensile properties of processed FDM polycarbonate material. Mater. Sci. Forum 2010, 654, 2556–2559. [Google Scholar] [CrossRef]
- Calabri, L.; Pugno, N.; Menozzi, C.; Valeri, S. AFM nanoindentation: Tip shape and tip radius of curvature effect on the hardness measurement. J. Phys. Condens. Matter 2008, 20, 474208. [Google Scholar] [CrossRef]
- Hengsberger, S.; Kulik, A.; Zysset, P.H. Nanoindentation discriminates the elastic properties of individual human bone lamellae under dry and physiological conditions. Bone 2002, 30, 178–184. [Google Scholar] [CrossRef]
- Sun, J.-y.; Tong, J. Fracture toughness properties of three different biomaterials measured by nanoindentation. J. Bionic Eng. 2007, 4, 11–17. [Google Scholar] [CrossRef]
- Gindl, W.; Gupta, H.S. Cell-wall hardness and Young’s modulus of melamine-modified spruce wood by nano-indentation. Compos. Part A Appl. Sci. Manuf. 2002, 33, 1141–1145. [Google Scholar] [CrossRef]
- Ramesh Babu, S.; Jaskari, M.; Järvenpää, A.; Porter, D. The effect of hot-mounting on the microstructure of an As-Quenched auto-tempered low-carbon martensitic steel. Metals 2019, 9, 550. [Google Scholar] [CrossRef]
- Chusuei, C.C.; Gwynn, L.K.; Schreifels, J.A. Two-level liquid-nitrogen-cooled sample mounting assembly for surface analysis. Rev. Sci. Instrum. 1992, 63, 3218–3219. [Google Scholar] [CrossRef]
- Islam, M.T.; Chaudhry, A.; Tang, S.; Tasciotti, E.; Righetti, R. A new method for estimating the effective Poisson’s ratio in ultrasound poroelastography. IEEE Trans. Med. Imaging 2018, 37, 1178–1191. [Google Scholar] [CrossRef] [PubMed]
- Nightingale, K. Acoustic radiation force impulse (ARFI) imaging: A review. Curr. Med. Imaging Rev. 2011, 7, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Gennisson, J.-L.; Deffieux, T.; Fink, M.; Tanter, M. Ultrasound elastography: Principles and techniques. Diagn. Interv. Imaging 2013, 94, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, P.; Singh, S.B.; Chakraborty, M. Elastic modulus of biomedical titanium alloys by nano-indentation and ultrasonic techniques—A comparative study. Mater. Sci. Eng. A 2008, 489, 419–425. [Google Scholar] [CrossRef]
- Kupperman, D.S.; Reimann, K.J.; Abrego-Lopez, J. Ultrasonic NDE of cast stainless steel. NDT Int. 1987, 20, 145–152. [Google Scholar]
- Wang, T.; Shukla, S.; Nene, S.S.; Frank, M.; Wheeler, R.W.; Mishra, R.S. Towards Obtaining Sound Butt Joint Between Metallurgically Immiscible Pure Cu and Stainless Steel Through Friction Stir Welding. Metall. Mater. Trans. A 2018, 49, 2578–2582. [Google Scholar] [CrossRef]
- Jin, Y.; Heo, H.; Walker, E.; Krokhin, A.; Choi, T.Y.; Neogi, A. The effects of temperature and frequency dispersion on sound speed in bulk poly (Vinyl Alcohol) poly (N-isopropylacrylamide) hydrogels caused by the phase transition. Ultrasonics 2020, 104, 105931. [Google Scholar] [CrossRef]
- Walker, J.M.; Myers, A.M.; Schluchter, M.D.; Goldberg, V.M.; Caplan, A.I.; Berilla, J.A.; Mansour, J.M.; Welter, J.F. Nondestructive evaluation of hydrogel mechanical properties using ultrasound. Ann. Biomed. Eng. 2011, 39, 2521. [Google Scholar] [CrossRef]
- Jin, Y.; Yang, T.; Ju, S.; Zhang, H.; Choi, T.-Y.; Neogi, A. Thermally Tunable Dynamic and Static Elastic Properties of Hydrogel Due to Volumetric Phase Transition. Polymers 2020, 12, 1462. [Google Scholar] [CrossRef]
- Jin, Y.; Walker, E.; Krokhin, A.; Heo, H.; Choi, T.-Y.; Neogi, A. Enhanced instantaneous elastography in tissues and hard materials using bulk modulus and density determined without externally applied material deformation. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2019, 67, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Walker, E.; Heo, H.; Krokhin, A.; Choi, T.-Y.; Neogi, A. Nondestructive ultrasonic evaluation of fused deposition modeling based additively manufactured 3D-printed structures. Smart Mater. Struct. 2020, 29, 045020. [Google Scholar] [CrossRef]
- Jin, Y.; Yang, T.; Heo, H.; Krokhin, A.; Shi, S.Q.; Dahotre, N.; Choi, T.Y.; Neogi, A. Novel 2D Dynamic Elasticity Maps for Inspection of Anisotropic Properties in Fused Deposition Modeling Objects. Polymers 2020, 12, 1966. [Google Scholar] [CrossRef] [PubMed]
- PolyJet 3D Printers Systems and Materials-Stratasys. Available online: https://www.stratasys.com/-/media/files/printer-spec-sheets/polyjet-systems-and-materials-overview-en-a4.pdf (accessed on 10 October 2020).
- Walker, E.L.; Reyes-Contreras, D.; Jin, Y.; Neogi, A. Tunable Hybrid Phononic Crystal Lens Using Thermo- Acoustic Polymers. ACS Omega 2019, 4, 16585–16590. [Google Scholar] [CrossRef] [PubMed]
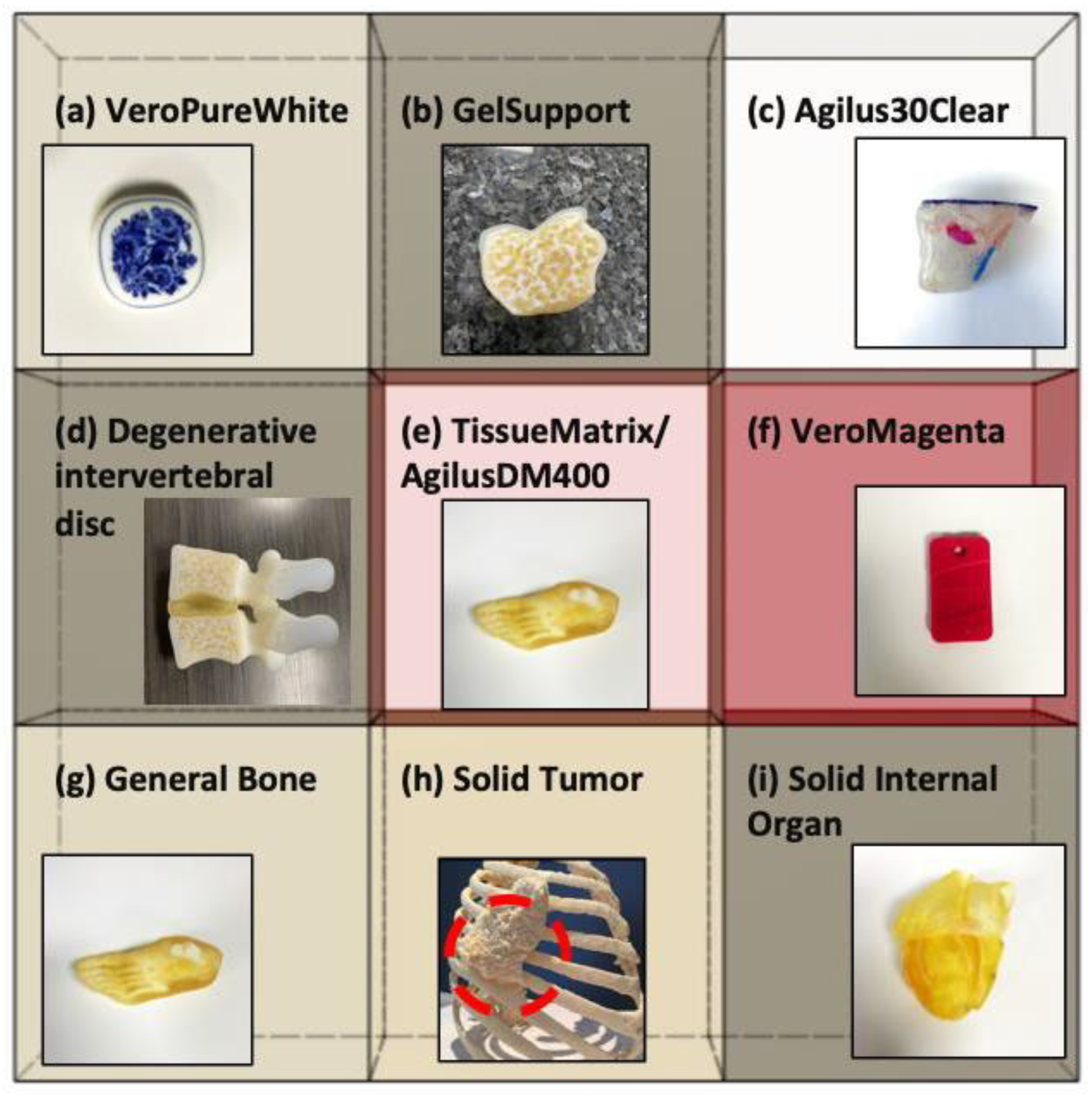

| Material | Dynamic Bulk Modulus, Kdyn (GPa) | Reference Modulus of Elasticity, Eref_static (GPa) [35] | Effective Density, Ρeff (kg/m3) | Reference Polymerized Density, Ρref (kg/m3) [35] | Average Speed of Sound (m/s) |
|---|---|---|---|---|---|
| (a) VeroPureWhite | 3.35 ± 0.13 | 2.0–3.0 | 1452 ± 70 | 1170–1180 | 1590 ± 40 |
| (b) GelSupport | 2.55 ± 0.09 | N/A | 1176 ± 40 | N/A | 1625 ±18 |
| (c) Agilus30Clear | 3.47 ± 0.17 | N/A | 1532 ± 66 | 1140–1150 | 1478 ± 26 |
| (d) Degenerative intervertebral disc | 2.99 ± 0.16 | N/A | 1274 ± 80 | N/A | 1642 ± 54 |
| (e) TissueMatrix/AgilusDM400 | 3.08 ± 0.14 | N/A | 1342 ± 75 | N/A | 1600 ± 38 |
| (f) VeroMagenta | 3.69 ± 0.06 | 2.0–3.0 | 1609 ± 33 | 1170–1180 | 1425 ± 15 |
| (g) General Bone | 3.62 ± 0.05 | N/A | 1625 ± 30 | N/A | 1370 ± 11 |
| (h) Solid Tumor | 3.26 ± 0.12 | N/A | 1412 ± 61 | N/A | 1635 ± 29 |
| (i) Solid internal organ | 3.25 ± 0.12 | N/A | 1403 ± 63 | N/A | 1651 ± 37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heo, H.; Jin, Y.; Yang, D.; Wier, C.; Minard, A.; Dahotre, N.B.; Neogi, A. Manufacturing and Characterization of Hybrid Bulk Voxelated Biomaterials Printed by Digital Anatomy 3D Printing. Polymers 2021, 13, 123. https://doi.org/10.3390/polym13010123
Heo H, Jin Y, Yang D, Wier C, Minard A, Dahotre NB, Neogi A. Manufacturing and Characterization of Hybrid Bulk Voxelated Biomaterials Printed by Digital Anatomy 3D Printing. Polymers. 2021; 13(1):123. https://doi.org/10.3390/polym13010123
Chicago/Turabian StyleHeo, Hyeonu, Yuqi Jin, David Yang, Christopher Wier, Aaron Minard, Narendra B. Dahotre, and Arup Neogi. 2021. "Manufacturing and Characterization of Hybrid Bulk Voxelated Biomaterials Printed by Digital Anatomy 3D Printing" Polymers 13, no. 1: 123. https://doi.org/10.3390/polym13010123
APA StyleHeo, H., Jin, Y., Yang, D., Wier, C., Minard, A., Dahotre, N. B., & Neogi, A. (2021). Manufacturing and Characterization of Hybrid Bulk Voxelated Biomaterials Printed by Digital Anatomy 3D Printing. Polymers, 13(1), 123. https://doi.org/10.3390/polym13010123