Biocompatible Nanocomposites Based on Poly(styrene-block-isobutylene-block-styrene) and Carbon Nanotubes for Biomedical Application
Abstract
1. Introduction
2. Materials and Methods
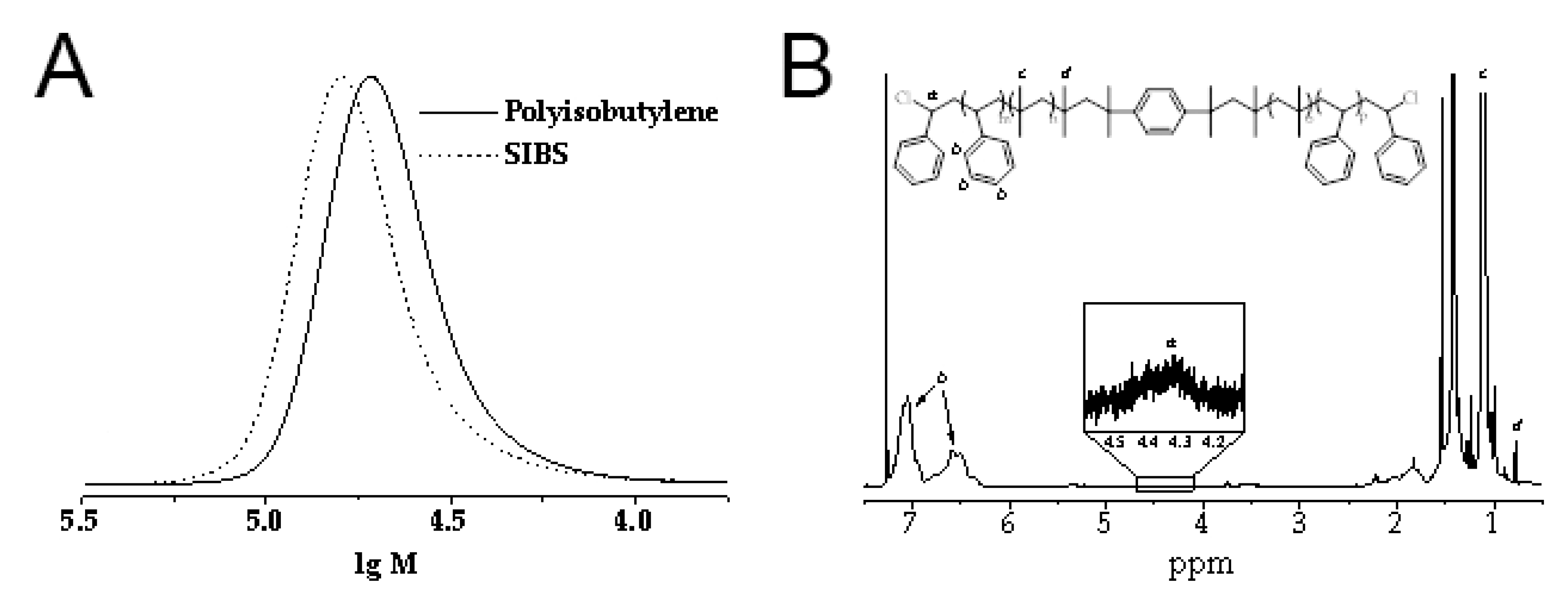
2.1. Synthesis of Poly(styrene-block-isobutylene-block-styrene)
2.2. Preparation of Nanocomposite Films
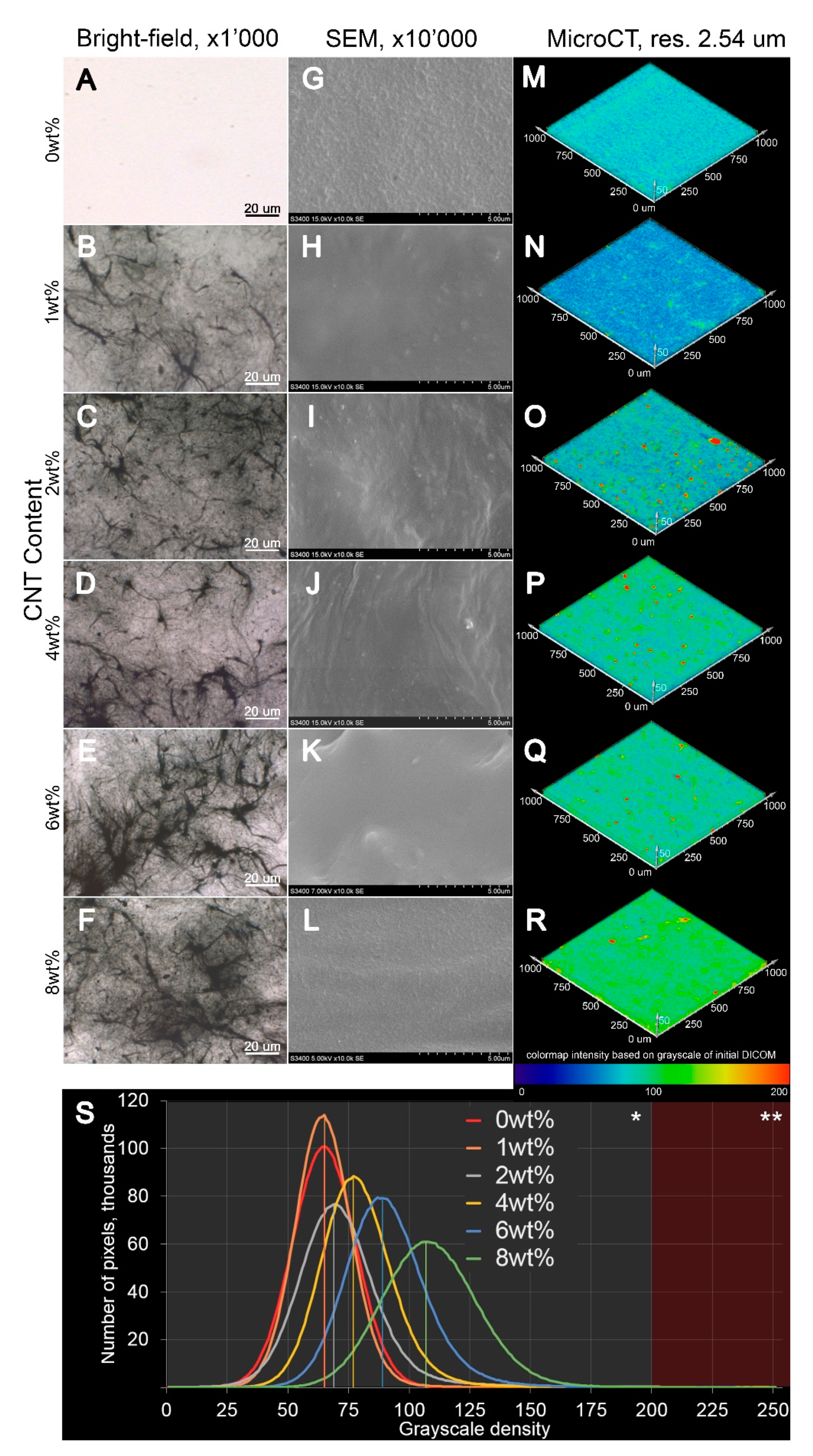
2.3. Microscopy
2.4. X-ray Microtomography (Micro-CT)
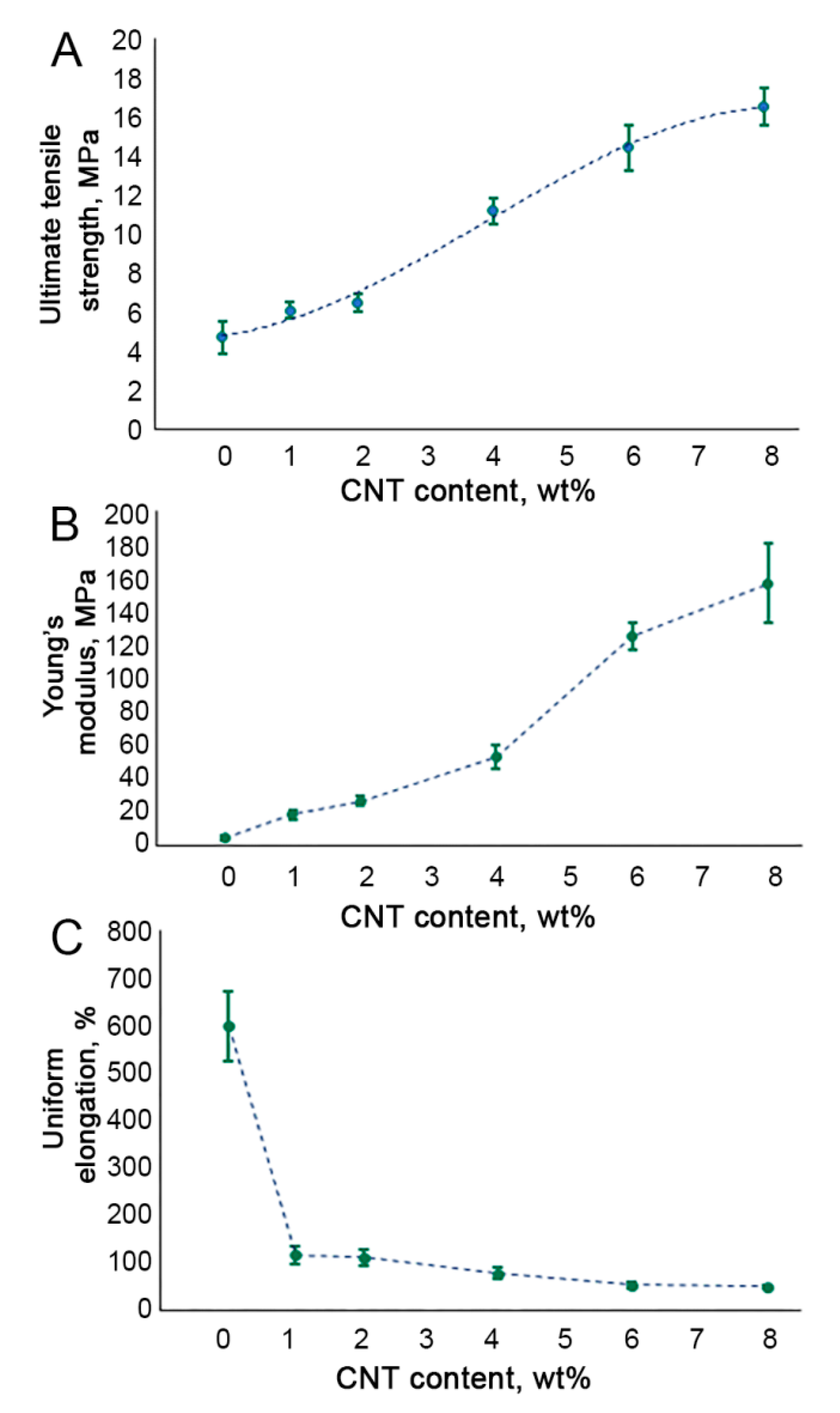
2.5. Tensile Testing
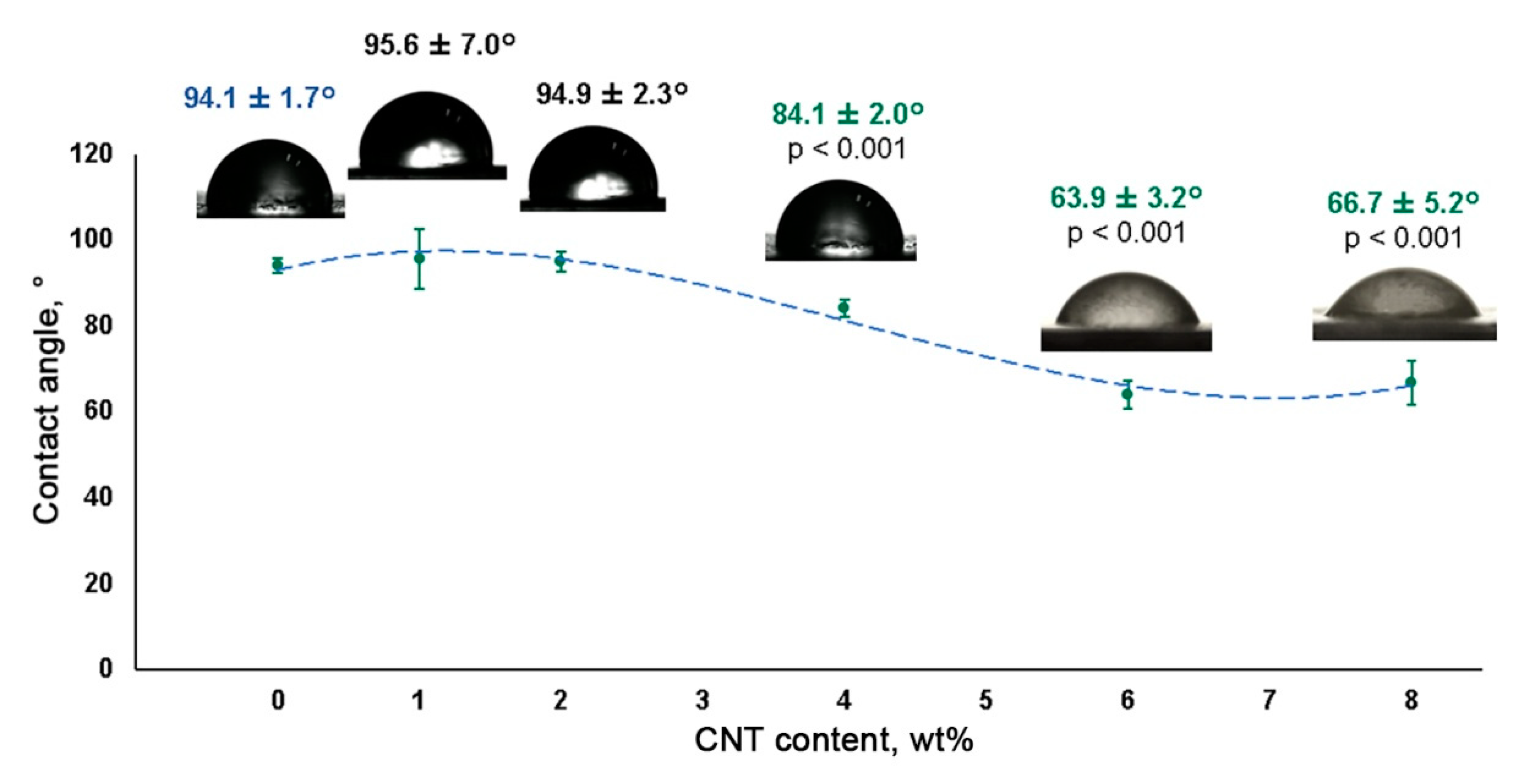
2.6. Contact Angle Measurement
2.7. Conductivity Assessment
2.8. Cytotoxicity Assessment
2.9. In Vitro Oxidation Assay
2.10. Statistical Analysis
3. Results
3.1. Synthesis of CNT-Modified SIBS Films and Analysis of Their Structure
3.2. Tensile Testing of CNT-Modified SIBS Films
3.3. Hydrophobicity Assessment of CNT-Modified SIBS Films
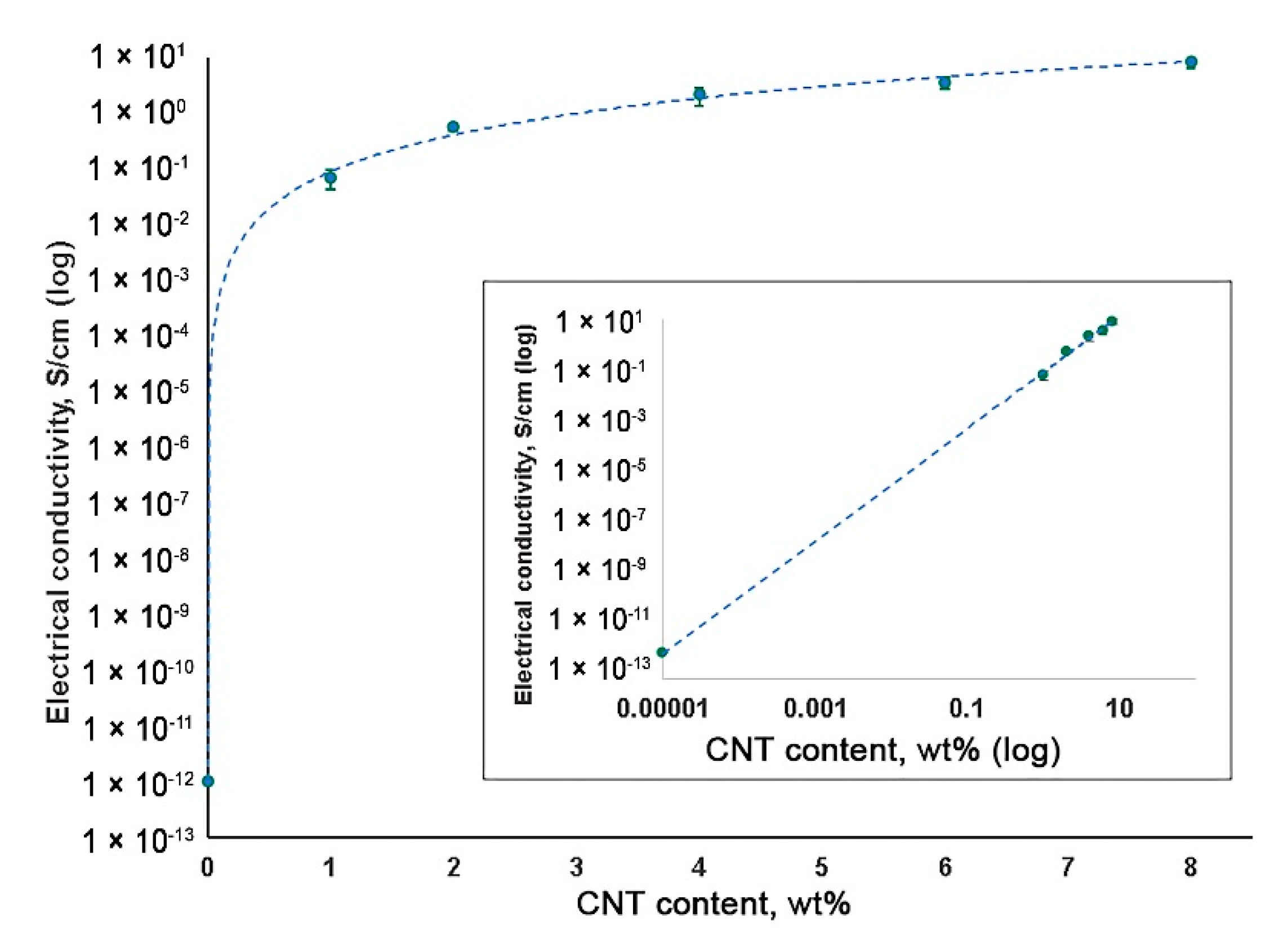
3.4. Electrical Conductivity Measurement of CNT-Modified SIBS Films
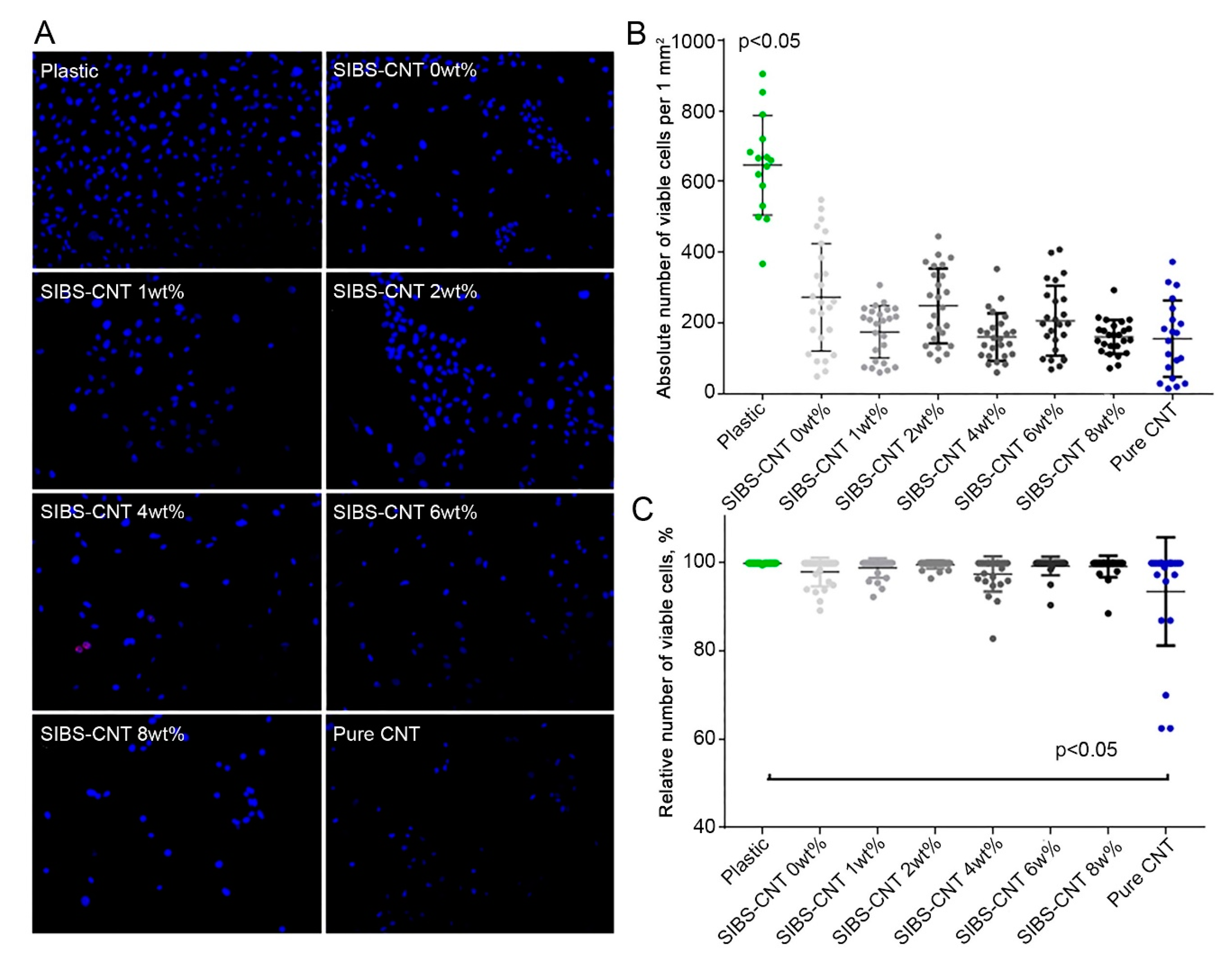
3.5. Cytotoxicity of CNT-Modified SIBS Films
3.6. In Vitro Oxidative Stability of CNT-Modified SIBS Films
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: A systematic analysis for the Global Burden of Disease Study 2017 [published correction appears in Lancet. 2019 Jun 22;393(10190):e44] [published correction appears in Lancet. 2018 Nov 17;392(10160):2170]. Lancet 2018, 392, 1736–1788. [Google Scholar] [CrossRef]
- Teo, A.J.T.; Mishra, A.; Park, I.; Kim, Y.-J.; Park, W.-T.; Yoon, Y.-J. Polymeric Biomaterials for Medical Implants and Devices. ACS Biomater. Sci. Eng. 2016, 2, 454–472. [Google Scholar] [CrossRef]
- Jaganathan, S.K.; Supriyanto, E.; Murugesan, S.; Balaji, A.; Asokan, M.K. Biomaterials in Cardiovascular Research: Applications and Clinical Implications. BioMed Res. Int. 2014, 2014, 459465. [Google Scholar] [CrossRef]
- Wu, G.; Li, P.; Feng, H.; Zhang, X.; Chu, P.K. Engineering and functionalization of biomaterials via surface modification. J. Mater. Chem. B 2015, 3, 2024–2042. [Google Scholar] [CrossRef]
- Narayan, R. Nanobiomaterials; Woodhead Publishing: Cambridge, UK, 2018; pp. 357–384. [Google Scholar] [CrossRef]
- Keledi, G.; Hári, J.; Pukánszky, B. Polymer nanocomposites: Structure, interaction, and functionality. Nanoscale 2012, 4, 1919–1938. [Google Scholar] [CrossRef] [PubMed]
- Hussain, F.; Hojjati, M.; Okamoto, M.; Gorga, R.E. Review article: Polymer-matrix Nanocomposites, Processing, Manufacturing, and Application: An Overview. J. Compos. Mater. 2006, 40, 1511–1575. [Google Scholar] [CrossRef]
- Maiti, D.; Tong, X.; Mou, X.; Yang, K. Carbon-Based Nanomaterials for Biomedical Applications: A Recent Study. Front. Pharmacol. 2019, 9, 1401. [Google Scholar] [CrossRef]
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, M.; Hanifehpour, Y.; Joo, S.W. Carbon nanotubes: Properties, synthesis, purification, and medical applications. Nanoscale Res. Lett. 2014, 9, 393. [Google Scholar] [CrossRef]
- Sheheri, S.Z.; Amshany, Z.M.; Sulami, Q.A.; Tashkandi, N.Y.; Hussein, M.A.; Shishtawy, R.M. The preparation of carbon nanofillers and their role on the performance of variable polymer nanocomposites. Des. Monomers Polym. 2019, 22, 8–53. [Google Scholar] [CrossRef]
- Kalakonda, P.; Banne, S.; Kalakonda, P. Enhanced mechanical properties of multiwalled carbon nanotubes/thermoplastic polyurethane nanocomposites. Nanomater. Nanotechnol. 2019, 9, 184798041984085. [Google Scholar] [CrossRef]
- Crosby, A.J.; Lee, J. Polymer Nanocomposites: The “Nano” Effect on Mechanical Properties. Polym. Rev. 2007, 47, 217–229. [Google Scholar] [CrossRef]
- Tjong, S.C. Structural and mechanical properties of polymer nanocomposites. Mater. Sci. Eng. R Rep. 2006, 53, 73–197. [Google Scholar] [CrossRef]
- Bhattacharya, M. Polymer Nanocomposites—A Comparison between Carbon Nanotubes, Graphene, and Clay as Nanofillers. Materials 2016, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Jumaili, A.; Alancherry, S.; Bazaka, K.; Jacob, M. Review on the Antimicrobial Properties of Carbon Nanostructures. Materials 2017, 10, 1066. [Google Scholar] [CrossRef]
- Pinchuk, L.; Wilson, G.J.; Barry, J.J.; Schoephoerster, R.T.; Parel, J.M.; Kennedy, J.P. Medical applications of poly(styrene-block-isobutylene-block-styrene) (“SIBS”). Biomaterials 2008, 29, 448–460. [Google Scholar] [CrossRef]
- Ovcharenko, E.; Rezvova, M.; Nikishau, P.; Kostjuk, S.; Glushkova, T.; Antonova, L.; Trebushat, D.; Akentieva, T.; Shishkova, D.; Krivikina, E.; et al. Polyisobutylene-Based Thermoplastic Elastomers for Manufacturing Polymeric Heart Valve Leaflets: In Vitro and In Vivo Results. Appl. Sci. 2019, 9, 4773. [Google Scholar] [CrossRef]
- Kaszas, G.; Puskas, J.E.; Kennedy, J.P.; Hager, W.G. Polyisobutylene–containing block polymers by sequential monomer addition. II. Polystyrene–polyisobutylene–polystyrene triblock polymers: Synthesis, characterization, and physical properties. J. Polym. Sci. A Polym. Chem. 1991, 29, 427–435. [Google Scholar] [CrossRef]
- Lu, Y.; Santino, L.M.; Acharya, S.; Anandarajah, H.; D’Arcy, J.M. Studying Electrical Conductivity Using a 3D Printed Four-Point Probe Station. J. Chem. Educ. 2017, 94, 950–955. [Google Scholar] [CrossRef]
- Christenson, E.M.; Anderson, J.M.; Hiltner, A. Oxidative mechanisms of poly(carbonate urethane) and poly(ether urethane) biodegradation: In vivo and in vitro correlations. J. Biomed. Mater. Res. A 2004, 70, 245–255. [Google Scholar] [CrossRef]
- Hsu, S.; Tang, C.-M.; Tseng, H.-J. Biostability and biocompatibility of poly(ester urethane)–gold nanocomposites. Acta Biomater. 2008, 4, 1797–1808. [Google Scholar] [CrossRef]
- Yuan, S.; Zhao, J.; Luan, S.; Yan, S.; Zheng, W.; Yin, J. Nuclease-Functionalized Poly(Styrene-b-isobutylene-b-styrene) Surface with Anti-Infection and Tissue Integration Bifunctions. ACS Appl. Mater. Interfaces 2014, 6, 18078–18086. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.A.; Obaid, A.Y.; El-Shishtawy, R.M.; Mohamed, S.A. Synthesis of nanocomposites of polypyrrole/carbon nanotubes/silver nano particles and their application in water disinfection. RSC Adv. 2017, 7, 16878–16884. [Google Scholar] [CrossRef]
- Kanduč, M.; Schlaich, A.; Schneck, E.; Netz, R.R. Water-Mediated Interactions between Hydrophilic and Hydrophobic Surfaces. Langmuir 2016, 32, 8767–8782. [Google Scholar] [CrossRef] [PubMed]
- Solazzo, M.; O’Brien, F.J.; Nicolosi, V.; Monaghan, M.G. The rationale and emergence of electroconductive biomaterial scaffolds in cardiac tissue engineering. APL Bioeng. 2019, 3, 041501. [Google Scholar] [CrossRef]
- Fray, M.E.; Prowans, P.; Puskas, J.E.; Altsta, V. Biocompatibility and Fatigue Properties of Polystyrene-Polyisobutylene-Polystyrene, an Emerging Thermoplastic Elastomeric Biomaterial. Biomacromolecules 2006, 7, 844–850. [Google Scholar] [CrossRef]
- Kwee, T.; Taylor, S.J.; Mauritz, K.A.; Storey, R.F. Morphology and mechanical and dynamic mechanical properties of linear and star poly(styrene-b-isobutylene-b-styrene) block copolymers. Polymer 2005, 46, 4480–4491. [Google Scholar] [CrossRef]
- Spitalsky, Z.; Tasis, D.; Papagelis, K.; Galiotis, C. Carbon nanotube–polymer composites: Chemistry, processing, mechanical and electrical properties. Prog. Polym. Sci. 2010, 35, 357–401. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Li, Z.; Liu, M.; Kinloch, I.A.; Young, R.J. Mechanisms of mechanical reinforcement by graphene and carbon nanotubes in polymer nanocomposites. Nanoscale 2020, 12, 2228–2267. [Google Scholar] [CrossRef]
- Kinloch, I.A.; Suhr, J.; Lou, J.; Young, R.J.; Ajayan, P.M. Composites with carbon nanotubes and graphene: An outlook. Science 2018, 362, 547–553. [Google Scholar] [CrossRef]
- Cadek, M.; Coleman, J.N.; Ryan, K.P.; Nicolosi, V.; Bister, G.; Fonseca, A.; Nagy, J.B.; Szostak, K.; Béguin, F.; Blau, W.J. Reinforcement of Polymers with Carbon Nanotubes: The Role of Nanotube Surface Area. Nano Lett. 2004, 4, 353–356. [Google Scholar] [CrossRef]
- Chen, J.; Liu, B.; Gao, X.; Xu, D. A review of the interfacial characteristics of polymer nanocomposites containing carbon nanotubes. RSC Adv. 2018, 8, 28048–28085. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Terentjev, E.M. Dispersion of Carbon Nanotubes: Mixing, Sonication, Stabilization, and Composite Properties. Polymers 2012, 4, 275–295. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Terentjev, E.M. Dispersion and rheology of carbon nanotubes in polymers. Int. J. Mater. Form. 2008, 1, 63–74. [Google Scholar] [CrossRef]
- Pedroni, L.G.; Araujo, J.R.; Felisberti, M.I.; Nogueira, A.F. Nanocomposites based on MWCNT and styrene–butadiene–styrene block copolymers: Effect of the preparation method on dispersion and polymer–filler interactions. Cmpos. Sci. Technol. 2012, 72, 1487–1492. [Google Scholar] [CrossRef]
- Tarfaoui, M.; Lafdi, K.; El Moumen, A. Mechanical properties of carbon nanotubes based polymer composites. Compos. Part B-Eng. 2016, 103, 113–121. [Google Scholar] [CrossRef]
- Ranade, S.V.; Miller, K.M.; Richard, R.E.; Chan, A.K.; Allen, M.J.; Helmus, M.N. Physical characterization of controlled release of paclitaxel from the TAXUS™ Express2™ drug-eluting stent. J. Biomed. Mater. Res. A 2004, 71, 625–634. [Google Scholar] [CrossRef]
- Peigney, A.; Laurent, C.; Flahaut, E.; Bacsa, R.R.; Rousset, A. Specific surface area of carbon nanotubes and bundles of carbon nanotubes. Carbon 2001, 39, 507–514. [Google Scholar] [CrossRef]
- Zhao, W.; Li, T.; Li, Y.; O’Brien, D.J.; Terrones, M.; Wei, B.; Suhr, J.; Lu, L.X. Mechanical properties of nanocomposites reinforced by carbon nanotube sponges. J. Mater. 2018, 4, 157–164. [Google Scholar] [CrossRef]
- Cao, J.; Lu, Y.; Chen, H.; Zhang, L.; Xiong, C. Preparation, mechanical properties and in vitro cytocompatibility of multi-walled carbon nanotubes/poly(etheretherketone) nanocomposites. J. Biomater. Sci. Polym. Ed. 2018, 29, 428–447. [Google Scholar] [CrossRef]
- Yadav, M.D.; Dasgupta, K.; Patwardhan, A.W.; Joshi, J.B. High performance fibers from carbon nanotubes: Synthesis, characterization and applications in composites. Ind. Eng. Chem. Res. 2017, 56, 12407–12437. [Google Scholar] [CrossRef]
- Zhang, S.; Nguyen, N.; Leonhardt, B.; Jolowsky, C.; Hao, A.; Park, J.G.; Liang, R. Carbon-nanotube-based electrical conductors: Fabrication, optimization, and applications. Adv. Electron. Mater. 2019, 5, 1800811. [Google Scholar] [CrossRef]
- Salah, N.; Alfawzan, A.M.; Saeed, A.; Alshahrie, A.; Allafi, W. Effective reinforcements for thermoplastics based on carbon nanotubes of oil fly ash. Sci. Rep. 2019, 9, 20288. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Ragaert, K.; Swieszkowski, W.; Selimović, Š.; Paul, A.; Camci-Unal, G.; Mohammad, R.K.; Khademhosseini, A. Biomechanical properties of native and tissue engineered heart valve constructs. J. Biomech. 2014, 47, 1949–1963. [Google Scholar] [CrossRef] [PubMed]
- McKenna, K.A.; Hinds, M.T.; Sarao, R.C.; Wu, P.-C.; Maslen, C.L.; Glanville, R.W.; Babcock, D.; Gregory, K.W. Mechanical property characterization of electrospun recombinant human tropoelastin for vascular graft biomaterials. Acta Biomater. 2012, 8, 225–233. [Google Scholar] [CrossRef]
- Ngo, B.K.D.; Grunlan, M.A. Protein Resistant Polymeric Biomaterials. ACS Macro Lett. 2017, 6, 992–1000. [Google Scholar] [CrossRef]
- Chen, G.; Tang, W.; Wang, X.; Zhao, X.; Chen, C.; Zhu, Z. Applications of Hydrogels with Special Physical Properties in Biomedicine. Polymers 2019, 11, 1420. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Patel, A.; Dolatshahi-Pirouz, A.; Zhang, H.; Rangarajan, K.; Iviglia, G.; Shin, S.-R.; Hussain, M.A.; Khademhosseini, A. Elastomeric nanocomposite scaffolds made from poly(glycerol sebacate) chemically crosslinked with carbon nanotubes. Biomater. Sci. 2015, 3, 46–58. [Google Scholar] [CrossRef]
- Shokraei, N.; Asadpour, S.; Shokraei, S.; Nasrollahzadeh Sabet, M.; Faridi-Majidi, R.; Ghanbari, H. Development of electrically conductive hybrid nanofibers based on CNT-polyurethane nanocomposite for cardiac tissue engineering. Microsc. Res. Tech. 2019, 82, 1316–1325. [Google Scholar] [CrossRef]
- Aqeel, S.M.; Wang, Z.; Than, L.; Sreenivasulu, G.; Zeng, X. Poly(vinylidene fluoride)/poly(acrylonitrile)—Based superior hydrophobic piezoelectric solid derived by aligned carbon nanotubes in electrospinning: Fabrication, phase conversion and surface energy. RSC Adv. 2015, 5, 76383–76391. [Google Scholar] [CrossRef]
- Gilmore, K.J.; Moulton, S.E.; Wallace, G.G. Incorporation of carbon nanotubes into the biomedical polymer poly(styrene-β-isobutylene-β-styrene). Carbon 2007, 45, 402–410. [Google Scholar] [CrossRef]
- Kaur, T.; Thirugnanam, A. Tailoring in vitro biological and mechanical properties of polyvinyl alcohol reinforced with threshold carbon nanotube concentration for improved cellular response. RSC Adv. 2016, 6, 39982–39992. [Google Scholar] [CrossRef]
- Marroquin, J.B.; Rhee, K.; Park, S. Chitosan nanocomposite films: Enhanced electrical conductivity, thermal stability, and mechanical properties. Carbohydr. Polym. 2013, 92, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Peng, W.; Zare, Y.; Hui, D.; Rhee, K.Y. Predicting the electrical conductivity in polymer carbon nanotube nanocomposites based on the volume fractions and resistances of the nanoparticle, interphase, and tunneling regions in conductive networks. RSC Adv. 2018, 8, 19001–19010. [Google Scholar] [CrossRef]
- Cui, Z.; Yang, B.; Liac, R.-K. Application of Biomaterials in Cardiac Repair and Regeneration Engineering. Engineering 2016, 2, 141–148. [Google Scholar] [CrossRef]
- Walker, B.W.; Lara, R.P.; Yu, C.; Sani, E.S.; Kimball, W.; Joyce, S.; Annabi, N. Engineering a naturally-derived adhesive and conductive cardiopatch. Biomaterials 2019, 207, 89–101. [Google Scholar] [CrossRef]
- Smith, A.S.T.; Yoo, H.; Yi, H.; Ahn, E.H.; Lee, J.H.; Shao, G.; Nagornyak, E.; Laflamme, M.A.; Murry, C.E.; Kim, D.H. Micro- and nano-patterned conductive graphene-PEG hybrid scaffolds for cardiac tissue engineering. Chem. Commun. (Camb.) 2017, 53, 7412–7415. [Google Scholar] [CrossRef]
- Thompson, B.C.; Murray, E.; Wallace, G.G. Graphite Oxide to Graphene. Biomaterials to Bionics. Adv. Mater. 2015, 27, 7563–7582. [Google Scholar] [CrossRef]
- Ahadian, S.; Davenport, H.L.; Estili, M.; Yee, B.; Smith, N.; Xu, Z.; Sun, Y.; Radisic, M. Moldable elastomeric polyester-carbon nanotube scaffolds for cardiac tissue engineering. Acta Biomater. 2017, 52, 81–91. [Google Scholar] [CrossRef]
- Bandaru, P.R. Electrical properties and applications of carbon nanotube structures. J. Nanosci. Nanotechnol. 2007, 7, 1239–1267. [Google Scholar] [CrossRef]
- Pok, S.; Vitale, F.; Eichmann, S.L.; Benavides, O.M.; Pasquali, M.; Jacot, J.G. Biocompatible Carbon Nanotube-Chitosan Scaffold Matching the Electrical Conductivity of the Heart. ACS Nano 2014, 8, 9822–9832. [Google Scholar] [CrossRef]
- Mora, A.; Verma, P.; Kumar, S. Electrical conductivity of CNT/polymer composites: 3D printing, measurements and modeling. Compos. Part B-Eng. 2020, 183, 107600. [Google Scholar] [CrossRef]
- Herren, B.; Larson, P.; Saha, M.C.; Liu, Y. Enhanced Electrical Conductivity of Carbon Nanotube-Based Elastomer Nanocomposites Prepared by Microwave Curing. Polymers 2019, 11, 1212. [Google Scholar] [CrossRef] [PubMed]
- Kota, A.K.; Cipriano, B.H.; Duesterberg, M.K.; Gershon, A.L.; Powell, D.; Raghavan, S.R.; Bruck, H.A. Electrical and Rheological Percolation in Polystyrene/MWCNT Nanocomposites. Macromolecules 2007, 40, 7400–7406. [Google Scholar] [CrossRef]
- Pinchuk, L.; Riss, I.; Batlle, J.F.; Kato, Y.P.; Martin, J.B.; Arrieta, E.; Palmberg, P.; Parrish, R.K.; Weber, B.A.; Kwon, Y.; et al. The development of a micro-shunt made from poly(styrene-block-isobutylene-block-styrene) to treat glaucoma. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 211–221. [Google Scholar] [CrossRef]
- Christenson, E.M.; Patel, S.; Anderson, J.M.; Hiltner, A. Enzymatic degradation of poly(ether urethane) and poly(carbonate urethane) by cholesterol esterase. Biomaterials 2006, 27, 3920–3926. [Google Scholar] [CrossRef] [PubMed]
- Wepasnick, K.A.; Smith, B.A.; Schrote, K.E.; Wilson, H.K.; Diegelmann, S.R.; Fairbrother, D.H. Surface and structural characterization of multi-walled carbon nanotubes following different oxidative treatments. Carbon 2011, 49, 24–36. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezvova, M.A.; Yuzhalin, A.E.; Glushkova, T.V.; Makarevich, M.I.; Nikishau, P.A.; Kostjuk, S.V.; Klyshnikov, K.Y.; Matveeva, V.G.; Khanova, M.Y.; Ovcharenko, E.A. Biocompatible Nanocomposites Based on Poly(styrene-block-isobutylene-block-styrene) and Carbon Nanotubes for Biomedical Application. Polymers 2020, 12, 2158. https://doi.org/10.3390/polym12092158
Rezvova MA, Yuzhalin AE, Glushkova TV, Makarevich MI, Nikishau PA, Kostjuk SV, Klyshnikov KY, Matveeva VG, Khanova MY, Ovcharenko EA. Biocompatible Nanocomposites Based on Poly(styrene-block-isobutylene-block-styrene) and Carbon Nanotubes for Biomedical Application. Polymers. 2020; 12(9):2158. https://doi.org/10.3390/polym12092158
Chicago/Turabian StyleRezvova, Maria A., Arseniy E. Yuzhalin, Tatiana V. Glushkova, Miraslau I. Makarevich, Pavel A. Nikishau, Sergei V. Kostjuk, Kirill Yu. Klyshnikov, Vera G. Matveeva, Mariam Yu. Khanova, and Evgeny A. Ovcharenko. 2020. "Biocompatible Nanocomposites Based on Poly(styrene-block-isobutylene-block-styrene) and Carbon Nanotubes for Biomedical Application" Polymers 12, no. 9: 2158. https://doi.org/10.3390/polym12092158
APA StyleRezvova, M. A., Yuzhalin, A. E., Glushkova, T. V., Makarevich, M. I., Nikishau, P. A., Kostjuk, S. V., Klyshnikov, K. Y., Matveeva, V. G., Khanova, M. Y., & Ovcharenko, E. A. (2020). Biocompatible Nanocomposites Based on Poly(styrene-block-isobutylene-block-styrene) and Carbon Nanotubes for Biomedical Application. Polymers, 12(9), 2158. https://doi.org/10.3390/polym12092158







