High-Barrier Polyimide Containing Carbazole Moiety: Synthesis, Gas Barrier Properties, and Molecular Simulations
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Instrumentation
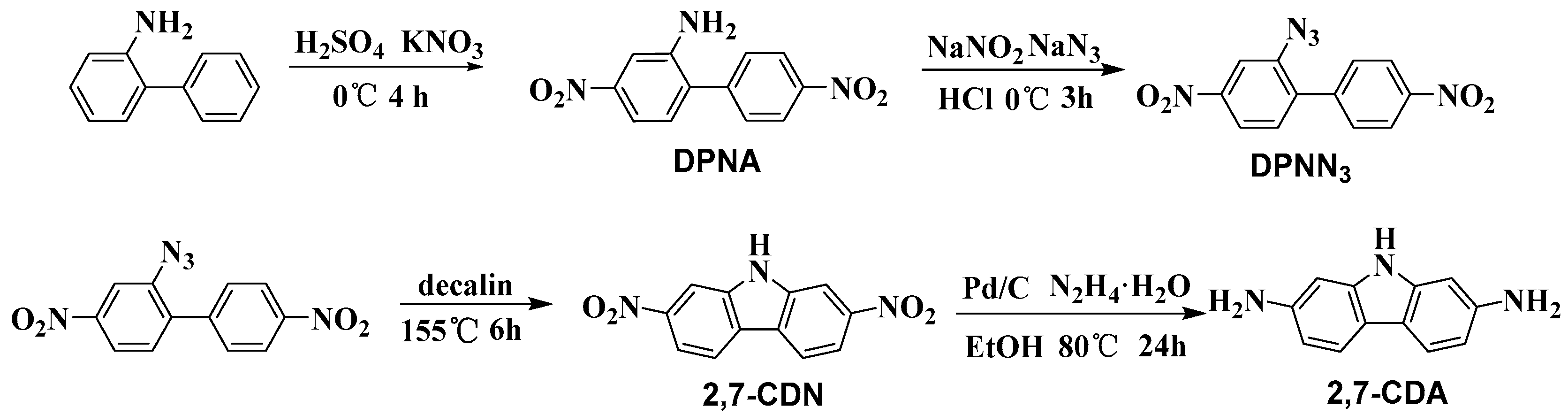
2.3. Synthesis of 4,4′-Dinitro-[1,1′-Biphenyl]-2-Amine (DPNA)
2.4. Synthesis of 2-Azido-4,4′-Dinitro-1,1′-Biphenyl (DPNN3)
2.5. Synthesis of 2,7-Dinitro-9H-Carbazole (2,7-CDN)
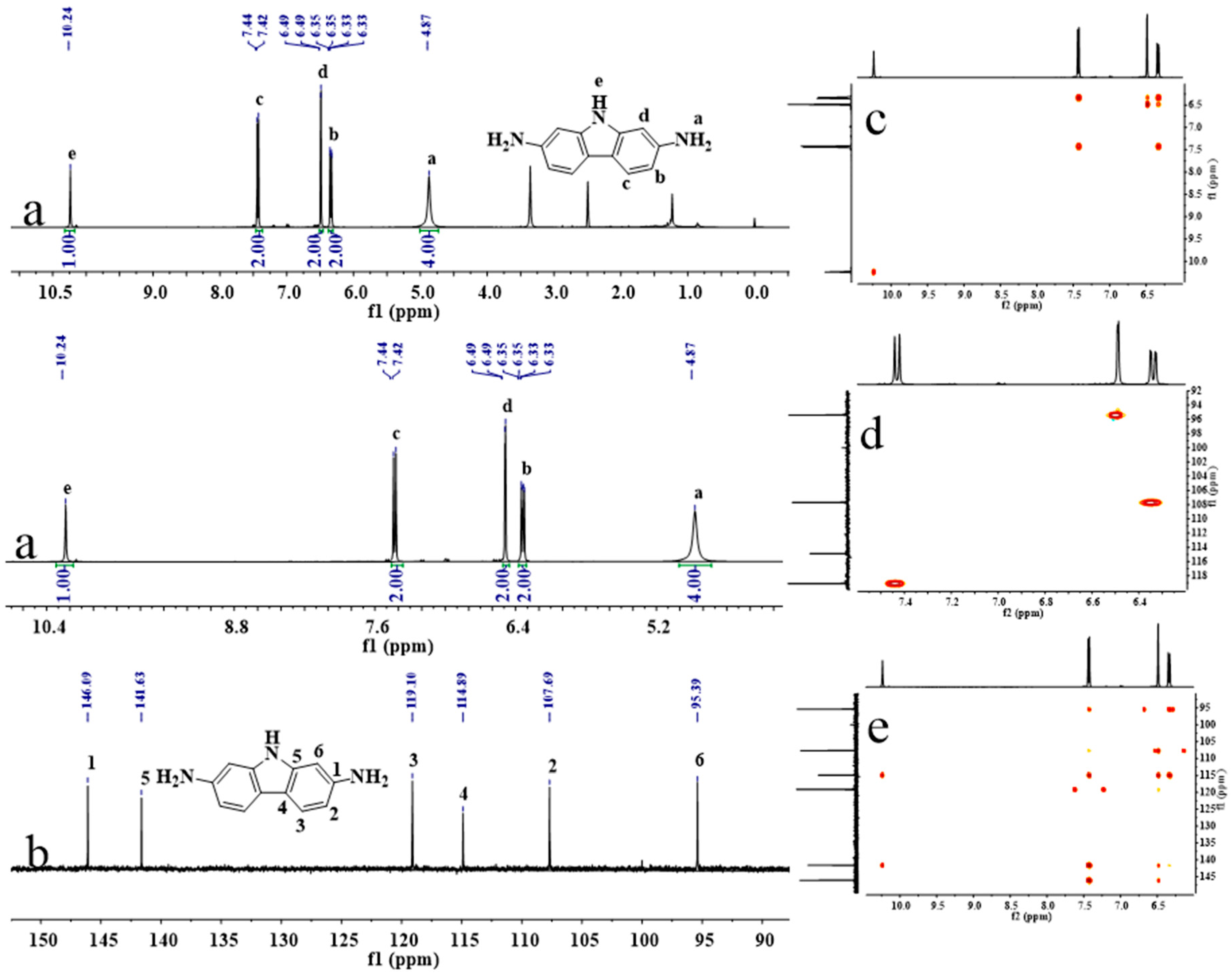
2.6. Synthesis of 9H-Carbazole-2,7-Diamine (2,7-CDA)
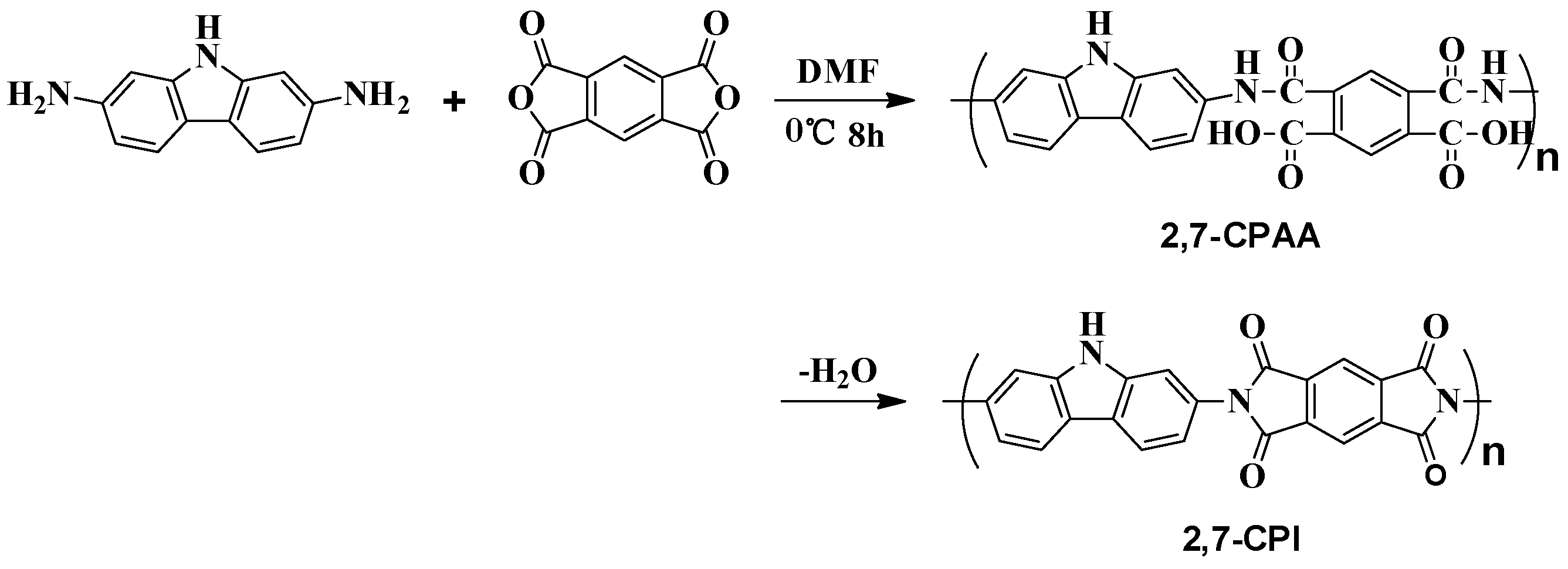
2.7. Synthesis of Polyimides
2.8. Molecular Simulation
2.8.1. Construction of Polymer Microstructures
2.8.2. Free Volume
2.8.3. Radial Distribution Functions
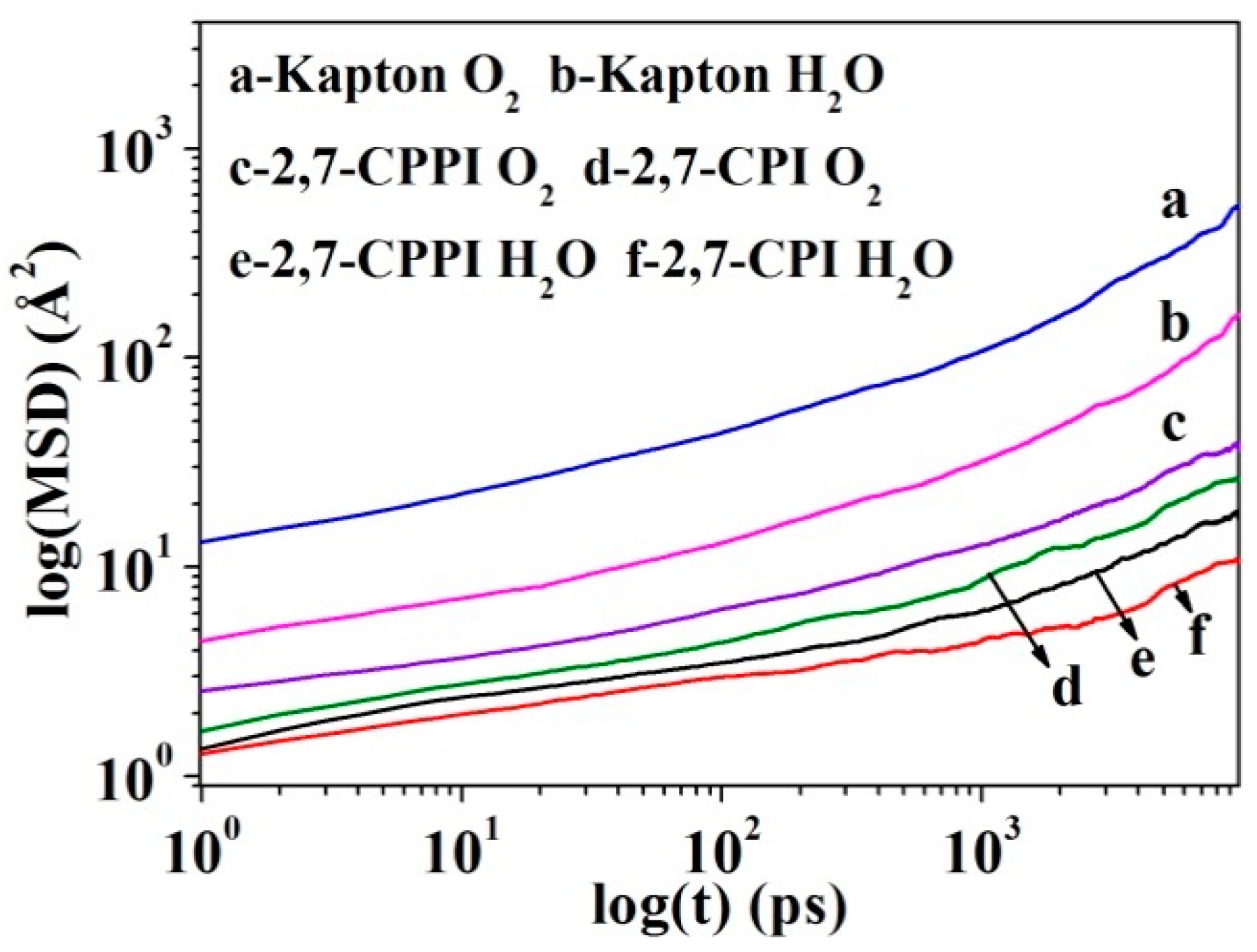
2.8.4. Diffusion Coefficients
2.8.5. Determination of Local Mobility of Polymer Chains
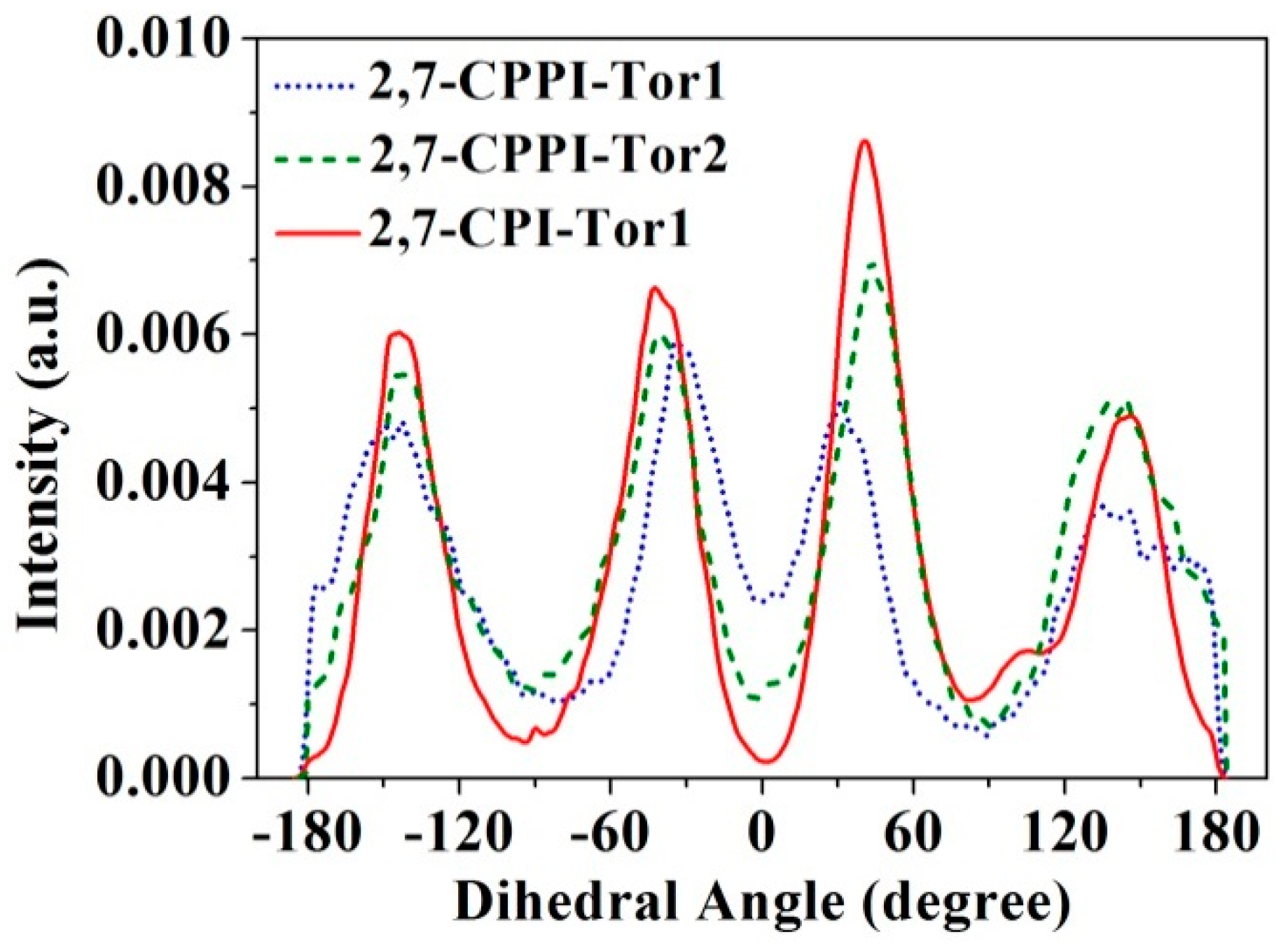
2.8.6. Dihedral Angle Profiles
2.8.7. Sorption Isotherm
3. Results and Discussion
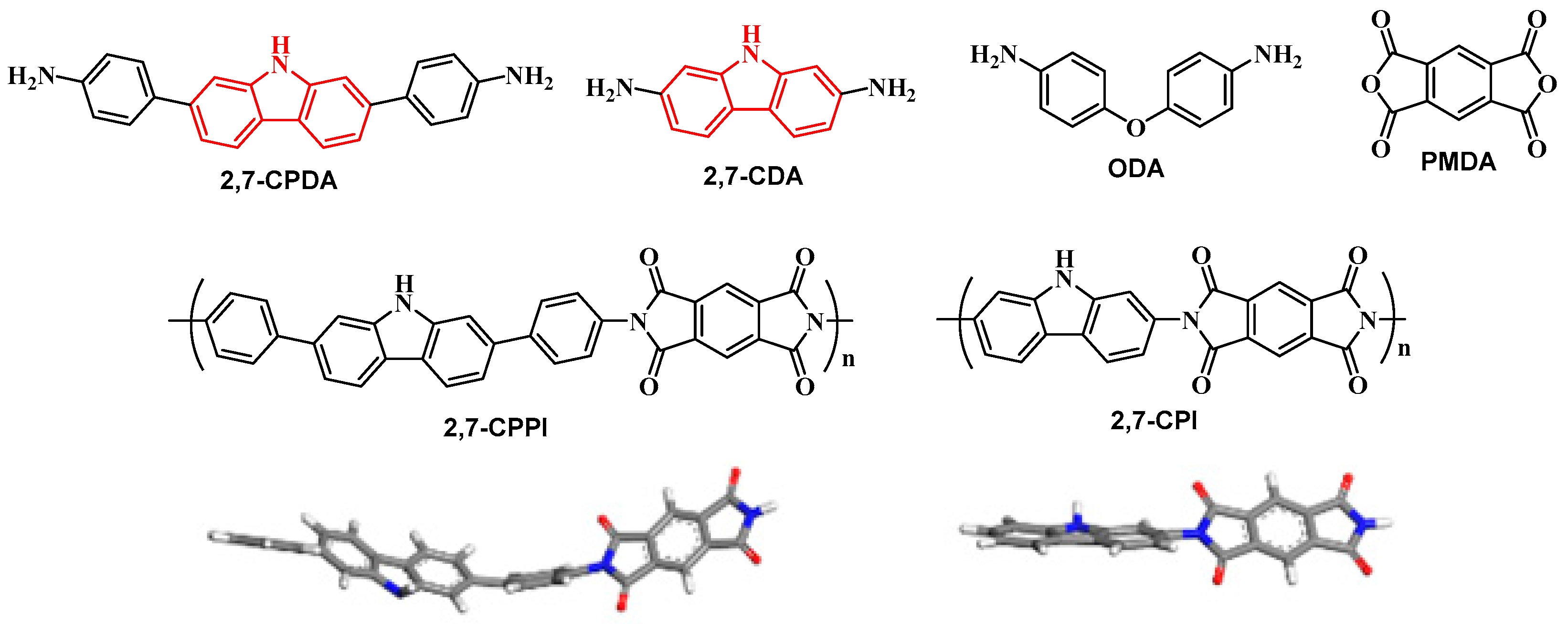
3.1. Synthesis and Characterization of Monomers and Polyimides
3.2. Thermal and Mechanical Properties
3.3. Barrier Properties
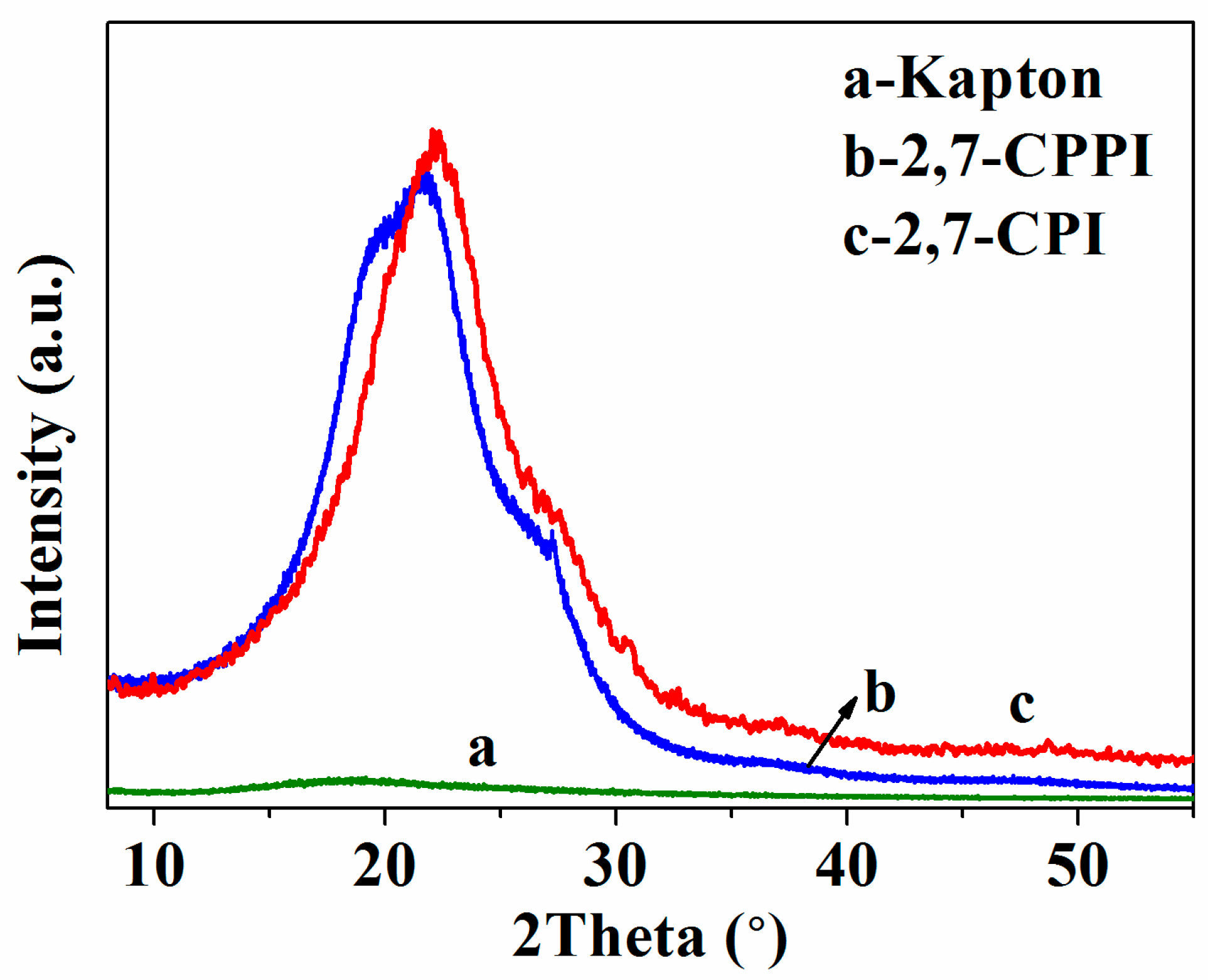
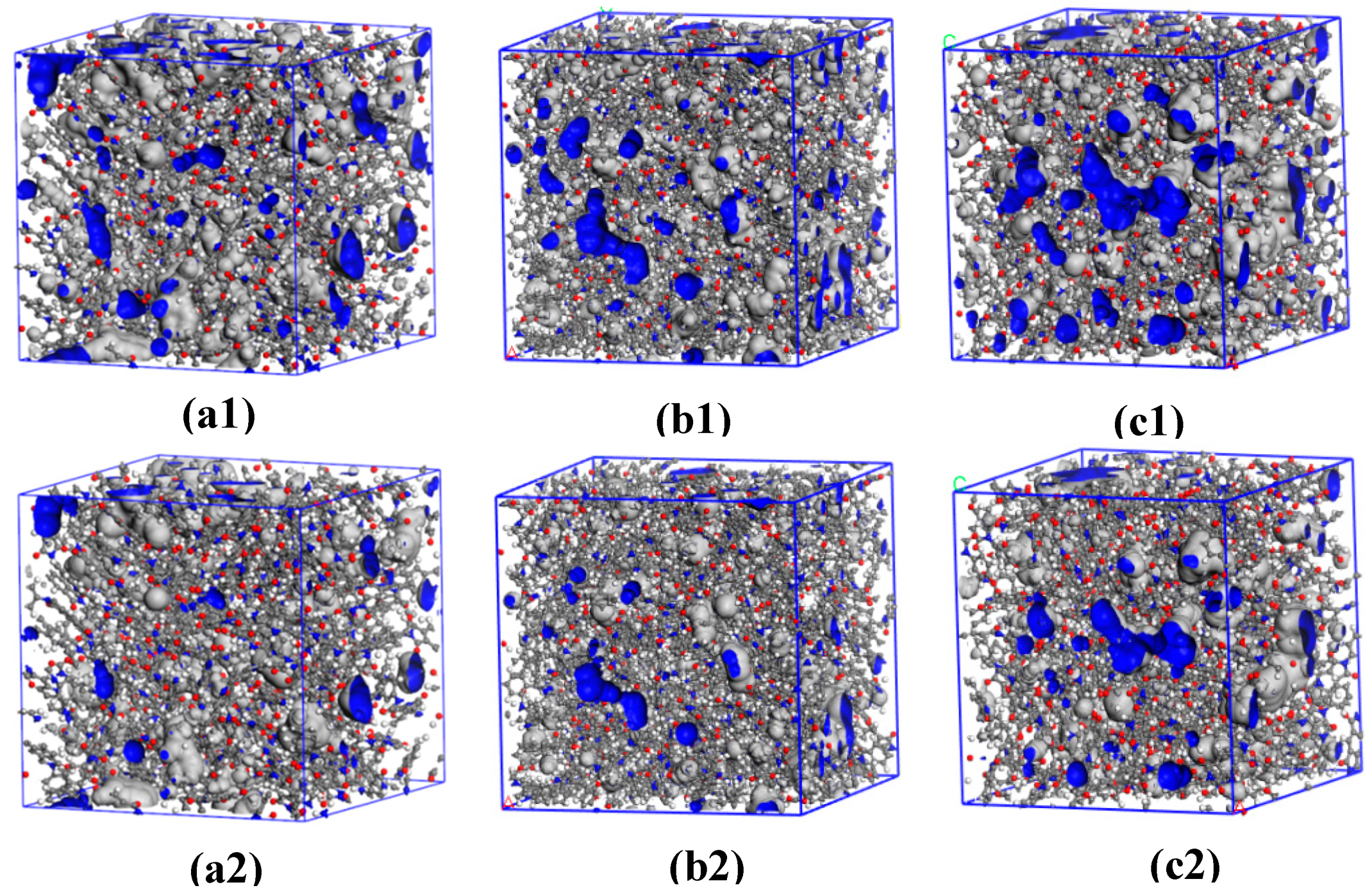
3.4. Aggregation Structures Analysis
3.5. Hydrogen Bonds Analysis
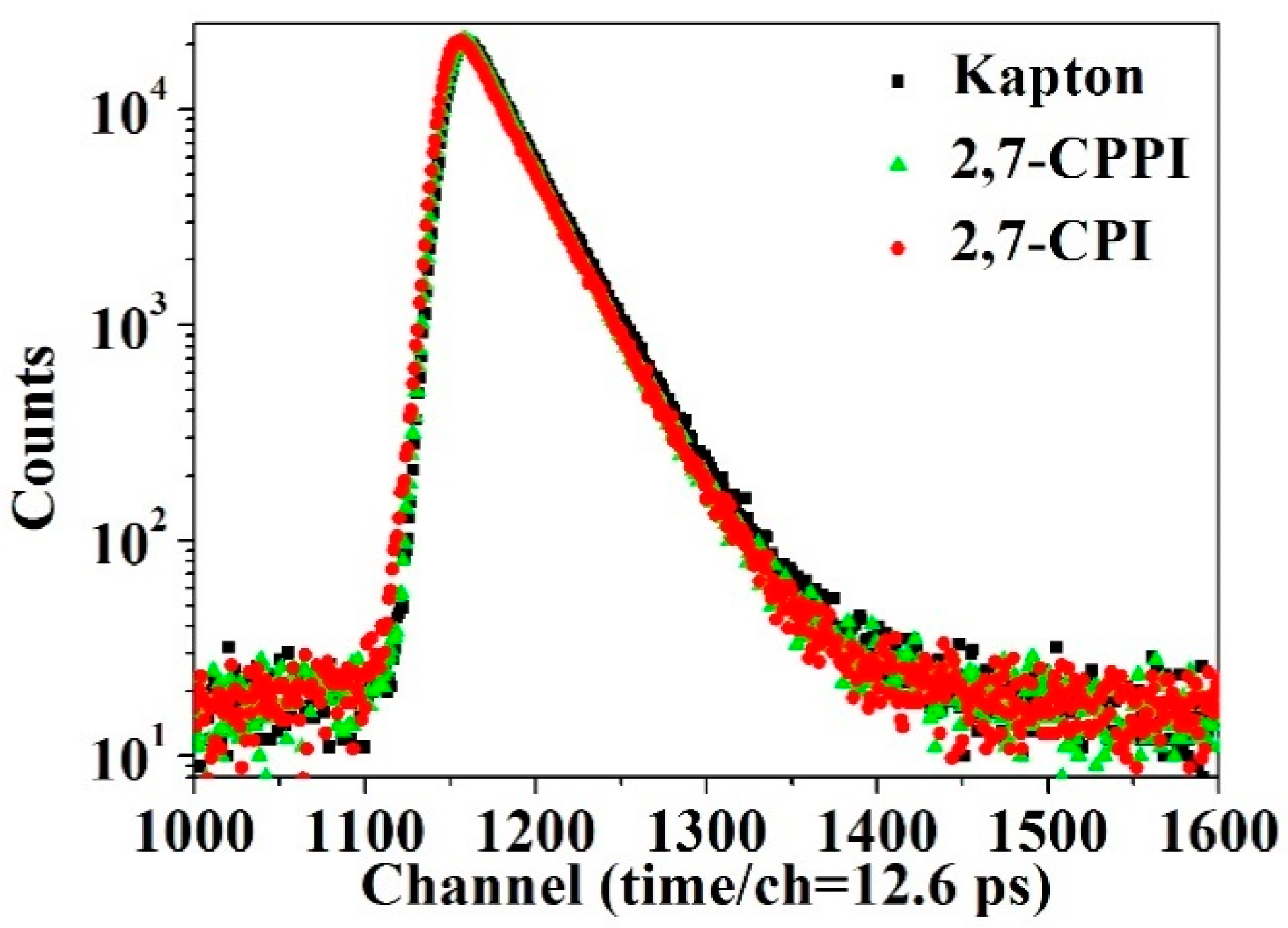
3.6. Free Volume and Cavity Size Distribution Analysis
3.7. Local Mobility of Polymer Chains
3.8. Gas Transport Behaviour
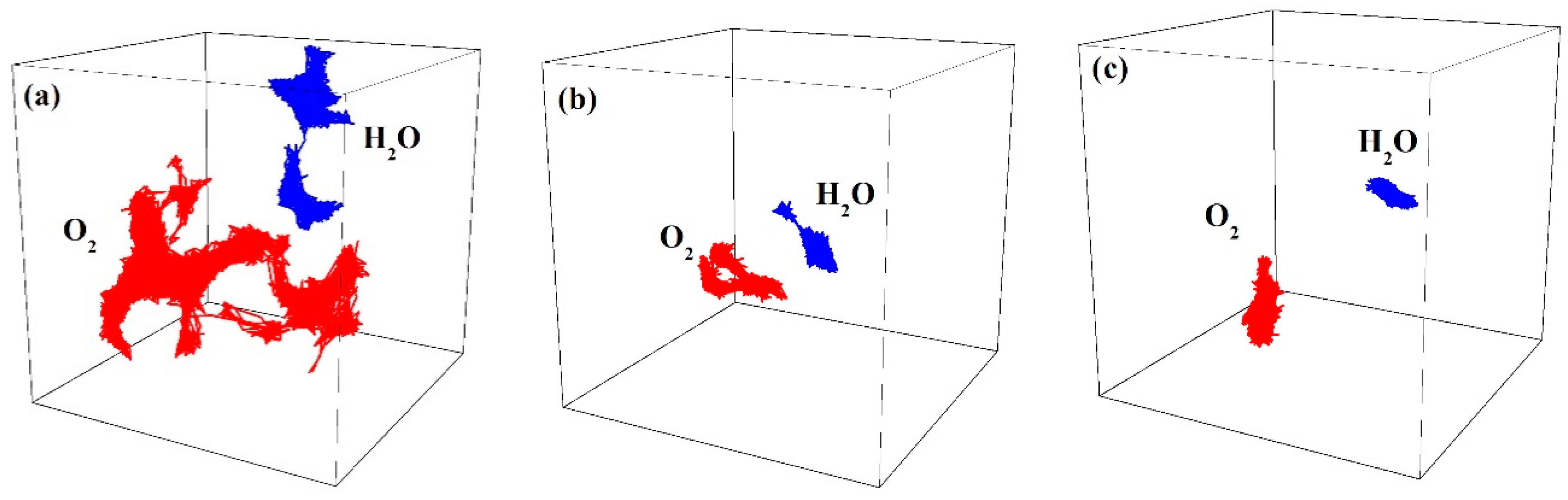
3.8.1. Gas Diffusion
3.8.2. Gas Solubility
3.8.3. Gas Permeability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huang, Y.; Hsiang, E.-L.; Deng, M.-Y.; Wu, S.-T. Mini-LED, Micro-LED and OLED displays: Present status and future perspectives. Light. Sci. Appl. 2020, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.-J.; Shen, Y.; Xie, F.-M.; Chen, J.-D.; Li, Y.-Q.; Tang, J.-X. Recent advances in organic light-emitting diodes: Toward smart lighting and displays. Mater. Chem. Front. 2020, 4, 788–820. [Google Scholar] [CrossRef]
- Choi, M.; Park, Y.J.; Sharma, B.K.; Bae, S.-R.; Kim, S.Y.; Ahn, J.-H. Flexible active-matrix organic light-emitting diode display enabled by MoS2thin-film transistor. Sci. Adv. 2018, 4, eaas8721. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-Y.; Su, P.-S.; Lee, C.-T. Micromesh-structured flexible polymer white organic light-emitting diodes using single emissive layer of blended polymer and quantum dots. Org. Electron. 2020, 82, 105722. [Google Scholar] [CrossRef]
- Mamo, M.D.; Noh, Y.-Y.; Shin, E.S. Self-aligned patterning of conductive films on plastic substrates for electrodes of flexible electronics. J. Mater. Chem. C 2017, 5, 10900–10906. [Google Scholar] [CrossRef]
- Macdonald, W.A.; Looney, M.K.; MacKerron, D.; Eveson, R.; Adam, R.; Hashimoto, K.; Rakos, K. Latest advances in substrates for flexible electronics. J. Soc. Inf. Disp. 2007, 15, 1075–1083. [Google Scholar] [CrossRef]
- Thomschke, M.; Reineke, S.; Lüssem, B.; Leo, K. Highly Efficient White Top-Emitting Organic Light-Emitting Diodes Comprising Laminated Microlens Films. Nano Lett. 2011, 12, 424–428. [Google Scholar] [CrossRef]
- Gardonio, S.; Gregoratti, L.; Melpignano, P.; Aballe, L.; Biondo, V.; Zamboni, R.; Murgia, M.; Caria, S.; Kiskinova, M. Degradation of organic light-emitting diodes under different environment at high drive conditions. Org. Electron. 2007, 8, 37–43. [Google Scholar] [CrossRef]
- Choi, M.-C.; Kim, Y.; Ha, C.-S. Polymers for flexible displays: From material selection to device applications. Prog. Polym. Sci. 2008, 33, 581–630. [Google Scholar] [CrossRef]
- Gao, X.; Lin, L.; Liu, Y.; Huang, X. LTPS TFT Process on Polyimide Substrate for Flexible AMOLED. J. Disp. Technol. 2015, 11, 666–669. [Google Scholar] [CrossRef]
- Salleo, A.; Street, R.A. Publisher’s Note: Light-induced bias stress reversal in polyfluorene thin-film transistors. J. Appl. Phys. 2003, 94, 4231. [Google Scholar] [CrossRef]
- Kao, S.-C.; Li, L.-J.; Hsieh, M.-C.; Zhang, S.; Tsai, P.-M.; Sun, Z.-Y.; Wang, D. 71-1: Invited Paper: The Challenges of Flexible OLED Display Development. SID Symp. Dig. Tech. Pap. 2017, 48, 1034–1037. [Google Scholar] [CrossRef]
- Tetsuka, H.; Ebina, T.; Tsunoda, T.; Nanjo, H.; Mizukami, F. Flexible organic electroluminescent devices based on transparent clay films. Nanotechnology 2007, 18, 355701. [Google Scholar] [CrossRef]
- Lee, J.; Lee, Y.; Kang, T.; Chu, H.; Kwag, J. Alleviation of abnormal NBTI phenomenon in LTPS TFTs on polyimide substrate for flexible AMOLED. J. Soc. Inf. Disp. 2020, 28, 333–341. [Google Scholar] [CrossRef]
- Park, J.-S.; Kim, T.; Stryakhilev, D.; Lee, J.-S.; An, S.-G.; Pyo, Y.-S.; Lee, D.-B.; Mo, Y.G.; Jin, D.U.; Chung, H.K. Flexible full color organic light-emitting diode display on polyimide plastic substrate driven by amorphous indium gallium zinc oxide thin-film transistors. Appl. Phys. Lett. 2009, 95, 13503. [Google Scholar] [CrossRef]
- Seo, S.-W.; Chung, H.K.; Chae, H.; Seo, S.J.; Cho, S.M. Flexible organic/inorganic moisture barrier using plasma-polymerized layer. Nano 2013, 8, 1350041. [Google Scholar] [CrossRef]
- Kwon, J.H.; Jeong, E.G.; Jeon, Y.; Kim, D.-G.; Lee, S.; Choi, K.C. Design of Highly Water Resistant, Impermeable, and Flexible Thin-Film Encapsulation Based on Inorganic/Organic Hybrid Layers. ACS Appl. Mater. Interfaces 2018, 11, 3251–3261. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Chang, C.-J.; Lu, H.-H.; Liao, Y.-F.; Tseng, I.-H. Properties of magnetron-sputtered moisture barrier layer on transparent polyimide/graphene nanocomposite film. Thin Solid Films 2013, 544, 324–330. [Google Scholar] [CrossRef]
- Sanaeepur, H.; Amooghin, A.E.; Bandehali, S.; Moghadassi, A.; Matsuura, T.; Van Der Bruggen, B. Polyimides in membrane gas separation: Monomer’s molecular design and structural engineering. Prog. Polym. Sci. 2019, 91, 80–125. [Google Scholar] [CrossRef]
- Madkour, T.; Madkour, T.M. Development of the molecular design rules of ultra-permeable poly[1-(trimethylsilyl)-1-propyne] membranes. Polymer 2000, 41, 7489–7497. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, J.; Tan, J.; Zeng, Y.; Liu, J.; Zhang, H.; Pei, Y.; Xiang, X.; Liu, Y. Intrinsic high-barrier polyimide with low free volume derived from a novel diamine monomer containing rigid planar moiety. Polymer 2017, 114, 289–297. [Google Scholar] [CrossRef]
- Yang, Y.; Nair, A.K.N.; Sun, S. Sorption and Diffusion of Methane and Carbon Dioxide in Amorphous Poly(alkyl acrylates): A Molecular Simulation Study. J. Phys. Chem. B 2020, 124, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Velioğlu, S.; Ahunbay, M.G.; Tantekin-Ersolmaz, S.B. Propylene/propane plasticization in polyimide membranes. J. Membr. Sci. 2016, 501, 179–190. [Google Scholar] [CrossRef]
- Sun, H. COMPASS: An ab Initio Force-Field Optimized for Condensed-Phase ApplicationsOverview with Details on Alkane and Benzene Compounds. J. Phys. Chem. B 1998, 102, 7338–7364. [Google Scholar] [CrossRef]
- McQuaid, M.J.; Sun, H.; Rigby, D. Development and validation of COMPASS force field parameters for molecules with aliphatic azide chains. J. Comput. Chem. 2003, 25, 61–71. [Google Scholar] [CrossRef]
- Zhu, J.; Zhao, X.; Liu, L.; Song, M.; Wu, S. Quantitative relationships between intermolecular interaction and damping parameters of irganox-1035/NBR hybrids: A combination of experiments, molecular dynamics simulations, and linear regression analyses. J. Appl. Polym. Sci. 2018, 135, 46202. [Google Scholar] [CrossRef]
- Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511. [Google Scholar] [CrossRef]
- Ban, S.; Vlugt, T.J. Zeolite microporosity studied by molecular simulation. Mol. Simul. 2009, 35, 1105–1115. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Gubbins, K.E. Fast Method for Computing Pore Size Distributions of Model Materials. Langmuir 2006, 22, 7726–7731. [Google Scholar] [CrossRef]
- Zeng, J.; Zhang, Y.; Dai, Y.; Chen, S. Molecular dynamics simulation of nitrobenzene in heterocyclic ionic liquids. J. Mol. Liq. 2014, 198, 274–279. [Google Scholar] [CrossRef]
- Pant, P.V.K.; Boyd, R.H. Molecular-dynamics simulation of diffusion of small penetrants in polymers. Macromolecules 1993, 26, 679–686. [Google Scholar] [CrossRef]
- Müller-Plathe, F. Molecular dynamics simulation of gas transport in amorphous polypropylene. J. Chem. Phys. 1992, 96, 3200–3205. [Google Scholar] [CrossRef]
- Akkermans, R.L.; Spenley, N.A.; Robertson, S.H. Monte Carlo methods in Materials Studio. Mol. Simul. 2013, 39, 1153–1164. [Google Scholar] [CrossRef]
- Fried, J.; Sadat-Akhavi, M.; Mark, J. Molecular simulation of gas permeability: Poly(2,6-dimethyl-1,4-phenylene oxide). J. Membr. Sci. 1998, 149, 115–126. [Google Scholar] [CrossRef]
- Liu, J.; Tan, J.-H.; Zeng, Y.; Liu, Y.; Zeng, K.-J.; Liu, Y.-J.; Wu, R.-M.; Chen, H. Synthesis and characterization of high-barrier polyimide containing rigid planar moieties and amide groups. Polym. Test. 2017, 61, 83–92. [Google Scholar] [CrossRef]
- Tseng, I.-H.; Liao, Y.-F.; Chiang, J.-C.; Tsai, M.-H. Transparent polyimide/graphene oxide nanocomposite with improved moisture barrier property. Mater. Chem. Phys. 2012, 136, 247–253. [Google Scholar] [CrossRef]
- Sykes, G.F.; Clair, A.K.S. The effect of molecular structure on the gas transmission rates of aromatic polyimides. J. Appl. Polym. Sci. 1986, 32, 3725–3735. [Google Scholar] [CrossRef]
- Inagaki, N.; Cech, V.; Narushima, K.; Takechi, Y. Oxygen and water vapor gas barrier poly(ethylene naphthalate) films by deposition of SiOx plasma polymers from mixture of tetramethoxysilane and oxygen. J. Appl. Polym. Sci. 2007, 104, 915–925. [Google Scholar] [CrossRef]
- Kim, S.-W.; Cha, S.-H. Thermal, mechanical, and gas barrier properties of ethylene-vinyl alcohol copolymer-based nanocomposites for food packaging films: Effects of nanoclay loading. J. Appl. Polym. Sci. 2013, 131, 40289. [Google Scholar] [CrossRef]
- Gargalaka, J.G., Jr.; Couto, R.A.A.; Constantino, V.R.L.; Toma, H.E.; Araki, K. Influence of the relative amounts of crystalline and amorphous phases on the mechanical properties of polyamide-6 nanocomposites. J. Appl. Polym. Sci. 2012, 125, 3239–3249. [Google Scholar] [CrossRef]
- Ebina, T.; Mizukami, S. Flexible Transparent Clay Films with Heat-Resistant and High Gas-Barrier Properties. Adv. Mater. 2007, 19, 2450–2453. [Google Scholar] [CrossRef]
- Bai, H.; Huang, C.; Xiu, H.; Zhang, Q.; Deng, H.; Wang, K.; Chen, F.; Fu, Q. Significantly Improving Oxygen Barrier Properties of Polylactide via Constructing Parallel-Aligned Shish-Kebab-Like Crystals with Well-Interlocked Boundaries. Biomacromolecules 2014, 15, 1507–1514. [Google Scholar] [CrossRef] [PubMed]
- La Cruz, D.S.-D.; Green, M.D.; Ye, Y.; Elabd, Y.A.; Long, T.E.; Winey, K.I. Correlating backbone-to-backbone distance to ionic conductivity in amorphous polymerized ionic liquids. J. Polym. Sci. Part. B Polym. Phys. 2011, 50, 338–346. [Google Scholar] [CrossRef]
- Mattozzi, A.; Hedenqvist, M.; Gedde, U. Diffusivity of n-hexane in poly(ethylene-stat-octene)s assessed by molecular dynamics simulation. Polymer 2007, 48, 5174–5180. [Google Scholar] [CrossRef]
- Fong, C.; Dong, A.W.; Hill, A.J.; Boyd, B.; Drummond, C.J. Positron annihilation lifetime spectroscopy (PALS): A probe for molecular organisation in self-assembled biomimetic systems. Phys. Chem. Chem. Phys. 2015, 17, 17527–17540. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.-S.; Chen, H.; Awad, S.; Yuan, J.-P.; Hung, W.-S.; Lee, K.-R.; Lai, J.-Y.; Hu, C.-C.; Jean, Y.C. Determination of Free-Volume Properties in Polymers Without Orthopositronium Components in Positron Annihilation Lifetime Spectroscopy. Macromolecules 2011, 44, 6818–6826. [Google Scholar] [CrossRef]
- Chen, Y.-R.; Chen, L.-H.; Chang, K.-S.; Chen, T.-H.; Lin, Y.-F.; Tung, K.-L. Structural characteristics and transport behavior of triptycene-based PIMs membranes: A combination study using ab initio calculation and molecular simulations. J. Membr. Sci. 2016, 514, 114–124. [Google Scholar] [CrossRef]
- Park, S.; Lee, A.S.; Do, Y.S.; Kim, J.F.; Hwang, S.S.; Lee, Y.M.; Lee, J.-H.; Lee, J.S. Side-chain engineering of ladder-structured polysilsesquioxane membranes for gas separations. J. Membr. Sci. 2016, 516, 202–214. [Google Scholar] [CrossRef]
- Neyertz, S.; Brown, D.; Pandiyan, S.; Van Der Vegt, N.F.A. Carbon Dioxide Diffusion and Plasticization in Fluorinated Polyimides. Macromol. 2010, 43, 7813–7827. [Google Scholar] [CrossRef]
- Mozaffari, F.; Eslami, H.; Moghadasi, J. Molecular dynamics simulation of diffusion and permeation of gases in polystyrene. Polymer 2010, 51, 300–307. [Google Scholar] [CrossRef]
- Zhang, K.; Yu, Q.; Zhu, L.; Liu, S.; Chi, Z.; Chen, X.; Zhang, Y.; Xu, J. The Preparations and Water Vapor Barrier Properties of Polyimide Films Containing Amide Moieties. Polymer 2017, 9, 677. [Google Scholar] [CrossRef] [PubMed]
- Petropoulos, J.H. A generalized, topologically consistent, dual-mode transport model for glassy polymer-gas systems. J. Polym. Sci. Part B 1989, 27, 603–620. [Google Scholar] [CrossRef]






| PI | Tga (°C) | Td5% (°C) | Td10% (°C) | CTE b (ppm∙K−1) | Tensile Strength (MPa) | Tensile Modulus (GPa) |
|---|---|---|---|---|---|---|
| Kapton c | 374 | 550 | 571 | 38.0 | 121 ± 3.0 | 2.3 ± 0.2 |
| 2,7-CPPI d | 437 | 556 | 580 | 2.89 | 143.8 ± 3.5 | 4.5 ± 0.2 |
| 2,7-CPI | 467 | 550 | 590 | 3.40 | 134 ± 3.0 | 5.7 ± 0.2 |
| PIs | WVP (g·mil·m−2·day−1) | OP (cm3·mil·m−2·day−1) | WVTR (g·m−2·day−1) | OTR (cm3·m−2·day−1) |
|---|---|---|---|---|
| Kapton a | 114.5 ± 2.12 | 330.7 ± 2.50 | 38.8 ± 0.72 | 112.0 ± 0.80 |
| 2,7-CPPI a | 0.3 ± 0.02 | 0.5 ± 0.03 | 0.1 ± 0.01 | 0.2 ± 0.01 |
| 2,7-CPI | 0.15 ± 0.01 | 0.41 ± 0.02 | 0.05 ± 0.003 | 0.14 ± 0.007 |
| PIs | Density (g.cm−3) | 2θ (°) | d-Spacing (Å) | CED (J/cm3) | N a (H-bonds) | Rb (Å) | FFVb (%) | FFVc (O2,%) | FFVc (H2O,%) | FFV0c (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Kapton | 1.42 | 18.64 | 4.76 | 454 | 0 | 2.60 | 11.50 | 10.48 | 15.80 | 38.51 |
| 2,7-CPPI | 1.57 | 21.91 | 4.05 | 570 | 49 | 2.14 | 6.82 | 6.29 | 11.51 | 35.33 |
| 2,7-CPI | 1.58 | 22.11 | 4.01 | 613 | 60 | 2.14 | 6.09 | 5.48 | 10.33 | 34.16 |
| PIs | D a | S b | P c | |||
|---|---|---|---|---|---|---|
| H2O | O2 | H2O | O2 | H2O | O2 | |
| Kapton | 12.8 | 89.4 | 3.92 | 0.056 | 50.18 | 5.00 |
| 2,7-CPPI | 3.0 | 6.13 | 0.64 | 0.013 | 1.92 | 0.08 |
| 2,7-CPI | 2.1 | 5.32 | 0.27 | 0.008 | 0.57 | 0.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Tang, A.; Tan, J.; Zhao, X.; Chen, C.; Wu, D.; Li, Y.; He, P.; Zhang, H. High-Barrier Polyimide Containing Carbazole Moiety: Synthesis, Gas Barrier Properties, and Molecular Simulations. Polymers 2020, 12, 2048. https://doi.org/10.3390/polym12092048
Liu Y, Tang A, Tan J, Zhao X, Chen C, Wu D, Li Y, He P, Zhang H. High-Barrier Polyimide Containing Carbazole Moiety: Synthesis, Gas Barrier Properties, and Molecular Simulations. Polymers. 2020; 12(9):2048. https://doi.org/10.3390/polym12092048
Chicago/Turabian StyleLiu, Yiwu, Ao Tang, Jinghua Tan, Xianqing Zhao, Chengliang Chen, Ding Wu, Yuhui Li, Pan He, and Hailiang Zhang. 2020. "High-Barrier Polyimide Containing Carbazole Moiety: Synthesis, Gas Barrier Properties, and Molecular Simulations" Polymers 12, no. 9: 2048. https://doi.org/10.3390/polym12092048
APA StyleLiu, Y., Tang, A., Tan, J., Zhao, X., Chen, C., Wu, D., Li, Y., He, P., & Zhang, H. (2020). High-Barrier Polyimide Containing Carbazole Moiety: Synthesis, Gas Barrier Properties, and Molecular Simulations. Polymers, 12(9), 2048. https://doi.org/10.3390/polym12092048







